Fluorometric Quantification of Human Platelet Polyphosphate Using 4′,6-Diamidine-2-phenylindole Dihydrochloride: Applications in the Japanese Population
Abstract
:1. Introduction
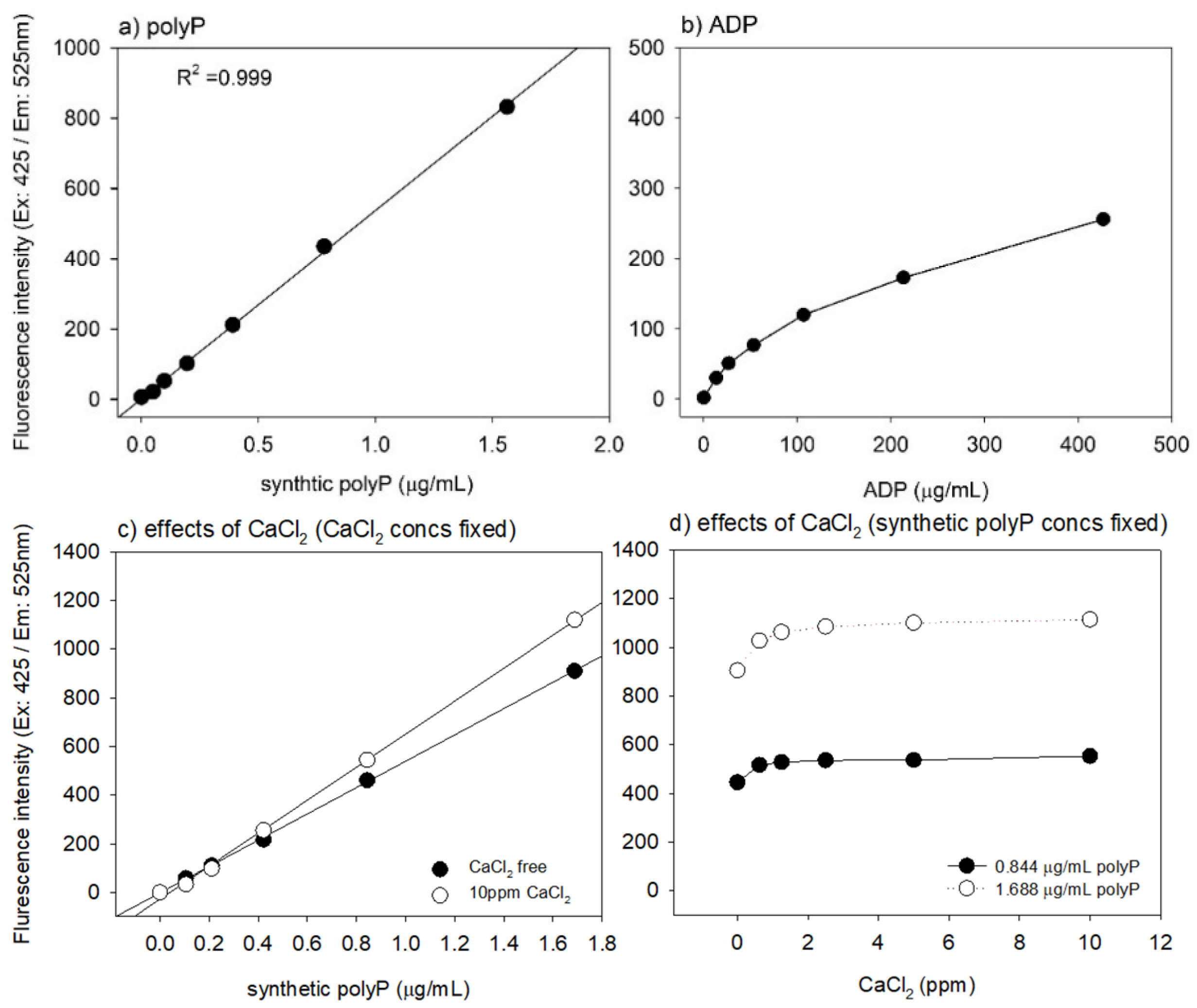
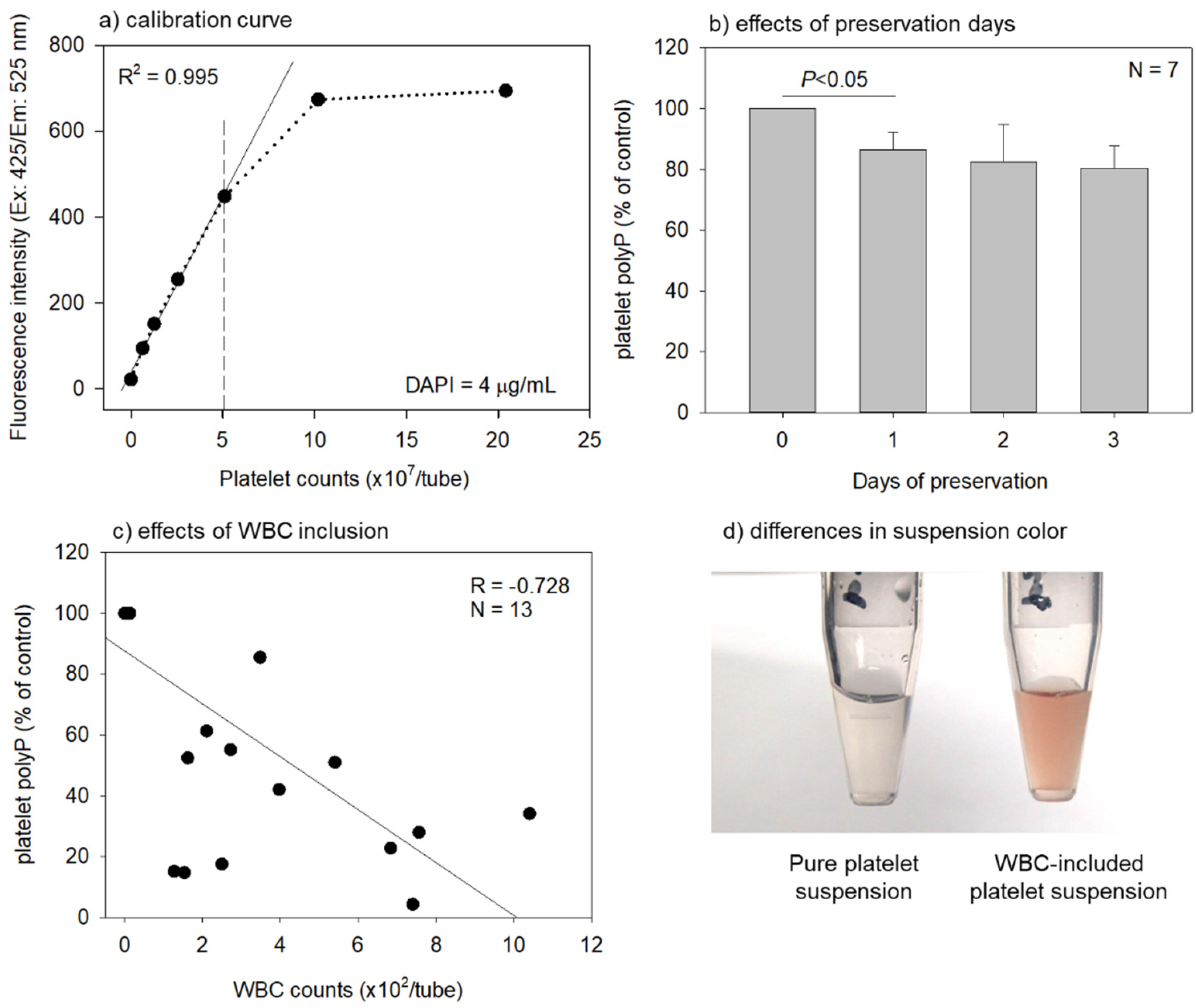
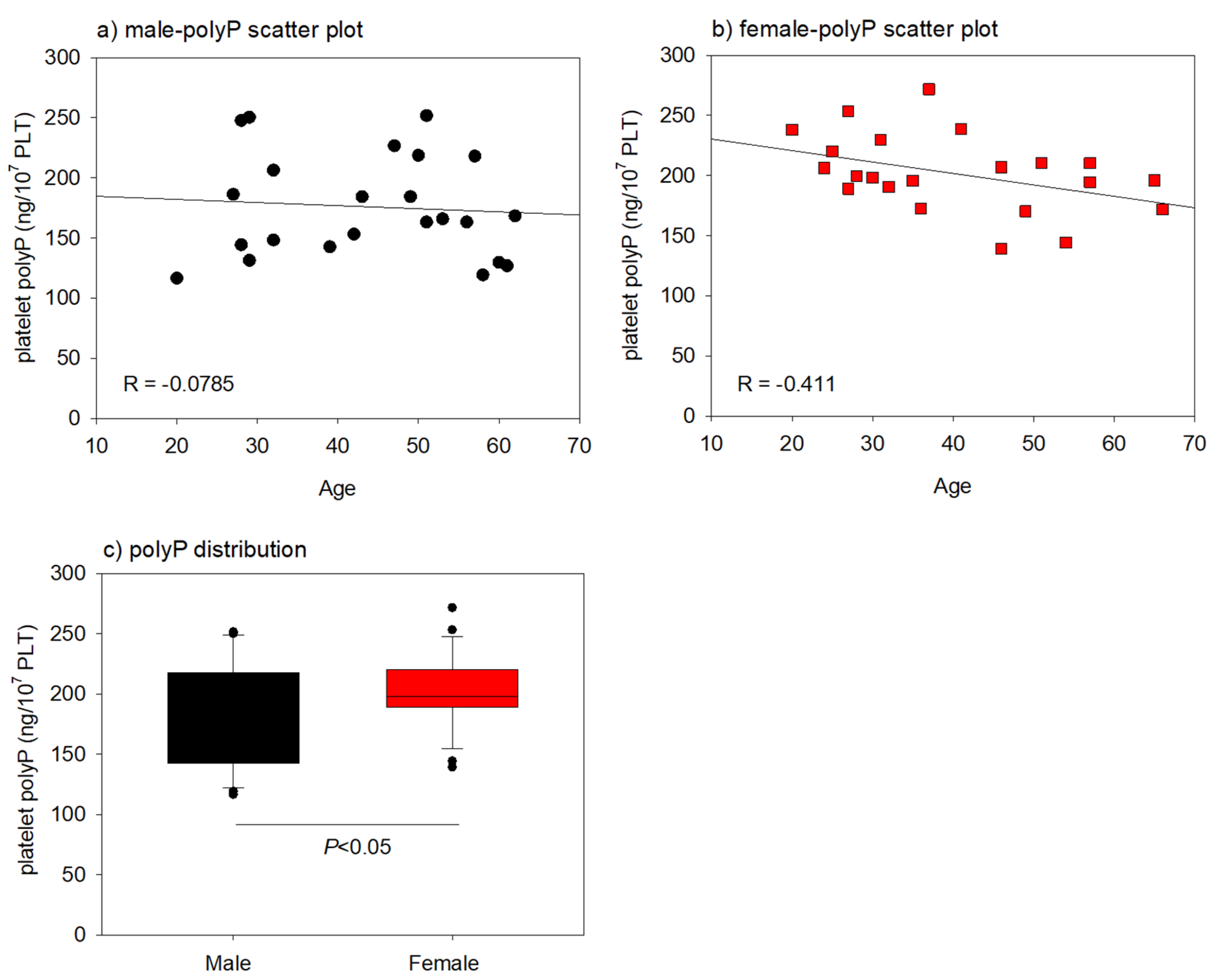
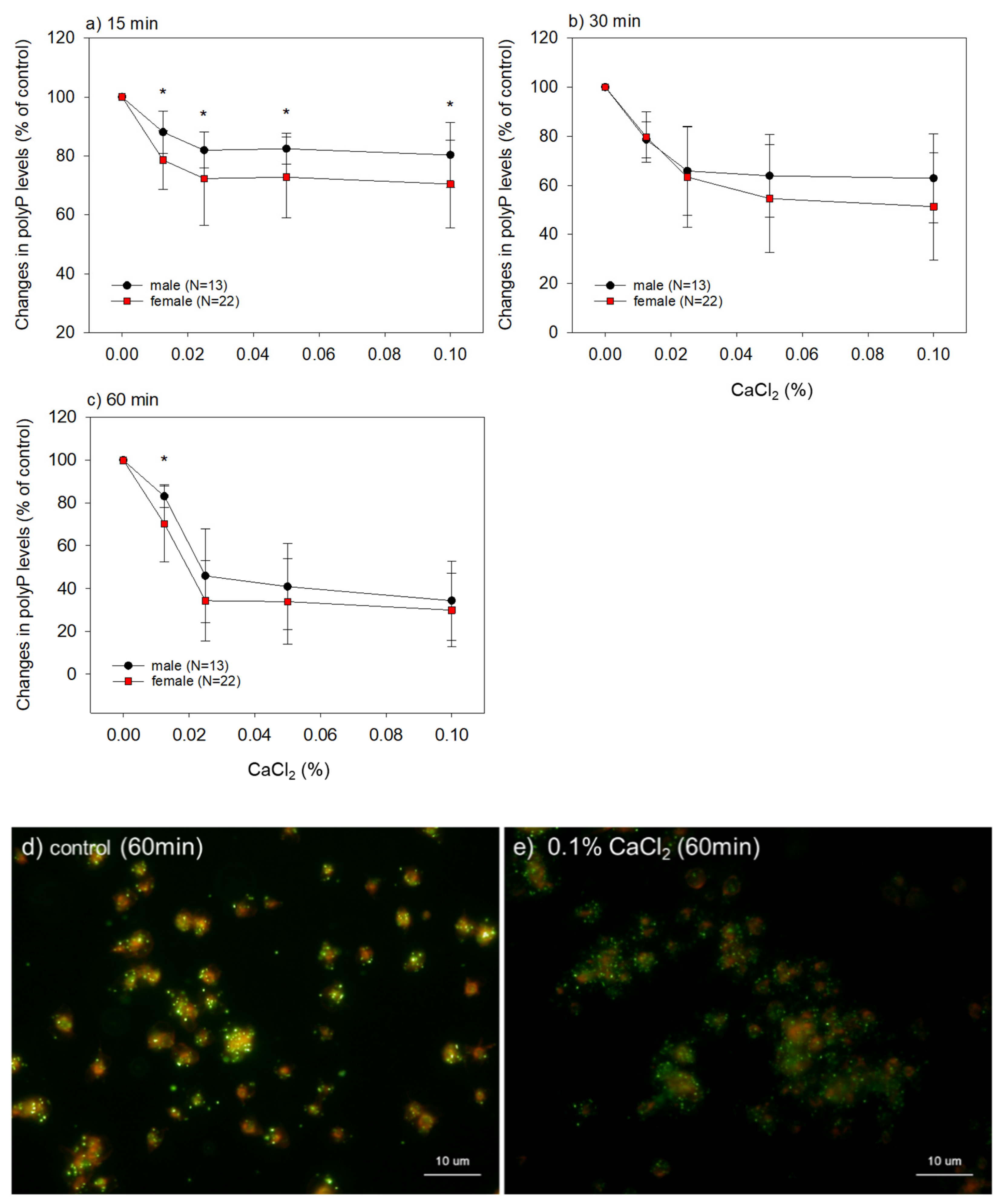
2. Results
3. Discussion
3.1. Optimization and Validation of the Quantification Protocol
3.2. Interpretation of the Main Findings of Gender and Age Differences
3.3. Relevance to the Quality of Platelet Concentrates
4. Materials and Methods
4.1. Blood Collection
4.2. Sample Calculation
4.3. Preparation of Platelet Suspension and Activation
4.4. Quantification of Platelet polyP Levels
4.5. Cytochemical Staining of polyP
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| polyP | polyphosphate |
| DAPI | 4′,6-diamidino-2-phenylindole |
| PC | platelet concentrate |
| R2 | coefficient of determination |
| R | correlation coefficient |
| ACD-A | A-formulation of acid-citrate-dextrose |
| PLT | platelet |
| WBC | white blood cell |
| RBC | red blood cell |
| PRP | platelet-rich plasma |
| HGB | hemoglobin |
References
- Nickel, K.F.; Ronquist, G.; Langer, F.; Labberton, L.; Fuchs, T.A.; Bokemeyer, C.; Sauter, G.; Graefen, M.; Mackman, N.; Stavrou, E.X.; et al. The polyphosphate-factor XII pathway drives coagulation in prostate cancer-associated thrombosis. Blood 2015, 126, 1379–1389. [Google Scholar] [CrossRef] [Green Version]
