Lipid Metabolism Is Dysregulated before, during and after Pregnancy in a Mouse Model of Gestational Diabetes
Abstract
1. Introduction
2. Results
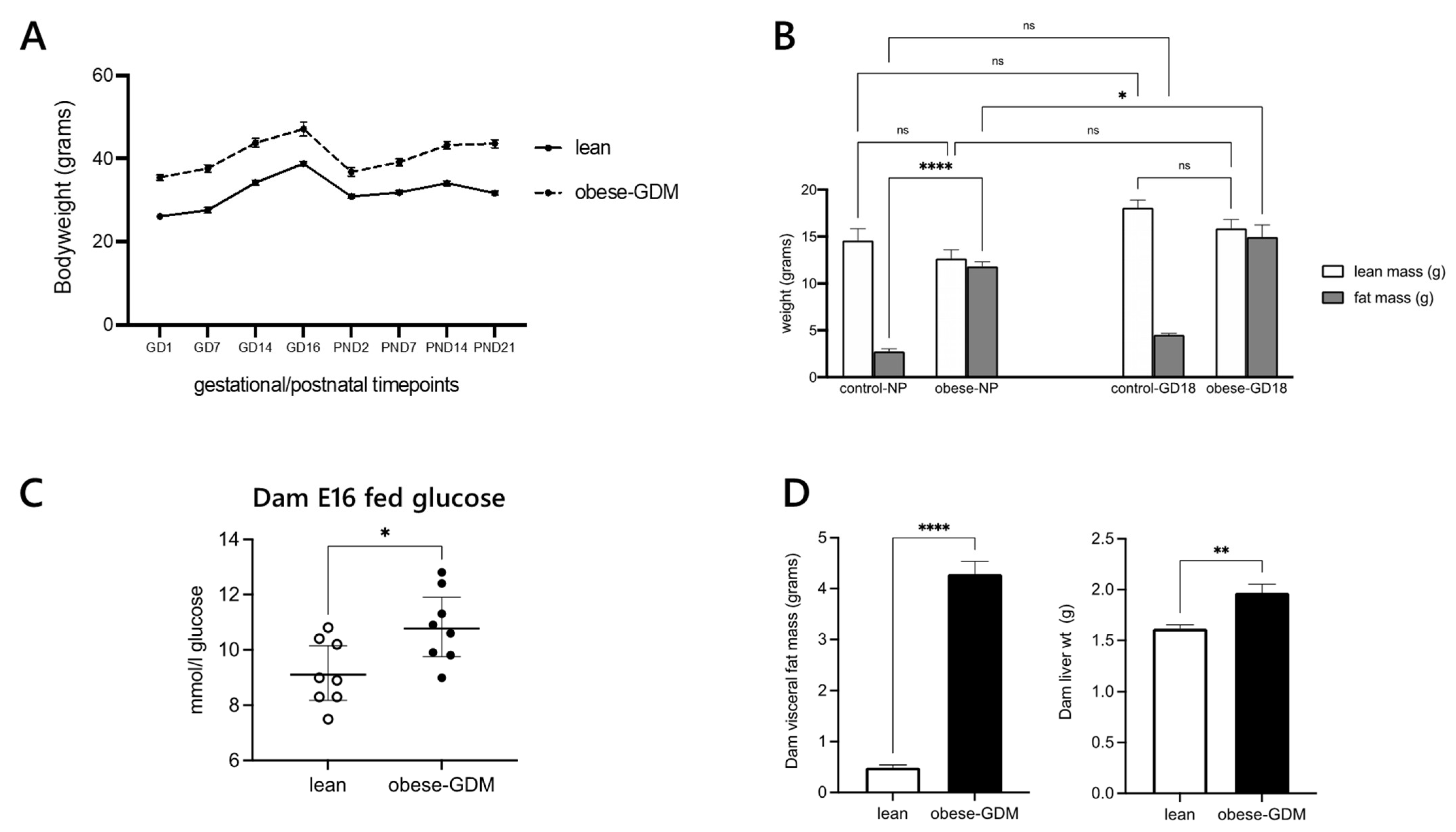
2.1. Metabolic Phenotyping
