Programmed Cell Death in the Small Intestine: Implications for the Pathogenesis of Celiac Disease
Abstract
1. Introduction
1.1. Programmed Cell Death (PCD)
1.2. Celiac Disease: A Complex Small Intestine Pathology
1.2.1. Antigen-Specific CD4+ T Cells
1.2.2. Cytotoxic Mechanisms
1.2.3. Innate Immunity
2. Apoptosis and Cell Shedding from the Epithelium
2.1. Intrinsic Apoptosis
2.2. Extrinsic Apoptosis
2.3. Common Executioner Pathway of Apoptosis
3. Non-Apoptotic Forms of Programmed Cell Death and Their Implications for CD
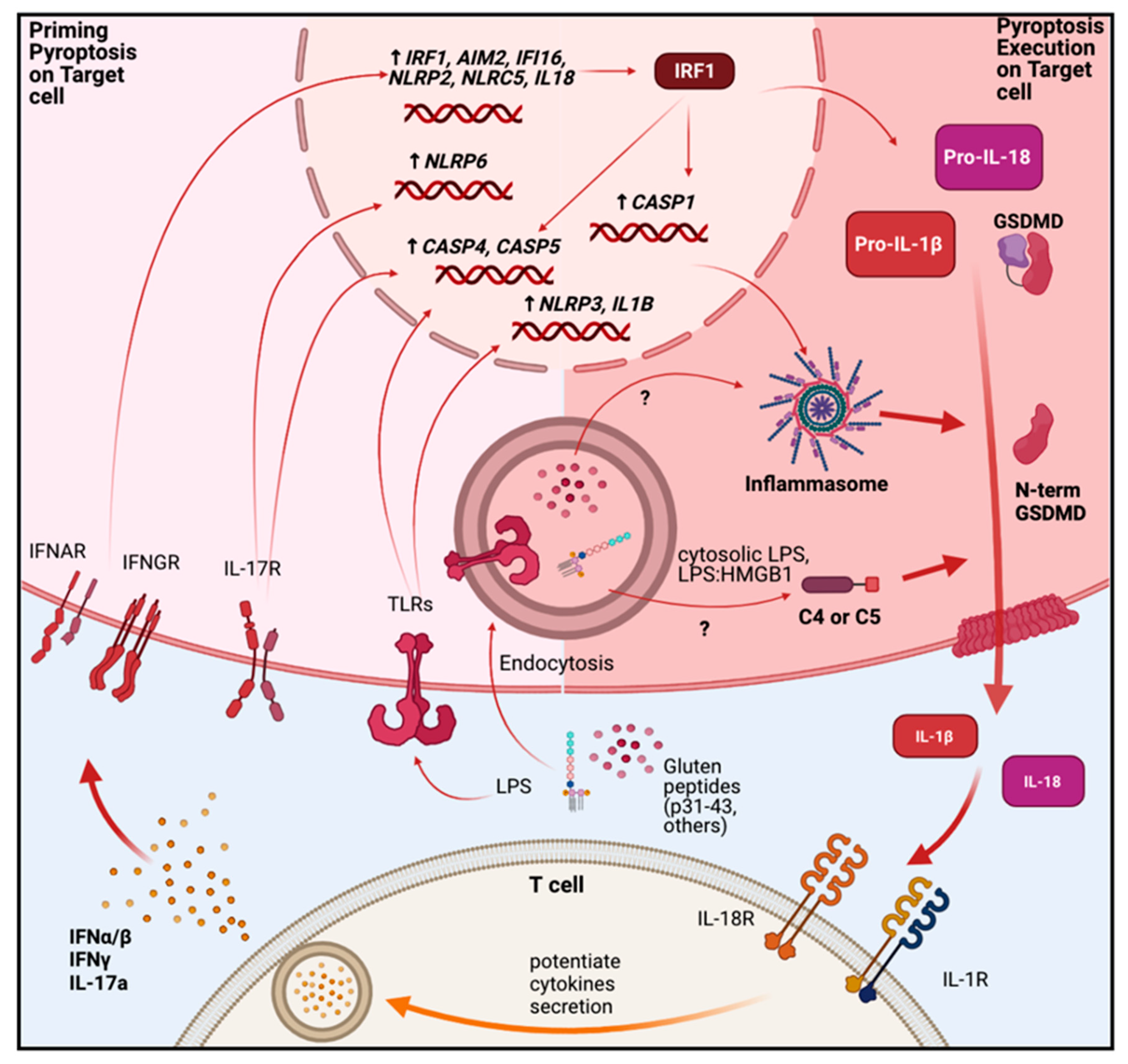
3.1. Pyroptosis
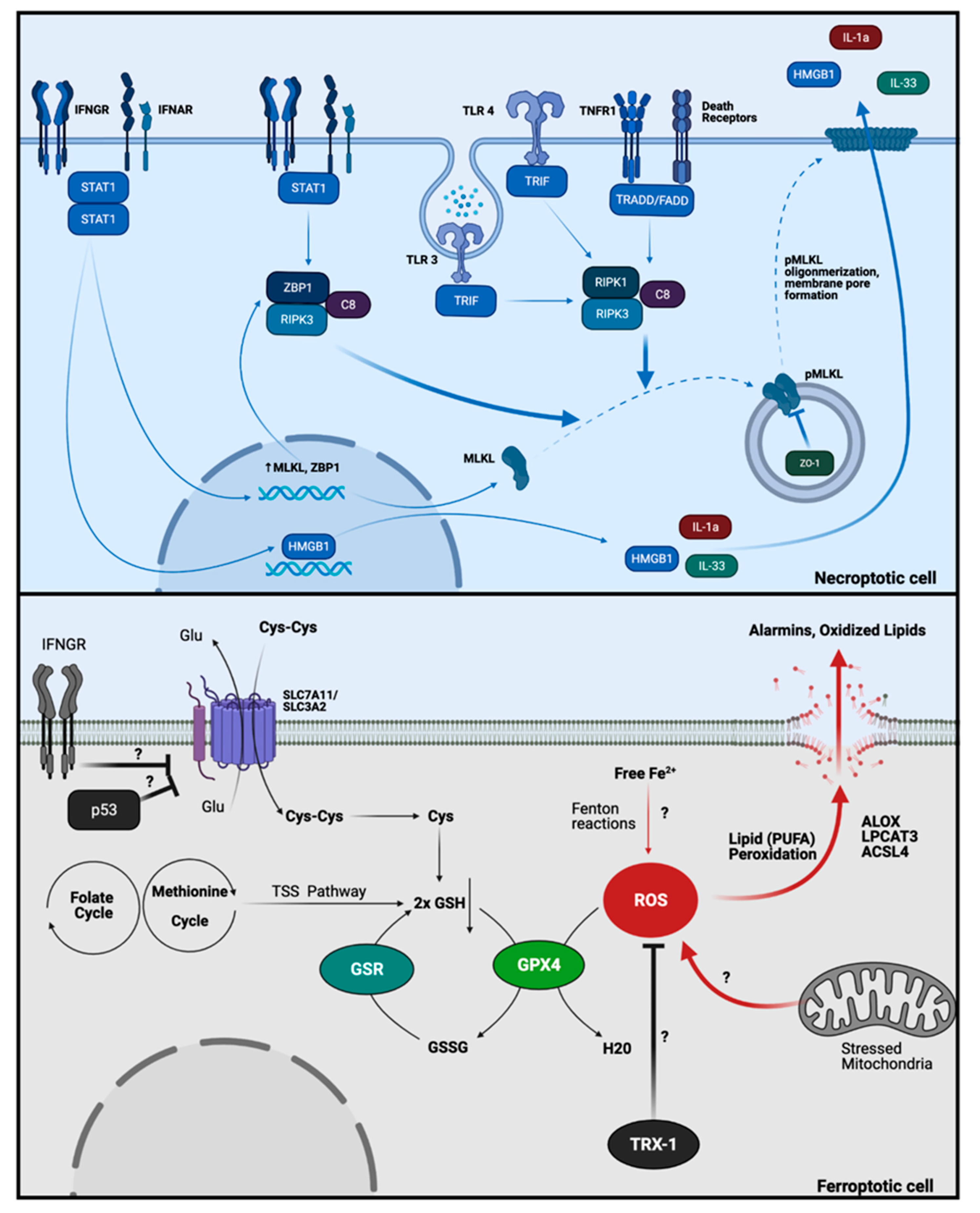
3.2. Necroptosis
3.3. Ferroptosis
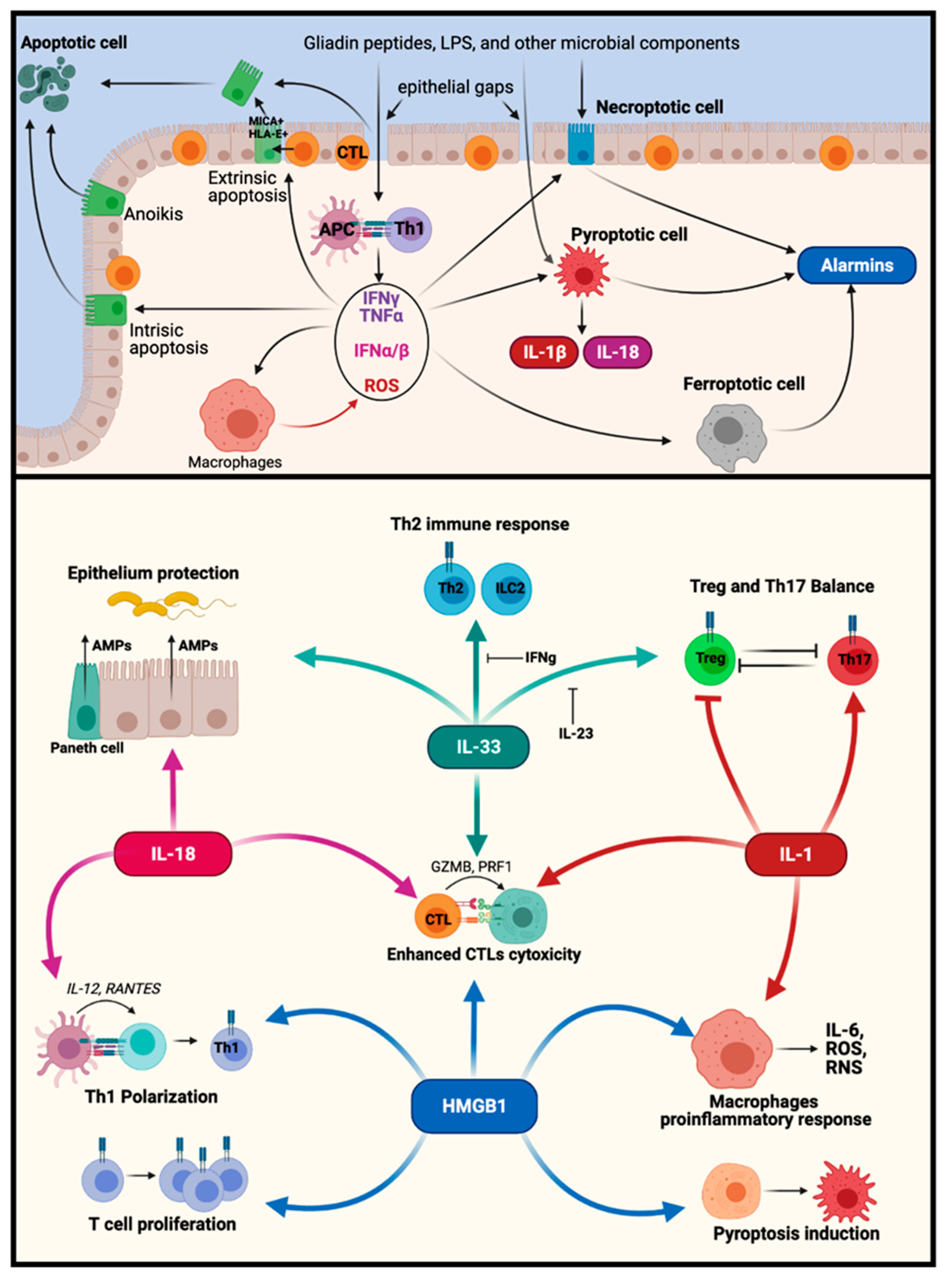
4. Alarmins and DAMPs
4.1. HMGB1
4.2. IL-33
5. Potential Interplay between Different PCDs
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Rider, P.; Voronov, E.D.; Charles, A.; Ron, N. Alarmins: Feel the Stress. J. Immunol. 2017, 198, 1395–1402. [Google Scholar] [CrossRef]
- Patankar, J.V.; Becker, C. Cell death in the gut epithelium and implications for chronic inflammation. Nat. Rev. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef]
- Evavold, C.L.; Ruan, J.; Tan, Y.; Xia, S.; Wu, H.; Kagan, J.C. The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages. Immunity 2018, 48, 35–44.e6. [Google Scholar] [CrossRef]
- Abadie, V.; Sollid, L.M.; Barreiro, L.B.; Jabri, B. Integration of genetic and immunological insights into a model of celiac disease pathogenesis. Annu. Rev. Immunol. 2011, 29, 493–525. [Google Scholar] [CrossRef]
- Sollid, L.M.; Lundin, K.E.A. Celiac disease. Autoimmune Dis. 2019, 849–869. [Google Scholar] [CrossRef]
- Lindfors, K.; Ciacci, C.; Kurppa, K.; Lundin, K.E.A.; Makharia, G.K.; Mearin, M.L.; Murray, J.A.; Verdu, E.F.; Kaukinen, K. Coeliac disease. Nat. Rev. Dis. Primers 2019, 5, 1–18. [Google Scholar] [CrossRef]
- Heyman, M.; Menard, S. Pathways of gliadin transport in celiac disease. Ann. N. Y. Acad. Sci. 2009, 1165, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Bouziat, R.; Hinterleitner, R.; Brown, J.J.; Stencel-Baerenwald, J.E.; Ikizler, M.; Mayassi, T.; Meisel, M.; Kim, S.M.; Discepolo, V.; Pruijssers, A.J.; et al. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science 2017, 356, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Mazzarella, G. Effector and suppressor T cells in celiac disease. World J. Gastroenterol. 2015, 21, 7349–7356. [Google Scholar] [CrossRef] [PubMed]
- Jabri, B.; Sollid, L.M. T Cells in Celiac Disease. J. Immunol. 2017, 198, 3005–3014. [Google Scholar] [CrossRef] [PubMed]
- Gianfrani, C.; Troncone, R.; Mugione, P.; Cosentini, E.; de Pascale, M.; Faruolo, C.; Senger, S.; Terrazzano, G.; Southwood, S.; Auricchio, S.; et al. Celiac Disease Association with CD8+ T Cell Responses: Identification of a Novel Gliadin-Derived HLA-A2-Restricted Epitope. J. Immunol. 2003, 170, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- Mazzarella, G.; Stefanile, R.; Camarca, A.; Giliberti, P.; Cosentini, E.; Marano, C.; Iaquinto, G.; Giardullo, N.; Auricchio, S.; Sette, A.; et al. Gliadin Activates HLA Class I-Restricted CD8+ T Cells in Celiac Disease Intestinal Mucosa and Induces the Enterocyte Apoptosis. Gastroenterology 2008, 134, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Picascia, S.; Sidney, J.; Camarca, A.; Mazzarella, G.; Giardullo, N.; Greco, L.; Auricchio, R.; Auricchio, S.; Troncone, R.; Sette, A.; et al. Gliadin-Specific CD8+ T Cell Responses Restricted by HLA Class I A*0101 and B*0801 Molecules in Celiac Disease Patients. J. Immunol. 2017, 198, 1838–1845. [Google Scholar] [CrossRef] [PubMed]