- Pavlov, E.; Zakharian, E.; Bladen, C.; Diao, C.T.; Grimbly, C.; Reusch, R.N.; French, R.J. A Large, Voltage-Dependent Channel, Isolated from Mitochondria by Water-Free Chloroform Extraction. Biophys. J. 2005, 88, 2614–2625. [Google Scholar] [CrossRef] [Green Version]
- Kumble, K.D.; Kornberg, A. Inorganic Polyphosphate in Mammalian Cells and Tissues. J. Biol. Chem. 1995, 270, 5818–5822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mailer, R.K.W.; Hänel, L.; Allende, M.; Renné, T. Polyphosphate as a Target for Interference with Inflammation and Thrombosis. Front. Med. 2019, 6, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, F.A.; Lea, C.R.; Oldfield, E.; Docampo, R. Human Platelet Dense Granules Contain Polyphosphate and Are Similar to Acidocalcisomes of Bacteria and Unicellular Eukaryotes. J. Biol. Chem. 2004, 279, 44250–44257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.A.; Morrissey, J.H. Polyphosphate enhances fibrin clot structure. Blood 2008, 112, 2810–2816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labberton, L.; Kenne, E.; Long, A.T.; Nickel, K.F.; Di Gennaro, A.; Rigg, R.A.; Hernandez, J.S.; Butler, L.; Maas, C.; Stavrou, E.; et al. Neutralizing blood-borne polyphosphate in vivo provides safe thromboprotection. Nat. Commun. 2016, 7, 12616. [Google Scholar] [CrossRef]
- Dinarvand, P.; Hassanian, S.M.; Qureshi, S.H.; Manithody, C.; Eissenberg, J.C.; Yang, L.; Rezaie, A.R. Polyphosphate amplifies proinflammatory responses of nuclear proteins through interaction with receptor for advanced glycation end products and P2Y1 purinergic receptor. Blood 2014, 123, 935–945. [Google Scholar] [CrossRef]
