The Hexosamine Biosynthetic Pathway as a Therapeutic Target after Cartilage Trauma: Modification of Chondrocyte Survival and Metabolism by Glucosamine Derivatives and PUGNAc in an Ex Vivo Model
Abstract
:1. Introduction
2. Results
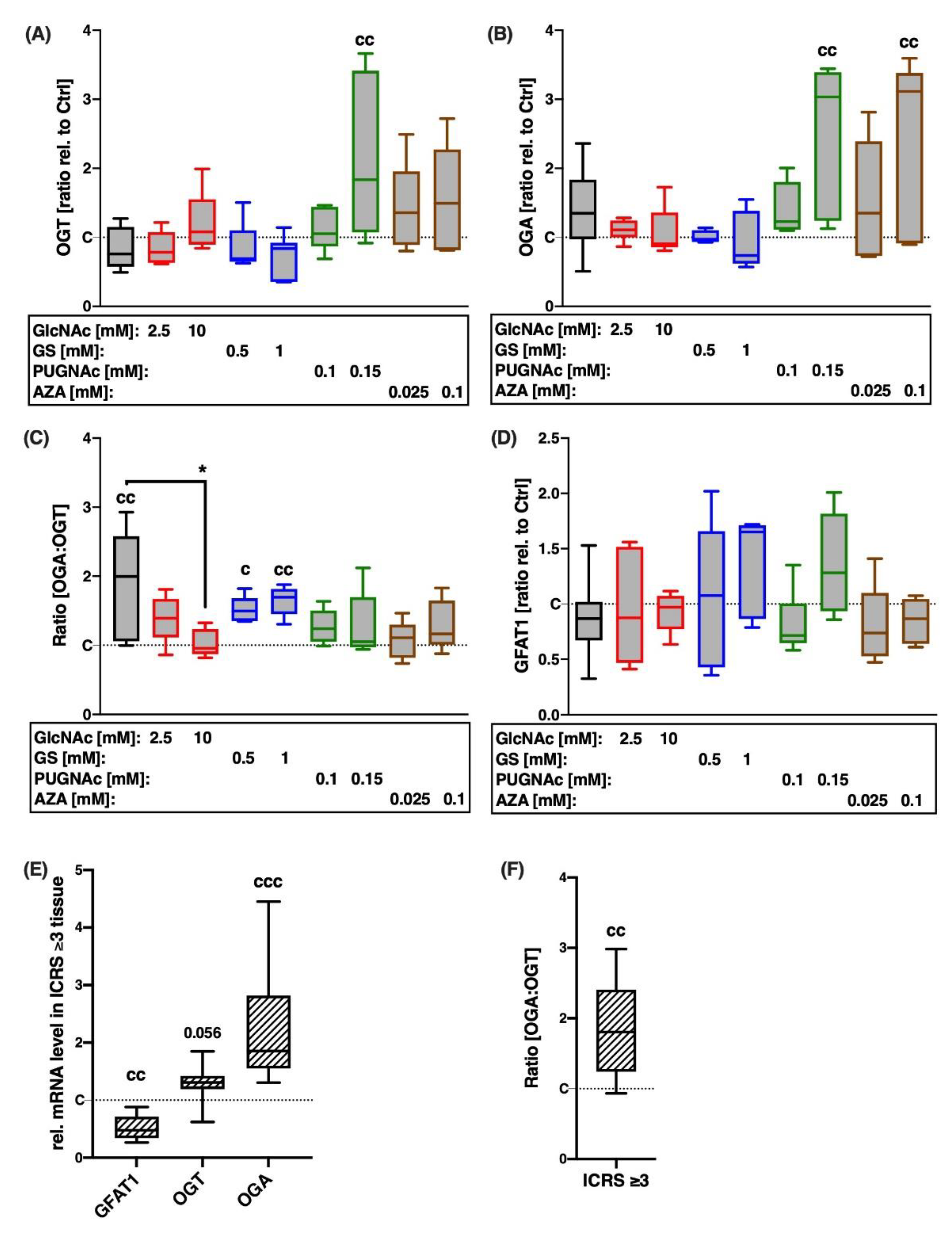
2.1. Gene Expression of HBP-Related Enzymes Is Altered after Cartilage Trauma and in Highly Degenerated Tissue
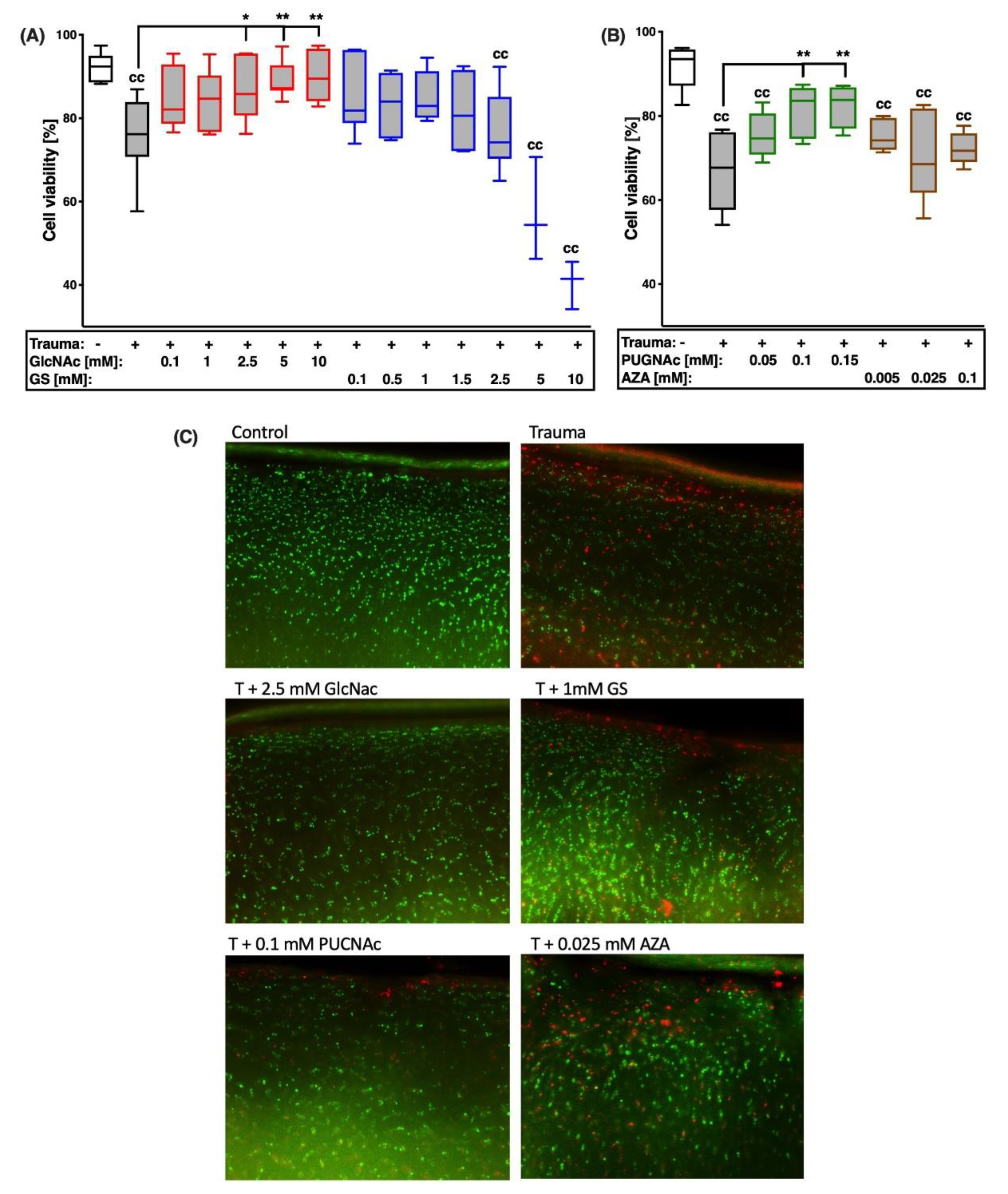
2.2. GlcNAc and PUGNAc Exert Cell Protective Effects after Cartilage Trauma
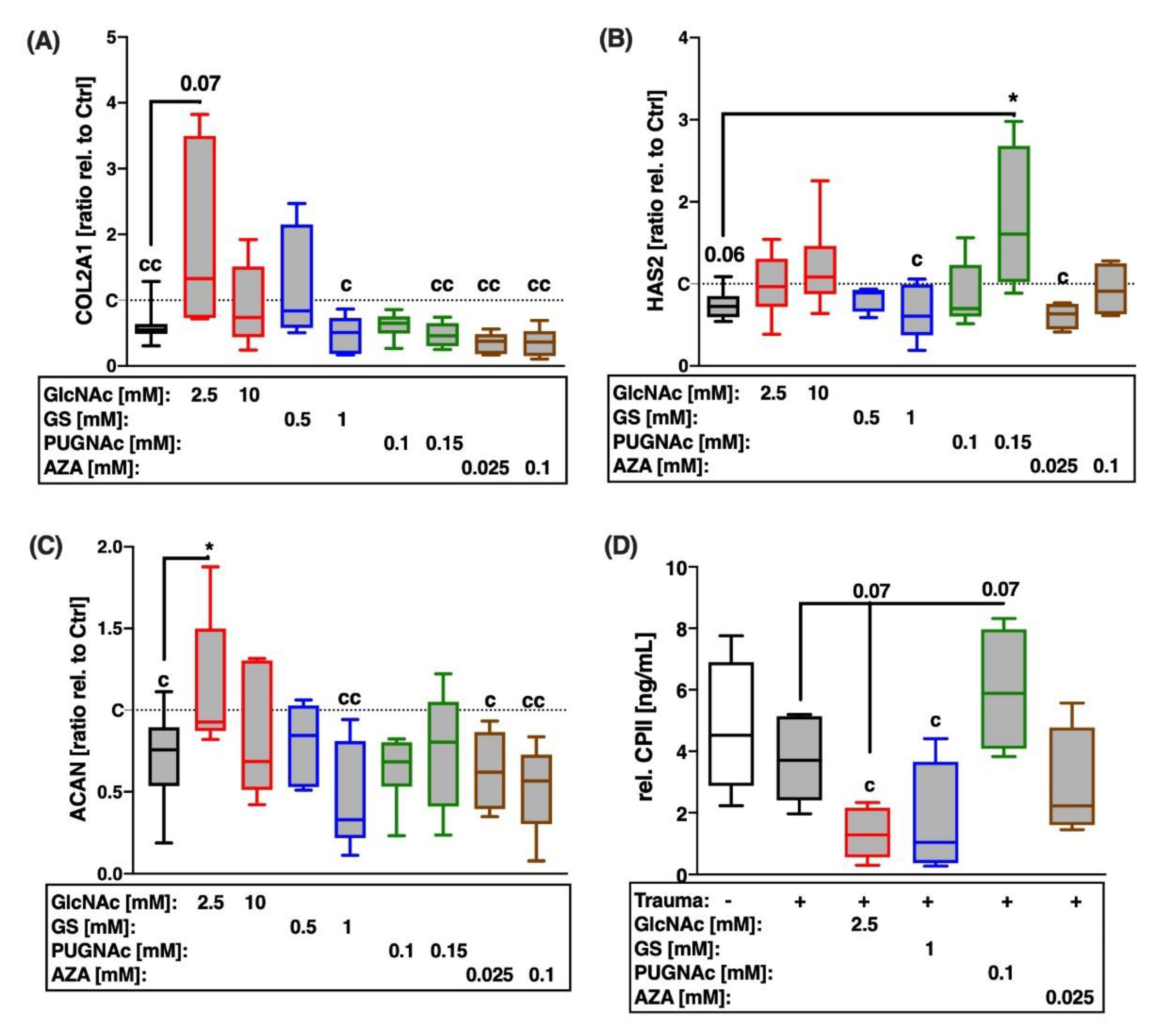
2.3. While PUGNAc Revealed Chondroanabolic Effects, Glucosamine Derivatives GlcNAc and GS Suppressed Type II Collagen Synthesis after Cartilage Trauma
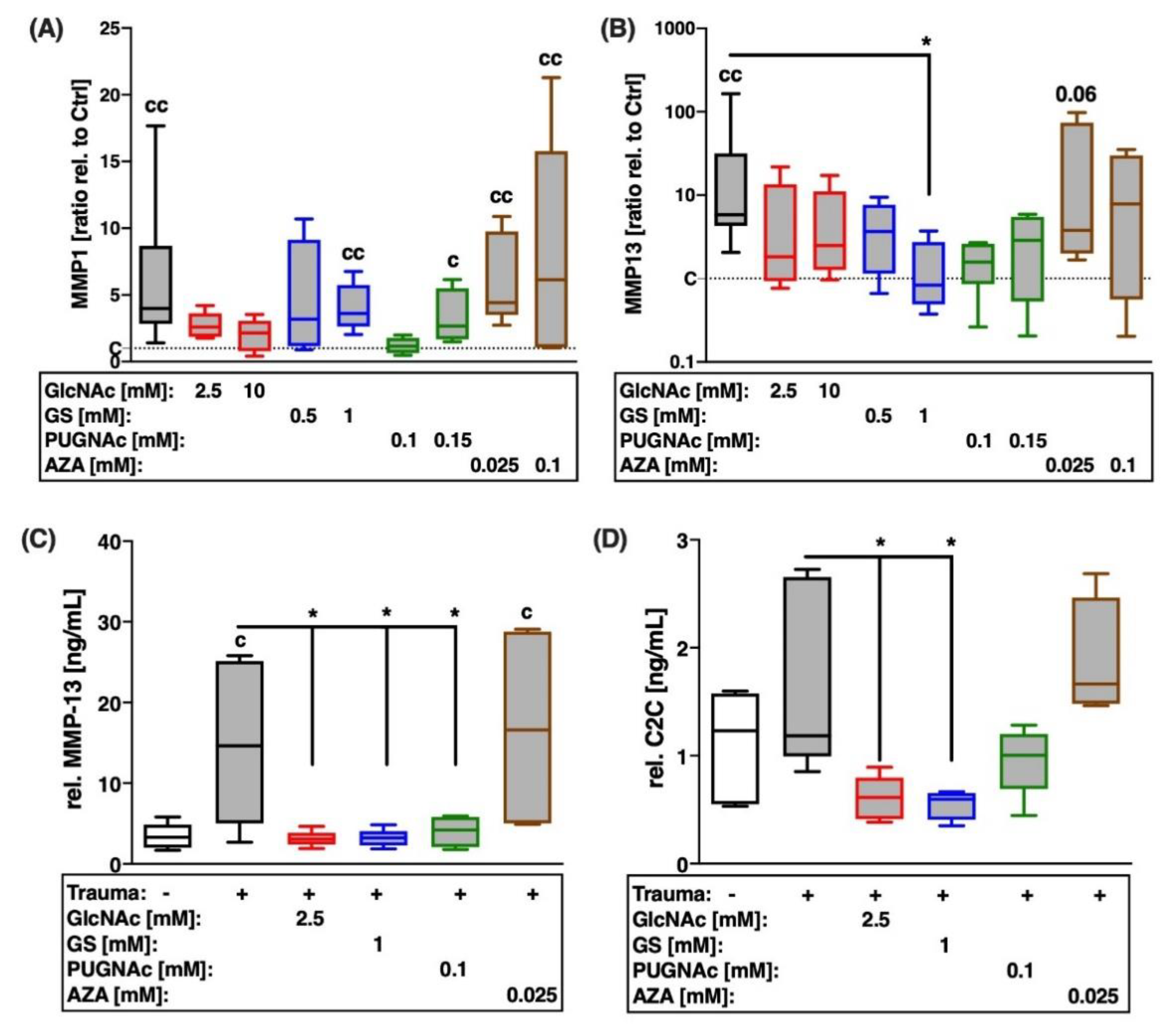
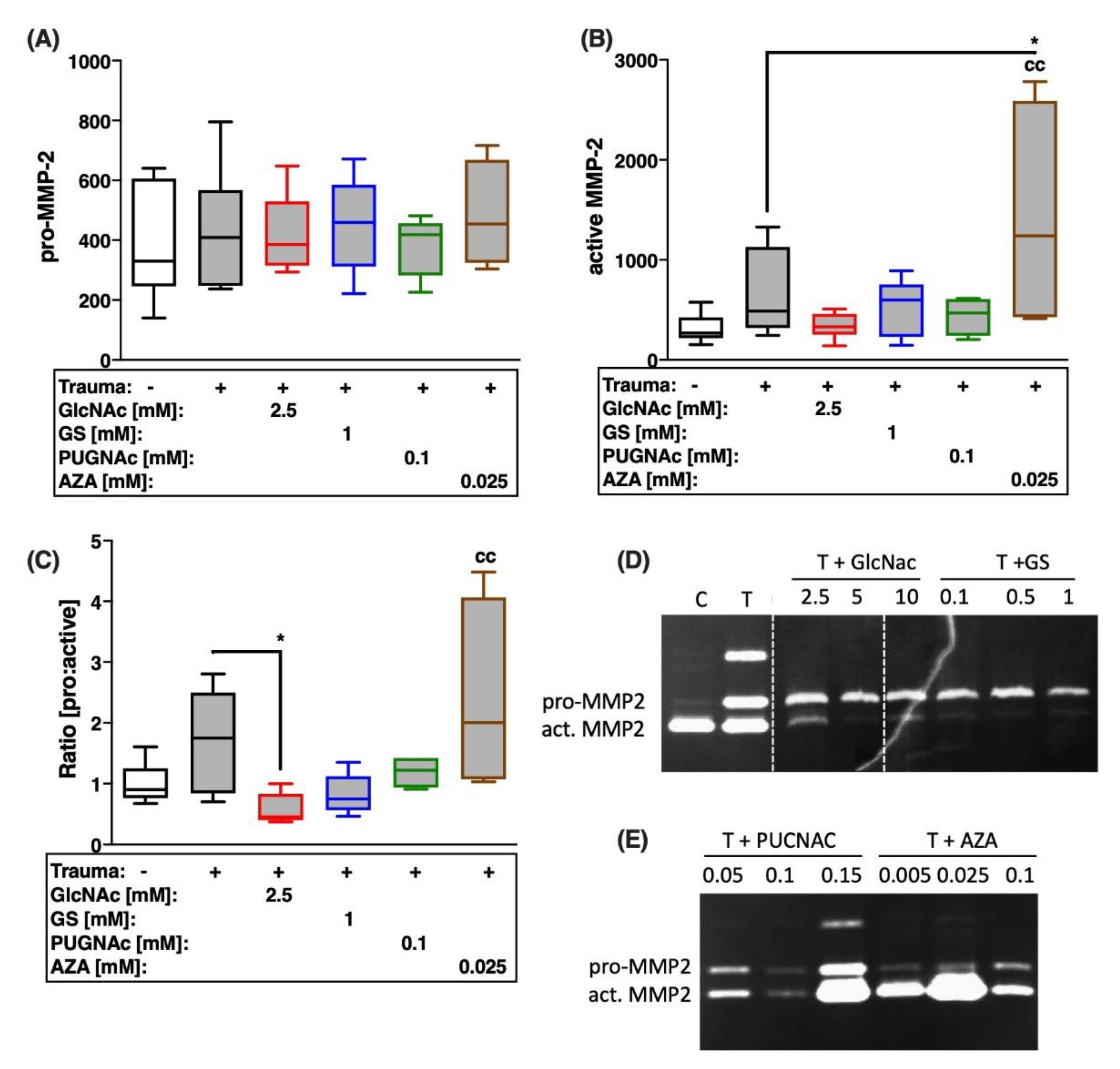
2.4. Trauma-Induced Expression of MMPs and Subsequent Type II Collagen Degradation Are Markedly Decreased after Treatment with Glucosamines or PUGNAc
2.5. Trauma-Induced Expression of Aggrecanases and Subsequent Aggrecan Degradation Are Largely Decreased after Treatment with Glucosamines or PUGNAc
3. Discussion
4. Materials and Methods
4.1. Specimen Preparation and Cultivation Conditions
4.2. Impact Loading and Subsequent Treatment
4.3. Live/Dead Cell Cytotoxicity Assay
4.4. mRNA Isolation and cDNA Synthesis
4.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.6. Culture Media Analysis by Means of Commercial ELISA Kits
4.7. Gelatin Zymography
4.8. Safranin-O Staining
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hunter, D.J.; Schofield, D.; Callander, E. The individual and socioeconomic impact of osteoarthritis. Nat. Rev. Rheumatol. 2014, 10, 437–441. [Google Scholar] [CrossRef]