- Meresse, B.; Curran, S.A.; Ciszewski, C.; Orbelyan, G.; Setty, M.; Bhagat, G.; Jabri, B. Reprogramming of CTLs into Natural Killer-like Cells in Celiac Disease. J. Exp. Med. 2006, 203, 1343–1345. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, A.; Mcclure, J.P.; Townley, R.R.W. Intraepithelial Lymphocyte Counts in Small Intestinal Biopsies From Children With Diarrhoea. Acta Pædiatrica 1976, 65, 541–546. [Google Scholar] [CrossRef]
- Meresse, B.; Chen, Z.; Ciszewski, C.; Tretiakova, M.; Bhagat, G.; Krausz, T.N.; Raulet, D.H.; Lanier, L.L.; Groh, V.; Spies, T.; et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity 2004, 21, 357–366. [Google Scholar] [CrossRef]
- Abadie, V.; Jabri, B. IL-15: A central regulator of celiac disease immunopathology. Immunol. Rev. 2014, 260, 221–234. [Google Scholar] [CrossRef]
- Sarra, M.; Cupi, M.L.; Monteleone, I.; Franzè, E.; Ronchetti, G.; Di Sabatino, A.; Gentileschi, P.; Franceschilli, L.; Sileri, P.; Sica, G.; et al. IL-15 positively regulates IL-21 production in celiac disease mucosa. Mucosal Immunol. 2013, 6, 244–255. [Google Scholar] [CrossRef]
- Meresse, B.; Verdier, J.; Cerf-Bensussan, N. The cytokine interleukin 21: A new player in coeliac disease? Gut 2008, 57, 879–881. [Google Scholar] [CrossRef]
- Jarry, A.; Malard, F.; Bou-hanna, C.; Meurette, G.; Mohty, M.; Mosnier, J.F.; Laboisse, C.L.; Bossard, C. Interferon-Alpha Promotes Th1 Response and Epithelial Apoptosis via Inflammasome Activation in Human Intestinal Mucosa. Cmgh 2017, 3, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Mayassi, T.; Jabri, B. Human intraepithelial lymphocytes. Mucosal Immunol. 2018, 11, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Cheroutre, H.; Lambolez, F.; Mucida, D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat. Rev. Immunol. 2011, 11, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Mayassi, T.; Ladell, K.; Gudjonson, H.; McLaren, J.E.; Shaw, D.G.; Tran, M.T.; Rokicka, J.J.; Lawrence, I.; Grenier, J.C.; van Unen, V.; et al. Chronic Inflammation Permanently Reshapes Tissue-Resident Immunity in Celiac Disease. Cell 2019, 176, 967–981.e19. [Google Scholar] [CrossRef] [PubMed]
- Abadie, V.; Discepolo, V.; Jabri, B. Intraepithelial lymphocytes in celiac disease immunopathology. Semin. Immunopathol. 2012, 34, 551–556. [Google Scholar] [CrossRef]
- Hüe, S.; Mention, J.J.; Monteiro, R.C.; Zhang, S.L.; Cellier, C.; Schmitz, J.; Verkarre, V.; Fodil, N.; Bahram, S.; Cerf-Bensussan, N.; et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity 2004, 21, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, Y.L.; Bondar, C.; Guzman, L.; Cueto Rua, E.; Chopita, N.; Fuertes, M.; Zwirner, N.W.; Chirdo, F.G. Broad MICA/B Expression in the Small Bowel Mucosa: A Link between Cellular Stress and Celiac Disease. PLoS ONE 2013, 8, e73658. [Google Scholar] [CrossRef]
- Jabri, B.; De Serre, N.P.M.; Cellier, C.; Evans, K.; Gache, C.; Carvalho, C.; Mougenot, J.F.; Allez, M.; Jian, R.; Desreumaux, P.; et al. Selective expansion of intraepithelial lymphocytes expressing the HLA-E- specific natural killer receptor CD94 in celiac disease. Gastroenterology 2000, 118, 867–879. [Google Scholar] [CrossRef]
- Gustafson, K.S.; Ginder, G.D. Interferon-γ induction of the human leukocyte antigen-E gene is mediated through binding of a complex containing STAT1α to a distinct interferon-γ- responsive element. J. Biol. Chem. 1996, 271, 20035–20046. [Google Scholar] [CrossRef]
- Gobin, S.J.P.; van den Elsen, P.J. Transcriptional regulation of the MHC class Ib genes HLA-E, HLA-F and HLA-G. Hum. Immunol. 2000, 61, 1102–1107. [Google Scholar] [CrossRef]
- Meresse, B.; Meresse, B.; Ciszewski, C.; Setty, M.; Curran, S.; Tretiakova, M.; Krausz, T.; Lanier, L.; Ebert, E.; Green, P.H.; et al. Selective expression of NKG2C in intraepithelial lymphocytes(IEL): A basis for IEL proliferation and epithelial cell killing in celiac disease (CD). J. Pediatr. Gastroenterol. Nutr. 2005, 41, 495. [Google Scholar] [CrossRef]