- Müller, F.; Mutch, N.J.; Schenk, W.A.; Smith, S.; Esterl, L.; Spronk, H.M.; Schmidbauer, S.; Gahl, W.A.; Morrissey, J.; Renné, T. Platelet Polyphosphates Are Proinflammatory and Procoagulant Mediators In Vivo. Cell 2009, 139, 1143–1156. [Google Scholar] [CrossRef] [Green Version]
- Omelon, S.; Georgiou, J.; Henneman, Z.J.; Wise, L.M.; Sukhu, B.; Hunt, T.; Wynnyckyj, C.; Holmyard, D.; Bielecki, R.; Grynpas, M.D. Control of Vertebrate Skeletal Mineralization by Polyphosphates. PLoS ONE 2009, 4, e5634. [Google Scholar] [CrossRef] [Green Version]
- Żurawska-Płaksej, E.; Kuliczkowski, W.; Karolko, B.; Cielecka-Prynda, M.; Dębski, J.; Kaaz, K.; Mysiak, A.; Wróbel, T.; Podolak-Dawidziak, M.; Usnarska-Zubkiewicz, L. Platelet polyphosphate level is elevated in patients with chronic primary thrombocytopenia: A preliminary study. Adv. Clin. Exp. Med. 2020, 29, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Tarayre, C.; Nguyen, H.-T.; Brognaux, A.; Delepierre, A.; De Clercq, L.; Charlier, R.; Michels, E.; Meers, E.; Delvigne, F. Characterisation of Phosphate Accumulating Organisms and Techniques for Polyphosphate Detection: A Review. Sensors 2016, 16, 797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, P.; Van Mooy, B.A.S. Fluorometric Quantification of Polyphosphate in Environmental Plankton Samples: Extraction Protocols, Matrix Effects, and Nucleic Acid Interference. Appl. Environ. Microbiol. 2012, 79, 273–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aschar-Sobbi, R.; Abramov, A.Y.; Diao, C.; Kargacin, M.E.; Kargacin, G.J.; French, R.J.; Pavlov, E. High sensitivity, quantitative measurements of polyphosphate using a new DAPI-based approach. J. Fluoresc. 