Smoking and Neuropsychiatric Disease—Associations and Underlying Mechanisms
Abstract
:1. Introduction
2. Smoking-Induced Diseases
2.1. Cancer
2.2. Lung Disease
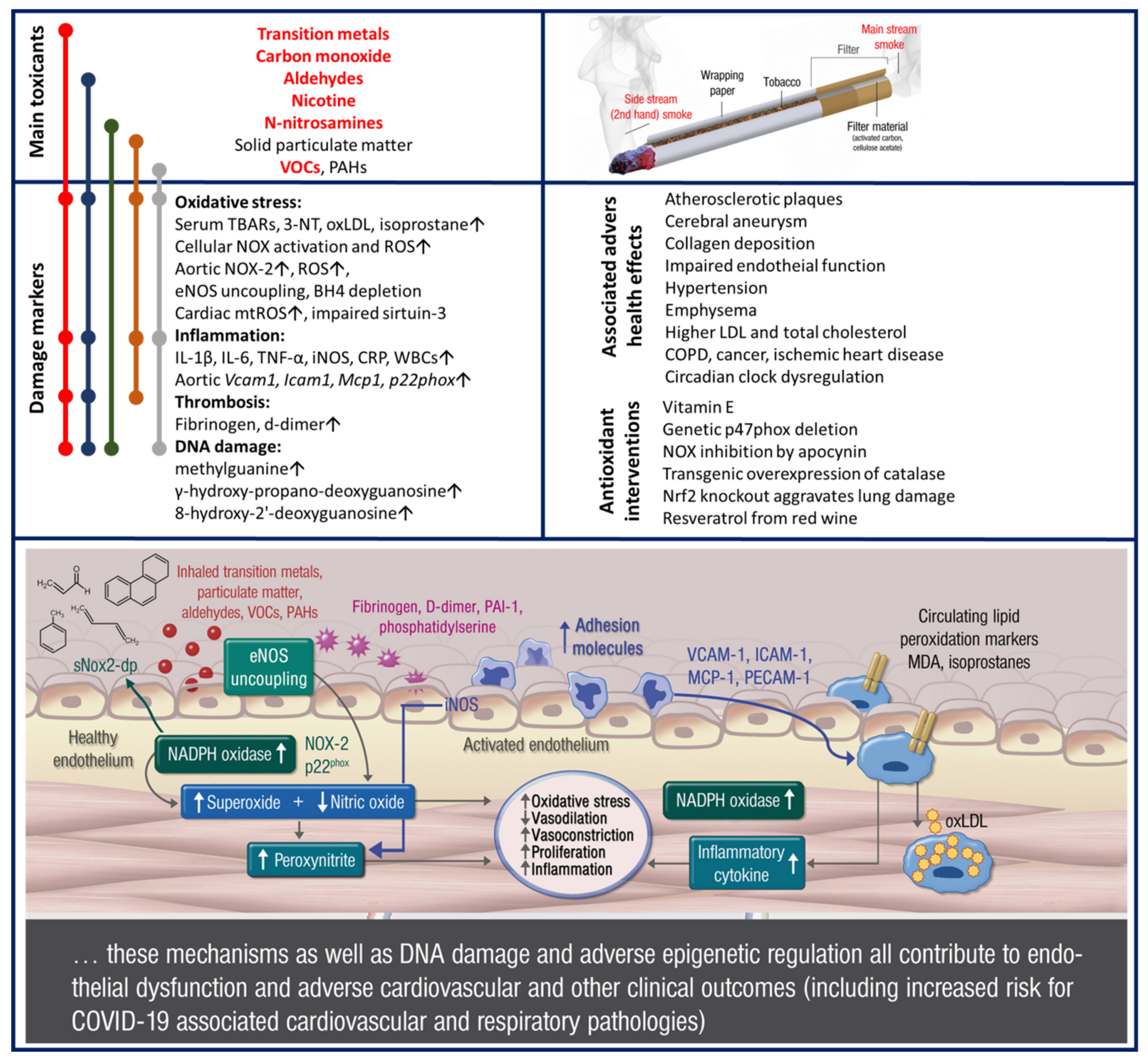
2.3. Cardiovascular Disease
2.4. Metabolic Disease
3. Smoking-Induced Disease Mechanisms
3.1. Oxidative Stress and Inflammation
3.2. Implications for Neuropsychiatric Disease
4. Smoking and Neuropsychiatric Disease Risk
4.1. Evidence from Observational/Epidemiological Studies
4.1.1. Depression
4.1.2. Anxiety Disorder
4.1.3. Suicidal Behavior
4.1.4. Dementia/Cognitive Decline
4.1.5. Schizophrenia/Psychosis
4.1.6. Other Neuropsychiatric Outcomes
4.2. Smoking and Brain Changes Affecting Neuropsychiatric Pathophysiology
5. Conclusions
Funding
Conflicts of Interest
Abbreviations
| ADHD | Attention-deficit/hyperactivity disorder |
| AMPK | AMP-activated protein kinase |
| BBB | Blood-brain barrier |
| CI | Confidence interval |
| COPD | Chronic obstructive pulmonary disease |
| DALYs | Disability-adjusted life years |
| DUOX1 | Dual oxidase 1 |
| GBD | Global Burden of Disease |
| GSH | Glutathione |
| HR | Hazard ratio |
| ICAM-1 | Intercellular adhesion molecule 1 |
| IL | Interleukin |
| IFN-γ | Interferon gamma |
| iNOS | Inducible nitric oxide synthase |
| IRR | Incidence rate ratio |
| LDL | Low-density lipoprotein |
| MCP-1 | Monocyte chemotactic protein 1 (CCL2) |
| MDA | Malondialdehyde |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NLR family pyrin domain containing 3 |
| NO | Nitric oxide |
| NOX | NADPH oxidase isoform |
| Nrf2 | Nuclear factor E2 related factor-2 |
| OR | Odds ratio |
| PPAR γ | Peroxisome proliferator-activated receptor gamma |
| p47phox | Regulatory subunit of NOX-2 and NOX-1 |
| ROS | Reactive oxygen species |
| RR | Relative risk |
| SOD | Superoxide dismutase |
| TGF- β | Transforming growth factor beta |
| TNFα | Tumor necrosis factor alpha |
| Trx | Thioredoxin |
| US | United States |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| WHO | World Health Organization |
References
- World Health Organization. Tobacco Free Initiative. Available online: https://www.who.int/tobacco/mpower/en/ (accessed on 29 March 2021).
- World Health Organization. Tobacco. Available online: https://www.who.int/news-room/fact-sheets/detail/tobacco (accessed on 29 March 2021).
- Drope, J.; Schluger, N.; Cahn, Z.; Drope, J.; Hamill, S.; Islami, F.; Liber, A.; Nargis, N.; Stoklosa, M. The Tobacco Atlas. Atlanta: American Cancer Society and Vital Strategies. 2018. Available online: https://tobaccoatlas.org/wp-content/uploads/2018/03/TobaccoAtlas_6thEdition_LoRes_Rev0318.pdf (accessed on 16 April 2021).
- Collaborators, G.R.F. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global. Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US). How Tobacco Smoke Causes Disease The Biology and Behavioral Basis for Smoking-Attributable Disease; Centers for Disease Control and Prevention (US): Rockville, MD, USA, 2010.
