Hybrid QM/MM Simulations Confirm Zn(II) Coordination Sphere That Includes Four Cysteines from the P2 × 4R Head Domain
Abstract
:1. Introduction
2. Results
2.1. Redox Potential of the Putative Zn(II) Binding-Site of P2X4R
2.2. QM/MM Molecular Dynamics Simulations of the WT and Mutant Head Domain of the P2X4R Protein
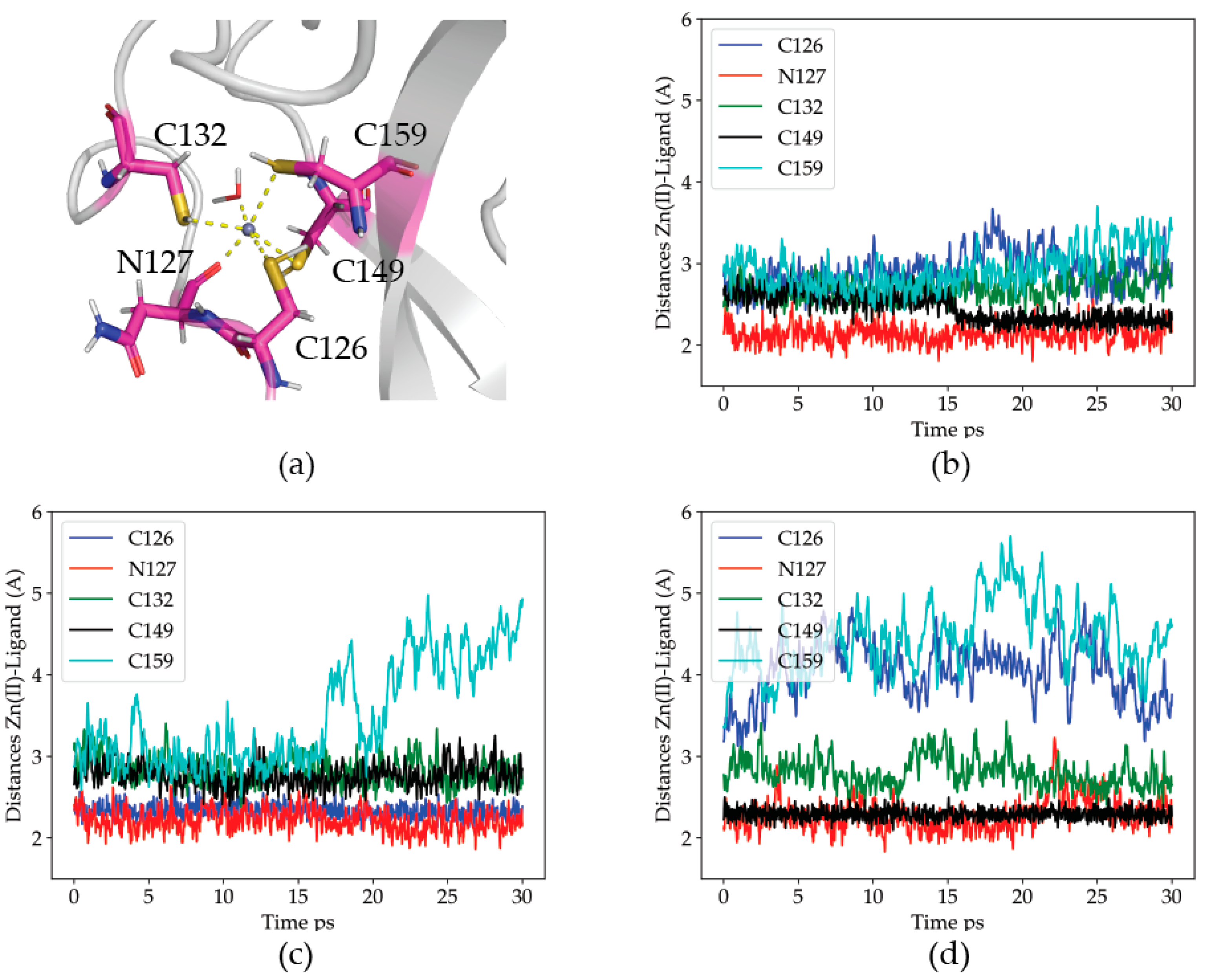
2.2.1. Dynamics of the Zn(II) Site in the Wild Type P2X4R
2.2.2. Dynamics of the C132A Mutant Zn(II) Site
2.2.3. Dynamics of the C132A/C159A Mutant of the Zn(II) Site
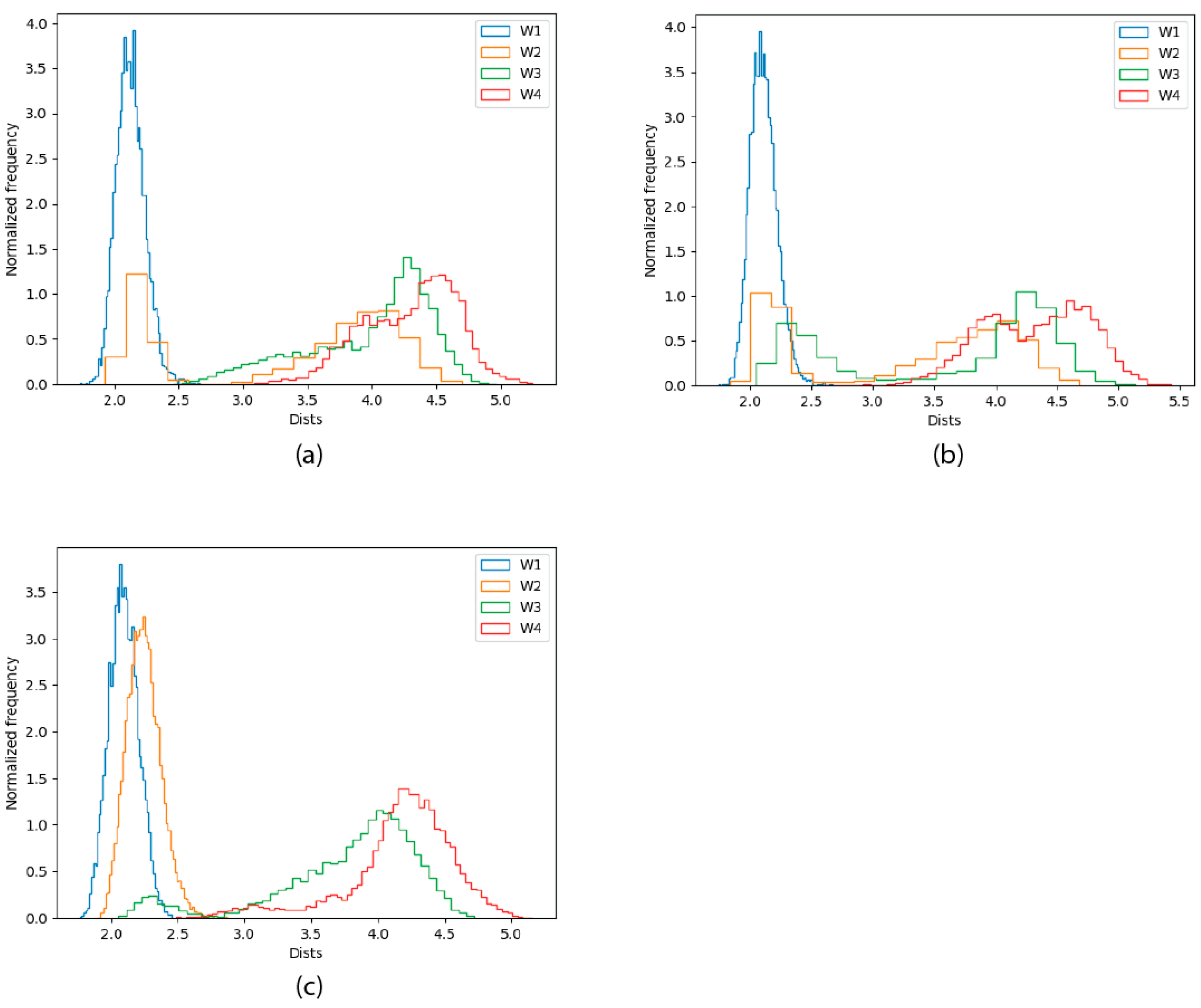
2.2.4. Solvation Analysis
2.2.5. Global Dynamical Features
3. Discussion
4. Materials and Methods
4.1. Estimation of Redox Potentials
4.2. Structural and Dynamical Characterization of the Putative Zn(II) Site of P2X4R in Its WT, and Mutant Variants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mulkidjanian, A.Y.; Galperin, M.Y. On the Origin of Life in the Zinc World. 2. Validation of the Hypothesis on the Photosynthesizing Zinc Sulfide Edifices as Cradles of Life on Earth. Biol. Direct 2009, 4, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vahrenkamp, H. Why Does Nature Use Zinc--a Personal View. Dalton Trans. 2007, 4751–4759. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.B.; Wenzel, H.J.; Kafer, K.E.; Schwartzkroin, P.A.; Palmiter, R.D. Elimination of Zinc from Synaptic Vesicles in the Intact Mouse Brain by Disruption of the ZnT3 Gene. Proc. Natl. Acad. Sci. USA 1999, 96, 1716–1721. [Google Scholar] [CrossRef] [Green Version]
- Palmiter, R.D.; Cole, T.B.; Quaife, C.J.; Findley, S.D. ZnT-3, a Putative Transporter of Zinc into Synaptic Vesicles. Proc. Natl. Acad. Sci. USA 1996, 93, 14934–14939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, J.; Noebels, J.L. Visualization of Transmitter Release with Zinc Fluorescence Detection at the Mouse Hippocampal Mossy Fibre Synapse. J. Physiol. 2005, 566, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Frederickson, C.J.; Koh, J.-Y.; Bush, A.I. The Neurobiology of Zinc in Health and Disease. Nat. Rev. Neurosci. 2005, 6, 449–462. [Google Scholar] [CrossRef]