2008, 18, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R.; Agrawalla, B.K.; Elustondo, P.A.; Gordon, J.; Shiba, T.; Abramov, A.; Chang, Y.-T.; Pavlov, E.V. In Situ Investigation of Mammalian Inorganic Polyphosphate Localization Using Novel Selective Fluorescent Probes JC-D7 and JC-D8. ACS Chem. Biol. 2014, 9, 2101–2110. [Google Scholar] [CrossRef]
- Lorenzo-Orts, L.; Couto, D.; Hothorn, M. Identity and functions of inorganic and inositol polyphosphates in plants. New Phytol. 2019, 225, 637–652. [Google Scholar] [CrossRef] [Green Version]
- Sato, A.; Aizawa, H.; Tsujino, T.; Isobe, K.; Watanabe, T.; Kitamura, Y.; Kawase, T. Fluorescent Cytochemical Detection of Polyphosphates Associated with Human Platelets. Int. J. Mol. Sci. 2021, 22, 1040. [Google Scholar] [CrossRef]
- Kawase, T. Platelet-rich plasma and its derivatives as promising bioactive materials for regenerative medicine: Basic principles and concepts underlying recent advances. Odontology 2015, 103, 126–135. [Google Scholar] [CrossRef]
- Kawase, T.; Mubarak, S.; Mourão, C.F. The Platelet Concentrates Therapy: From the Biased Past to the Anticipated Future. Bioengineering 2020, 7, 82. [Google Scholar] [CrossRef]
- Kawase, T.; Okuda, K. Comprehensive Quality Control of the Regenerative Therapy Using Platelet Concentrates: The Current Situation and Prospects in Japan. BioMed Res. Int. 2018, 2018, 6389157. [Google Scholar] [CrossRef]
