Porphylipoprotein Accumulation and Porphylipoprotein Photodynamic Therapy Effects Involving Cancer Cell-Specific Cytotoxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Photosensitizers
2.2. Cell Culture
2.3. siRNA-Mediated Downregulation of HCP1
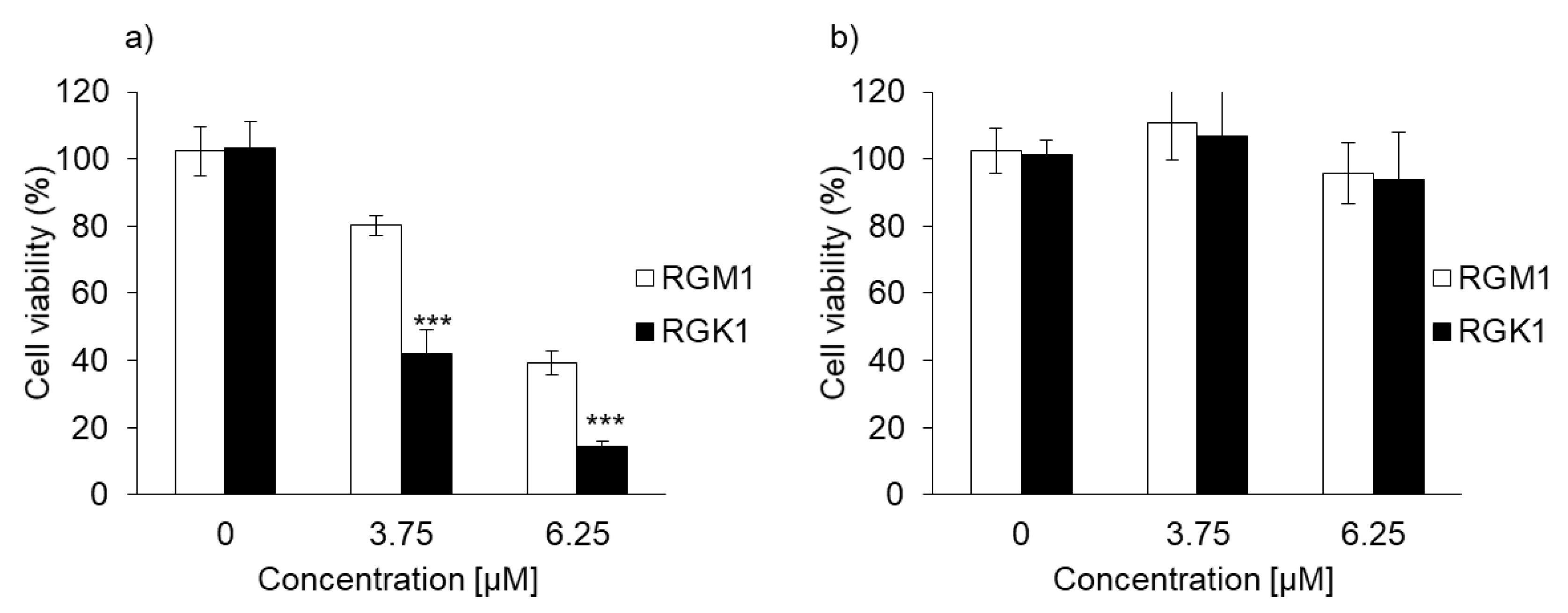
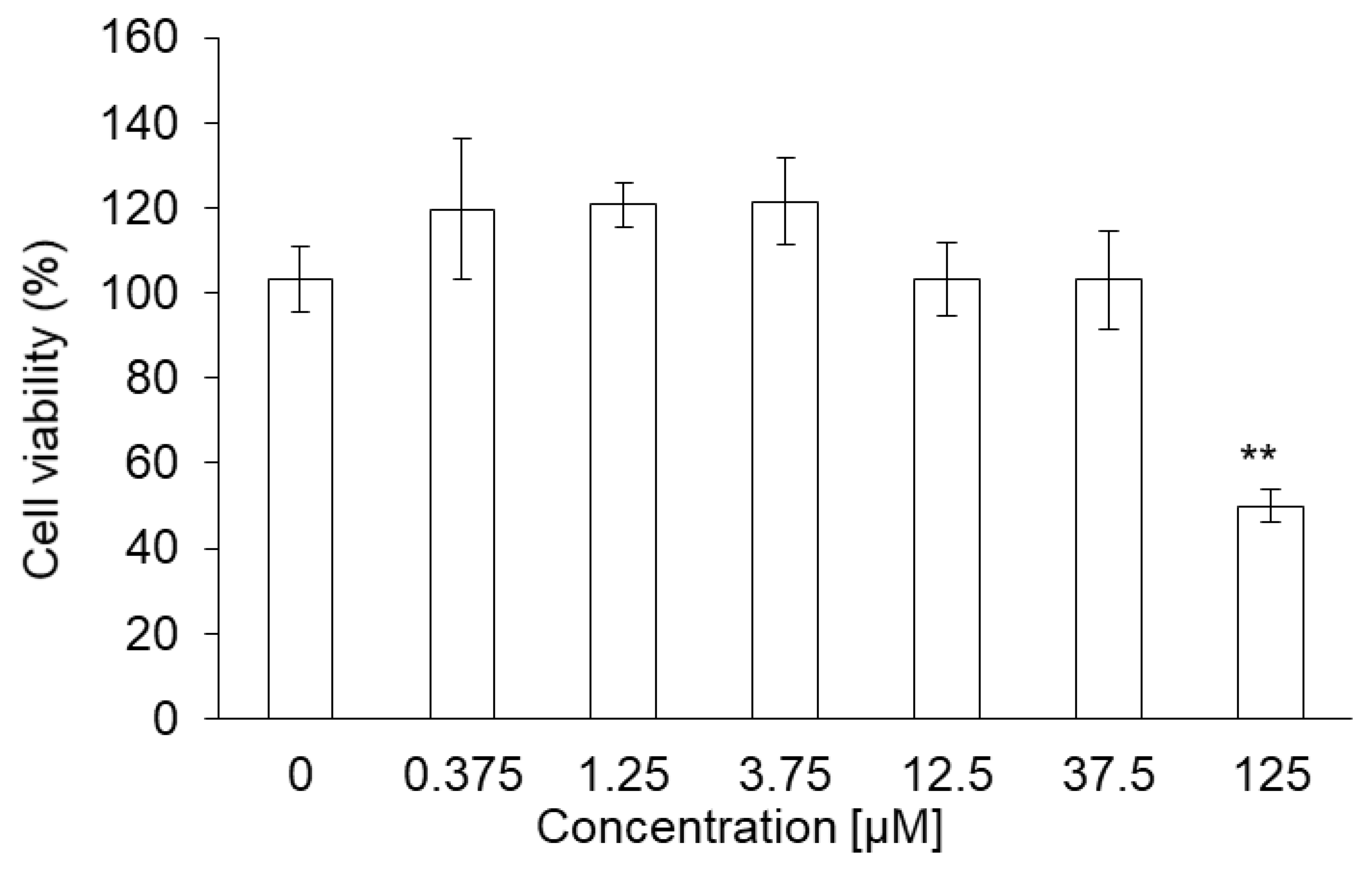
2.4. Cell Viability Assay
2.5. Localization of PLP in the Cells
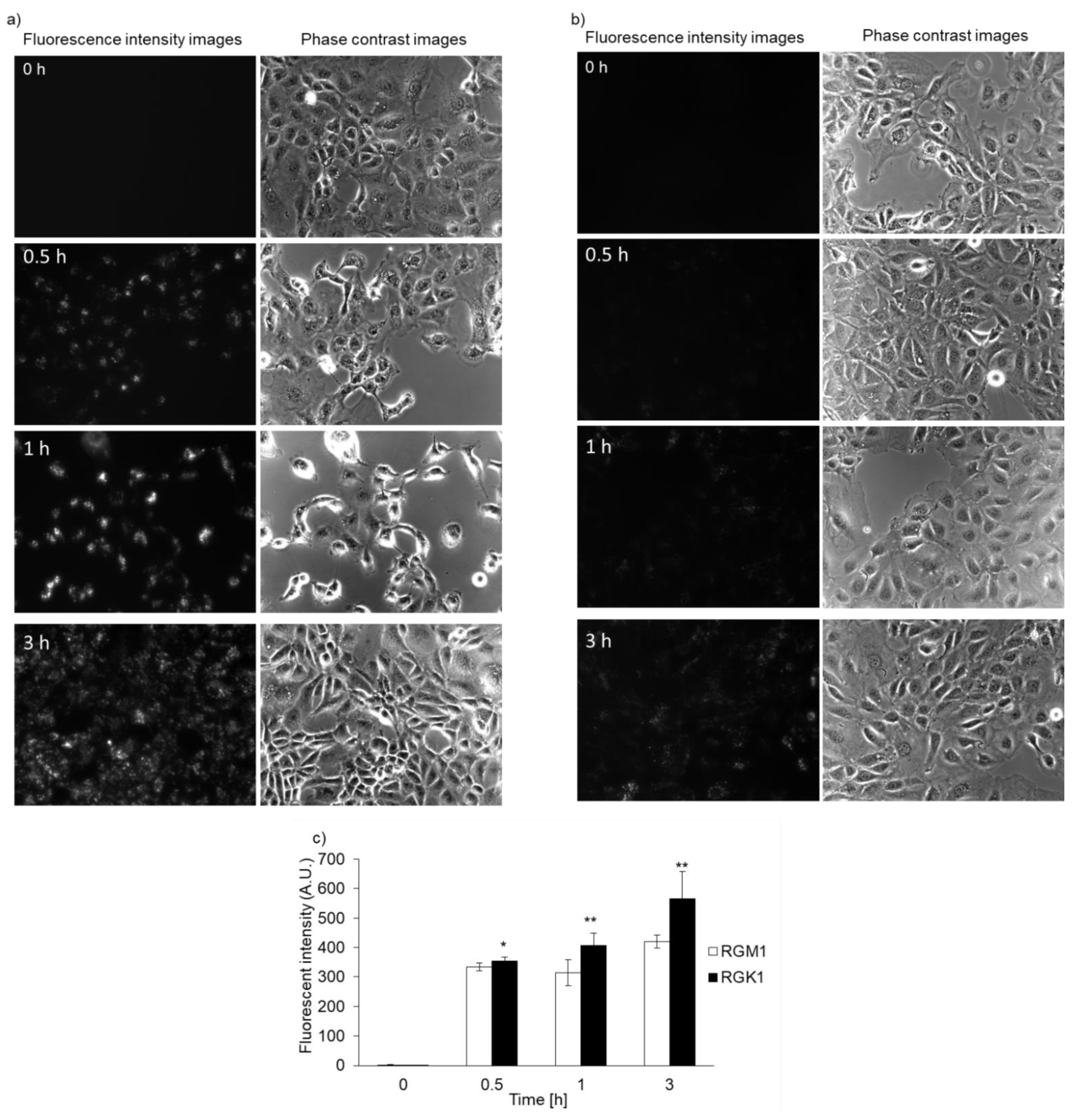
2.6. Intracellular Accumulation of PLP
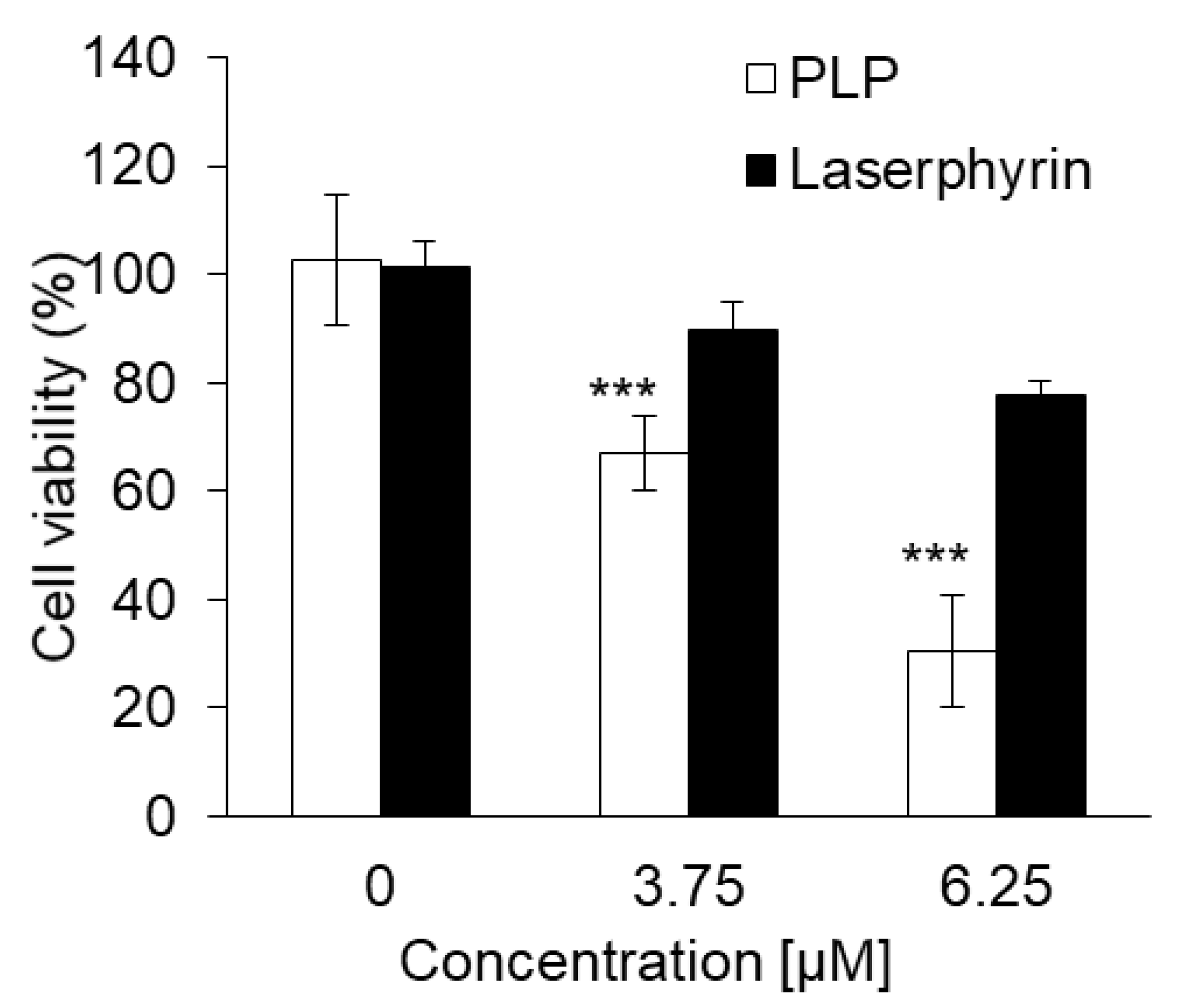
2.7. PDT Treatment and Cell Viability Assay
2.8. Effect of HCP1 Expression on the Intracellular Accumulation of PLP
2.9. PDT Treatment of Tumor-Bearing Mice
3. Results
3.1. Cytotoxicity of PLP and Laserphyrin in Cancer and Normal Cells
3.2. Intracellular PLP Accumulation Is Accelerated in Cancer Cells Compared with Normal Cells
3.3. PLP PDT Is More Cytotoxic Than Laserphyrin PDT
3.4. Relationship between PLP Uptake and HCP1 Expression
3.5. Cytotoxicity of PLP in NOZ Cells
3.6. Intracellular PLP Accumulation in NOZ Cells
3.7. Cytotoxic Effect of PLP and Laserphyrin PDT on NOZ Cells
3.8. Inhibitory Effects of PLP PDT and Laserphyrin PDT on the Growth of NOZ Tumors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ushio-Fukai, M.; Nakamura, Y. Reactive oxygen species and angiogenesis: Nadph oxidase as target for cancer therapy. Cancer Lett. 2008, 266, 37–52. [Google Scholar] [CrossRef] [Green Version]
- Dougherty, T.J.; Marcus, S.L. Photodynamic therapy. Eur. J. Cancer 1992, 28, 1734–1742. [Google Scholar] [CrossRef]