- Gumá, M.; Busch, L.K.; Salazar-Fontana, L.I.; Bellosillo, B.; Morte, C.; García, P.; López-Botet, M. The CD94/NKG2C killer lectin-like receptor constitutes an alternative activation pathway for a subset of CD8+ T cells. Eur. J. Immunol. 2005, 35, 2071–2080. [Google Scholar] [CrossRef] [PubMed]
- Caminero, A.; Verdu, E.F. Celiac disease: Should we care about microbes? Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G161–G170. [Google Scholar] [CrossRef] [PubMed]
- Junker, Y.; Zeissig, S.; Kim, S.J.; Barisani, D.; Wieser, H.; Leffler, D.A.; Zevallos, V.; Libermann, T.A.; Dillon, S.; Freitag, T.L.; et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J. Exp. Med. 2012, 209, 2395–2408. [Google Scholar] [CrossRef] [PubMed]
- Chirdo, F.G.; Auricchio, S.; Troncone, R.; Barone, M.V. The Gliadin p31-43 Peptide: Inducer of Multiple Proinflammatory Effects, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; Volume 358. [Google Scholar]
- Abadie, V.; Kim, S.M.; Lejeune, T.; Palanski, B.A.; Ernest, J.D.; Tastet, O.; Voisine, J.; Discepolo, V.; Marietta, E.V.; Hawash, M.B.F.; et al. IL-15, gluten and HLA-DQ8 drive tissue destruction in coeliac disease. Nature 2020, 578, 600–604. [Google Scholar] [CrossRef]
- Falcigno, L.; Calvanese, L.; Conte, M.; Nanayakkara, M.; Barone, M.V.; D’auria, G. Structural perspective of gliadin peptides active in celiac disease. Int. J. Mol. Sci. 2020, 21, 9301. [Google Scholar] [CrossRef]
- Paolella, G.; Lepretti, M.; Martucciello, S.; Nanayakkara, M.; Auricchio, S.; Esposito, C.; Barone, M.V.; Caputo, I. The toxic alpha-gliadin peptide 31-43 enters cells without a surface membrane receptor. Cell Biol. Int. 2018, 42, 112–120. [Google Scholar] [CrossRef]
- Maiuri, L.; Villella, V.R.; Raia, V.; Kroemer, G. The gliadin-CFTR connection: New perspectives for the treatment of celiac disease. Ital. J. Pediatr. 2019, 45, 1–4. [Google Scholar] [CrossRef]
- Gómez Castro, M.F.; Miculán, E.; Herrera, M.G.; Ruera, C.; Perez, F.; Prieto, E.D.; Barrera, E.; Pantano, S.; Carasi, P.; Chirdo, F.G. p31-43 Gliadin Peptide Forms Oligomers and Induces NLRP3 Inflammasome/Caspase 1- Dependent Mucosal Damage in Small Intestine. Front. Immunol. 2019, 10, 31. [Google Scholar] [CrossRef]
- Herrera, M.G.; Gómez Castro, M.F.; Prieto, E.; Barrera, E.; Dodero, V.I.; Pantano, S.; Chirdo, F. Structural conformation and self-assembly process of p31-43 gliadin peptide in aqueous solution. Implications for celiac disease. FEBS J. 2020, 287, 2134–2149. [Google Scholar] [CrossRef]
- Ruera, C.N.; Miculán, E.; Pérez, F.; Ducca, G.; Carasi, P.; Chirdo, F.G. Sterile inflammation drives multiple programmed cell death pathways in the gut. J. Leukoc. Biol. 2021, 109, 211–221. [Google Scholar] [CrossRef]
- Williams, J.M.; Duckworth, C.A.; Burkitt, M.D.; Watson, A.J.M.; Campbell, B.J.; Pritchard, D.M. Epithelial Cell Shedding and Barrier Function: A Matter of Life and Death at the Small Intestinal Villus Tip. Vet. Pathol. 2015, 52, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Gudipaty, S.A.; Rosenblatt, J. Epithelial cell extrusion: Pathways and pathologies. Semin. Cell Dev. Biol. 2017, 67, 132–140. [Google Scholar] [CrossRef]
- Eisenhoffer, G.T.; Loftus, P.D.; Yoshigi, M.; Otsuna, H.; Chien, C.; Morcos, P.A.; Rosenblatt, J. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 2012, 484, 546–549. [Google Scholar] [CrossRef]
- Lubkov, V.; Bar-Sagi, D. E-cadherin-mediated cell coupling is required for apoptotic cell extrusion. Curr. Biol. 2014, 24, 868–874. [Google Scholar] [CrossRef]
- Kiesslich, R.; Goetz, M.; Angus, E.M.; Hu, Q.; Guan, Y.; Potten, C.; Allen, T.; Neurath, M.F.; Shroyer, N.F.; Montrose, M.H.; et al. Identification of Epithelial Gaps in Human Small and Large Intestine by Confocal Endomicroscopy. Gastroenterology 2007, 133, 1769–1778. [Google Scholar] [CrossRef]