- Kawase, T.; Okuda, K.; Wolff, L.F.; Yoshie, H. Platelet-rich plasma-derived fibrin clot formation stimulates collagen synthesis in periodontal ligament and osteoblastic cells in vitro. J. Periodontol. 2003, 74, 858–864. [Google Scholar] [CrossRef]
- Kawase, T.; Takahashi, A.; Watanabe, T.; Tsujino, T. Proposal for point-of-care testing of platelet-rich plasma quality. Int. J. Growth Factors Stem Cells Dent. 2019, 2, 13. [Google Scholar] [CrossRef]
- Kawase, T.; Tanaka, T. An updated proposal for terminology and classification of platelet-rich fibrin. Regen. Ther. 2017, 7, 80–81. [Google Scholar] [CrossRef]
- Kawase, T.; Watanabe, T.; Okuda, K. Platelet-rich plasma and its derived platelet concentrates: What dentists involved in cell-based regenerative therapy should know. Nihon Shishubyo Gakkai Kaishi 2017, 59, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Okuda, K.; Kawase, T.; Momose, M.; Murata, M.; Saito, Y.; Suzuki, H.; Wolff, L.F.; Yoshie, H. Platelet-rich plasma contains high levels of platelet-derived growth factor and transforming growth factor-beta and modulates the proliferation of periodontally related cells in vitro. J. Periodontol. 2003, 74, 849–857. [Google Scholar] [CrossRef]
- Mutch, N.J.; Engel, R.; De Willige, S.U.; Philippou, H.; Ariëns, R.A.S. Polyphosphate modifies the fibrin network and down-regulates fibrinolysis by attenuating binding of tPA and plasminogen to fibrin. Blood 2010, 115, 3980–3988. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, M.; Li, P.; Teng, H.; Fan, D.; Du, W.; Guo, Z. Progress and Applications of Polyphosphate in Bone and Cartilage Regeneration. BioMed Res. Int. 2019, 2019, 5141204. [Google Scholar] [CrossRef]
