Knockdown of a β-Adrenergic-Like Octopamine Receptor Affects Locomotion and Reproduction of Tribolium castaneum
Abstract
:1. Introduction
2. Results
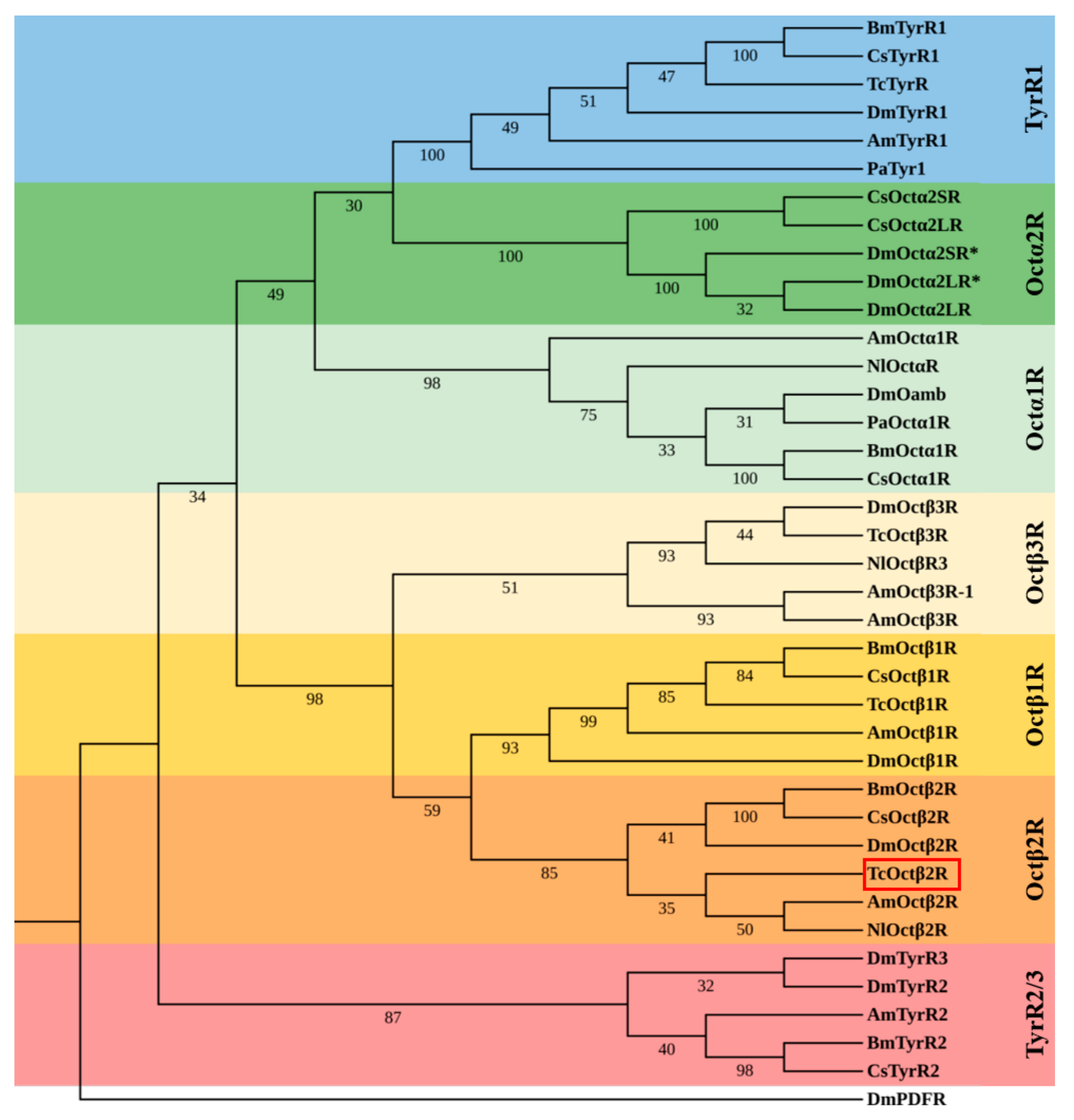
2.1. Molecular Cloning and Sequence Analysis
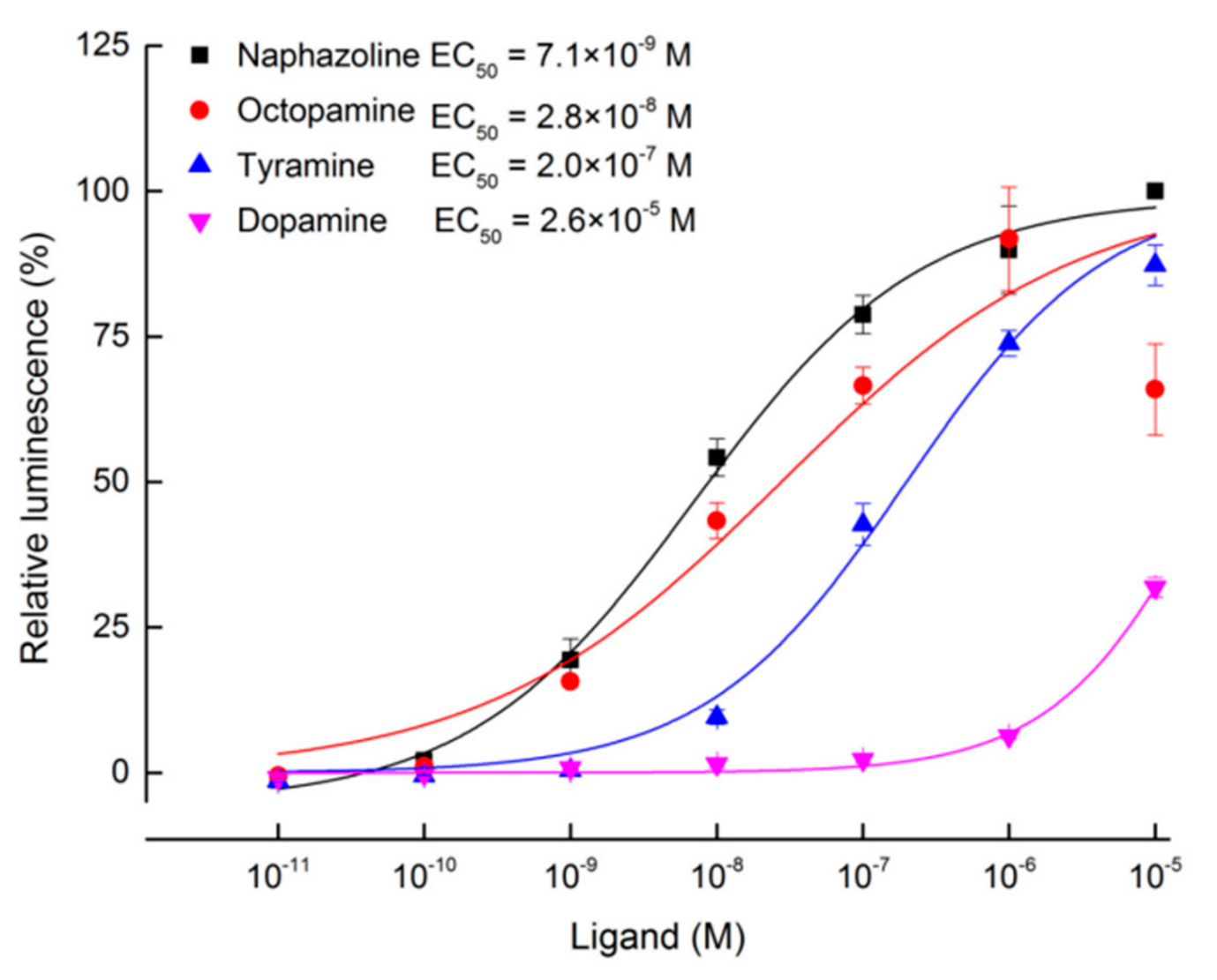
2.2. Heterologous Expression and Functional Assay
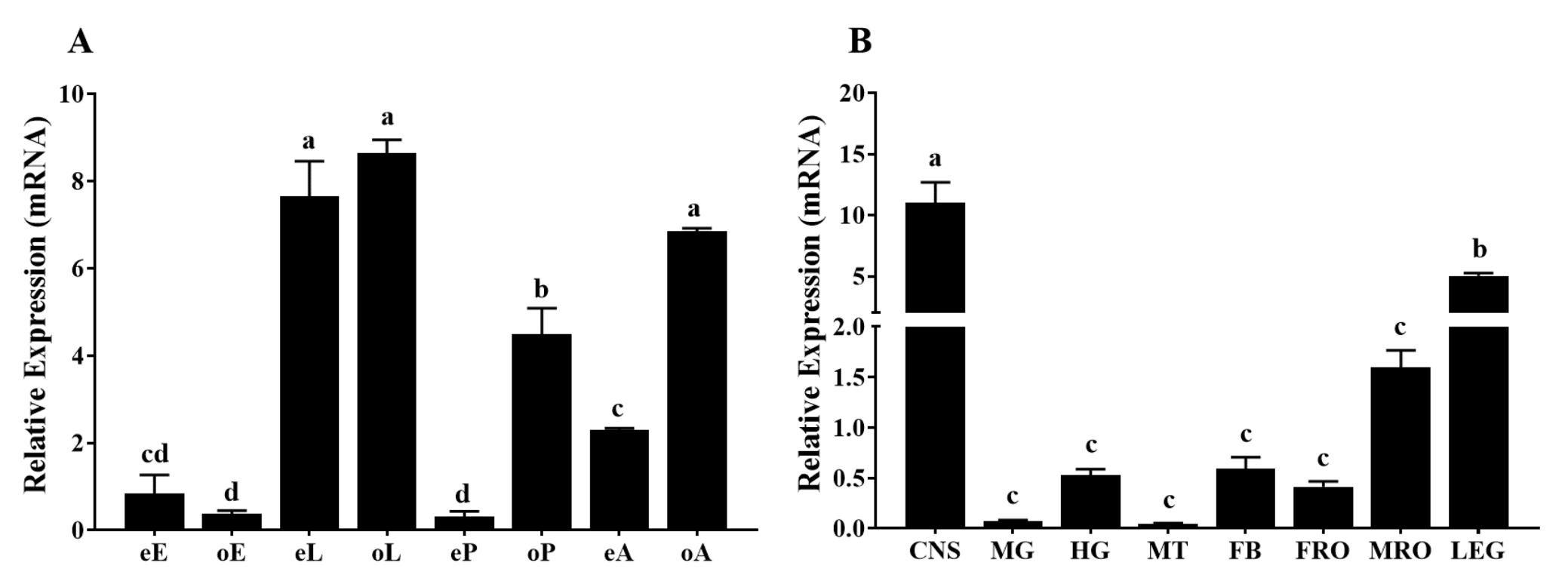
2.3. Spatial and Temporal Expression Profiles
2.4. Effect of TcOctβ2R Knockdown on Mobility
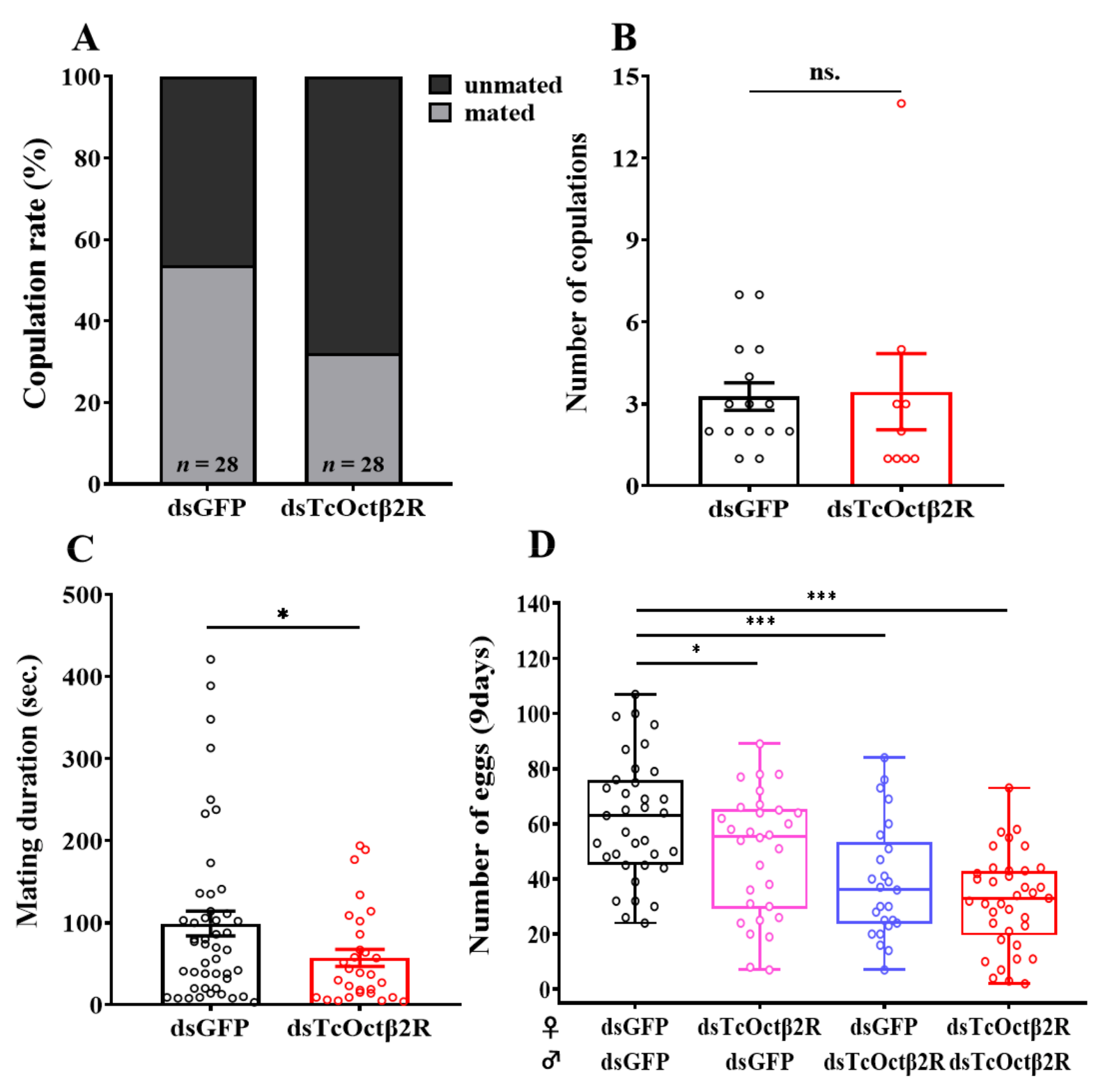
2.5. Effect of TcOctβ2R Knockdown on Mating Behavior and Reproduction
3. Discussion
4. Materials and Methods
4.1. Test Insects
4.2. Primers, Plasmids, and Chemicals
4.3. Molecular Cloning and Sequence Analysis
4.4. Heterologous Expression and Functional Assay
4.5. Quantitative Reverse Transcription PCR (qRT-PCR)
4.6. RNA Interference
4.7. Mobility Assay after RNAi
4.8. Mating Behavior and Fecundity Assay after RNAi
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erspamer, V.; Boretti, G. Identification and characterization, by paper chromatography, of enteramine, octopamine, tyramine, histamine and allied substances in extracts of posterior salivary glands of octopoda and in other tissue extracts of vertebrates and invertebrates. Arch. Int. Pharm. Ther. 1951, 88, 296–332. [Google Scholar]
- Chen, X.; Ohta, H.; Ozoe, F.; Miyazawa, K.; Huang, J.; Ozoe, Y. Functional and pharmacological characterization of a β-adrenergic-like octopamine receptor from the silkworm Bombyx mori. Insect Biochem. Mol. Biol. 2010, 40, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Audsley, N.; Down, R.E. G protein coupled receptors as targets for next generation pesticides. Insect Biochem. Mol. Biol. 2015, 67, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Schendzielorz, T.; Schirmer, K.; Stolte, P.; Stengl, M. Octopamine Regulates Antennal Sensory Neurons via Daytime-Dependent Changes in cAMP and IP3 Levels in the Hawkmoth Manduca sexta. PLoS ONE 2015, 10, e0121230. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.Y.H.; Wan, B.A.; Bland, T.; Montagnese, M.; McLachlan, A.D.; O’Kane, C.J.; Zhang, S.W.; Masuda-Nakagawa, L.M. Octopaminergic neurons have multiple targets in Drosophila larval mushroom body calyx and can modulate behavioral odor discrimination. Learn. Mem. 2021, 28, 53–71. [Google Scholar] [CrossRef]
- Ma, Z.Y.; Guo, X.J.; Lei, H.; Li, T.; Hao, S.G.; Kang, L. Octopamine and tyramine respectively regulate attractive and repulsive behavior in locust phase changes. Sci. Rep. 2015, 5, 8036. [Google Scholar] [CrossRef] [Green Version]
- Tao, J.; Ma, Y.C.; Yang, Z.S.; Zou, C.G.; Zhang, K.Q. Octopamine connects nutrient cues to lipid metabolism upon nutrient deprivation. Sci. Adv. 2016, 2, e1501372. [Google Scholar] [CrossRef] [Green Version]
- Classen, G.; Scholz, H. Octopamine Shifts the Behavioral Response from Indecision to Approach or Aversion in Drosophila melanogaster. Front. Behav. Neurosci. 2018, 12, 131. [Google Scholar] [CrossRef]
- Sabandal, J.M.; Sabandal, P.R.; Kim, Y.C.; Han, K.A. Concerted Actions of Octopamine and Dopamine Receptors Drive Olfactory Learning. J. Neurosci. 2020, 40, 4240–4250. [Google Scholar] [CrossRef]
- Certel, S.J.; Leung, A.; Lin, C.Y.; Perez, P.; Chiang, A.S.; Kravitz, E.A. Octopamine neuromodulatory effects on a social behavior decision-making network in Drosophila males. PLoS ONE 2010, 5, e13248. [Google Scholar] [CrossRef] [Green Version]