- Thomas, A.C.; Hubbard-Turner, T.; Wikstrom, E.A.; Palmieri-Smith, R.M. Epidemiology of Posttraumatic Osteoarthritis. J. Athl. Train. 2017, 52, 491–496. [Google Scholar] [CrossRef] [Green Version]
- Riegger, J.; Brenner, R.E. Pathomechanisms of Posttraumatic Osteoarthritis: Chondrocyte Behavior and Fate in a Precarious Environment. Int. J. Mol. Sci. 2020, 21, 1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruyère, O.; Burlet, N.; Delmas, P.D.; Rizzoli, R.; Cooper, C.; Reginster, J.Y. Evaluation of symptomatic slow-acting drugs in osteoarthritis using the GRADE system. BMC Musculoskelet. Disord. 2008, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.; Bello, L.; Chávez, M.; Añez, R.; Rojas, J.; Bermúdez, V. Glucosamine for Osteoarthritis: Biological Effects, Clinical Efficacy, and Safety on Glucose Metabolism. Arthritis 2014, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Shang, J.; Yao, Y.; Yin, X.; Liu, M.; Liu, H.; Zhou, Y. O-GlcNAcylation: A bridge between glucose and cell differentiation. J. Cell. Mol. Med. 2016, 20, 769–781. [Google Scholar] [CrossRef]
- Kiani, C.; Chen, L.; Wu, Y.J.; Yee, A.J.; Yang, B.B. Structure and function of aggrecan. Cell Res. 2002, 12, 19–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatham, J.C.; Nöt, L.G.; Fülöp, N.; Marchase, R.B. Hexosamine biosynthesis and protein O-glycosylation: The first line of defense against stress, ischemia, and trauma. Shock 2008, 29, 431–440. [Google Scholar] [CrossRef]