- Carney, B.C.; Simbulan-Rosenthal, C.M.; Gaur, A.; Browne, B.J.; Moghe, M.; Crooke, E.; Moffatt, L.T.; Shupp, J.W.; Rosenthal, D.S. Inorganic polyphosphate in platelet rich plasma accelerates re-epithelialization in vitro and in vivo. Regen. Ther. 2020, 15, 138–148. [Google Scholar] [CrossRef]
- Omelon, S.; Georgiou, J.; Habraken, W. A cautionary (spectral) tail: Red-shifted fluorescence by DAPI–DAPI interactions. Biochem. Soc. Trans. 2016, 44, 46–49. [Google Scholar] [CrossRef]
- Daniel, J.L. Inositol Phosphate Metabolism and Platelet Activation. Platelets 1990, 1, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P. The use of platelets in regenerative medicine and proposal for a new classification system: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1895–1900. [Google Scholar] [CrossRef]
- Lee, G.; Arepally, G.M. Anticoagulation techniques in apheresis: From heparin to citrate and beyond. J. Clin. Apher. 2012, 27, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Khalifa, A.R.M.; Abdel-Rahman, E.A.; Mahmoud, A.M.; Ali, M.H.; Noureldin, M.; Saber, S.H.; Mohsen, M.; Ali, S.S. Sex-specific differences in mitochondria biogenesis, morphology, respiratory function, and ROS homeostasis in young mouse heart and brain. Physiol. Rep. 2017, 5, e13125. [Google Scholar] [CrossRef]
- Watanabe, D.; Hatakeyama, K.; Ikegami, R.; Eshima, H.; Yagishita, K.; Poole, D.C.; Kano, Y. Sex differences in mitochondrial Ca(2+) handling in mouse fast-twitch skeletal muscle in vivo. J. Appl. Physiol. 2020, 128, 241–251. [Google Scholar] [CrossRef]