- Royal College of Physicians. Smoking and Health; RCP: London, UK, 1962. [Google Scholar]
- Samet, J.M. Tobacco smoking: The leading cause of preventable disease worldwide. Thorac. Surg. Clin. 2013, 23, 103–112. [Google Scholar] [CrossRef]
- Rodgman, A.; Perfetti, T.A. The Chemical Components of Tobacco and Tobacco Smoke, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Farsalinos, K.E.; Gillman, G.; Poulas, K.; Voudris, V. Tobacco-Specific Nitrosamines in Electronic Cigarettes: Comparison between Liquid and Aerosol Levels. Int. J. Environ. Res. Public Health 2015, 12, 9046–9053. [Google Scholar] [CrossRef] [Green Version]
- Bostrom, C.E.; Gerde, P.; Hanberg, A.; Jernstrom, B.; Johansson, C.; Kyrklund, T.; Rannug, A.; Tornqvist, M.; Victorin, K.; Westerholm, R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ. Health Perspect 2002, 110 (Suppl. 3), 451–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camara-Lemarroy, C.R.; Rodriguez-Gutierrez, R.; Monreal-Robles, R.; Gonzalez-Gonzalez, J.G. Acute toluene intoxication--clinical presentation, management and prognosis: A prospective observational study. BMC Emerg Med. 2015, 15, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed Laskar, A.; Younus, H. Aldehyde toxicity and metabolism: The role of aldehyde dehydrogenases in detoxification, drug resistance and carcinogenesis. Drug Metab. Rev. 2019, 51, 42–64. [Google Scholar] [CrossRef] [PubMed]
- Prockop, L.D.; Chichkova, R.I. Carbon monoxide intoxication: An updated review. J. Neurol. Sci. 2007, 262, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Ochsner, A.; Debakey, M. Primary pulmonary malignancy: Treatment by total pneumonectomy; analysis of 79 collected cases and presentation of 7 personal cases. Ochsner J. 1999, 1, 109–125. [Google Scholar]
- Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US). The Health Consequences of Smoking—50 Years of Progress; Centers for Disease Control and Prevention (US): Rockville, MD, USA, 2014.
- California Environmental Protection Agency: Air Resources Board. Proposed Identification of Environmental Tobacco Smoke as a Toxic Air Contaminant; California Environmental Protection Agency: Air Resources Board:: Sacramento, CA, USA, 2005.
- Collishaw, N.E.; Boyd, N.F.; Cantor, K.P.; Hammond, S.K.; Johnson, K.C.; Millar, J.; Miller, A.B.; Miller, M.; Palmer, J.R.; Salmon, A.G.; et al. Canadian Expert Panel on Tobacco Smoke and Breast Cancer Risk. Tobacco Control 2009, 20, e2. [Google Scholar]
- International Agency for Research on Cancer. Personal Habits and Indoor Combustions; International Agency for Research on Cancer: Lyon, France, 2012. [Google Scholar]
- Macacu, A.; Autier, P.; Boniol, M.; Boyle, P. Active and passive smoking and risk of breast cancer: A meta-analysis. Breast Cancer Res. Treat. 2015, 154, 213–224. [Google Scholar] [CrossRef] [Green Version]
- Emilio, S.; Luigi, V.; Riccardo, B.; Carlo, G. Lifestyle in urology: Cancer. Urologia 2019, 86, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Darcey, E.; Boyle, T. Tobacco smoking and survival after a prostate cancer diagnosis: A systematic review and meta-analysis. Cancer Treat. Rev. 2018, 70, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Cumberbatch, M.G.; Rota, M.; Catto, J.W.; La Vecchia, C. The Role of Tobacco Smoke in Bladder and Kidney Carcinogenesis: A Comparison of Exposures and Meta-analysis of Incidence and Mortality Risks. Eur. Urol 2016, 70, 458–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plummer, M.; Herrero, R.; Franceschi, S.; Meijer, C.J.; Snijders, P.; Bosch, F.X.; de Sanjose, S.; Munoz, N. Smoking and cervical cancer: Pooled analysis of the IARC multi-centric case-control study. Cancer Causes Control. 2003, 14, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Qin, W.; Xue, F.; Wei, X.; Guan, Q.; Jiang, W.; Wang, S.; Xu, M.; Yu, S. The relation of passive smoking with cervical cancer: A systematic review and meta-analysis. Medicine 2018, 97, e13061. [Google Scholar] [CrossRef]
- Korc, M.; Jeon, C.Y.; Edderkaoui, M.; Pandol, S.J.; Petrov, M.S.; Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Tobacco and alcohol as risk factors for pancreatic cancer. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Morales-Oyarvide, V.; Babic, A.; Clish, C.B.; Kraft, P.; Bao, Y.; Qian, Z.R.; Rubinson, D.A.; Ng, K.; Giovannucci, E.L.; et al. Cigarette Smoking and Pancreatic Cancer Survival. J. Clin. Oncol. 2017, 35, 1822–1828. [Google Scholar] [CrossRef]
- Zhang, Y.; He, J.; He, B.; Huang, R.; Li, M. Effect of tobacco on periodontal disease and oral cancer. Tob. Induc. Dis. 2019, 17, 40. [Google Scholar] [CrossRef]
- Jethwa, A.R.; Khariwala, S.S. Tobacco-related carcinogenesis in head and neck cancer. Cancer Metastasis Rev. 2017, 36, 411–423. [Google Scholar] [CrossRef]
- Praestegaard, C.; Jensen, A.; Jensen, S.M.; Nielsen, T.S.; Webb, P.M.; Nagle, C.M.; DeFazio, A.; Australian Ovarian Cancer Study Group; Hogdall, E.; Rossing, M.A.; et al. Cigarette smoking is associated with adverse survival among women with ovarian cancer: Results from a pooled analysis of 19 studies. Int. J. Cancer 2017, 140, 2422–2435. [Google Scholar] [CrossRef] [Green Version]
- Lundback, B.; Lindberg, A.; Lindstrom, M.; Ronmark, E.; Jonsson, A.C.; Jonsson, E.; Larsson, L.G.; Andersson, S.; Sandstrom, T.; Larsson, K.; et al. Not 15 but 50% of smokers develop COPD?--Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir. Med. 2003, 97, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tashkin, D.P. Smoking Cessation in Chronic Obstructive Pulmonary Disease. Semin. Respir. Crit. Care Med. 2015, 36, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Piipari, R.; Jaakkola, J.J.; Jaakkola, N.; Jaakkola, M.S. Smoking and asthma in adults. Eur. Respir. J. 2004, 24, 734–739. [Google Scholar] [CrossRef]
- Cockcroft, D.W. Environmental Causes of Asthma. Semin. Respir. Crit. Care Med. 2018, 39, 12–18. [Google Scholar] [CrossRef]
- Hagmeyer, L.; Randerath, W. Smoking-related interstitial lung disease. Dtsch. Arztebl. Int. 2015, 112, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Schonfeld, N.; Dirks, K.; Costabel, U.; Loddenkemper, R. A prospective clinical multicentre study on adult pulmonary Langerhans’ cell histiocytosis. Sarcoidosis Vasc. Diffuse Lung Dis. 2012, 29, 132–138. [Google Scholar]
- Fraig, M.; Shreesha, U.; Savici, D.; Katzenstein, A.L. Respiratory bronchiolitis: A clinicopathologic study in current smokers, ex-smokers, and never-smokers. Am. J. Surg Pathol. 2002, 26, 647–653. [Google Scholar] [CrossRef]
- Carrington, C.B.; Gaensler, E.A.; Coutu, R.E.; FitzGerald, M.X.; Gupta, R.G. Natural history and treated course of usual and desquamative interstitial pneumonia. N. Engl. J. Med. 1978, 298, 801–809. [Google Scholar] [CrossRef]