- Hart, G.W.; Slawson, C.; Ramirez-Correa, G.; Lagerlof, O. Cross Talk Between O-GlcNAcylation and Phosphorylation: Roles in Signaling, Transcription, and Chronic Disease. Annu. Rev. Biochem. 2011, 80, 825–858. [Google Scholar] [CrossRef] [Green Version]
- Tardio, L.; Andres, J.; Herrero-Beaumont, G.; Largo, R. Protein o-linked n-acetylglucosamine levels in the cartilage of patients with knee osteoarthritis. Ann. Rheum. Dis. 2011, 70, A86. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, P.S.; Lagerlöf, O.; Hart, G.W. Roles of O-GlcNAc in chronic diseases of aging. Mol. Aspects Med. 2016, 51, 1–15. [Google Scholar] [CrossRef]
- Tardio, L.; Andrés-Bergós, J.; Zachara, N.E.; Larrañaga-Vera, A.; Rodriguez-Villar, C.; Herrero-Beaumont, G. O-linked N-acetylglucosamine (O-GlcNAc) protein modification is increased in the cartilage of patients with knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 259–263. [Google Scholar] [CrossRef] [Green Version]
- Derfoul, A.; Miyoshi, A.D.; Freeman, D.E.; Tuan, R.S. Glucosamine promotes chondrogenic phenotype in both chondrocytes and mesenchymal stem cells and inhibits MMP-13 expression and matrix degradation. Osteoarthr. Cartil. 2007, 15, 646–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Zheng, W.; Chen, H.; Shao, X.; Lin, P.; Liu, X.; Ye, H. Glucosamine promotes chondrocyte proliferation via the Wnt/β-catenin signaling pathway. Int. J. Mol. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Uitterlinden, E.J.; Jahr, H.; Koevoet, J.L.M.; Jenniskens, Y.M.; Bierma-Zeinstra, S.M.A.; DeGroot, J.; Van Osch, G.J.V.M. Glucosamine decreases expression of anabolic and catabolic genes in human osteoarthritic cartilage explants. Osteoarthr. Cartil. 