Anti-Allergic Drug Suppressed Pancreatic Carcinogenesis via Down-Regulation of Cellular Proliferation
Abstract
:1. Introduction
2. Results
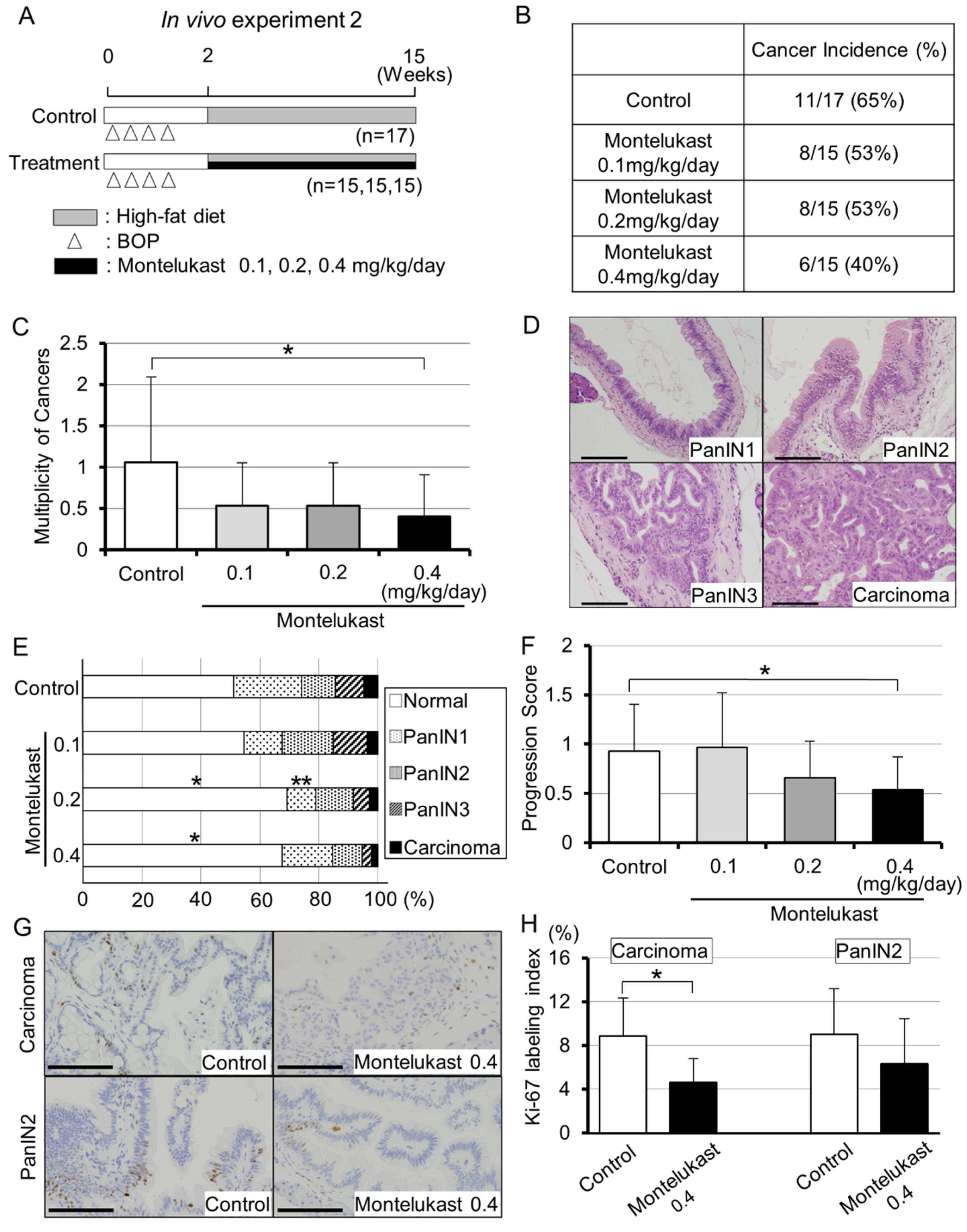
2.1. Animal Experiments Demonstrated the Suppressive Effects of Montelukast on Pancreatic Carcinogenesis in BOP-Treated Hamsters
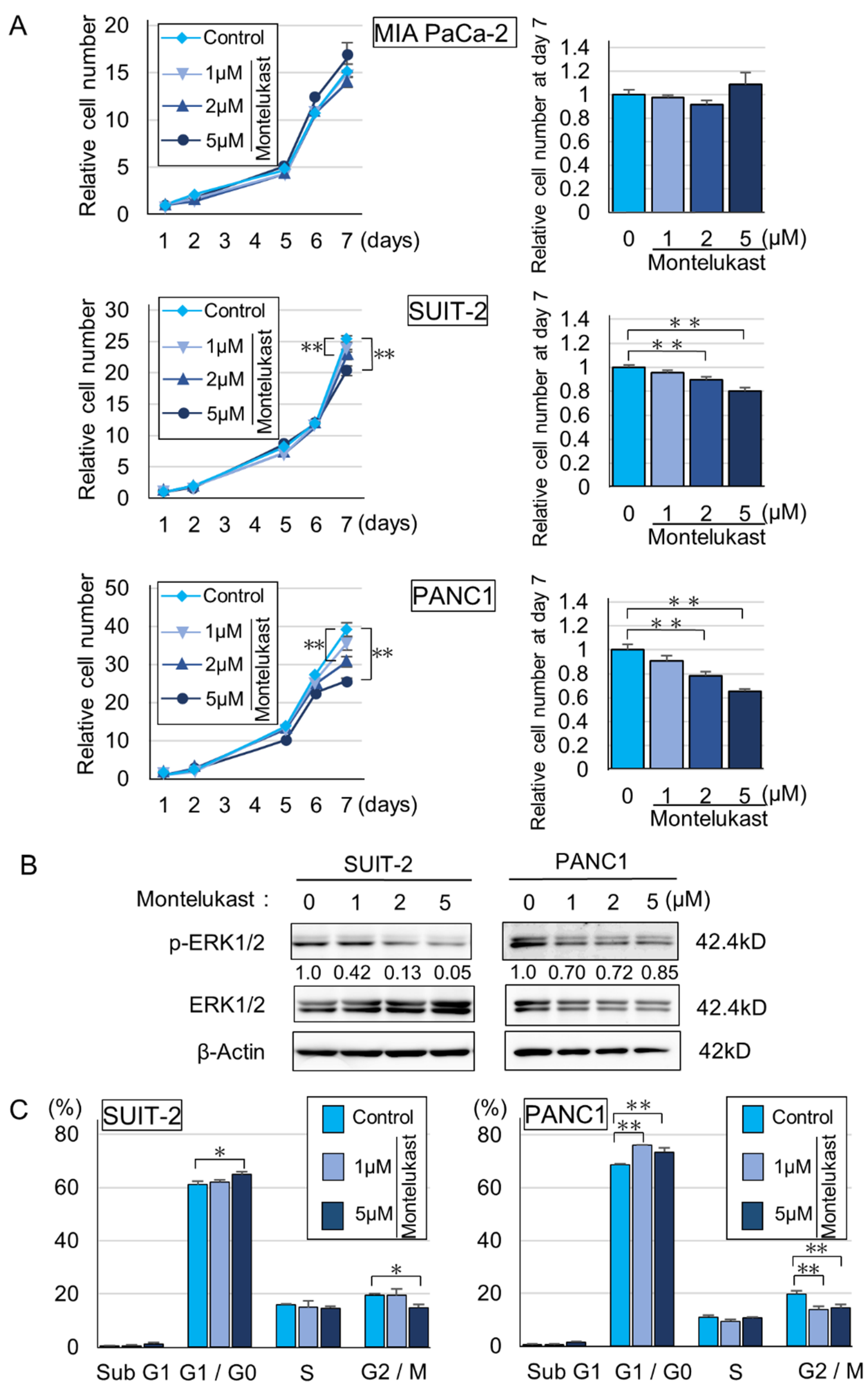
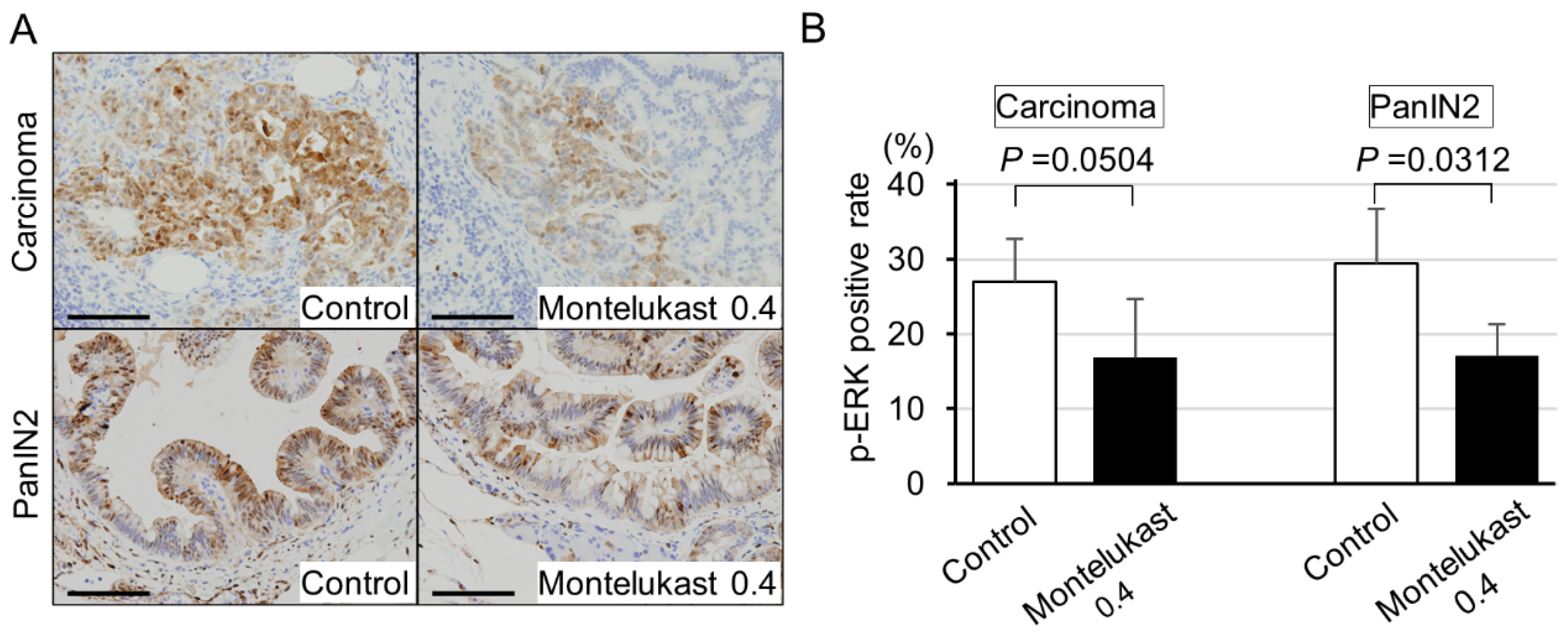
2.2. High-Dose Montelukast Significantly Inhibited Pancreatic Cancer Development by Suppressing Cellular Proliferation
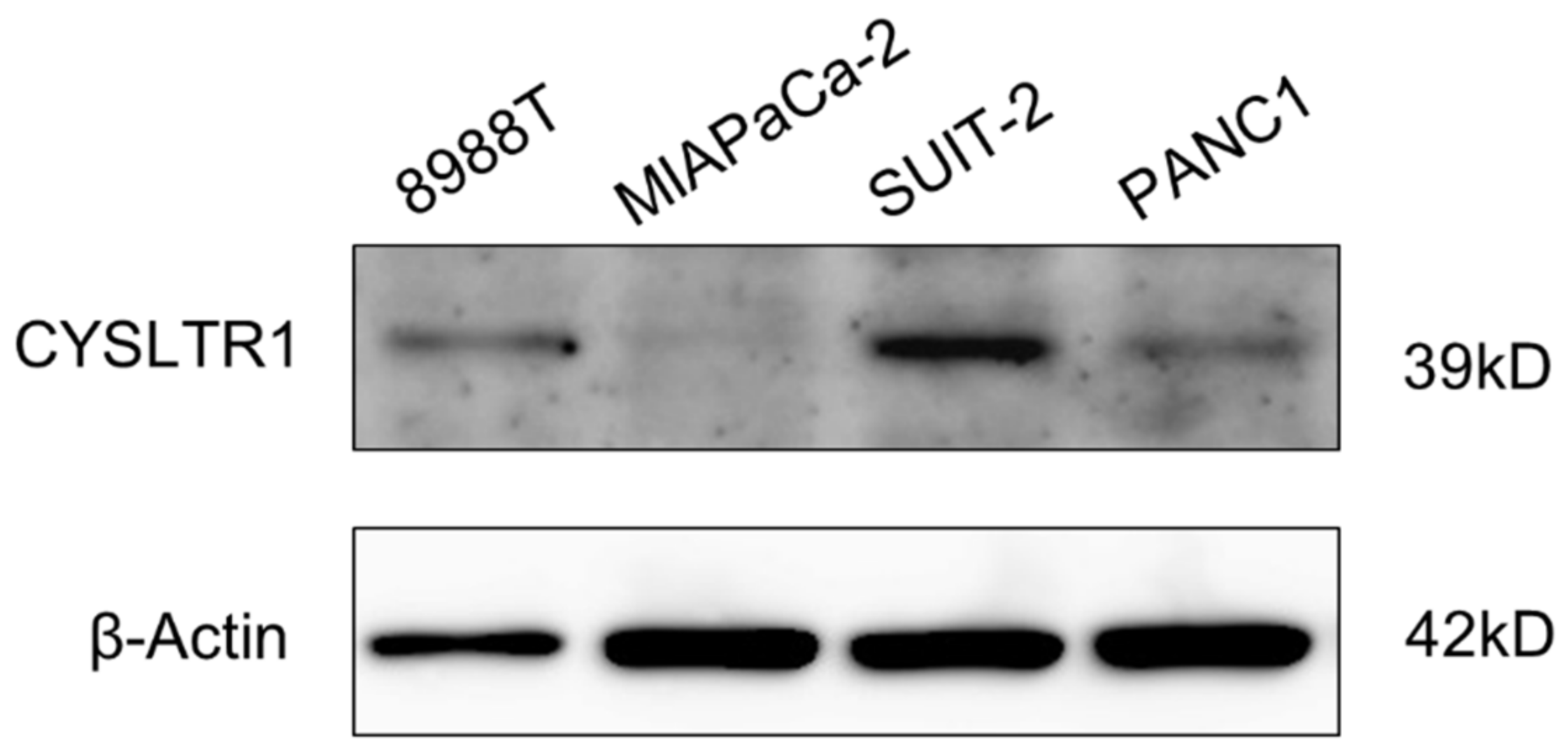
2.3. CYSLTR1 Regulated the Cellular Proliferation of PDAC Cells
2.4. High CYSLTR1 Expression Was Associated with Poor Prognosis in PDAC Patients
3. Discussion
4. Materials and Methods
4.1. Animals, Diet and Chemicals
4.2. Animal Experiments
4.3. Histopathological Examination
4.4. Immunohistochemistry
4.5. Cell Culture
4.6. Cell Cycle Analysis
4.7. Immunoblotting
4.8. Tissue Samples, Clinical Information and CYSLTR1 Expression in PDAC
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Rubio, P.; Zock, J.-P.; Rava, M.; Marquez, M.; Sharp, L.; Hidalgo, M.; Carrato, A.; Ilzarbe, L.; Michalski, C.; Molero, X.; et al. Reduced risk of pancreatic cancer associated with asthma and nasal allergies. Gut 2015, 66, 314–322. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Wu, P.-H.; Sheu, C.-C.; Hsu, Y.-L.; Chang, W.-A.; Hung, J.-Y.; Yang, C.-J.; Yang, Y.-H.; Kuo, P.-L.; Huang, M.-S. Cysteinyl Leukotriene Receptor Antagonists Decrease Cancer Risk in Asthma Patients. Sci. Rep. 2016, 6, 23979. [Google Scholar] [CrossRef]