- Bruewer, M.; Luegering, A.; Kucharzik, T.; Parkos, C.A.; Madara, J.L.; Hopkins, A.M.; Nusrat, A. Proinflammatory Cytokines Disrupt Epithelial Barrier Function by Apoptosis-Independent Mechanisms. J. Immunol. 2003, 171, 6164–6172. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Finamore, A.; Ara, C.; di Sabatino, A.; Mengheri, E.; Corazza, G.R. Altered expression, localization, and phosphorylation of epithelial junctional proteins in celiac disease. Am. J. Clin. Pathol. 2006, 125, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Karayiannakis, A.J.; Syrigos, K.N.; Efstathiou, J.; Valizadeh, A.; Noda, M.; Playford, R.J.; Kmiot, W.; Pignatelli, M. Expression of catenins and E-cadherin during epithelial restitution in inflammatory bowel disease. J. Pathol. 1998, 185, 413–418. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef] [PubMed]
- Afonina, I.S.; Cullen, S.P.; Martin, S.J. Cytotoxic and non-cytotoxic roles of the CTL/NK protease granzyme B. Immunol. Rev. 2020, 235, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Shalimar, D.M.; Das, P.; Sreenivas, V.; Gupta, S.D.; Panda, S.K.; Makharia, G.K. Mechanism of villous atrophy in celiac disease: Role of apoptosis and epithelial regeneration. Arch. Pathol. Lab. Med. 2013, 137, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Cheravsky, A.C.; Rubio, A.E.; Vanzulli, S.; Rubinstein, N.; de Rosa, S.; Fainboim, L. Evidences of the involvement of Bak, a member of the Bcl-2 family of proteins, in active coeliac disease. Autoimmunity 2002, 35, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Weyman, C.M.; Liu, H.; Almassan, A.; Zhou, A. IFN-γ induces apoptosis in HL-60 cells through decreased Bcl-2 and increased Bak expression. J. Interf. Cytokine Res. 2008, 28, 65–72. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Mirandola, P.; Ponti, C.; Gobbi, G.; Sponzilli, I.; Vaccarezza, M.; Cocco, L.; Zauli, G.; Secchiero, P.; Manzoli, F.A.; Vitale, M. Activated human NK and CD8+ T cells express both TNF-related apoptosis-inducing ligand (TRAIL) and TRAIL receptors but are resistant to TRAIL-mediated cytotoxicity. Blood 2004, 104, 2418–2424. [Google Scholar] [CrossRef]
- Prager, I.; Liesche, C.; Van Ooijen, H.; Urlaub, D.; Verron, Q.; Sandström, N.; Fasbender, F.; Claus, M.; Eils, R.; Beaudouin, J.; et al. NK cells switch from granzyme B to death receptor-mediated cytotoxicity during serial killing. J. Exp. Med. 2019, 216, 2113–2127. [Google Scholar] [CrossRef] [PubMed]
- Brincks, E.L.; Katewa, A.; Kucaba, T.A.; Griffith, T.S.; Legge, K.L. CD8 T Cells Utilize TRAIL to Control Influenza Virus Infection. J. Immunol. 2008, 181, 4918–4925. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Di Sabatino, A.; Parroni, R.; Muzi, P.; D’Alò, S.; Ventura, T.; Pistoia, M.A.; Cifone, M.G.; Corazza, G.R. Increased Enterocyte Apoptosis and Fas-Fas Ligand System in Celiac Disease. Am. J. Clin. Pathol. 2001, 115, 494–503. [Google Scholar] [CrossRef][Green Version]
- Giovannini, C.; Matarrese, P.; Scazzocchio, B.; Varì, R.; D’Archivio, M.; Straface, E.; Masella, R.; Malorni, W.; De Vincenzi, M. Wheat gliadin induces apoptosis of intestinal cells via an autocrine mechanism involving Fas-Fas ligand pathway. FEBS Lett. 2003, 540, 117–124. [Google Scholar] [CrossRef]
- Siegmund, D.; Wicovsky, A.; Schmitz, I.; Schulze-Osthoff, K.; Kreuz, S.; Leverkus, M.; Dittrich-Breiholz, O.; Kracht, M.; Wajant, H. Death Receptor-Induced Signaling Pathways Are Differentially Regulated by Gamma Interferon Upstream of Caspase 8 Processing. Mol. Cell. Biol. 2005, 25, 6363–6379. [Google Scholar] [CrossRef] [PubMed]
- Kotredes, K.P.; Gamero, A.M. Interferons as inducers of apoptosis in malignant cells. J. Interferon Cytokine Res. 2013, 33, 162–170. [Google Scholar] [CrossRef]
- Ruera, N.C.; Perez, F.; Miculan, E.; Ducca, G.; Guzman, L.; Garbi, L.; Carasi, P.; Chirdo, F. Inflammatory cell death pathways in celiac disease. in preparation.