2006, 14, 250–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valvason, C.; Musacchio, E.; Pozzuoli, A.; Ramonda, R.; Aldegheri, R.; Punzi, L. Influence of glucosamine sulphate on oxidative stress in human osteoarthritic chondrocytes: Effects on HO-1, p22Phox and iNOS expression. Rheumatology 2008, 47, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Altman, R.D.; Abramson, S.; Bruyere, O.; Clegg, D.; Herrero-Beaumont, G.; Maheu, E.; Reginster, J.Y. Commentary: Osteoarthritis of the knee and glucosamine. Osteoarthr. Cartil. 2006, 14, 963–966. [Google Scholar] [CrossRef] [Green Version]
- Fransen, M.; Agaliotis, M.; Nairn, L.; Votrubec, M.; Bridgett, L.; Su, S.; Day, R. Glucosamine and chondroitin for knee osteoarthritis: A double-blind randomised placebo-controlled clinical trial evaluating single and combination regimens. Ann. Rheum. Dis. 2015, 74, 851–858. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.-H.; Park, S.; Ahn, C.; Song, J.; Kim, D.; Jin, E.-J. Beneficial reward-to-risk action of glucosamine during pathogenesis of osteoarthritis. Eur. J. Med. Res. 2015, 20, 89. [Google Scholar] [CrossRef] [Green Version]
- Jordan, K.M. EULAR Recommendations 2003: An evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann. Rheum. Dis. 2003, 62, 1145–1155. [Google Scholar] [CrossRef]
- Jerosch, J. Effects of Glucosamine and Chondroitin Sulfate on Cartilage Metabolism in OA: Outlook on Other Nutrient Partners Especially Omega-3 Fatty Acids. Int. J. Rheumatol. 2011, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Caramés, B.; Taniguchi, N.; Otsuki, S.; Blanco, F.J.; Lotz, M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010, 62, 791–801. [Google Scholar] [CrossRef] [Green Version]