- Silaidos, C.; Pilatus, U.; Grewal, R.; Matura, S.; Lienerth, B.; Pantel, J.; Eckert, G.P. Sex-associated differences in mitochondrial function in human peripheral blood mononuclear cells (PBMCs) and brain. Biol. Sex Differ. 2018, 9, 34. [Google Scholar] [CrossRef]
- Miotto, P.M.; McGlory, C.; Holloway, T.M.; Phillips, S.M.; Holloway, G.P. Sex differences in mitochondrial respiratory function in human skeletal muscle. Am. J. Physiol. Integr. Comp. Physiol. 2018, 314, R909–R915. [Google Scholar] [CrossRef]
- Boengler, K.; Kosiol, M.; Mayr, M.; Schulz, R.; Rohrbach, S. Mitochondria and ageing: Role in heart, skeletal muscle and adipose tissue. J. Cachexia Sarcopenia Muscle 2017, 8, 349–369. [Google Scholar] [CrossRef] [Green Version]
- Middeldorp, S. Thrombosis in women: What are the knowledge gaps in 2013? J. Thromb. Haemost. 2013, 11, 180–191. [Google Scholar] [CrossRef] [Green Version]
- Roach, R.E.J.; Lijfering, W.M.; Helmerhorst, F.M.; Cannegieter, S.C.; Rosendaal, F.R.; Vlieg, A.V.H. The risk of venous thrombosis in women over 50 years old using oral contraception or postmenopausal hormone therapy. J. Thromb. Haemost. 2012, 11, 124–131. [Google Scholar] [CrossRef]
- Rosendaal, F.; Helmerhorst, F.; Vandenbroucke, J.P. Female Hormones and Thrombosis. Arter. Thromb. Vasc. Biol. 2002, 22, 201–210. [Google Scholar] [CrossRef] [Green Version]
- American Heart Association. Understand Your Risk for Excessive Blood Clotting. Available online: https://www.heart.org/en/health-topics/venous-thromboembolism/understand-your-risk-for-excessive-blood-clotting (accessed on 11 May 2021).