- Moss, S.F.; Attia, L.; Scholes, J.V.; Walters, J.R.F.; Holt, P.R. Increased small intestinal apoptosis in coeliac disease. Gut 1996, 39, 811–817. [Google Scholar] [CrossRef]
- Araya, R.E.; Gomez Castro, M.F.; Carasi, P.; McCarville, J.L.; Jury, J.; Mowat, A.M.; Verdu, E.F.; Chirdo, F.G.; Florencia, M.; Castro, G.; et al. Mechanisms of innate immune activation by gluten peptide p31-43 in mice. Am. J. Physiol. Liver Physiol. 2016, 311, G40–G49. [Google Scholar] [CrossRef]
- Kuriakose, T.; Kanneganti, T.D. Gasdermin D Flashes an Exit Signal for IL-1. Immunity 2018, 48, 1–3. [Google Scholar] [CrossRef]
- Sarhan, M.; Land, W.G.; Tonnus, W.; Hugo, C.P.; Linkermann, A. Origin and consequences of necroinflammation. Physiol. Rev. 2018, 98, 727–780. [Google Scholar] [CrossRef] [PubMed]
- Kesavardhana, S.; Kanneganti, T.D. Mechanisms governing inflammasome activation, assembly and pyroptosis induction. Int. Immunol. 2017, 29, 201–210. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Tang, Y.; Li, W.; Wang, X.; Zhang, R.; Zhang, X.; Zhao, X.; Liu, J.; Tang, C.; Liu, Z.; et al. The Endotoxin Delivery Protein HMGB1 Mediates Caspase-11-Dependent Lethality in Sepsis. Immunity 2018, 49, 740–753.e7. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, M.; He, X.; Ouyang, D. A mini-review on ion fluxes that regulate NLRP3 inflammasome activation. Acta Biochim. Biophys. Sin. 2021, 53, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Moretti, S.; Mrakic-Sposta, S.; Roncoroni, L.; Vezzoli, A.; Dellanoce, C.; Monguzzi, E.; Branchi, F.; Ferretti, F.; Lombardo, V.; Doneda, L.; et al. Oxidative stress as a biomarker for monitoring treated celiac disease article. Clin. Transl. Gastroenterol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Kasapovi, J.; Peji, S.; Gavrilovi, L.; Radlovi, N.; Saii, Z.; Pajovi, S. Antioxidant status of the celiac mucosa: Implications for disease pathogenesis. In Celiac Disease—From Pathophysiology to Advanced Therapies; InTech: London, UK, 2012. [Google Scholar] [CrossRef]
- Stojiljković, V.; Pejić, S.; Kasapović, J.; Gavrilović, L.; Stojiljković, S.; Nikolić, D.; Pajović, S.B. Glutathione redox cycle in small intestinal mucosa and peripheral blood of pediatric celiac disease patients. An. Acad. Bras. Cienc. 2012, 84, 175–184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perez, F.; Ruera, C.N.; Miculan, E.; Carasi, P.; Dubois-Camacho, K.; Garbi, L.; Guzman, L.; Hermoso, M.A.; Chirdo, F.G.; Miculán, E.G.; et al. IL-33 Alarmin and Its Active Proinflammatory Fragments Are Released in Small Intestine in Celiac Disease. Front. Immunol. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Manti, S.; Cuppari, C.; Tardino, L.; Parisi, G.; Spina, M.; Salpietro, C.; Leonardi, S. HMGB1 as a new biomarker of celiac disease in children: A multicenter study. Nutrition 2017, 37, 18–21. [Google Scholar] [CrossRef]
- Pietz, G.; De, R.; Hedberg, M.; Sjöberg, V.; Sandström, O.; Hernell, O.; Hammarström, S.; Hammarström, M.-L.L. Immunopathology of childhood celiac disease—Key role of intestinal epithelial cells. PLoS ONE 2017, 12, e0185025. [Google Scholar] [CrossRef] [PubMed]
- Cornut, M.; Bourdonnay, E.; Henry, T. Transcriptional regulation of inflammasomes. Int. J. Mol. Sci. 2020, 21, 8087. [Google Scholar] [CrossRef]
- León, A.J.; Garrote, J.A.; Blanco-Quirós, A.; Calvo, C.; Fernández-Salazar, L.; Del Villar, A.; Barrera, A.; Arranz, E.; León, A.J.; Garrote, J.A.; et al. Interleukin 18 maintains a long-standing inflammation in coeliac disease patients. Clin. Exp. Immunol. 2006, 146, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Merendino, R.A.; Di Pasquale, G.; Sturniolo, G.C.; Ruello, A.; Albanese, V.; Minciullo, P.L.; Di Mauro, S.; Gangemi, S. Relationship between IL-18 and sICAM-1 serum levels in patients affected by coeliac disease: Preliminary considerations. Immunol. Lett. 2003, 85, 257–260. [Google Scholar] [CrossRef]
- Harris, K.M.; Fasano, A.; Mann, D.L. Cutting Edge: IL-1 Controls the IL-23 Response Induced by Gliadin, the Etiologic Agent in Celiac Disease. J. Immunol. 2008, 181, 4457–4460. [Google Scholar] [CrossRef]
- Manavalan, J.S.; Hernandez, L.; Shah, J.G.; Konikkara, J.; Naiyer, A.J.; Lee, A.R.; Ciaccio, E.; Minaya, M.T.; Green, P.H.R.R.; Bhagat, G. Serum cytokine elevations in celiac disease: Association with disease presentation. Hum. Immunol. 2010, 71, 50–57. [Google Scholar] [CrossRef]
- Palová-Jelínková, L.; Dáňová, K.; Drašarová, H.; Dvořák, M.; Funda, D.P.; Fundová, P.; Kotrbová-Kozak, A.; Černá, M.; Kamanová, J.; Martin, S.F.; et al. Pepsin Digest of Wheat Gliadin Fraction Increases Production of IL-1β via TLR4/MyD88/TRIF/MAPK/NF-κB Signaling Pathway and an NLRP3 Inflammasome Activation. PLoS ONE 2013, 8, e62426. [Google Scholar] [CrossRef]
- Pontillo, A.; Vendramin, A.; Catamo, E.; Fabris, A.; Crovella, S. The missense variation Q705K in CIAS1/NALP3/NLRP3 gene and an NLRP1 haplotype are associated with celiac disease. Am. J. Gastroenterol. 2011, 106, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.L.; Crusius, J.B.A.; Cherñavsky, A.; Sugai, E.; Sambuelli, A.; Vazquez, H.; Mauriño, E.; Peña, A.S.; Bai, J.C. The IL-1 gene family and bone involvement in celiac disease. Immunogenetics 2005, 57, 618–620. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.F.; Liao, Y.C.; Wu, S.L.; Tsai, F.J.; Lee, C.C.; Hua, C.S. Association of interleukin-1 beta and receptor antagonist gene polymorphisms with late onset Alzheimer’s disease in Taiwan Chinese. Eur. J. Neurol. 2005, 12, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Sultana, Z.; Bankura, B.; Pattanayak, A.K.; Sengupta, D.; Sengupta, M.; Saha, M.L.; Panda, C.K.; Das, M. Association of Interleukin-1 beta and tumor necrosis factor-alpha genetic polymorphisms with gastric cancer in India. Environ. Mol. Mutagen. 2018, 59, 653–667. [Google Scholar] [CrossRef]
- Kim, S.H.; Mok, J.W.; Kim, H.S.; Joo, C.K. Association of -31T>C and -511 C>T polymorphisms in the interleukin 1 beta (IL1B) promoter in Korean keratoconus patients. Mol. Vis. 2008, 14, 2109–2116. Available online: http://www.molvis.org/molvis/v14/a247 (accessed on 24 April 2021).