- Baumgartner, K.B.; Samet, J.M.; Stidley, C.A.; Colby, T.V.; Waldron, J.A. Cigarette smoking: A risk factor for idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 1997, 155, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Nabel, E.G. Cardiovascular disease. N. Engl. J. Med. 2003, 349, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Sciarretta, S.; Violi, F.; Nocella, C.; Loffredo, L.; Perri, L.; Peruzzi, M.; Marullo, A.G.; De Falco, E.; Chimenti, I.; et al. Acute Impact of Tobacco vs Electronic Cigarette Smoking on Oxidative Stress and Vascular Function. Chest 2016, 150, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Munzel, T.; Hahad, O.; Kuntic, M.; Keaney, J.F.; Deanfield, J.E.; Daiber, A. Effects of tobacco cigarettes, e-cigarettes, and waterpipe smoking on endothelial function and clinical outcomes. Eur. Heart J. 2020, 41, 4057–4070. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, T.; Yla-Herttuala, S.; Luoma, J.; Kurz, S.; Munzel, T.; Just, H.; Olschewski, M.; Drexler, H. Cigarette smoking potentiates endothelial dysfunction of forearm resistance vessels in patients with hypercholesterolemia. Role of oxidized LDL. Circulation 1996, 93, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Lavi, S.; Prasad, A.; Yang, E.H.; Mathew, V.; Simari, R.D.; Rihal, C.S.; Lerman, L.O.; Lerman, A. Smoking is associated with epicardial coronary endothelial dysfunction and elevated white blood cell count in patients with chest pain and early coronary artery disease. Circulation 2007, 115, 2621–2627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murohara, T.; Kugiyama, K.; Ohgushi, M.; Sugiyama, S.; Yasue, H. Cigarette smoke extract contracts isolated porcine coronary arteries by superoxide anion-mediated degradation of EDRF. Am. J. Physiol. 1994, 266, H874–H880. [Google Scholar] [CrossRef] [PubMed]
- Howard, G.; Wagenknecht, L.E.; Burke, G.L.; Diez-Roux, A.; Evans, G.W.; McGovern, P.; Nieto, F.J.; Tell, G.S. Cigarette smoking and progression of atherosclerosis: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA 1998, 279, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Benowitz, N.L.; Burbank, A.D. Cardiovascular toxicity of nicotine: Implications for electronic cigarette use. Trends Cardiovasc. Med. 2016, 26, 515–523. [Google Scholar] [CrossRef] [Green Version]
- Jatoi, N.A.; Jerrard-Dunne, P.; Feely, J.; Mahmud, A. Impact of smoking and smoking cessation on arterial stiffness and aortic wave reflection in hypertension. Hypertension 2007, 49, 981–985. [Google Scholar] [CrossRef] [Green Version]
- Hackshaw, A.; Morris, J.K.; Boniface, S.; Tang, J.L.; Milenkovic, D. Low cigarette consumption and risk of coronary heart disease and stroke: Meta-analysis of 141 cohort studies in 55 study reports. BMJ 2018, 360, j5855. [Google Scholar] [CrossRef] [Green Version]
- Iso, H.; Date, C.; Yamamoto, A.; Toyoshima, H.; Watanabe, Y.; Kikuchi, S.; Koizumi, A.; Wada, Y.; Kondo, T.; Inaba, Y.; et al. Smoking cessation and mortality from cardiovascular disease among Japanese men and women: The JACC Study. Am. J. Epidemiol. 2005, 161, 170–179. [Google Scholar] [CrossRef]
- Venkatason, P.; Salleh, N.M.; Zubairi, Y.; Hafidz, I.; Ahmad, W.A.; Han, S.K.; Zuhdi, A.S. The bizzare phenomenon of smokers’ paradox in the immediate outcome post acute myocardial infarction: An insight into the Malaysian National Cardiovascular Database-Acute Coronary Syndrome (NCVD-ACS) registry year 2006–2013. Springerplus 2016, 5, 534. [Google Scholar] [CrossRef] [Green Version]
- Heeringa, J.; Kors, J.A.; Hofman, A.; van Rooij, F.J.; Witteman, J.C. Cigarette smoking and risk of atrial fibrillation: The Rotterdam Study. Am. Heart J. 2008, 156, 1163–1169. [Google Scholar] [CrossRef]
- Rimm, E.B.; Chan, J.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C. Prospective study of cigarette smoking, alcohol use, and the risk of diabetes in men. BMJ 1995, 310, 555–559. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Shu, X.O.; Li, H.; Cai, H.; Liu, Q.; Zheng, W.; Xiang, Y.B.; Villegas, R. Physical activity, smoking, and alcohol consumption in association with incidence of type 2 diabetes among middle-aged and elderly Chinese men. PLoS ONE 2013, 8, e77919. [Google Scholar] [CrossRef] [Green Version]
- Sargeant, L.A.; Khaw, K.T.; Bingham, S.; Day, N.E.; Luben, R.N.; Oakes, S.; Welch, A.; Wareham, N.J. Cigarette smoking and glycaemia: The EPIC-Norfolk Study. European Prospective Investigation into Cancer. Int. J. Epidemiol. 2001, 30, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Rossouw, J.; Tong, E.; Giovino, G.A.; Lee, C.C.; Chen, C.; Ockene, J.K.; Qi, L.; Margolis, K.L. Smoking and diabetes: Does the increased risk ever go away? Am. J. Epidemiol. 2013, 178, 937–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahad, O.; Wild, P.S.; Prochaska, J.H.; Schulz, A.; Hermanns, I.; Lackner, K.J.; Pfeiffer, N.; Schmidtmann, I.; Beutel, M.; Gori, T.; et al. Endothelial Function Assessed by Digital Volume Plethysmography Predicts the Development and Progression of Type 2 Diabetes Mellitus. J. Am. Heart Assoc. 2019, 8, e012509. [Google Scholar] [CrossRef] [PubMed]
- Pryor, W.A.; Stone, K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann. N. Y. Acad. Sci. 1993, 686, 12–27. [Google Scholar] [CrossRef]
- Messner, B.; Bernhard, D. Smoking and cardiovascular disease: Mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb. Vasc. Biol. 2014, 34, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Szabo, C.; Ischiropoulos, H.; Radi, R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug. Discov. 2007, 6, 662–680. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Matsuno, S.; Kagota, S.; Haginaka, J.; Kunitomo, M. Peroxynitrite-mediated oxidative modification of low-density lipoprotein by aqueous extracts of cigarette smoke and the preventive effect of fluvastatin. Atherosclerosis 2004, 172, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Holvoet, P.; De Keyzer, D.; Jacobs, D.R., Jr. Oxidized LDL and the metabolic syndrome. Future Lipidol. 2008, 3, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Jaimes, E.A.; DeMaster, E.G.; Tian, R.X.; Raij, L. Stable compounds of cigarette smoke induce endothelial superoxide anion production via NADPH oxidase activation. Arterioscler Thromb. Vasc. Biol. 2004, 24, 1031–1036. [Google Scholar] [CrossRef] [Green Version]
- Dikalov, S.; Itani, H.; Richmond, B.; Vergeade, A.; Rahman, S.M.J.; Boutaud, O.; Blackwell, T.; Massion, P.P.; Harrison, D.G.; Dikalova, A. Tobacco smoking induces cardiovascular mitochondrial oxidative stress, promotes endothelial dysfunction, and enhances hypertension. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H639–H646. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Steven, S.; Euler, G.; Schulz, R. Vascular and cardiac oxidative stress and inflammation as targets for cardioprotection. Curr. Pharm. Des. 2021. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstadter, J.; Kroller-Schon, S.; Munzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid. Med. Cell Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kianoush, S.; Yakoob, M.Y.; Al-Rifai, M.; DeFilippis, A.P.; Bittencourt, M.S.; Duncan, B.B.; Bensenor, I.M.; Bhatnagar, A.; Lotufo, P.A.; Blaha, M.J. Associations of Cigarette Smoking With Subclinical Inflammation and Atherosclerosis: ELSA-Brasil (The Brazilian Longitudinal Study of Adult Health). J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Tibuakuu, M.; Kamimura, D.; Kianoush, S.; DeFilippis, A.P.; Al Rifai, M.; Reynolds, L.M.; White, W.B.; Butler, K.R.; Mosley, T.H.; Turner, S.T.; et al. The association between cigarette smoking and inflammation: The Genetic Epidemiology Network of Arteriopathy (GENOA) study. PLoS ONE 2017, 12, e0184914. [Google Scholar] [CrossRef]
- Gualano, R.C.; Hansen, M.J.; Vlahos, R.; Jones, J.E.; Park-Jones, R.A.; Deliyannis, G.; Turner, S.J.; Duca, K.A.; Anderson, G.P. Cigarette smoke worsens lung inflammation and impairs resolution of influenza infection in mice. Respir. Res. 2008, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Sopori, M. Effects of cigarette smoke on the immune system. Nat. Rev. Immunol. 2002, 2, 372–377. [Google Scholar] [CrossRef]