- Kato, A.; Naiki-Ito, A.; Nakazawa, T.; Hayashi, K.; Naitoh, I.; Miyabe, K.; Shimizu, S.; Kondo, H.; Nishi, Y.; Yoshida, M.; et al. Chemopreventive effect of resveratrol and apocynin on pancreatic carcinogenesis via modulation of nuclear phosphorylated GSK3β and ERK1/2. Oncotarget 2015, 6, 42963–42975. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.-J.; Chang, W.-A.; Tsai, P.-H.; Wu, C.-Y.; Ho, Y.-W.; Yen, M.-C.; Lin, Y.-S.; Kuo, P.-L.; Hsu, Y.-L. Montelukast Induces Apoptosis-Inducing Factor-Mediated Cell Death of Lung Cancer Cells. Int. J. Mol. Sci. 2017, 18, 1353. [Google Scholar] [CrossRef] [Green Version]
- Matsuyama, M.; Hayama, T.; Funao, K.; Kawahito, Y.; Sano, H.; Takemoto, Y.; Nakatani, T.; Yoshimura, R. Overexpression of cysteinyl LT1 receptor in prostate cancer and CysLT1R antagonist inhibits prostate can-cer cell growth through apoptosis. Oncol. Rep. 2007, 18, 99–104. [Google Scholar]
- Funao, K.; Matsuyama, M.; Naganuma, T.; Kawahito, Y.; Sano, H.; Nakatani, T.; Yoshimura, R. The cyste-inylLT1 receptor in human renal cell carcinoma. Mol. Med. Rep. 2008, 1, 185–189. [Google Scholar]
- Matsuyama, M.; Funao, K.; Kawahito, Y.; Sano, H.; Chargui, J.; Touraine, J.-L.; Nakatani, T.; Yoshimura, R. Expression of cysteinylLT1 receptor in human testicular cancer and growth reduction by its antagonist through apoptosis. Mol. Med. Rep. 2009, 2, 163–167. [Google Scholar] [CrossRef] [Green Version]