- van Straten, D.; Mashayekhi, V.; de Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic photodynamic therapy: Basic principles, current clinical status and future directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part three-photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagn. Photodyn. Ther. 2005, 2, 91–106. [Google Scholar] [CrossRef] [Green Version]
- Misawa, J.; Moriwaki, S.; Kohno, E.; Hirano, T.; Tokura, Y.; Takigawa, M. The role of low-density lipoprotein receptors in sensitivity to killing by photofrin-mediated photodynamic therapy in cultured human tumor cell lines. J. Dermatol. Sci. 2005, 40, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Kakehashi, A.; Ueno, N.; Chakrabarti, B. Molecular mechanisms of photochemically induced posterior vitreous detachment. Ophthalmic Res. 1994, 26, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Shayeghi, M.; Latunde-Dada, G.O.; Oakhill, J.S.; Laftah, A.H.; Takeuchi, K.; Halliday, N.; Khan, Y.; Warley, A.; McCann, F.E.; Hider, R.C.; et al. Identification of an intestinal heme transporter. Cell 2005, 122, 789–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiyama, K.; Matsui, H.; Tamura, M.; Shimokawa, O.; Hiyama, M.; Kaneko, T.; Nagano, Y.; Hyodo, I.; Tanaka, J.; Miwa, Y.; et al. Cancer cells uptake porphyrins via heme carrier protein 1. J. Porphyr. Phthalocyanines 2012, 16, 36–43. [Google Scholar]
- Krishnamurthy, P.; Xie, T.; Schuetz, J.D. The role of transporters in cellular heme and porphyrin homeostasis. Pharmacol. Ther. 2007, 114, 345–358. [Google Scholar] [CrossRef]
- Chandel, N.S. Mitochondrial regulation of oxygen sensing. Adv. Exp. Med. Biol. 2010, 661, 339–354. [Google Scholar]