- Marger, L.; Schubert, C.R.; Bertrand, D. Zinc: An Underappreciated Modulatory Factor of Brain Function. Biochem. Pharmacol. 2014, 91, 426–435. [Google Scholar] [CrossRef]
- Peralta, F.A.; Huidobro-Toro, J.P. Zinc as Allosteric Ion Channel Modulator: Ionotropic Receptors as Metalloproteins. Int. J. Mol. Sci. 2016, 17, 1059. [Google Scholar] [CrossRef] [Green Version]
- Bitanihirwe, B.K.Y.; Cunningham, M.G. Zinc: The Brain’s Dark Horse. Synapse 2009, 63, 1029–1049. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Castillo, C.; Morales, B.; Huidobro-Toro, J.P. Zinc and Copper Modulate Differentially the P2X4 Receptor. J. Neurochem. 2000, 74, 1529–1537. [Google Scholar] [CrossRef] [Green Version]
- Xiong, K.; Peoples, R.W.; Montgomery, J.P.; Chiang, Y.; Stewart, R.R.; Weight, F.F.; Li, C. Differential Modulation by Copper and Zinc of P2X2 and P2X4 Receptor Function. J. Neurophysiol. 1999, 81, 2088–2094. [Google Scholar] [CrossRef]
- Wildman, S.S.; King, B.F.; Burnstock, G. Zn2+ Modulation of ATP-Responses at Recombinant P2X2 Receptors and Its Dependence on Extracellular pH. Br. J. Pharmacol. 1998, 123, 1214–1220. [Google Scholar] [CrossRef] [Green Version]
- Acuña-Castillo, C.; Coddou, C.; Bull, P.; Brito, J.; Huidobro-Toro, J.P. Differential Role of Extracellular Histidines in Copper, Zinc, Magnesium and Proton Modulation of the P2X7 Purinergic Receptor. J. Neurochem. 2007, 101, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, K.; Ohno, Y. Effects of Neuroamines and Divalent Cations on Cloned and Mutated ATP-Gated Channels. Eur. J. Pharmacol. 1997, 325, 101–108. [Google Scholar] [CrossRef]
- Garcia-Guzman, M.; Soto, F.; Gomez-Hernandez, J.M.; Lund, P.E.; Stühmer, W. Characterization of Recombinant Human P2X4 Receptor Reveals Pharmacological Differences to the Rat Homologue. Mol. Pharmacol. 1997, 51, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Wildman, S.S.; King, B.F.; Burnstock, G. Modulatory Activity of Extracellular H+ and Zn2+ on ATP-Responses at rP2X1 and rP2X3 Receptors. Br. J. Pharmacol. 1999, 128, 486–492. [Google Scholar] [CrossRef] [Green Version]
- Virginio, C.; Church, D.; North, R.A.; Surprenant, A. Effects of Divalent Cations, Protons and Calmidazolium at the Rat P2X7 Receptor. Neuropharmacology 1997, 36, 1285–1294. [Google Scholar] [CrossRef]