- Matsuyama, M.; Funao, K.; Hayama, T.; Tanaka, T.; Kawahito, Y.; Sano, H.; Takemoto, Y.; Nakatani, T.; Yoshimura, R. Relationship Between Cysteinyl-Leukotriene-1 Receptor and Human Transitional Cell Carcinoma in Bladder. Urology 2009, 73, 916–921. [Google Scholar] [CrossRef]
- Matsuyama, M.; Yoshimura, R. Cysteinyl-leukotriene1 receptor is a potent target for the prevention and treatment of human urological cancer. Mol. Med. Rep. 2011, 3, 245–251. [Google Scholar] [CrossRef]
- Shah, R.R.; Stonier, P.D. Repurposing old drugs in oncology: Opportunities with clinical and regulatory challenges ahead. J. Clin. Pharm. Ther. 2019, 44, 6–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, S.; Uemura, H.; Seeni, A.; Tang, M.; Komiya, M.; Long, N.; Ishiguro, H.; Kubota, Y.; Shirai, T. Therapeutic targeting of angiotensin II receptor type 1 to regulate androgen receptor in prostate cancer. Prostate 2012, 72, 1559–1572. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Isayama, H.; Sasaki, T.; Takahara, N.; Saito, K.; Ishigaki, K.; Hamada, T.; Mizuno, S.; Miyabayashi, K.; Yamamoto, K.; et al. The inhibition of renin-angiotensin system in advanced pancreatic cancer: An exploratory analysis in 349 patients. J. Cancer Res. Clin. Oncol. 2015, 141, 933–939. [Google Scholar] [CrossRef]