- Kobayashi, I.; Kawano, S.; Tsuji, S.; Matsui, H.; Nakama, A.; Sawaoka, H.; Masuda, E.; Takei, Y.; Nagano, K.; Fusamoto, H.; et al. Rgm1, a cell line derived from normal gastric mucosa of rat. Vitr. Cell. Dev. Biol. Anim. 1996, 32, 259–261. [Google Scholar] [CrossRef]
- Shimokawa, O.; Matsui, H.; Nagano, Y.; Kaneko, T.; Shibahara, T.; Nakahara, A.; Hyodo, I.; Yanaka, A.; Majima, H.J.; Nakamura, Y.; et al. Neoplastic transformation and induction of h+, k+-adenosine triphosphatase by n-methyl-n′-nitro-n-nitrosoguanidine in the gastric epithelial rgm-1 cell line. In Vitro Cell. Dev. Biol. Anim. 2008, 44, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Matsui, H.; Tomita, T.; Sadakata, H.; Indo, H.P.; Majima, H.J.; Kaneko, T.; Hyodo, I. Mitochondrial reactive oxygen species accelerate gastric cancer cell invasion. J. Clin. Biochem. Nutr. 2014, 54, 12–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, H.; Matsui, H.; Tamura, M.; Majima, H.J.; Indo, H.P.; Hyodo, I. Mitochondrial reactive oxygen species accelerate the expression of heme carrier protein 1 and enhance photodynamic cancer therapy effect. J. Clin. Biochem. Nutr. 2014, 55, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Yoon, I.; Li, J.Z.; Shim, Y.K. Advance in photosensitizers and light delivery for photodynamic therapy. Clin. Endosc. 2013, 46, 7–23. [Google Scholar] [CrossRef]
- Cui, L.; Lin, Q.; Jin, C.S.; Jiang, W.; Huang, H.; Ding, L.; Muhanna, N.; Irish, J.C.; Wang, F.; Chen, J.; et al. A pegylation-free biomimetic porphyrin nanoplatform for personalized cancer theranostics. ACS Nano 2015, 9, 4484–4495. [Google Scholar] [CrossRef] [PubMed]