- Liu, X.; Surprenant, A.; Mao, H.-J.; Roger, S.; Xia, R.; Bradley, H.; Jiang, L.-H. Identification of Key Residues Coordinating Functional Inhibition of P2X7 Receptors by Zinc and Copper. Mol. Pharmacol. 2008, 73, 252–259. [Google Scholar] [CrossRef] [Green Version]
- Wildman, S.S.; Brown, S.G.; Rahman, M.; Noel, C.A.; Churchill, L.; Burnstock, G.; Unwin, R.J.; King, B.F. Sensitization by Extracellular Ca(2+) of Rat P2X(5) Receptor and Its Pharmacological Properties Compared with Rat P2X(1). Mol. Pharmacol. 2002, 62, 957–966. [Google Scholar] [CrossRef]
- Peralta, F.A.; Huidobro-Toro, J.P. New Insights of the Zn(II)-Induced P2 × 4R Positive Allosteric Modulation: Role of Head Receptor Domain SS2/SS3, E160 and D170. Int. J. Mol. Sci. 2020, 21, 6940. [Google Scholar] [CrossRef] [PubMed]
- Igawa, T.; Abe, Y.; Tsuda, M.; Inoue, K.; Ueda, T. Solution Structure of the Rat P2X4 Receptor Head Domain Involved in Inhibitory Metal Binding. FEBS Lett. 2015, 589, 680–686. [Google Scholar] [CrossRef] [Green Version]
- Coddou, C.; Acuña-Castillo, C.; Bull, P.; Huidobro-Toro, J.P. Dissecting the Facilitator and Inhibitor Allosteric Metal Sites of the P2X4 Receptor Channel: Critical Roles of CYS132 for Zinc Potentiation and ASP138 for Copper Inhibition. J. Biol. Chem. 2007, 282, 36879–36886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.-Y.; Xiong, K.-M.; Wu, Y.-X.; Liu, Y.-W.; Chen, L.; Stewart, R.R.; Peoples, R.W.; Yi, C.-L. Conserved Extracellular Cysteines Differentially Regulate the Potentiation Produced by Zn2+ in Rat P2X4 Receptors. Eur. J. Pharmacol. 2013, 707, 11–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latapiat, V.; Rodríguez, F.E.; Godoy, F.; Montenegro, F.A.; Barrera, N.P.; Huidobro-Toro, J.P. P2X4 Receptor in Silico and Electrophysiological Approaches Reveal Insights of Ivermectin and Zinc Allosteric Modulation. Front. Pharmacol. 