- Koon, A.C.; Ashley, J.; Barria, R.; DasGupta, S.; Brain, R.; Waddell, S.; Alkema, M.J.; Budnik, V. Autoregulatory and paracrine control of synaptic and behavioral plasticity by octopaminergic signaling. Nat. Neurosci. 2011, 14, 190–275. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Yu, Y.; Zhang, V.; Tian, Y.; Qi, W.; Wang, L. Octopamine mediates starvation-induced hyperactivity in adult Drosophila. Proc. Natl. Acad. Sci. USA 2015, 112, 5219–5224. [Google Scholar] [CrossRef] [Green Version]
- Sujkowski, A.; Gretzinger, A.; Soave, N.; Todi, S.V.; Wessells, R. Alpha-and beta-adrenergic octopamine receptors in muscle and heart are required for Drosophila exercise adaptations. PLoS Genet. 2020, 16, e1008778. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, X.; He, T.; Wu, X.; Lv, P.; Zhu, A.J.; Du, J. Epigenetic regulator Stuxnet modulates octopamine effect on sleep through a Stuxnet-Polycomb-Oct beta 2R cascade. Embo Rep. 2021, 22, e47910. [Google Scholar] [CrossRef]
- Yoshinari, Y.; Ameku, T.; Kondo, S.; Tanimoto, H.; Kuraishi, T.; Shimada-Niwa, Y.; Niwa, R. Neuronal octopamine signaling regulates mating-induced germline stem cell increase in female Drosophila melanogaster. Elife 2020, 9, e57101. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fink, C.; El-Kholy, S.; Roeder, T. The Octopamine Receptor oct beta 2R is Essential for Ovulation and Fertilization in the Fruit Fly Drosophila melanogaster. Arch. Insect Biochem. Physiol. 2015, 88, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Hana, S.; Lange, A.B. Octopamine and tyramine regulate the activity of reproductive visceral muscles in the adult female blood-feeding bug, Rhodnius prolixus. J. Exp. Biol. 2017, 220, 1830–1836. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.F.; Jv, X.M.; Li, J.; Xu, G.J.; Cai, X.Y.; Gao, C.F. Pharmacological characterisation and functional roles for egg-laying of a beta-adrenergic-like octopamine receptor in the brown planthopper Nilaparvata lugens. Insect Biochem. Mol. Biol. 2017, 87, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Sabandal, P.R.; Fernandez, A.; Sabandal, J.M.; Lee, H.G.; Evans, P.; Han, K.A. The Octopamine Receptor Oct beta 2R Regulates Ovulation in Drosophila melanogaster. PLoS ONE 2014, 9, e104441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, K.A.; Millar, N.S.; Davis, R.L. A novel octopamine receptor with preferential expression in Drosophila mushroom bodies. J. Neurosci. 1998, 18, 3650–3658. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.F.; Xu, G.; Qi, Y.X.; Xia, R.Y.; Huang, J.; Ye, G.Y. Two splicing variants of a novel family of octopamine receptors with different signaling properties. J. Neurochem. 2014, 129, 37–47. [Google Scholar] [CrossRef]
- Evans, P.D.; Maqueira, B. Insect octopamine receptors: A new classification scheme based on studies of cloned Drosophila G-protein coupled receptors. Invertebr. Neurosci. 2005, 5, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Tahira, F. Review of octopamine in insect nervous systems. Open Access Insect Physiol. 2012, 2012, 1–17. [Google Scholar]
- Hana, S.; Lange, A.B. Cloning and Functional Characterization of Oct beta 2-Receptor and Tyr1-Receptor in the Chagas Disease Vector, Rhodnius prolixus. Front. Physiol. 2020, 8, 649. [Google Scholar] [CrossRef]