- Muhanna, N.; Chan, H.H.L.; Townson, J.L.; Jin, C.S.; Ding, L.; Valic, M.S.; Douglas, C.M.; MacLaughlin, C.M.; Chen, J.; Zheng, G.; et al. Photodynamic therapy enables tumor-specific ablation in preclinical models of thyroid cancer. Endocr. Relat. Cancer 2020, 27, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Muhanna, N.; Cui, L.; Chan, H.; Burgess, L.; Jin, C.S.; MacDonald, T.D.; Huynh, E.; Wang, F.; Chen, J.; Irish, J.C.; et al. Multimodal image-guided surgical and photodynamic interventions in head and neck cancer: From primary tumor to metastatic drainage. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 961–970. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.K.; Weber, G.F.; Crespi, C.L.; Waxman, D.J. Differential activation of cyclophosphamide and ifosphamide by cytochromes p-450 2b and 3a in human liver microsomes. Cancer Res. 1993, 53, 5629–5637. [Google Scholar]
- Ito, H.; Matsui, H.; Hirayama, A.; Indo, H.P.; Majima, H.J.; Hyodo, I. Reactive oxygen species induced by non-steroidal anti-inflammatory drugs enhance the effects of photodynamic therapy in gastric cancer cells. J. Clin. Biochem. Nutr. 2016, 58, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Euhus, D.M.; Hudd, C.; LaRegina, M.C.; Johnson, F.E. Tumor measurement in the nude mouse. J. Surg. Oncol. 1986, 31, 229–234. [Google Scholar] [CrossRef]
- Tomayko, M.M.; Reynolds, C.P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother. Pharmacol. 1989, 24, 148–154. [Google Scholar] [CrossRef]
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef] [Green Version]