2017, 8, 918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawate, T.; Michel, J.C.; Birdsong, W.T.; Gouaux, E. Crystal Structure of the ATP-Gated P2X(4) Ion Channel in the Closed State. Nature 2009, 460, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Gouaux, E. Molecular Mechanism of ATP Binding and Ion Channel Activation in P2X Receptors. Nature 2012, 485, 207–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasuya, G.; Fujiwara, Y.; Takemoto, M.; Dohmae, N.; Nakada-Nakura, Y.; Ishitani, R.; Hattori, M.; Nureki, O. Structural Insights into Divalent Cation Modulations of ATP-Gated P2X Receptor Channels. Cell Rep. 2016, 14, 932–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansoor, S.E.; Lü, W.; Oosterheert, W.; Shekhar, M.; Tajkhorshid, E.; Gouaux, E. X-Ray Structures Define Human P2X(3) Receptor Gating Cycle and Antagonist Action. Nature 2016, 538, 66–71. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, A.E.; Yoshioka, C.; Mansoor, S.E. Full-Length P2X7 Structures Reveal How Palmitoylation Prevents Channel Desensitization. Cell 2019, 179, 659–670.e13. [Google Scholar] [CrossRef]
- Cunden, L.S.; Brophy, M.B.; Rodriguez, G.E.; Flaxman, H.A.; Nolan, E.M. Biochemical and Functional Evaluation of the Intramolecular Disulfide Bonds in the Zinc-Chelating Antimicrobial Protein Human S100A7 (Psoriasin). Biochemistry 2017, 56, 5726–5738. [Google Scholar] [CrossRef] [Green Version]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB-An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehlert, S.; Stahn, M.; Spicher, S.; Grimme, S. A Robust and Efficient Implicit Solvation Model for Fast Semiempirical Methods. ChemRxiv 2021. [Google Scholar] [CrossRef]
- Bannwarth, C.; Caldeweyher, E.; Ehlert, S.; Hansen, A.; Pracht, P.; Seibert, J.; Spicher, S.; Grimme, S. Extended Tight-binding Quantum Chemistry Methods. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2021, 11. [Google Scholar] [CrossRef]
- Kromann, J.C.; Welford, A.; Christensen, A.S.; Jensen, J.H. Random versus Systematic Errors in Reaction Enthalpies Computed Using Semiempirical and Minimal Basis Set Methods. ACS Omega 2018, 3, 4372–4377. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1-2, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved Side-Chain Torsion Potentials for the Amber ff99SB Protein Force Field. Proteins 2010, 78, 1950–1958. [Google Scholar] [CrossRef] [Green Version]