- Wu, S.F.; Yao, Y.; Huang, J.; Ye, G.Y. Characterization of a β-adrenergic-like octopamine receptor from the rice stem borer (Chilo suppressalis). J. Exp. Biol. 2012, 215, 2646–2652. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.T.; Ma, H.H.; Deng, X.L.; Zhu, H.; Liu, J.; Zhou, Y.; Zhou, X.M. Pharmacological characterization of a beta-adrenergic-like octopamine receptor in Plutella xylostella. Arch. Insect Biochem. Physiol. 2018, 98, e21466. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ohta, H.; Sasaki, K.; Ozoe, F.; Ozoe, Y. Amino acid residues involved in the interaction with the intrinsic agonist (R)-octopamine in the beta-adrenergic-like octopamine receptor from the silkworm Bombyx mori. J. Pestic. Sci. 2011, 36, 473–480. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Chang, X.F.; Gu, G.X.; Jia, W.X.; Guo, L.; Huang, J.; Ye, G.Y. Molecular and pharmacological characterization of a beta-adrenergic-like octopamine receptor from the green rice leafhopper Nephotettix cincticeps. Insect Biochem. Mol. Biol. 2020, 120, 103337. [Google Scholar] [CrossRef]
- Cunningham, C.B.; Douthit, M.K.; Moore, A.J. Octopaminergic gene expression and flexible social behaviour in the subsocial burying beetle Nicrophorus vespilloides. Insect Mol. Biol. 2014, 23, 391–404. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, C.B.; Douthit, M.K.; Moore, A.J. Expression of octopaminergic receptor genes in 4 nonneural tissues in female Nicrophorus vespilloides beetles. Insect Sci. 2015, 22, 495–502. [Google Scholar] [CrossRef]
- Nishi, Y.; Sasaki, K.; Miyatake, T. Biogenic amines, caffeine and tonic immobility in Tribolium castaneum. J. Insect Physiol. 2010, 56, 622–628. [Google Scholar] [CrossRef]
- Ohtani, A.; Arai, Y.; Ozoe, F.; Ohta, H.; Narusuye, K.; Huang, J.; Enomoto, K.; Kataoka, H.; Hirota, A.; Ozoe, Y. Molecular cloning and heterologous expression of an α-adrenergic-like octopamine receptor from the silkworm Bombyx mori. Insect Mol. Biol. 2006, 15, 763–772. [Google Scholar] [CrossRef]