- de Groen, P.C.; Gores, G.J.; LaRusso, N.F.; Gunderson, L.L.; Nagorney, D.M. Biliary tract cancers. N. Engl. J. Med. 1999, 341, 1368–1378. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Kondo, S.; Zen, Y.; Yonemori, A.; Kubota, K.; Kawakami, H.; Tanaka, E.; Hirano, S.; Itoh, T.; Nakanuma, Y. Impact of residual in situ carcinoma on postoperative survival in 125 patients with extrahepatic bile duct carcinoma. J. Hepato-Biliary-Pancreat. Sci. 2010, 17, 166–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoepf, T. Photodynamic therapy of cholangiocarcinoma. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2008, 10, 161–163. [Google Scholar] [CrossRef] [Green Version]
- Kasuya, K.; Oka, K.; Soya, R.; Tsuchiya, T.; Itoi, T.; Tsuchida, A. Photodynamic therapy for biliary tract organ via a novel ultra-small composite optical fiberscope. Exp. Ther. Med. 2017, 14, 4344–4348. [Google Scholar] [CrossRef]
- Wagner, A.; Kiesslich, T.; Neureiter, D.; Friesenbichler, P.; Puespoek, A.; Denzer, U.W.; Wolkersdörfer, G.W.; Emmanuel, K.; Lohse, A.W.; Berr, F. Photodynamic therapy for hilar bile duct cancer: Clinical evidence for improved tumoricidal tissue penetration by temoporfin. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2013, 12, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Ayaru, L.; Bown, S.G.; Pereira, S.P. Photodynamic therapy for pancreatic and biliary tract carcinoma. Int. J. Gastrointest. Cancer 2005, 35, 1–13. [Google Scholar] [CrossRef]