- Fritz, I.; Wagner, P.; Broberg, P.; Einefors, R.; Olsson, H. Desloratadine and loratadine stand out among common H1-antihistamines for association with improved breast cancer survival. Acta Oncol. 2020, 59, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, K.Y.; Liu, Q.; Wang, G.; Jiang, W.; Meng, Q.; Yi, Y.; Yang, Y.; Wang, R.; Zhu, S.; et al. Antihistamine Drug Ebastine Inhibits Cancer Growth by Targeting Polycomb Group Protein EZH2. Mol. Cancer Ther. 2020, 19, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Tsuruda, Y.; Matsumoto, Y.; Uchida, H.; Nakayama, K.I.; Mimori, K. Drug repositioning in cancer: The current situation in Japan. Cancer Sci. 2020, 111, 1039–1046. [Google Scholar] [CrossRef] [Green Version]
- Pantziarka, P.; Verbaanderd, C.; Sukhatme, V.; Capistrano, I.R.; Crispino, S.; Gyawali, B.; Rooman, I.; Van Nuffel, A.; Meheus, L.; Sukhatme, V.P.; et al. ReDO_DB: The repurposing drugs in oncology database. Ecancermedicalscience 2018, 12, 886. [Google Scholar] [CrossRef] [Green Version]
- Hennig, R.; Osman, T.; Esposito, I.; Giese, N.; Rao, S.M.; Ding, X.-Z.; Tong, W.-G.; Büchler, M.W.; Yokomizo, T.; Friess, H.; et al. BLT2 is expressed in PanINs, IPMNs, pancreatic cancer and stimulates tumour cell proliferation. Br. J. Cancer 2008, 99, 1064–1073. [Google Scholar] [CrossRef] [Green Version]
- Massoumi, R.; Sjölander, A. The Role of Leukotriene Receptor Signaling in Inflammation and Cancer. Sci. World J. 2007, 7, 1413–1421. [Google Scholar] [CrossRef]
- Magnusson, C.; Mezhybovska, M.; Lörinc, E.; Fernebro, E.; Nilbert, M.; Sjölander, A. Low expression of CysLT1R and high expression of CysLT2R mediate good prognosis in colorectal cancer. Eur. J. Cancer 2010, 46, 826–835. [Google Scholar] [CrossRef]