- Escarcega, R.O.; Lipinski, M.J.; Garcia-Carrasco, M.; Mendoza-Pinto, C.; Galvez-Romero, J.L.; Cervera, R. Inflammation and atherosclerosis: Cardiovascular evaluation in patients with autoimmune diseases. Autoimmun. Rev. 2018, 17, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Iho, S.; Tanaka, Y.; Takauji, R.; Kobayashi, C.; Muramatsu, I.; Iwasaki, H.; Nakamura, K.; Sasaki, Y.; Nakao, K.; Takahashi, T. Nicotine induces human neutrophils to produce IL-8 through the generation of peroxynitrite and subsequent activation of NF-kappaB. J. Leukoc. Biol. 2003, 74, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Lowery, C.L., 3rd; Elliott, C.; Cooper, A.; Hadden, C.; Sonon, R.N.; Azadi, P.; Williams, D.K.; Marsh, J.D.; Woulfe, D.S.; Kilic, F. Cigarette Smoking-Associated Alterations in Serotonin/Adrenalin Signaling Pathways of Platelets. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Bortolasci, C.C.; Vargas, H.O.; Souza-Nogueira, A.; Barbosa, D.S.; Moreira, E.G.; Nunes, S.O.; Berk, M.; Dodd, S.; Maes, M. Lowered plasma paraoxonase (PON)1 activity is a trait marker of major depression and PON1 Q192R gene polymorphism-smoking interactions differentially predict the odds of major depression and bipolar disorder. J. Affect. Disord. 2014, 159, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Forlenza, M.J.; Miller, G.E. Increased serum levels of 8-hydroxy-2’-deoxyguanosine in clinical depression. Psychosom. Med. 2006, 68, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maes, M.; Mihaylova, I.; Kubera, M.; Leunis, J.C.; Geffard, M. IgM-mediated autoimmune responses directed against multiple neoepitopes in depression: New pathways that underpin the inflammatory and neuroprogressive pathophysiology. J. Affect. Disord. 2011, 135, 414–418. [Google Scholar] [CrossRef]
- Parker, W.; Hornik, C.D.; Bilbo, S.; Holzknecht, Z.E.; Gentry, L.; Rao, R.; Lin, S.S.; Herbert, M.R.; Nevison, C.D. The role of oxidative stress, inflammation and acetaminophen exposure from birth to early childhood in the induction of autism. J. Int. Med. Res. 2017, 45, 407–438. [Google Scholar] [CrossRef]
- Wilson, C.B.; McLaughlin, L.D.; Nair, A.; Ebenezer, P.J.; Dange, R.; Francis, J. Inflammation and oxidative stress are elevated in the brain, blood, and adrenal glands during the progression of post-traumatic stress disorder in a predator exposure animal model. PLoS ONE 2013, 8, e76146. [Google Scholar] [CrossRef]
- Blanc, E.M.; Keller, J.N.; Fernandez, S.; Mattson, M.P. 4-hydroxynonenal, a lipid peroxidation product, impairs glutamate transport in cortical astrocytes. Glia 1998, 22, 149–160. [Google Scholar] [CrossRef]
- Sultana, R.; Perluigi, M.; Butterfield, D.A. Protein oxidation and lipid peroxidation in brain of subjects with Alzheimer’s disease: Insights into mechanism of neurodegeneration from redox proteomics. Antioxid. Redox. Signal. 2006, 8, 2021–2037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picklo, M.J.; Amarnath, V.; McIntyre, J.O.; Graham, D.G.; Montine, T.J. 4-Hydroxy-2(E)-nonenal inhibits CNS mitochondrial respiration at multiple sites. J. Neurochem. 1999, 72, 1617–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deas, E.; Cremades, N.; Angelova, P.R.; Ludtmann, M.H.; Yao, Z.; Chen, S.; Horrocks, M.H.; Banushi, B.; Little, D.; Devine, M.J.; et al. Alpha-Synuclein Oligomers Interact with Metal Ions to Induce Oxidative Stress and Neuronal Death in Parkinson’s Disease. Antioxid. Redox. Signal. 2016, 24, 376–391. [Google Scholar] [CrossRef] [Green Version]
- Tonnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [Green Version]
- Reichenberg, A.; Yirmiya, R.; Schuld, A.; Kraus, T.; Haack, M.; Morag, A.; Pollmacher, T. Cytokine-associated emotional and cognitive disturbances in humans. Arch. Gen. Psychiatry 2001, 58, 445–452. [Google Scholar] [CrossRef]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L.; et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef] [Green Version]
- Goldsmith, D.R.; Rapaport, M.H.; Miller, B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: Comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry 2016, 21, 1696–1709. [Google Scholar] [CrossRef] [PubMed]
- Attwells, S.; Setiawan, E.; Wilson, A.A.; Rusjan, P.M.; Mizrahi, R.; Miler, L.; Xu, C.; Richter, M.A.; Kahn, A.; Kish, S.J.; et al. Inflammation in the Neurocircuitry of Obsessive-Compulsive Disorder. JAMA Psychiatry 2017, 74, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Anand, D.; Colpo, G.D.; Zeni, G.; Zeni, C.P.; Teixeira, A.L. Attention-Deficit/Hyperactivity Disorder And Inflammation: What Does Current Knowledge Tell Us? A Systematic Review. Front. Psychiatry 2017, 8, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oades, R.D. An exploration of the associations of pregnancy and perinatal features with cytokines and tryptophan/kynurenine metabolism in children with attention-deficit hyperactivity disorder (ADHD). Atten. Defic. Hyperact. Disord. 2011, 3, 301–318. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Zusso, M.; Giusti, P. An Inflammation-Centric View of Neurological Disease: Beyond the Neuron. Front. Cell Neurosci. 2018, 12, 72. [Google Scholar] [CrossRef]
- Joseph, N.; Zhang-James, Y.; Perl, A.; Faraone, S.V. Oxidative Stress and ADHD: A Meta-Analysis. J. Atten. Disord. 2015, 19, 915–924. [Google Scholar] [CrossRef]
- Guilarte, T.R.; Opler, M.; Pletnikov, M. Is lead exposure in early life an environmental risk factor for Schizophrenia? Neurobiological connections and testable hypotheses. Neurotoxicology 2012, 33, 560–574. [Google Scholar] [CrossRef] [Green Version]
- Dome, P.; Lazary, J.; Kalapos, M.P.; Rihmer, Z. Smoking, nicotine and neuropsychiatric disorders. Neurosci. Biobehav. Rev. 2010, 34, 295–342. [Google Scholar] [CrossRef]
- Jacobsen, L.K.; D’Souza, D.C.; Mencl, W.E.; Pugh, K.R.; Skudlarski, P.; Krystal, J.H. Nicotine effects on brain function and functional connectivity in schizophrenia. Biol. Psychiatry 2004, 55, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.C.; Warner-Cohen, J.; Matute, M.; Butler, E.; Kelly, E.; Vaidhyanathaswamy, S.; Khan, A. Effects of nicotine nasal spray on cognitive function in schizophrenia. Neuropsychopharmacology 2006, 31, 637–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, E.D.; Conners, C.K.; Silva, D.; Canu, W.; March, J. Effects of chronic nicotine and methylphenidate in adults with attention deficit/hyperactivity disorder. Exp. Clin. PsychoPharmacol. 2001, 9, 83–90. [Google Scholar] [CrossRef]
- Potter, A.S.; Newhouse, P.A. Acute nicotine improves cognitive deficits in young adults with attention-deficit/hyperactivity disorder. Pharmacol. Biochem. Behav. 2008, 88, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.; Martin, J. Smoking and drug interactions. Aust. Prescr. 2013, 36, 102–104. [Google Scholar] [CrossRef]
- Bondolfi, G.; Morel, F.; Crettol, S.; Rachid, F.; Baumann, P.; Eap, C.B. Increased clozapine plasma concentrations and side effects induced by smoking cessation in 2 CYP1A2 genotyped patients. Ther. Drug Monit. 2005, 27, 539–543. [Google Scholar] [CrossRef]
- Mihailescu, S.; Drucker-Colin, R. Nicotine, brain nicotinic receptors, and neuropsychiatric disorders. Arch. Med. Res. 2000, 31, 131–144. [Google Scholar] [CrossRef]
- Newhouse, P.A.; Hughes, J.R. The role of nicotine and nicotinic mechanisms in neuropsychiatric disease. Br. J. Addict. 1991, 86, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Mackay, J.; Schluger, N.; Gomeshtapeh, F.I.; Drope, J. The Tobacco Atlas. Atlanta: American Cancer Society. 2015. Available online: https://ncdalliance.org/sites/default/files/resource_files/TA5_2015_WEB.pdf (accessed on 16 April 2021).