- Field, M.J. The pDynamo Program for Molecular Simulations Using Hybrid Quantum Chemical and Molecular Mechanical Potentials. J. Chem. Theory Comput. 2008, 4, 1151–1161. [Google Scholar] [CrossRef]
- Shirts, M.R.; Klein, C.; Swails, J.M.; Yin, J.; Gilson, M.K.; Mobley, D.L.; Case, D.A.; Zhong, E.D. Lessons Learned from Comparing Molecular Dynamics Engines on the SAMPL5 Dataset. J. Comput. Aided Mol. Des. 2017, 31, 147–161. [Google Scholar] [CrossRef] [Green Version]
- Mera-Adasme, R.; Domínguez, M.; Denis-Alpizar, O. A Benchmark for the Size of the QM System Required for Accurate Hybrid QM/MM Calculations on the Metal Site of the Protein Copper, Zinc Superoxide Dismutase. J. Mol. Model. 2019, 25, 176. [Google Scholar] [CrossRef]
- Domínguez, M.; Jiménez, V.; Savasci, G.; Pesonen, J.; Mera-Adasme, R. GoChem: A Composable Library for Multi-Scale Computational Chemistry Data Analysis. Manuscript in preparation. 2021. [Google Scholar]
- Schrödinger, LLC. The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC; Schrödinger, Inc.: New York, NY, USA, 2015. [Google Scholar]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peralta, F.A.; Huidobro-Toro, J.P.; Mera-Adasme, R. Hybrid QM/MM Simulations Confirm Zn(II) Coordination Sphere That Includes Four Cysteines from the P2 × 4R Head Domain. Int. J. Mol. Sci. 2021, 22, 7288. https://doi.org/10.3390/ijms22147288
Peralta FA, Huidobro-Toro JP, Mera-Adasme R. Hybrid QM/MM Simulations Confirm Zn(II) Coordination Sphere That Includes Four Cysteines from the P2 × 4R Head Domain. International Journal of Molecular Sciences. 2021; 22(14):7288. https://doi.org/10.3390/ijms22147288
Chicago/Turabian StylePeralta, Francisco Andrés, J. Pablo Huidobro-Toro, and Raúl Mera-Adasme. 2021. "Hybrid QM/MM Simulations Confirm Zn(II) Coordination Sphere That Includes Four Cysteines from the P2 × 4R Head Domain" International Journal of Molecular Sciences 22, no. 14: 7288. https://doi.org/10.3390/ijms22147288
APA StylePeralta, F. A., Huidobro-Toro, J. P., & Mera-Adasme, R. (2021). Hybrid QM/MM Simulations Confirm Zn(II) Coordination Sphere That Includes Four Cysteines from the P2 × 4R Head Domain. International Journal of Molecular Sciences, 22(14), 7288. https://doi.org/10.3390/ijms22147288