- Balfanz, S.; Strunker, T.; Frings, S.; Baumann, A. A family of octopamine receptors that specifically induce cyclic AMP production or Ca2+ release in Drosophila melanogaster. J. Neurochem. 2005, 94, 1168. [Google Scholar] [CrossRef]
- Bischof, L.J.; Enan, E.E. Cloning, expression and functional analysis of an octopamine receptor from Periplaneta americana. Insect Biochem. Mol. Biol. 2004, 34, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Balfanz, S.; Jordan, N.; Langenstück, T.; Breuer, J.; Bergmeier, V.; Baumann, A. Molecular, pharmacological, and signaling properties of octopamine receptors from honeybee (Apis mellifera) brain. J. Neurochem. 2014, 129, 284–296. [Google Scholar] [CrossRef]
- Li, H.M.; Jiang, H.B.; Gui, S.H.; Liu, X.Q.; Liu, H.; Lu, X.P.; Smagghe, G.; Wang, J.J. Characterization of a beta-Adrenergic-Like Octopamine Receptor in the Oriental Fruit Fly, Bactrocera dorsalis (Hendel). Int. J. Mol. Sci. 2016, 17, 1577. [Google Scholar] [CrossRef] [Green Version]
- Maqueira, B.; Chatwin, H.; Evans, P.D. Identification and characterization of a novel family of Drosophila beta-adrenergic-like octopamine G-protein coupled receptors. J. Neurochem. 2005, 94, 547–560. [Google Scholar] [CrossRef]
- Guo, Y.F.; Qiu, J.R.; Chen, T.; Gao, S.J.; Bu, S.H.; Wang, R.; Wang, J.D. Characterization and functional analysis of a beta-adrenergic-like octopamine receptor from the oriental armyworm (Mythimna separata Walker). Arch. Insect Biochem. Physiol. 2021, 106, e21772. [Google Scholar] [CrossRef]