- Magnusson, C.; Liu, J.; Ehrnström, R.; Manjer, J.; Jirström, K.; Andersson, T.; Sjölander, A. Cysteinyl leukotriene receptor expression pattern affects migration of breast cancer cells and survival of breast cancer patients. Int. J. Cancer 2010, 129, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Venerito, M.; Kuester, D.; Harms, C.; Schubert, D.; Wex, T.; Malfertheiner, P. Upregulation of Leukotriene Receptors in Gastric Cancer. Cancers 2011, 3, 3156–3168. [Google Scholar] [CrossRef] [Green Version]
- Savari, S.; Liu, M.; Zhang, Y.; Sime, W.; Sjölander, A. CysLT1R Antagonists Inhibit Tumor Growth in a Xenograft Model of Colon Cancer. PLoS ONE 2013, 8, e73466. [Google Scholar] [CrossRef] [Green Version]
- Kuo, P.-L.; Huang, M.-S.; Hung, J.-Y.; Chou, S.-H.; Chiang, S.-Y.; Huang, Y.-F.; Yang, C.-J.; Tsai, M.-J.; Chang, W.-A.; Hsu, Y.-L. Synergistic effect of lung tumor-associated dendritic cell-derived HB-EGF and CXCL5 on cancer progression. Int. J. Cancer 2014, 135, 96–108. [Google Scholar] [CrossRef]
- Roberts, P.J.; Der, C.J. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007, 26, 3291–3310. [Google Scholar] [CrossRef] [Green Version]
- Savari, S. Cysteinyl leukotrienes and their receptors: Bridging inflammation and colorectal cancer. World J. Gastroenterol. 2014, 20, 968–977. [Google Scholar] [CrossRef]