- Boksa, P. Smoking, psychiatric illness and the brain. J. Psychiatry Neurosci. 2017, 42, 147–149. [Google Scholar] [CrossRef] [Green Version]
- Beutel, M.E.; Brahler, E.; Wiltink, J.; Kerahrodi, J.G.; Burghardt, J.; Michal, M.; Schulz, A.; Wild, P.S.; Munzel, T.; Schmidtmann, I.; et al. New onset of depression in aging women and men: Contributions of social, psychological, behavioral, and somatic predictors in the community. Psychol. Med. 2019, 49, 1148–1155. [Google Scholar] [CrossRef]
- Cabello, M.; Miret, M.; Caballero, F.F.; Chatterji, S.; Naidoo, N.; Kowal, P.; D’Este, C.; Ayuso-Mateos, J.L. The role of unhealthy lifestyles in the incidence and persistence of depression: A longitudinal general population study in four emerging countries. Glob. Health 2017, 13, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flensborg-Madsen, T.; von Scholten, M.B.; Flachs, E.M.; Mortensen, E.L.; Prescott, E.; Tolstrup, J.S. Tobacco smoking as a risk factor for depression. A 26-year population-based follow-up study. J. Psychiatr. Res. 2011, 45, 143–149. [Google Scholar] [CrossRef]
- Klungsoyr, O.; Nygard, J.F.; Sorensen, T.; Sandanger, I. Cigarette smoking and incidence of first depressive episode: An 11-year, population-based follow-up study. Am. J. Epidemiol. 2006, 163, 421–432. [Google Scholar] [CrossRef] [Green Version]
- Pasco, J.A.; Williams, L.J.; Jacka, F.N.; Ng, F.; Henry, M.J.; Nicholson, G.C.; Kotowicz, M.A.; Berk, M. Tobacco smoking as a risk factor for major depressive disorder: Population-based study. Br. J. Psychiatry 2008, 193, 322–326. [Google Scholar] [CrossRef]
- Goodman, E.; Capitman, J. Depressive symptoms and cigarette smoking among teens. Pediatrics 2000, 106, 748–755. [Google Scholar] [CrossRef] [Green Version]
- Kang, E.; Lee, J. A longitudinal study on the causal association between smoking and depression. J. Prev. Med. Public Health 2010, 43, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Munafo, M.R.; Hitsman, B.; Rende, R.; Metcalfe, C.; Niaura, R. Effects of progression to cigarette smoking on depressed mood in adolescents: Evidence from the National Longitudinal Study of Adolescent Health. Addiction 2008, 103, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Fluharty, M.; Taylor, A.E.; Grabski, M.; Munafo, M.R. The Association of Cigarette Smoking With Depression and Anxiety: A Systematic Review. Nicotine Tob. Res. 2017, 19, 3–13. [Google Scholar] [CrossRef]
- Leung, J.; Gartner, C.; Hall, W.; Lucke, J.; Dobson, A. A longitudinal study of the bi-directional relationship between tobacco smoking and psychological distress in a community sample of young Australian women. Psychol. Med. 2012, 42, 1273–1282. [Google Scholar] [CrossRef] [Green Version]
- Khaled, S.M.; Bulloch, A.G.; Williams, J.V.; Hill, J.C.; Lavorato, D.H.; Patten, S.B. Persistent heavy smoking as risk factor for major depression (MD) incidence--evidence from a longitudinal Canadian cohort of the National Population Health Survey. J. Psychiatr. Res. 2012, 46, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.G.; Cohen, P.; Pine, D.S.; Klein, D.F.; Kasen, S.; Brook, J.S. Association between cigarette smoking and anxiety disorders during adolescence and early adulthood. JAMA 2000, 284, 2348–2351. [Google Scholar] [CrossRef] [PubMed]
- Mojtabai, R.; Crum, R.M. Cigarette smoking and onset of mood and anxiety disorders. Am. J. Public Health 2013, 103, 1656–1665. [Google Scholar] [CrossRef]
- Cuijpers, P.; Smit, F.; ten Have, M.; de Graaf, R. Smoking is associated with first-ever incidence of mental disorders: A prospective population-based study. Addiction 2007, 102, 1303–1309. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, W.; von Soest, T. Smoking, nicotine dependence and mental health among young adults: A 13-year population-based longitudinal study. Addiction 2009, 104, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Kirkwood, B.R.; Pednekar, S.; Weiss, H.; Mabey, D. Risk factors for common mental disorders in women. Population-based longitudinal study. Br. J. Psychiatry 2006, 189, 547–555. [Google Scholar] [CrossRef]
- Taylor, A.E.; Fluharty, M.E.; Bjorngaard, J.H.; Gabrielsen, M.E.; Skorpen, F.; Marioni, R.E.; Campbell, A.; Engmann, J.; Mirza, S.S.; Loukola, A.; et al. Investigating the possible causal association of smoking with depression and anxiety using Mendelian randomisation meta-analysis: The CARTA consortium. BMJ Open 2014, 4, e006141. [Google Scholar] [CrossRef] [Green Version]
- Poorolajal, J.; Darvishi, N. Smoking and Suicide: A Meta-Analysis. PLoS ONE 2016, 11, e0156348. [Google Scholar] [CrossRef]
- Li, D.; Yang, X.; Ge, Z.; Hao, Y.; Wang, Q.; Liu, F.; Gu, D.; Huang, J. Cigarette smoking and risk of completed suicide: A meta-analysis of prospective cohort studies. J. Psychiatr. Res. 2012, 46, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Breslau, N.; Schultz, L.R.; Johnson, E.O.; Peterson, E.L.; Davis, G.C. Smoking and the risk of suicidal behavior: A prospective study of a community sample. Arch. Gen. Psychiatry 2005, 62, 328–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, M.; O’Reilly, E.J.; Mirzaei, F.; Okereke, O.I.; Unger, L.; Miller, M.; Ascherio, A. Cigarette smoking and completed suicide: Results from 3 prospective cohorts of American adults. J. Affect. Disord. 2013, 151, 1053–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evins, A.E.; Korhonen, T.; Kinnunen, T.H.; Kaprio, J. Prospective association between tobacco smoking and death by suicide: A competing risks hazard analysis in a large twin cohort with 35-year follow-up. Psychol. Med. 2017, 47, 2143–2154. [Google Scholar] [CrossRef]
- Holma, K.M.; Holma, I.; Ketokivi, M.; Oquendo, M.A.; Isometsa, E. The Relationship between Smoking and Suicidal Behavior in Psychiatric Patients with Major Depressive Disorder. Arch. Suicide Res. 2019, 23, 590–604. [Google Scholar] [CrossRef] [PubMed]
- McGee, R.; Williams, S.; Nada-Raja, S. Is cigarette smoking associated with suicidal ideation among young people? Am. J. Psychiatry 2005, 162, 619–620. [Google Scholar] [CrossRef]
- Bronisch, T.; Hofler, M.; Lieb, R. Smoking predicts suicidality: Findings from a prospective community study. J. Affect. Disord. 2008, 108, 135–145. [Google Scholar] [CrossRef]