- Leng, X.-H.; Hong, S.Y.; Larrucea, S.; Zhang, W.; Li, T.-T.; LόpezJ, A.; Bray, P.F. Platelets of Female Mice Are Intrinsically More Sensitive to Agonists Than Are Platelets of Males. Arter. Thromb. Vasc. Biol. 2004, 24, 376–381. [Google Scholar] [CrossRef]
- Eshel-Green, T.; Berny-Lang, M.; Conley, R.B.; Mccarty, O.J.T. Effect of sex difference on platelet adhesion, spreading and aggregate formation under flow. Thromb. Haemost. 2009, 102, 958–965. [Google Scholar] [CrossRef]
- Marx, R.E.; Carlson, E.R.; Eichstaedt, R.M.; Schimmele, S.R.; Strauss, J.E.; Georgeff, K.R. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 85, 638–646. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Tolba, E.; Schröder, H.C.; Wang, X. Polyphosphate: A Morphogenetically Active Implant Material Serving as Metabolic Fuel for Bone Regeneration. Macromol. Biosci. 2015, 15, 1182–1197. [Google Scholar] [CrossRef]
- Gomes, F.; Ramos, I.; Wendt, C.; Girard-Dias, W.; De Souza, W.; Machado, E.; Miranda, E.K. New insights into the in situ microscopic visualization and quantification of inorganic polyphosphate stores by 4′,6-diamidino-2-phenylindole (DAPI)-staining. Eur. J. Histochem. 2013, 57, e34. [Google Scholar] [CrossRef] [Green Version]
- Evanson, J.R.; Guyton, M.K.; Oliver, D.L.; Hire, J.M.; Topolski, R.L.; Zumbrun, S.D.; McPherson, J.C.; Bojescul, J.A. Gender and Age Differences in Growth Factor Concentrations from Platelet-Rich Plasma in Adults. Mil. Med. 2014, 179, 799–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniguchi, Y.; Yoshioka, T.; Sugaya, H.; Gosho, M.; Aoto, K.; Kanamori, A.; Yamazaki, M. Growth factor levels in leukocyte-poor platelet-rich plasma and correlations with donor age, gender, and platelets in the Japanese population. J. Exp. Orthop. 2019, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Weibrich, G.; Kleis, W.K.; Hafner, G.; Hitzler, W.E. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J. Cranio-Maxillofac. Surg. 2002, 30, 97–102. [Google Scholar] [CrossRef]
- Xiong, G.; Lingampalli, N.; Koltsov, J.C.; Leung, L.L.; Bhutani, N.; Robinson, W.H.; Chu, C.R. Men and Women Differ in the Biochemical Composition of Platelet-Rich Plasma. Am. J. Sports Med. 2018, 46, 409–419. [Google Scholar] [CrossRef]
- Schmidt, V.; Hilberg, T. ThromboFix™ Platelet Stabilizer: Advances in clinical platelet analyses by flow cytometry? Platelets 2006, 17, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Aizawa, H.; Kawabata, H.; Sato, A.; Watanabe, T.; Isobe, K.; Kitamura, Y.; Tanaka, T.; Kawase, T. Platelet adhesion on commercially pure titanium plates in vitro III: Effects of calcium phosphate-blasting on titanium plate biocompatibility. Int. J. Implant Dent. 2020, 74, 6. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, T.; Kitamura, Y.; Aizawa, H.; Masuki, H.; Tsujino, T.; Sato, A.; Kawabata, H.; Isobe, K.; Nakata, K.; Kawase, T. Fluorometric Quantification of Human Platelet Polyphosphate Using 4′,6-Diamidine-2-phenylindole Dihydrochloride: Applications in the Japanese Population. Int. J. Mol. Sci. 2021, 22, 7257. https://doi.org/10.3390/ijms22147257
Watanabe T, Kitamura Y, Aizawa H, Masuki H, Tsujino T, Sato A, Kawabata H, Isobe K, Nakata K, Kawase T. Fluorometric Quantification of Human Platelet Polyphosphate Using 4′,6-Diamidine-2-phenylindole Dihydrochloride: Applications in the Japanese Population. International Journal of Molecular Sciences. 2021; 22(14):7257. https://doi.org/10.3390/ijms22147257
Chicago/Turabian StyleWatanabe, Taisuke, Yutaka Kitamura, Hachidai Aizawa, Hideo Masuki, Tetsuhiro Tsujino, Atsushi Sato, Hideo Kawabata, Kazushige Isobe, Koh Nakata, and Tomoyuki Kawase. 2021. "Fluorometric Quantification of Human Platelet Polyphosphate Using 4′,6-Diamidine-2-phenylindole Dihydrochloride: Applications in the Japanese Population" International Journal of Molecular Sciences 22, no. 14: 7257. https://doi.org/10.3390/ijms22147257
APA StyleWatanabe, T., Kitamura, Y., Aizawa, H., Masuki, H., Tsujino, T., Sato, A., Kawabata, H., Isobe, K., Nakata, K., & Kawase, T. (2021). Fluorometric Quantification of Human Platelet Polyphosphate Using 4′,6-Diamidine-2-phenylindole Dihydrochloride: Applications in the Japanese Population. International Journal of Molecular Sciences, 22(14), 7257. https://doi.org/10.3390/ijms22147257