- Chen, M.-L.; Liao, N.; Zhao, H.; Huang, J.; Xie, Z.-F. Association between the IL1B (-511), IL1B (+3954), IL1RN (VNTR) Polymorphisms and Graves’ Disease Risk: A Meta-Analysis of 11 Case-Control Studies. PLoS ONE 2014, 9, e86077. [Google Scholar] [CrossRef]
- Tsuji-Takayama, K.; Aizawa, Y.; Okamoto, I.; Kojima, H.; Koide, K.; Takeuchi, M.; Ikegami, H.; Ohta, T.; Kurimoto, M. Interleukin-18 induces interferon-γ production through NF-κb and NFAT activation in murine T helper type 1 cells. Cell. Immunol. 1999, 196, 41–50. [Google Scholar] [CrossRef]
- Deknuydt, F.; Bioley, G.; Valmori, D.; Ayyoub, M. IL-1β and IL-2 convert human Treg into TH17 cells. Clin. Immunol. 2009, 131, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Revu, S.; Wu, J.; Henkel, M.; Rittenhouse, N.; Menk, A.; Delgoffe, G.M.; Poholek, A.C.; McGeachy, M.J. IL-23 and IL-1β Drive Human Th17 Cell Differentiation and Metabolic Reprogramming in Absence of CD28 Costimulation. Cell Rep. 2018, 22, 2642–2653. [Google Scholar] [CrossRef] [PubMed]
- Mailer, R.K.W. Alternative splicing of FOXP3-virtue and vice. Front. Immunol. 2018, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Mailer, R.K.W.; Falk, K.; Rötzschke, O. Absence of leucine zipper in the natural FOXP3Δ2Δ7 isoform does not affect dimerization but abrogates suppressive capacity. PLoS ONE 2009, 4, e6104. [Google Scholar] [CrossRef]
- Serena, G.; Yan, S.; Camhi, S.; Patel, S.; Lima, R.S.; Sapone, A.; Leonard, M.M.; Mukherjee, R.; Nath, B.J.; Lammers, K.M.; et al. Proinflammatory cytokine interferon-γ and microbiome-derived metabolites dictate epigenetic switch between forkhead box protein 3 isoforms in coeliac disease. Clin. Exp. Immunol. 2017, 187, 490–506. [Google Scholar] [CrossRef] [PubMed]
- López-Casado, M.A.; Lorite, P.; Palomeque, T.; Torres, M.I. Potential role of the IL-33/ST2 axis in celiac disease. Cell. Mol. Immunol. 2017, 14, 285–292. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Nakabayashi, O.; Nakano, H. FLIP the switch: Regulation of apoptosis and necroptosis by cFLIP. Int. J. Mol. Sci. 2015, 16, 30321–30341. [Google Scholar] [CrossRef]
- Osborn, S.L.; Diehl, G.; Han, S.J.; Xue, L.; Kurd, N.; Hsieh, K.; Cado, D.; Robey, E.A.; Winoto, A. Fas-associated death domain (FADD) is a negative regulator of T-cell receptor-mediated necroptosis. Proc. Natl. Acad. Sci. USA 2010, 107, 13034–13039. [Google Scholar] [CrossRef]
- Samson, A.L.; Zhang, Y.; Geoghegan, N.D.; Gavin, X.J.; Davies, K.A.; Mlodzianoski, M.J.; Whitehead, L.W.; Frank, D.; Garnish, S.E.; Fitzgibbon, C.; et al. MLKL trafficking and accumulation at the plasma membrane control the kinetics and threshold for necroptosis. Nat. Commun. 2020, 11, 1–17. [Google Scholar] [CrossRef]
- He, S.; Liang, Y.; Shao, F.; Wang, X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc. Natl. Acad. Sci. USA 2011, 108, 20054–20059. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liang, Y.; Zhao, S.; Ding, Y.; Zhuang, Q.; Shi, Q.; Ai, T.; Wu, S.Q.; Han, J. ZBP1 mediates interferon-induced necroptosis. Cell. Mol. Immunol. 2020, 17, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Kanneganti, T.D. The regulation of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (PANoptosis). Immunol. Rev. 2020, 297, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Günther, C.; Ruder, B.; Stolzer, I.; Dorner, H.; He, G.W.; Chiriac, M.T.; Aden, K.; Strigli, A.; Bittel, M.; Zeissig, S.; et al. Interferon Lambda Promotes Paneth Cell Death Via STAT1 Signaling in Mice and Is Increased in Inflamed Ileal Tissues of Patients With Crohn’s Disease. Gastroenterology. 2019, 157, 1310–1322.e13. [Google Scholar] [CrossRef] [PubMed]
- Pizzuti, D.; Bortolami, M.; Mazzon, E.; Buda, A.; Guariso, G.; D’Odorico, A.; Chiarelli, S.; D’Incà, R.; De Lazzari, F.; Martines, D. Transcriptional downregulation of tight junction protein ZO-1 in active coeliac disease is reversed after a gluten-free diet. Dig. Liver Dis. 2004, 36, 337–341. [Google Scholar] [CrossRef]
- Montalto, M.; Cuoco, L.; Ricci, R.; Maggiano, N.; Vecchio, F.M.; Gasbarrini, G. Immunohistochemical Analysis of ZO-1 in the Duodenal Mucosa of Patients with Untreated and Treated Celiac Disease. Digestion 2002, 65, 227–233. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef]
- Riegman, M.; Sagie, L.; Galed, C.; Levin, T.; Steinberg, N.; Dixon, S.J.; Wiesner, U.; Bradbury, M.S.; Niethammer, P.; Zaritsky, A.; et al. Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture. Nat. Cell Biol. 2020, 22, 1042–1048. [Google Scholar] [CrossRef]
- Yan, B.; Ai, Y.; Sun, Q.; Ma, Y.; Cao, Y.; Wang, J.; Zhang, Z.; Wang, X. Membrane Damage during Ferroptosis Is Caused by Oxidation of Phospholipids Catalyzed by the Oxidoreductases POR and CYB5R1. Mol. Cell 2021, 81, 355–369.e10. [Google Scholar] [CrossRef]
- Martin-Sanchez, D.; Ruiz-Andres, O.; Poveda, J.; Carrasco, S.; Cannata-Ortiz, P.; Sanchez-Niño, M.D.; Ruiz Ortega, M.; Egido, J.; Linkermann, A.; Ortiz, A.; et al. Ferroptosis, but not necroptosis, is important in nephrotoxic folic acid-induced AKI. J. Am. Soc. Nephrol. 2017, 28, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, J.; Song, X.; Qu, L.; Wei, R.; He, F.; Wang, K.; Luo, B. RIP3 induces ischemic neuronal DNA degradation and programmed necrosis in rat via AIF. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Maluf, S.W.; Filho, D.W.; Parisotto, E.B.; de Medeiros, G. da S.; Pereira, C.H.J.; Maraslis, F.T.; Schoeller, C.C.D.; da Rosa, J.S.; Fröde, T.S. DNA damage, oxidative stress, and inflammation in children with celiac disease. Genet. Mol. Biol. 2020, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stazi, A.V.; Trinti, B. Selenium status and over-expression of interleukin-15 in celiac disease and autoimmune thyroid diseases. Ann. Ist. Super. Sanita 2010, 46, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Green, M.; Choi, J.E.; Gijón, M.; Kennedy, P.D.; Johnson, J.K.; Liao, P.; Lang, X.; Kryczek, I.; Sell, A.; et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 2019, 569, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef]