- Anstey, K.J.; von Sanden, C.; Salim, A.; O’Kearney, R. Smoking as a risk factor for dementia and cognitive decline: A meta-analysis of prospective studies. Am. J. Epidemiol. 2007, 166, 367–378. [Google Scholar] [CrossRef]
- Zhong, G.; Wang, Y.; Zhang, Y.; Guo, J.J.; Zhao, Y. Smoking is associated with an increased risk of dementia: A meta-analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS ONE 2015, 10, e0118333. [Google Scholar] [CrossRef] [Green Version]
- Beydoun, M.A.; Beydoun, H.A.; Gamaldo, A.A.; Teel, A.; Zonderman, A.B.; Wang, Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: Systematic review and meta-analysis. BMC Public Health 2014, 14, 643. [Google Scholar] [CrossRef] [Green Version]
- Li, X.Y.; Zhang, M.; Xu, W.; Li, J.Q.; Cao, X.P.; Yu, J.T.; Tan, L. Midlife Modifiable Risk Factors for Dementia: A Systematic Review and Meta-analysis of 34 Prospective Cohort Studies. Curr. Alzheimer. Res. 2019, 16, 1254–1268. [Google Scholar] [CrossRef]
- Lu, Y.; Sugawara, Y.; Zhang, S.; Tomata, Y.; Tsuji, I. Smoking cessation and incident dementia in elderly Japanese: The Ohsaki Cohort 2006 Study. Eur. J. Epidemiol. 2020, 35, 851–860. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.; Choi, S.; Park, S.M. Effect of smoking cessation on the risk of dementia: A longitudinal study. Ann. Clin. Transl. Neurol. 2018, 5, 1192–1199. [Google Scholar] [CrossRef]
- Otuyama, L.J.; Oliveira, D.; Locatelli, D.; Machado, D.A.; Noto, A.R.; Galduroz, J.C.F.; Prince, M.J.; Ferri, C.P. Tobacco smoking and risk for dementia: Evidence from the 10/66 population-based longitudinal study. Aging Ment. Health 2020, 24, 1796–1806. [Google Scholar] [CrossRef] [Green Version]
- Gurillo, P.; Jauhar, S.; Murray, R.M.; MacCabe, J.H. Does tobacco use cause psychosis? Systematic review and meta-analysis. Lancet Psychiatry 2015, 2, 718–725. [Google Scholar] [CrossRef] [Green Version]
- Hunter, A.; Murray, R.; Asher, L.; Leonardi-Bee, J. The Effects of Tobacco Smoking, and Prenatal Tobacco Smoke Exposure, on Risk of Schizophrenia: A Systematic Review and Meta-Analysis. Nicotine Tob. Res. 2020, 22, 3–10. [Google Scholar] [CrossRef]
- Mustonen, A.; Ahokas, T.; Nordstrom, T.; Murray, G.K.; Maki, P.; Jaaskelainen, E.; Heiskala, A.; McGrath, J.J.; Scott, J.G.; Miettunen, J.; et al. Smokin’ hot: Adolescent smoking and the risk of psychosis. Acta Psychiatr. Scand. 2018, 138, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kendler, K.S.; Lonn, S.L.; Sundquist, J.; Sundquist, K. Smoking and schizophrenia in population cohorts of Swedish women and men: A prospective co-relative control study. Am. J. Psychiatry 2015, 172, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Wootton, R.E.; Richmond, R.C.; Stuijfzand, B.G.; Lawn, R.B.; Sallis, H.M.; Taylor, G.M.J.; Hemani, G.; Jones, H.J.; Zammit, S.; Davey Smith, G.; et al. Evidence for causal effects of lifetime smoking on risk for depression and schizophrenia: A Mendelian randomisation study. Psychol. Med. 2020, 50, 2435–2443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dworetzky, B.A.; Bromfield, E.B.; Townsend, M.K.; Kang, J.H. A prospective study of smoking, caffeine, and alcohol as risk factors for seizures or epilepsy in young adult women: Data from the Nurses’ Health Study II. Epilepsia 2010, 51, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Mappin-Kasirer, B.; Pan, H.; Lewington, S.; Kizza, J.; Gray, R.; Clarke, R.; Peto, R. Tobacco smoking and the risk of Parkinson disease: A 65-year follow-up of 30,000 male British doctors. Neurology 2020, 94, e2132–e2138. [Google Scholar] [CrossRef]
- Gallo, V.; Vineis, P.; Cancellieri, M.; Chiodini, P.; Barker, R.A.; Brayne, C.; Pearce, N.; Vermeulen, R.; Panico, S.; Bueno-de-Mesquita, B.; et al. Exploring causality of the association between smoking and Parkinson’s disease. Int. J. Epidemiol. 2019, 48, 912–925. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Lu, Y.; Cheng, H.; Wang, C.; Chan, P. The impact of long-term exposure to ambient air pollution and second-hand smoke on the onset of Parkinson disease: A review and meta-analysis. Public Health 2020, 179, 100–110. [Google Scholar] [CrossRef]
- Rosen, B.N.; Lee, B.K.; Lee, N.L.; Yang, Y.; Burstyn, I. Maternal Smoking and Autism Spectrum Disorder: A Meta-analysis. J. Autism. Dev. Disord. 2015, 45, 1689–1698. [Google Scholar] [CrossRef]
- Jung, Y.; Lee, A.M.; McKee, S.A.; Picciotto, M.R. Maternal smoking and autism spectrum disorder: Meta-analysis with population smoking metrics as moderators. Sci. Rep. 2017, 7, 4315. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.L.; Lehti, V.; Lampi, K.M.; Helenius, H.; Suominen, A.; Gissler, M.; Brown, A.S.; Sourander, A. Smoking during pregnancy and risk of autism spectrum disorder in a Finnish National Birth Cohort. Paediatr. Perinat. Epidemiol. 2013, 27, 266–274. [Google Scholar] [CrossRef]
- Caramaschi, D.; Taylor, A.E.; Richmond, R.C.; Havdahl, K.A.; Golding, J.; Relton, C.L.; Munafo, M.R.; Davey Smith, G.; Rai, D. Maternal smoking during pregnancy and autism: Using causal inference methods in a birth cohort study. Transl. Psychiatry 2018, 8, 262. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Wang, Y.; Zhang, L.; Zheng, Z.; Zhu, T.; Qu, Y.; Mu, D. Maternal Smoking and Attention-Deficit/Hyperactivity Disorder in Offspring: A Meta-analysis. Pediatrics 2018, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, T.; Hu, W.; Zhou, X.; Lin, H.; Lan, L.; Hang, B.; Lv, W.; Geng, Q.; Xia, Y. Prenatal exposure to maternal smoking during pregnancy and attention-deficit/hyperactivity disorder in offspring: A meta-analysis. Reprod. Toxicol. 2018, 76, 63–70. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, J.; Zhu, L.H.; Hua, L.L.; Ke, F.F. Maternal Smoking during Pregnancy and ADHD: Results from a Systematic Review and Meta-Analysis of Prospective Cohort Studies. J. Atten. Disord. 2020, 24, 1637–1647. [Google Scholar] [CrossRef]
- Joelsson, P.; Chudal, R.; Talati, A.; Suominen, A.; Brown, A.S.; Sourander, A. Prenatal smoking exposure and neuropsychiatric comorbidity of ADHD: A finnish nationwide population-based cohort study. BMC Psychiatry 2016, 16, 306. [Google Scholar] [CrossRef] [Green Version]