- Paruchuri, S.; Hallberg, B.; Juhas, M.; Larsson, C.; Sjölander, A. Leukotriene D(4) activates MAPK through a Ras-independent but PKCepsilon-dependent pathway in intestinal epithelial cells. J. Cell Sci. 2002, 115, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.-M.; Fang, S.-H.; Qian, X.-D.; Liu, L.-Y.; Xu, L.-H.; Shi, W.-Z.; Zhang, L.-H.; Lu, Y.-B.; Zhang, W.-P.; Wei, E.-Q. Leukotriene D4 stimulates the migration but not proliferation of endothelial cells mediated by the cysteinyl leukotriene cyslt(1) receptor via the extracellular signal-regulated kinase pathway. J. Pharmacol. Sci. 2009, 109, 285–292. [Google Scholar] [CrossRef] [Green Version]
- Hori, M.; Kitahashi, T.; Imai, T.; Ishigamori, R.; Takasu, S.; Mutoh, M.; Sugimura, T.; Wakabayashi, K.; Takahashi, M. Enhancement of Carcinogenesis and Fatty Infiltration in the Pancreas in N-Nitrosobis(2-Oxopropyl)Amine-Treated Hamsters by High-Fat Diet. Pancreas 2011, 40, 1234–1240. [Google Scholar] [CrossRef]
- Kato, H.; Naiki-Ito, A.; Suzuki, S.; Inaguma, S.; Komura, M.; Nakao, K.; Naiki, T.; Kachi, K.; Kato, A.; Matsuo, Y.; et al. DPYD, down-regulated by the potentially chemopreventive agent luteolin, interacts with STAT3 in pancreatic cancer. Carcinogenesis 2021. [Google Scholar] [CrossRef] [PubMed]
- Inaguma, S.; Lasota, J.; Felisiak-Golabek, A.; Kowalik, A.; Wang, Z.; Zieba, S.; Kalisz, J.; Ikeda, H.; Miettinen, M. Histopathological and genotypic characterization of metastatic colorectal carcinoma with PD-L1 (CD274)-expression: Possible roles of tumour micro environmental factors for CD274 expression. J. Pathol. Clin. Res. 2017, 3, 268–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inaguma, S.; Riku, M.; Hashimoto, M.; Murakami, H.; Saga, S.; Ikeda, H.; Kasai, K. GLI1 Interferes with the DNA Mismatch Repair System in Pancreatic Cancer through BHLHE41-Mediated Suppression of MLH1. Cancer Res. 2013, 73, 7313–7323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kachi, K.; Kato, H.; Naiki-Ito, A.; Komura, M.; Nagano-Matsuo, A.; Naitoh, I.; Hayashi, K.; Kataoka, H.; Inaguma, S.; Takahashi, S. Anti-Allergic Drug Suppressed Pancreatic Carcinogenesis via Down-Regulation of Cellular Proliferation. Int. J. Mol. Sci. 2021, 22, 7444. https://doi.org/10.3390/ijms22147444
Kachi K, Kato H, Naiki-Ito A, Komura M, Nagano-Matsuo A, Naitoh I, Hayashi K, Kataoka H, Inaguma S, Takahashi S. Anti-Allergic Drug Suppressed Pancreatic Carcinogenesis via Down-Regulation of Cellular Proliferation. International Journal of Molecular Sciences. 2021; 22(14):7444. https://doi.org/10.3390/ijms22147444
Chicago/Turabian StyleKachi, Kenta, Hiroyuki Kato, Aya Naiki-Ito, Masayuki Komura, Aya Nagano-Matsuo, Itaru Naitoh, Kazuki Hayashi, Hiromi Kataoka, Shingo Inaguma, and Satoru Takahashi. 2021. "Anti-Allergic Drug Suppressed Pancreatic Carcinogenesis via Down-Regulation of Cellular Proliferation" International Journal of Molecular Sciences 22, no. 14: 7444. https://doi.org/10.3390/ijms22147444