- Caramés, B.; Taniguchi, N.; Seino, D.; Blanco, F.J.; D’Lima, D.; Lotz, M. Mechanical injury suppresses autophagy regulators and pharmacologic activation of autophagy results in chondroprotection. Arthritis Rheum. 2012, 64, 1182–1192. [Google Scholar] [CrossRef] [Green Version]
- Caramés, B.; Kiosses, W.B.; Akasaki, Y.; Brinson, D.C.; Eap, W.; Koziol, J.; Lotz, M.K. Glucosamine Activates Autophagy In Vitro and In Vivo: Glucosamine-Induced Activation of Autophagy. Arthritis Rheum. 2013, 65, 1843–1852. [Google Scholar] [CrossRef]
- de Mattei, M.; Pellati, A.; Pasello, M.; de Terlizzi, F.; Massari, L.; Gemmati, D.; Caruso, A. High doses of glucosamine-HCl have detrimental effects on bovine articular cartilage explants cultured in vitro. Osteoarthr. Cartil. 2002, 10, 816–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, C.; Wang, L.; Zhu, X.; Lin, W.; Chen, X.; Huang, Z.; Yang, S. Glucosamine promotes osteoblast proliferation by modulating autophagy via the mammalian target of rapamycin pathway. Biomed. Pharm. 2018, 99, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Shikhman, A.R.; Brinson, D.C.; Valbracht, J.; Lotz, M.K. Differential metabolic effects of glucosamine and N-acetylglucosamine in human articular chondrocytes. Osteoarthr. Cartil. 2009, 17, 1022–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schegg, B.; Hülsmeier, A.J.; Rutschmann, C.; Maag, C.; Hennet, T. Core Glycosylation of Collagen Is Initiated by Two β(1-O)Galactosyltransferases. Mol. Cell. Biol. 2009, 29, 943–952. [Google Scholar] [CrossRef] [Green Version]