- Zorro, M.M.; Aguirre-Gamboa, R.; Mayassi, T.; Ciszewski, C.; Barisani, D.; Hu, S.; Weersma, R.K.; Withoff, S.; Li, Y.; Wijmenga, C.; et al. Tissue alarmins and adaptive cytokine induce dynamic and distinct transcriptional responses in tissue-resident intraepithelial cytotoxic T lymphocytes. J. Autoimmun. 2020, 108. [Google Scholar] [CrossRef]
- Jabri, B.; Abadie, V. IL-15 functions as a danger signal to regulate tissue-resident T cells and tissue destruction. Nat. Rev. Immunol. 2015, 15, 771–783. [Google Scholar] [CrossRef]
- Bianchi, M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol. 2007, 81, 1–5. [Google Scholar] [CrossRef]
- Linkermann, A.; Stockwell, B.R.; Krautwald, S.; Anders, H.J. Regulated cell death and inflammation: An auto-amplification loop causes organ failure. Nat. Rev. Immunol. 2014, 14, 759–767. [Google Scholar] [CrossRef]
- Yuan, F.; Gu, L.; Guo, S.; Wang, C.; Li, G.M. Evidence for involvement of HMGB1 protein in human DNA mismatch repair. J. Biol. Chem. 2004, 279, 20935–20940. [Google Scholar] [CrossRef]
- Lu, B.; Antoine, D.J.; Kwan, K.; Lundbäck, P.; Wähämaa, H.; Schierbeck, H.; Robinson, M.; Van Zoelen, M.A.D.; Yang, H.; Li, J.; et al. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc. Natl. Acad. Sci. USA 2020, 111, 3068–3073. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kwak, M.S.; Lee, B.; Shin, J.M.; Aum, S.; Park, I.H.; Lee, M.G.; Shin, J.S. Secretory autophagy machinery and vesicular trafficking are involved in HMGB1 secretion. Autophagy 2020, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hreggvidsdottir, H.S.; Palmblad, K.; Wang, H.; Ochani, M.; Li, J.; Lu, B.; Chavan, S.; Rosas-Ballina, M.; Al-Abed, Y.; et al. A critical cysteine is required for HMGB1 binding to toll-like receptor 4 and activation of macrophage cytokine release. Proc. Natl. Acad. Sci. USA 2010, 107, 11942–11947. [Google Scholar] [CrossRef]
- Kierdorf, K.; Fritz, G. RAGE regulation and signaling in inflammation and beyond. J. Leukoc. Biol. 2013, 94, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, E.; Fasth, A.E.R.; Palmblad, K.; Harris, H.E.; Andersson, U. High mobility group box chromosomal protein 1 acts as a proliferation signal for activated T lymphocytes. Immunobiology 2009, 214, 303–309. [Google Scholar] [CrossRef]
- Harris, H.E.; Andersson, U.; Pisetsky, D.S. HMGB1: A multifunctional alarmin driving autoimmune and inflammatory disease. Nat. Rev. Rheumatol. 2012, 8, 195–202. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, Y.; Wang, J.; Shi, X.; Liu, Q.; Liu, Z.; Li, Y.; Scott, M.J.; Xiao, G.; Li, S.; et al. Macrophage endocytosis of high-mobility group box 1 triggers pyroptosis. Cell Death Differ. 2014, 21, 1229–1239. [Google Scholar] [CrossRef]
- Manti, S.; Cuppari, C.; Parisi, G.F.; Tardino, L.; Salpietro, C.; Leonardi, S. HMGB1 values and response to HBV vaccine in children with celiac disease. Nutrition 2017, 42, 20–22. [Google Scholar] [CrossRef]
- Palone, F.; Vitali, R.; Trovato, C.M.; Montuori, M.; Negroni, A.; Mallardo, S.; Stronati, L. Faecal high mobility group box 1 in children with celiac disease: A pilot study. Dig. Liver Dis. 2018, 50, 916–919. [Google Scholar] [CrossRef]
- Piatek-Guziewicz, A.; Ptak-Belowska, A.; Przybylska-Felus, M.; Pasko, P.; Zagrodzki, P.; Brzozowski, T.; Mach, T.; Zwolinska-Wcislo, M. Intestinal parameters of oxidative imbalance in celiac adults with extraintestinal manifestations. World J. Gastroenterol. 2017, 23, 7849–7862. [Google Scholar] [CrossRef]
- Hadithi, M.; de Boer, H.; Meijer, J.W.R.; Willekens, F.; Kerckhaert, J.A.; Heijmans, R.; Peña, A.S.; Stehouwer, C.D.A.; Mulder, C.J.J. Coeliac disease in Dutch patients with Hashimoto’s thyroiditis and vice versa. World J. Gastroenterol. 2007, 13, 1715–1722. [Google Scholar] [CrossRef]
- Viljamaa, M.; Kaukinen, K.; Huhtala, H.; Kyrönpalo, S.; Rasmussen, M.; Collin, P. Coeliac disease, autoimmune diseases and gluten exposure. Scand. J. Gastroenterol. 2005, 40, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Elfström, P.; Sundström, J.; Ludvigsson, J.F. Systematic review with meta-analysis: Associations between coeliac disease and type 1 diabetes. Aliment. Pharmacol. Ther. 2014, 40, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, L.; Wang, F.; Zou, Y.; Li, J.J.; Luo, J.; Khan, F.; Sun, F.; Li, Y.; Liu, J.; et al. Extracellular HMGB1 exacerbates autoimmune progression and recurrence of type 1 diabetes by impairing regulatory T cell stability. Diabetologia 2020, 63, 987–1001. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Peng, S.; Liu, X.; Han, C.; Wang, X.; Jin, T.; Liu, S.; Wang, W.; Xie, X.; He, X.; et al. Glycyrrhizin, a Direct HMGB1 Antagonist, Ameliorates Inflammatory Infiltration in a Model of Autoimmune Thyroiditis via Inhibition of TLR2-HMGB1 Signaling. Thyroid 2017, 27, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, H.; Wu, J.; Cai, Z.Y.; Li, B.; Ni, H.; Qiu, X.; Chen, H.; Liu, W.; Yang, Z.H.; et al. Gut stem cell necroptosis by genome instability triggers bowel inflammation. Nature 2020, 580, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Messmer, D.; Yang, H.; Telusma, G.; Knoll, F.; Li, J.; Messmer, B.; Tracey, K.J.; Chiorazzi, N. High Mobility Group Box Protein 1: An Endogenous Signal for Dendritic Cell Maturation and Th1 Polarization. J. Immunol. 2004, 173, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liang, X.; Lotze, M.T. HMGB1: The central cytokine for all lymphoid cells. Front. Immunol. 2013, 4. [Google Scholar] [CrossRef]
- Schaper, F.; de Leeuw, K.; Horst, G.; Bootsma, H.; Limburg, P.C.; Heeringa, P.; Bijl, M.; Westra, J. High mobility group box 1 skews macrophage polarization and negatively influences phagocytosis of apoptotic cells. Rheumatology 2016, 55, 2260–2270. [Google Scholar] [CrossRef] [PubMed]
- Pichery, M.; Mirey, E.; Mercier, P.; Lefrancais, E.; Dujardin, A.; Ortega, N.; Girard, J.-P. Endogenous IL-33 Is Highly Expressed in Mouse Epithelial Barrier Tissues, Lymphoid Organs, Brain, Embryos, and Inflamed Tissues: In Situ Analysis Using a Novel Il-33–LacZ Gene Trap Reporter Strain. J. Immunol. 2012, 188, 3488–3495. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.T.; Martin, M.U. Interleukin 33 is a guardian of barriers and a local alarmin. Nat. Immunol. 2016, 17, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Bessa, J.; Meyer, C.A.; de Vera Mudry, M.C.; Schlicht, S.; Smith, S.H.; Iglesias, A.; Cote-Sierra, J. Altered subcellular localization of IL-33 leads to non-resolving lethal inflammation. J. Autoimmun. 2014, 55, 33–41. [Google Scholar] [CrossRef]