- Hahad, O.; Lelieveld, J.; Birklein, F.; Lieb, K.; Daiber, A.; Munzel, T. Ambient Air Pollution Increases the Risk of Cerebrovascular and Neuropsychiatric Disorders through Induction of Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 4306. [Google Scholar] [CrossRef]
- Halder, S.; Kar, R.; Chakraborty, S.; Bhattacharya, S.K.; Mediratta, P.K.; Banerjee, B.D. Cadmium level in brain correlates with memory impairment in F1 and F2 generation mice: Improvement with quercetin. Environ. Sci. Pollut Res. Int. 2019, 26, 9632–9639. [Google Scholar] [CrossRef] [PubMed]
- Kienzl, E.; Puchinger, L.; Jellinger, K.; Linert, W.; Stachelberger, H.; Jameson, R.F. The role of transition metals in the pathogenesis of Parkinson’s disease. J. Neurol. Sci. 1995, 134, 69–78. [Google Scholar] [CrossRef]
- Maher, P. Potentiation of glutathione loss and nerve cell death by the transition metals iron and copper: Implications for age-related neurodegenerative diseases. Free Radic. Biol. Med. 2018, 115, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yujie, Q.; Gong, S.; Zhang, H.; Ding, S. Learning and memory impairment of mice caused by gaseous formaldehyde. Environ. Res. 2020, 184, 109318. [Google Scholar] [CrossRef]
- Rana, I.; Rieswijk, L.; Steinmaus, C.; Zhang, L. Formaldehyde and Brain Disorders: A Meta-Analysis and Bioinformatics Approach. Neurotox. Res. 2021, 39, 924–948. [Google Scholar] [CrossRef]
- Wood, P.L.; Khan, M.A.; Kulow, S.R.; Mahmood, S.A.; Moskal, J.R. Neurotoxicity of reactive aldehydes: The concept of "aldehyde load" as demonstrated by neuroprotection with hydroxylamines. Brain Res. 2006, 1095, 190–199. [Google Scholar] [CrossRef]
- Wang, F.; Fangfang, Z.; Guo, X.; Chen, W.; Yao, W.; Liu, H.; Lyu, C.; Zhang, Y.; Fan, C. Effects of volatile organic compounds and carbon monoxide mixtures on learning and memory, oxidative stress, and monoamine neurotransmitters in the brains of mice. Toxicol. Ind. Health 2018, 34, 178–187. [Google Scholar] [CrossRef]
- Sivandzade, F.; Cucullo, L. Assessing the protective effect of rosiglitazone against electronic cigarette/tobacco smoke-induced blood-brain barrier impairment. BMC Neurosci. 2019, 20, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paquette, S.T.; Dawes, R.P.; Sundar, I.K.; Rahman, I.; Brown, E.B.; White, P.M. Chronic cigarette smoke exposure drives spiral ganglion neuron loss in mice. Sci. Rep. 2018, 8, 5746. [Google Scholar] [CrossRef] [Green Version]
- Starke, R.M.; Thompson, J.W.; Ali, M.S.; Pascale, C.L.; Martinez Lege, A.; Ding, D.; Chalouhi, N.; Hasan, D.M.; Jabbour, P.; Owens, G.K.; et al. Cigarette Smoke Initiates Oxidative Stress-Induced Cellular Phenotypic Modulation Leading to Cerebral Aneurysm Pathogenesis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 610–621. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.L.; Saad, S.; Pollock, C.; Oliver, B.; Al-Odat, I.; Zaky, A.A.; Jones, N.; Chen, H. Impact of maternal cigarette smoke exposure on brain inflammation and oxidative stress in male mice offspring. Sci. Rep. 2016, 6, 25881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanna, A.; Guo, M.; Mehra, M.; Royal, W., 3rd. Inflammation and oxidative stress induced by cigarette smoke in Lewis rat brains. J. NeuroImmunol. 2013, 254, 69–75. [Google Scholar] [CrossRef] [Green Version]
- La Maestra, S.; Kisby, G.E.; Micale, R.T.; Johnson, J.; Kow, Y.W.; Bao, G.; Sheppard, C.; Stanfield, S.; Tran, H.; Woltjer, R.L.; et al. Cigarette smoke induces DNA damage and alters base-excision repair and tau levels in the brain of neonatal mice. Toxicol. Sci. 2011, 123, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Tuon, T.; Valvassori, S.S.; Lopes-Borges, J.; Fries, G.R.; Silva, L.A.; Kapczinski, F.; Quevedo, J.; Pinho, R.A. Effects of moderate exercise on cigarette smoke exposure-induced hippocampal oxidative stress values and neurological behaviors in mice. Neurosci. Lett. 2010, 475, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Kaisar, M.A.; Villalba, H.; Prasad, S.; Liles, T.; Sifat, A.E.; Sajja, R.K.; Abbruscato, T.J.; Cucullo, L. Offsetting the impact of smoking and e-cigarette vaping on the cerebrovascular system and stroke injury: Is Metformin a viable countermeasure? Redox Biol. 2017, 13, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, W.H.; Kim, Y.Y.; Park, H.Y. Air Pollution and Central Nervous System Disease: A Review of the Impact of Fine Particulate Matter on Neurological Disorders. Front. Public Health 2020, 8, 575330. [Google Scholar] [CrossRef]
- Hussien, H.M.; Abd-Elmegied, A.; Ghareeb, D.A.; Hafez, H.S.; Ahmed, H.E.A.; El-Moneam, N.A. Neuroprotective effect of berberine against environmental heavy metals-induced neurotoxicity and Alzheimer’s-like disease in rats. Food Chem. Toxicol. 2018, 111, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Monnet-Tschudi, F.; Zurich, M.G.; Boschat, C.; Corbaz, A.; Honegger, P. Involvement of environmental mercury and lead in the etiology of neurodegenerative diseases. Rev. Environ. Health 2006, 21, 105–117. [Google Scholar] [CrossRef]
- Feron, V.J.; Arts, J.H.; Kuper, C.F.; Slootweg, P.J.; Woutersen, R.A. Health risks associated with inhaled nasal toxicants. Crit. Rev. Toxicol. 2001, 31, 313–347. [Google Scholar] [CrossRef]
- Bernardini, L.; Barbosa, E.; Charao, M.F.; Brucker, N. Formaldehyde toxicity reports from in vitro and in vivo studies: A review and updated data. Drug Chem. Toxicol. 2020. [Google Scholar] [CrossRef]
- Tin Tin Win, S.; Yamamoto, S.; Nakajima, D.; Furuyama, A.; Fukushima, A.; Ahmed, S.; Goto, S.; Fujimaki, H. Modulation of neurological related allergic reaction in mice exposed to low-level toluene. Toxicol. Appl. Pharmacol. 2007, 222, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.Y.; Kong, X.X.; Cheng, Y.; Du, L.; Wang, Z.C.; Yuan, C.; Cheng, J.B.; Yuan, Z.Q.; Zhang, H.Y.; Liao, Y.J. 1,2,4-Trimethoxybenzene selectively inhibits NLRP3 inflammasome activation and attenuates experimental autoimmune encephalomyelitis. Acta Pharmacol. Sin. 2021. [Google Scholar] [CrossRef] [PubMed]