- Gouze, J.-N.; Gouze, E.; Popp, M.P.; Bush, M.L.; Dacanay, E.A.; Kay, J.D.; Ghivizzani, S.C. Exogenous glucosamine globally protects chondrocytes from the arthritogenic effects of IL-1beta. Arthritis Res. Ther. 2006, 8, R173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandy, J.D.; Gamett, D.; Thompson, V.; Verscharen, C. Chondrocyte-mediated catabolism of aggrecan: Aggrecanase-dependent cleavage induced by interleukin-1 or retinoic acid can be inhibited by glucosamine. Biochem. J. 1998, 335, 59–66. [Google Scholar] [CrossRef] [Green Version]
- d’Abusco, S.A.; Calamia, V.; Cicione, C.; Grigolo, B.; Politi, L.; Scandurra, R. Glucosamine affects intracellular signalling through inhibition of mitogen-activated protein kinase phosphorylation in human chondrocytes. Arthritis Res. Ther. 2007, 9, R104. [Google Scholar] [CrossRef] [Green Version]
- Shikhman, A.R.; Kuhn, K.; Alaaeddine, N.; Lotz, M. N-Acetylglucosamine prevents IL-1β-mediated activation of human chondrocytes. J. Immunol. 2001, 166, 5155–5160. [Google Scholar] [CrossRef] [Green Version]
- Andrés-Bergós, J.; Tardio, L.; Larranaga-Vera, A.; Gómez, R.; Herrero-Beaumont, G.; Largo, R. The increase in O-linked N-acetylglucosamine protein modification stimulates chondrogenic differentiation both in vitro and in vivo. J. Biol. Chem. 2012, 287, 33615–33628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Feng, Z.; Zou, X.; Cao, K.; Xu, J.; Liu, J. Aging leads to elevation of O-GlcNAcylation and disruption of mitochondrial homeostasis in retina. Oxid. Med. Cell. Longev. 