- Lüthi, A.U.; Cullen, S.P.; McNeela, E.A.; Duriez, P.J.; Afonina, I.S.; Sheridan, C.; Brumatti, G.; Taylor, R.C.; Kersse, K.; Vandenabeele, P.; et al. Suppression of Interleukin-33 Bioactivity through Proteolysis by Apoptotic Caspases. Immunity 2009, 31, 84–98. [Google Scholar] [CrossRef]
- Molofsky, A.B.B.; Savage, A.K.K.; Locksley, R.M.M. Interleukin-33 in Tissue Homeostasis, Injury, and Inflammation. Immunity 2015, 42, 1005–1019. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Saluja, R.; Ketelaar, M.E.; Hawro, T.; Church, M.K.; Maurer, M.; Nawijn, M.C. The role of the IL-33/IL-1RL1 axis in mast cell and basophil activation in allergic disorders. Mol. Immunol. 2015, 63, 80–85. [Google Scholar] [CrossRef]
- Salimi, M.; Barlow, J.L.; Saunders, S.P.; Xue, L.; Gutowska-Owsiak, D.; Wang, X.; Huang, L.C.; Johnson, D.; Scanlon, S.T.; McKenzie, A.N.J.; et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 2013, 210, 2939–2950. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.; Bonilla, W.V.; Fröhlich, A.; Helmstetter, C.; Peine, M.; Hegazy, A.N.; Pinschewer, D.D.; Löhning, M. T-bet– and STAT4–dependent IL-33 receptor expression directly promotes antiviral Th1 cell responses. Proc. Natl. Acad. Sci. USA 2015, 112, 4056–4061. [Google Scholar] [CrossRef]
- Bonilla, W.V.; Fröhlich, A.; Senn, K.; Kallert, S.; Fernandez, M.; Fallon, P.G.; Klemenz, R.; Nakae, S.; Adler, H.; Merkler, D.; et al. The Alarmin Interleukin-33 Drives. Science 2012, 335, 984–989. [Google Scholar] [CrossRef]
- Schiering, C.; Krausgruber, T.; Chomka, A.; Fröhlich, A.; Adelmann, K.; Wohlfert, E.A.; Pott, J.; Griseri, T.; Bollrath, J.; Hegazy, A.N.; et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 2014, 513, 564–568. [Google Scholar] [CrossRef]
- Monticelli, L.A.; Osborne, L.C.; Noti, M.; Tran, S.V.; Zaiss, D.M.W.W.; Artis, D. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin–EGFR interactions. Proc. Natl. Acad. Sci. USA 2015, 112, 10762–10767. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, X.; Zhao, Y.; Chen, F.; Sun, M.; Yang, W.; Chen, L.; Yao, S.; Peniche, A.; Dann, S.M.; et al. Interleukin-33 Promotes REG3γ Expression in Intestinal Epithelial Cells and Regulates Gut Microbiota. Cmgh 2019, 8, 21–36. [Google Scholar] [CrossRef]
- Lefrançais, E.; Roga, S.; Gautier, V.; Gonzalez-de-Peredo, A.; Monsarrat, B.; Girard, J.-P.; Cayrol, C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc. Natl. Acad. Sci. USA 2012, 109, 1673–1678. [Google Scholar] [CrossRef]
- Lefranҫais, E.; Duval, A.; Mirey, E.; Roga, S.; Espinosa, E.; Cayrol, C.; Girard, J.P. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc. Natl. Acad. Sci. USA 2014, 111, 15502–15507. [Google Scholar] [CrossRef]
- Villarreal, D.O.; Wise, M.C.; Walters, J.N.; Reuschel, E.L.; Choi, M.J.; Obeng-Adjei, N.; Yan, J.; Morrow, M.P.; Weiner, D.B. Alarmin IL-33 Acts as an immunoadjuvant to enhance antigen-specific tumor immunity. Cancer Res. 2014, 74, 1789–1800. [Google Scholar] [CrossRef]
- Bakker, O.B.; Sánchez, A.D.R.; Borek, Z.A.; De Klein, N.; Li, Y.; Modderman, R.; Winkelaar, Y.K.; Johannesen, M.K.; Matarese, F.; Martens, J.H.A.; et al. Potential impact of celiac disease genetic risk factors on T cell receptor signaling in gluten—Specific CD4+ T cells. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Benhar, M. Oxidants, antioxidants and thiol Redox switches in the control of regulated cell death pathways. Antioxidants 2020, 9, 309. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Stamnaes, J.; Klöck, C.; DiRaimondo, T.R.; Sollid, L.M.; Khosla, C. Activation of extracellular transglutaminase 2 by thioredoxin. J. Biol. Chem. 2011, 286, 37866–37873. [Google Scholar] [CrossRef]
- Plugis, N.M.; Palanski, B.A.; Weng, C.H.; Albertelli, M.; Khosla, C. Thioredoxin-1 selectively activates transglutaminase 2 in the extracellular matrix of the small intestine: Implications for celiac disease. J. Biol. Chem. 2017, 292, 2000–2008. [Google Scholar] [CrossRef] [PubMed]
- Samir, P.; Malireddi, R.K.S.; Kanneganti, T.D. The PANoptosome: A Deadly Protein Complex Driving Pyroptosis, Apoptosis, and Necroptosis (PANoptosis). Front. Cell. Infect. Microbiol. 2020, 10. [Google Scholar] [CrossRef]
- Apelbaum, A.; Yarden, G.; Warszawski, S.; Harari, D.; Schreiber, G. Type I Interferons Induce Apoptosis by Balancing cFLIP and Caspase-8 Independent of Death Ligands. Mol. Cell. Biol. 2013, 33, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, J.; Liu, B.C.; Muendlein, H.I.; Weindel, C.G.; Smirnova, I.; Tang, A.Y.; Ilyukha, V.; Sorokin, M.; Buzdin, A.; Fitzgerald, K.A.; et al. Constitutive interferon signaling maintains critical threshold of MLKL expression to license necroptosis. Cell Death Differ. 2019, 26, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Therrien, A.; Kelly, C.P.; Silvester, J.A. Celiac Disease: Extraintestinal Manifestations and Associated Conditions. J. Clin. Gastroenterol. 2020, 54, 8–21. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez, F.; Ruera, C.N.; Miculan, E.; Carasi, P.; Chirdo, F.G. Programmed Cell Death in the Small Intestine: Implications for the Pathogenesis of Celiac Disease. Int. J. Mol. Sci. 2021, 22, 7426. https://doi.org/10.3390/ijms22147426
Perez F, Ruera CN, Miculan E, Carasi P, Chirdo FG. Programmed Cell Death in the Small Intestine: Implications for the Pathogenesis of Celiac Disease. International Journal of Molecular Sciences. 2021; 22(14):7426. https://doi.org/10.3390/ijms22147426
Chicago/Turabian StylePerez, Federico, Carolina Nayme Ruera, Emanuel Miculan, Paula Carasi, and Fernando Gabriel Chirdo. 2021. "Programmed Cell Death in the Small Intestine: Implications for the Pathogenesis of Celiac Disease" International Journal of Molecular Sciences 22, no. 14: 7426. https://doi.org/10.3390/ijms22147426
APA StylePerez, F., Ruera, C. N., Miculan, E., Carasi, P., & Chirdo, F. G. (2021). Programmed Cell Death in the Small Intestine: Implications for the Pathogenesis of Celiac Disease. International Journal of Molecular Sciences, 22(14), 7426. https://doi.org/10.3390/ijms22147426