- Ortner, M.E.; Caca, K.; Berr, F.; Liebetruth, J.; Mansmann, U.; Huster, D.; Voderholzer, W.; Schachschal, G.; Mössner, J.; Lochs, H. Successful photodynamic therapy for nonresectable cholangiocarcinoma: A randomized prospective study. Gastroenterology 2003, 125, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Denzer, U.W.; Neureiter, D.; Kiesslich, T.; Puespoeck, A.; Rauws, E.A.J.; Emmanuel, K.; Degenhardt, N.; Frick, U.; Beuers, U.; et al. Temoporfin improves efficacy of photodynamic therapy in advanced biliary tract carcinoma: A multicenter prospective phase ii study. Hepatology 2015, 62, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, M.; Miki, Y.; Akimoto, J.; Haraoka, J.; Aizawa, K.; Hirano, K.; Beppu, M. Photodynamic therapy with talaporfin sodium induces dose-dependent apoptotic cell death in human glioma cell lines. Photodiagn. Photodyn. Ther. 2013, 10, 103–110. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurokawa, H.; Ito, H.; Matsui, H. Porphylipoprotein Accumulation and Porphylipoprotein Photodynamic Therapy Effects Involving Cancer Cell-Specific Cytotoxicity. Int. J. Mol. Sci. 2021, 22, 7306. https://doi.org/10.3390/ijms22147306
Kurokawa H, Ito H, Matsui H. Porphylipoprotein Accumulation and Porphylipoprotein Photodynamic Therapy Effects Involving Cancer Cell-Specific Cytotoxicity. International Journal of Molecular Sciences. 2021; 22(14):7306. https://doi.org/10.3390/ijms22147306
Chicago/Turabian StyleKurokawa, Hiromi, Hiromu Ito, and Hirofumi Matsui. 2021. "Porphylipoprotein Accumulation and Porphylipoprotein Photodynamic Therapy Effects Involving Cancer Cell-Specific Cytotoxicity" International Journal of Molecular Sciences 22, no. 14: 7306. https://doi.org/10.3390/ijms22147306