2014, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groves, J.A.; Lee, A.; Yildirir, G.; Zachara, N.E. Dynamic O-GlcNAcylation and its roles in the cellular stress response and homeostasis. Cell Stress Chaperones 2013, 18, 535–558. [Google Scholar] [CrossRef] [Green Version]
- Martinez, M.R.; Dias, T.B.; Natov, P.S.; Zachara, N.E. Stress-induced O-GlcNAcylation: An adaptive process of injured cells. Biochem. Soc. Trans. 2017, 45, 237–249. [Google Scholar] [CrossRef]
- Champattanachai, V.; Marchase, R.B.; Chatham, J.C. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc. Am. J. Physiol. Cell Physiol. 2007, 292, C178–C187. [Google Scholar] [CrossRef] [Green Version]
- Ngoh, G.A.; Facundo, H.T.; Hamid, T.; Dillmann, W.; Zachara, N.E.; Jones, S.P. Unique Hexosaminidase Reduces Metabolic Survival Signal and Sensitizes Cardiac Myocytes to Hypoxia/Reoxygenation Injury. Circ. Res. 2009, 104, 41–49. [Google Scholar] [CrossRef]
- Honda, A.; Tsuboi, I.; Kimata, K.; Hirabayashi, Y.; Yamada, K.; Mori, Y. Abnormal synthesis of cartilage-characteristic proteoglycan in azaserine-induced micromelial limbs. Biochem. J. 1989, 261, 627–635. [Google Scholar] [CrossRef] [Green Version]
- Riegger, J.; Joos, H.; Palm, H.-G.; Friemert, B.; Reichel, H.; Ignatius, A.; Brenner, R.E. Antioxidative therapy in an ex vivo human cartilage trauma-model: Attenuation of trauma-induced cell loss and ECM-destructive enzymes by N-acetyl cysteine. Osteoarthr. Cartil. 2016, 24, 2171–2180. [Google Scholar] [CrossRef] [PubMed]