- Verlinden, H.; Vleugels, R.; Marchal, E.; Badisco, L.; Tobback, J.; Pflüger, H.J.; Blenau, W.; Broeck, J.V. The cloning, phylogenetic relationship and distribution pattern of two new putative GPCR-type octopamine receptors in the desert locust (Schistocerca gregaria). J. Insect Physiol. 2010, 56, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Koon, A.C.; James, A.; Romina, B.; Shamik, D.G.; Ruth, B.; Scott, W.; Alkema, M.J.; Vivian, B. Autoregulatory and paracrine control of synaptic and behavioral plasticity by octopaminergic signaling. J. Neurosci. 2012, 32, 6312–6322. [Google Scholar] [CrossRef] [Green Version]
- Yuya, O.; Yuko, S.N.; Ryusuke, N.; Yasunari, K.; Yoshiki, H.; Kazutaka, A.; Hitoshi, U.; Kimiko, Y.K.; Satoru, K. Autocrine regulation of ecdysone synthesis by β3-octopamine receptor in the prothoracic gland is essential for Drosophila metamorphosis. Proc. Natl. Acad. Sci. USA 2015, 112, 1452–1457. [Google Scholar]
- Lorenz, M.W.; Gaede, G. Hormonal regulation of energy metabolism in insects as a driving force for performance. Integr. Comp. Biol. 2009, 49, 380–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezával, C.; Nojima, T.; Neville, M.C.; Lin Andrew, C.; Goodwin, S.F. Sexually Dimorphic Octopaminergic Neurons Modulate Female Postmating Behaviors in Drosophila. Curr. Biol. 2014, 24, 725–730. [Google Scholar] [CrossRef] [Green Version]
- Himuro, C.; Fujisaki, K. Effects of mating duration on female reproductive traits of the seed bug Togo hemipterus (Heteroptera: Lygaeidae). Appl. Entomol. Zool. 2015, 50, 491–496. [Google Scholar] [CrossRef]
- Wu, S.F.; Jv, X.M.; Huang, J.M.; Gao, C.F. Molecular features and expression profiles of octopamine receptors in the brown planthopper, Nilaparvata lugens. Pest. Manag. Sci. 2019, 75, 2663–2671. [Google Scholar] [CrossRef]