- Prasedya, E.S.; Ambana, Y.; Martyasari, N.W.R.; Aprizal, Y.; Nurrijawati; Sunarpi. Short-term E-cigarette toxicity effects on brain cognitive memory functions and inflammatory responses in mice. Toxicol. Res. 2020, 36, 267–273. [Google Scholar] [CrossRef]
- Yang, X.; Guo, A.L.; Pang, Y.P.; Cheng, X.J.; Xu, T.; Li, X.R.; Liu, J.; Zhang, Y.Y.; Liu, Y. Astaxanthin Attenuates Environmental Tobacco Smoke-Induced Cognitive Deficits: A Critical Role of p38 MAPK. Mar. Drugs 2019, 17, 24. [Google Scholar] [CrossRef] [Green Version]
- Adhami, N.; Chen, Y.; Martins-Green, M. Biomarkers of disease can be detected in mice as early as 4 weeks after initiation of exposure to third-hand smoke levels equivalent to those found in homes of smokers. Clin. Sci. 2017, 131, 2409–2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Li, X.; Gao, S.; Wang, D.; Gao, X.; Li, Y.; Wang, X.; Cui, Z.; Ma, H.; Liu, Q.; et al. Exposure to Cigarette Smoke Augments Post-ischemic Brain Injury and Inflammation via Mobilization of Neutrophils and Monocytes. Front. Immunol. 2019, 10, 2576. [Google Scholar] [CrossRef]
- Croze, M.L.; Zimmer, L. Ozone Atmospheric Pollution and Alzheimer’s Disease: From Epidemiological Facts to Molecular Mechanisms. J. Alzheimers Dis. 2018, 62, 503–522. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gonzalez, I.; Estrada, L.D.; Sanchez-Mejias, E.; Soto, C. Smoking exacerbates amyloid pathology in a mouse model of Alzheimer’s disease. Nat. Commun. 2013, 4, 1495. [Google Scholar] [CrossRef] [Green Version]
- Allen, J.L.; Klocke, C.; Morris-Schaffer, K.; Conrad, K.; Sobolewski, M.; Cory-Slechta, D.A. Cognitive Effects of Air Pollution Exposures and Potential Mechanistic Underpinnings. Curr. Environ. Health Rep. 2017, 4, 180–191. [Google Scholar] [CrossRef]
- Heldt, N.A.; Reichenbach, N.; McGary, H.M.; Persidsky, Y. Effects of Electronic Nicotine Delivery Systems and Cigarettes on Systemic Circulation and Blood-Brain Barrier: Implications for Cognitive Decline. Am. J. Pathol. 2021, 191, 243–255. [Google Scholar] [CrossRef]
- Pelch, K.E.; Bolden, A.L.; Kwiatkowski, C.F. Environmental Chemicals and Autism: A Scoping Review of the Human and Animal Research. Environ. Health Perspect 2019, 127, 46001. [Google Scholar] [CrossRef] [Green Version]
- Allen, J.L.; Oberdorster, G.; Morris-Schaffer, K.; Wong, C.; Klocke, C.; Sobolewski, M.; Conrad, K.; Mayer-Proschel, M.; Cory-Slechta, D.A. Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: Parallels with neuropathological and behavioral features of autism and oTher. neurodevelopmental disorders. Neurotoxicology 2017, 59, 140–154. [Google Scholar] [CrossRef] [Green Version]
- Jayaraj, R.L.; Rodriguez, E.A.; Wang, Y.; Block, M.L. Outdoor Ambient Air Pollution and Neurodegenerative Diseases: The Neuroinflammation Hypothesis. Curr. Environ. Health Rep. 2017, 4, 166–179. [Google Scholar] [CrossRef] [PubMed]
- D’Angiulli, A. Severe Urban Outdoor Air Pollution and Children’s Structural and Functional Brain Development, From Evidence to Precautionary Strategic Action. Front. Public Health 2018, 6, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Nakagawa, S. Planetary Health and the Future of Human Capacity: The Increasing Impact of Planetary Distress on the Human Brain. Challenges 2018, 9, 41. [Google Scholar] [CrossRef] [Green Version]
- Block, M.L.; Calderon-Garciduenas, L. Air pollution: Mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009, 32, 506–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, G.C.; Vilalta, A. How microglia kill neurons. Brain Res. 2015, 1628, 288–297. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Haslund-Vinding, J.; McBean, G.; Jaquet, V.; Vilhardt, F. NADPH oxidases in oxidant production by microglia: Activating receptors, pharmacology and association with disease. Br. J. Pharmacol. 2017, 174, 1733–1749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBean, G.J.; Lopez, M.G.; Wallner, F.K. Redox-based therapeutics in neurodegenerative disease. Br. J. Pharmacol. 2017, 174, 1750–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilhardt, F.; Haslund-Vinding, J.; Jaquet, V.; McBean, G. Microglia antioxidant systems and redox signalling. Br. J. Pharmacol. 2017, 174, 1719–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levesque, S.; Taetzsch, T.; Lull, M.E.; Johnson, J.A.; McGraw, C.; Block, M.L. The role of MAC1 in diesel exhaust particle-induced microglial activation and loss of dopaminergic neuron function. J. Neurochem. 2013, 125, 756–765. [Google Scholar] [CrossRef] [Green Version]
- Block, M.L.; Wu, X.; Pei, Z.; Li, G.; Wang, T.; Qin, L.; Wilson, B.; Yang, J.; Hong, J.S.; Veronesi, B. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: The role of microglia, phagocytosis, and NADPH oxidase. FASEB J. 2004, 18, 1618–1620. [Google Scholar] [CrossRef] [Green Version]
- Hughes, K.; Bellis, M.A.; Hardcastle, K.A.; Sethi, D.; Butchart, A.; Mikton, C.; Jones, L.; Dunne, M.P. The effect of multiple adverse childhood experiences on health: A systematic review and meta-analysis. Lancet Public Health 2017, 2, e356–e366. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hahad, O.; Daiber, A.; Michal, M.; Kuntic, M.; Lieb, K.; Beutel, M.; Münzel, T. Smoking and Neuropsychiatric Disease—Associations and Underlying Mechanisms. Int. J. Mol. Sci. 2021, 22, 7272. https://doi.org/10.3390/ijms22147272
Hahad O, Daiber A, Michal M, Kuntic M, Lieb K, Beutel M, Münzel T. Smoking and Neuropsychiatric Disease—Associations and Underlying Mechanisms. International Journal of Molecular Sciences. 2021; 22(14):7272. https://doi.org/10.3390/ijms22147272
Chicago/Turabian StyleHahad, Omar, Andreas Daiber, Matthias Michal, Marin Kuntic, Klaus Lieb, Manfred Beutel, and Thomas Münzel. 2021. "Smoking and Neuropsychiatric Disease—Associations and Underlying Mechanisms" International Journal of Molecular Sciences 22, no. 14: 7272. https://doi.org/10.3390/ijms22147272
APA StyleHahad, O., Daiber, A., Michal, M., Kuntic, M., Lieb, K., Beutel, M., & Münzel, T. (2021). Smoking and Neuropsychiatric Disease—Associations and Underlying Mechanisms. International Journal of Molecular Sciences, 22(14), 7272. https://doi.org/10.3390/ijms22147272