- Riegger, J.; Joos, H.; Palm, H.-G.; Friemert, B.; Reichel, H.; Ignatius, A.; Brenner, R.E. Striking a new path in reducing cartilage breakdown: Combination of antioxidative therapy and chondroanabolic stimulation after blunt cartilage trauma. J. Cell. Mol. Med. 2018, 22, 77–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riegger, J.; Baumert, J.; Zaucke, F.; Brenner, R.E. The Hexosamine Biosynthetic Pathway as a Therapeutic Target after Cartilage Trauma: Modification of Chondrocyte Survival and Metabolism by Glucosamine Derivatives and PUGNAc in an Ex Vivo Model. Int. J. Mol. Sci. 2021, 22, 7247. https://doi.org/10.3390/ijms22147247
Riegger J, Baumert J, Zaucke F, Brenner RE. The Hexosamine Biosynthetic Pathway as a Therapeutic Target after Cartilage Trauma: Modification of Chondrocyte Survival and Metabolism by Glucosamine Derivatives and PUGNAc in an Ex Vivo Model. International Journal of Molecular Sciences. 2021; 22(14):7247. https://doi.org/10.3390/ijms22147247
Chicago/Turabian StyleRiegger, Jana, Julia Baumert, Frank Zaucke, and Rolf E. Brenner. 2021. "The Hexosamine Biosynthetic Pathway as a Therapeutic Target after Cartilage Trauma: Modification of Chondrocyte Survival and Metabolism by Glucosamine Derivatives and PUGNAc in an Ex Vivo Model" International Journal of Molecular Sciences 22, no. 14: 7247. https://doi.org/10.3390/ijms22147247
APA StyleRiegger, J., Baumert, J., Zaucke, F., & Brenner, R. E. (2021). The Hexosamine Biosynthetic Pathway as a Therapeutic Target after Cartilage Trauma: Modification of Chondrocyte Survival and Metabolism by Glucosamine Derivatives and PUGNAc in an Ex Vivo Model. International Journal of Molecular Sciences, 22(14), 7247. https://doi.org/10.3390/ijms22147247