- Šimo, L.; Juraj, K.; Žitňan, D.; Park, Y. Evidence for D1 dopamine receptor activation by a paracrine signal of dopamine in tick salivary glands. PLoS ONE 2011, 6, e16158. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Kim, H.G.; Park, Y. Alternatively spliced orcokinin isoforms and their functions in Tribolium castaneum. Insect Biochem. Mol. Biol. 2015, 65, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Jiang, H.B.; Chen, X.F.; Xiong, Y.; Lu, X.P.; Pei, Y.X.; Smagghe, G.; Wang, J.J. How Tyramine β-Hydroxylase Controls the Production of Octopamine, Modulating the Mobility of Beetles. Int. J. Mol. Sci. 2018, 19, 846. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.-S.; Liu, X.-Q.; Liu, G.-G.; Huang, Q.-Q.; Wang, J.-J.; Jiang, H.-B. Knockdown of a β-Adrenergic-Like Octopamine Receptor Affects Locomotion and Reproduction of Tribolium castaneum. Int. J. Mol. Sci. 2021, 22, 7252. https://doi.org/10.3390/ijms22147252
Zheng L-S, Liu X-Q, Liu G-G, Huang Q-Q, Wang J-J, Jiang H-B. Knockdown of a β-Adrenergic-Like Octopamine Receptor Affects Locomotion and Reproduction of Tribolium castaneum. International Journal of Molecular Sciences. 2021; 22(14):7252. https://doi.org/10.3390/ijms22147252
Chicago/Turabian StyleZheng, Li-Sha, Xiao-Qiang Liu, Ge-Ge Liu, Qian-Qiao Huang, Jin-Jun Wang, and Hong-Bo Jiang. 2021. "Knockdown of a β-Adrenergic-Like Octopamine Receptor Affects Locomotion and Reproduction of Tribolium castaneum" International Journal of Molecular Sciences 22, no. 14: 7252. https://doi.org/10.3390/ijms22147252
APA StyleZheng, L. -S., Liu, X. -Q., Liu, G. -G., Huang, Q. -Q., Wang, J. -J., & Jiang, H. -B. (2021). Knockdown of a β-Adrenergic-Like Octopamine Receptor Affects Locomotion and Reproduction of Tribolium castaneum. International Journal of Molecular Sciences, 22(14), 7252. https://doi.org/10.3390/ijms22147252