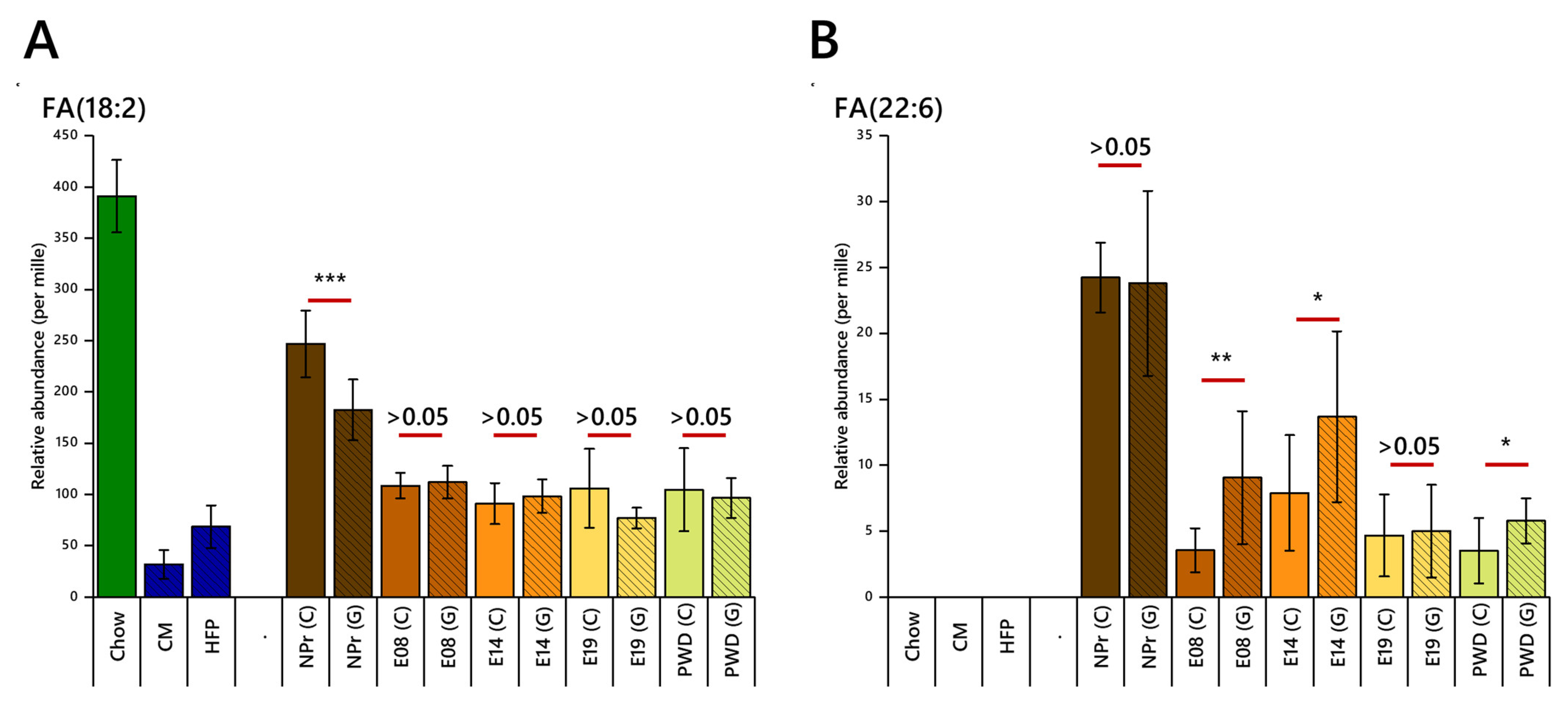
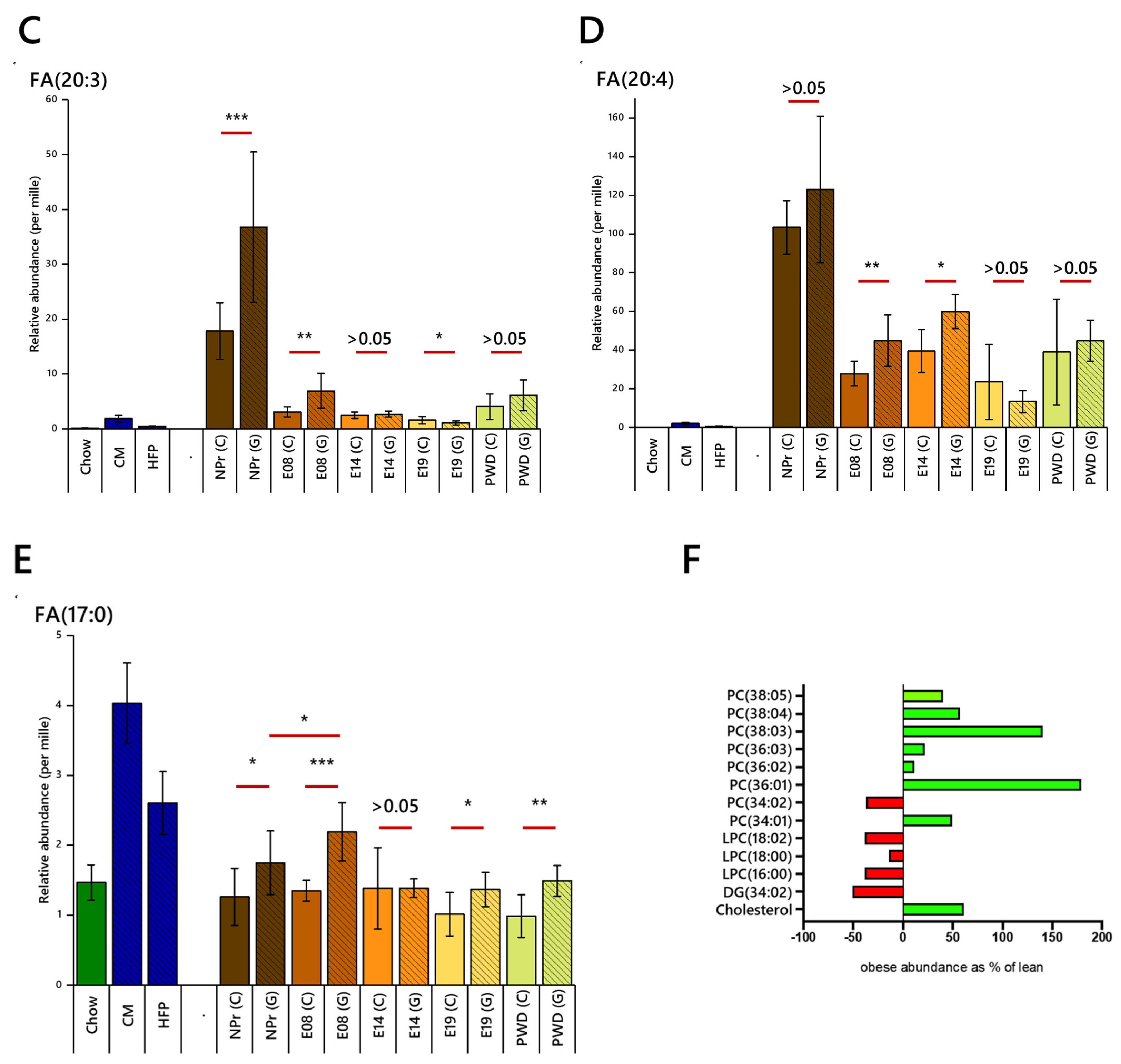
2.2. Fatty Acid Regulation
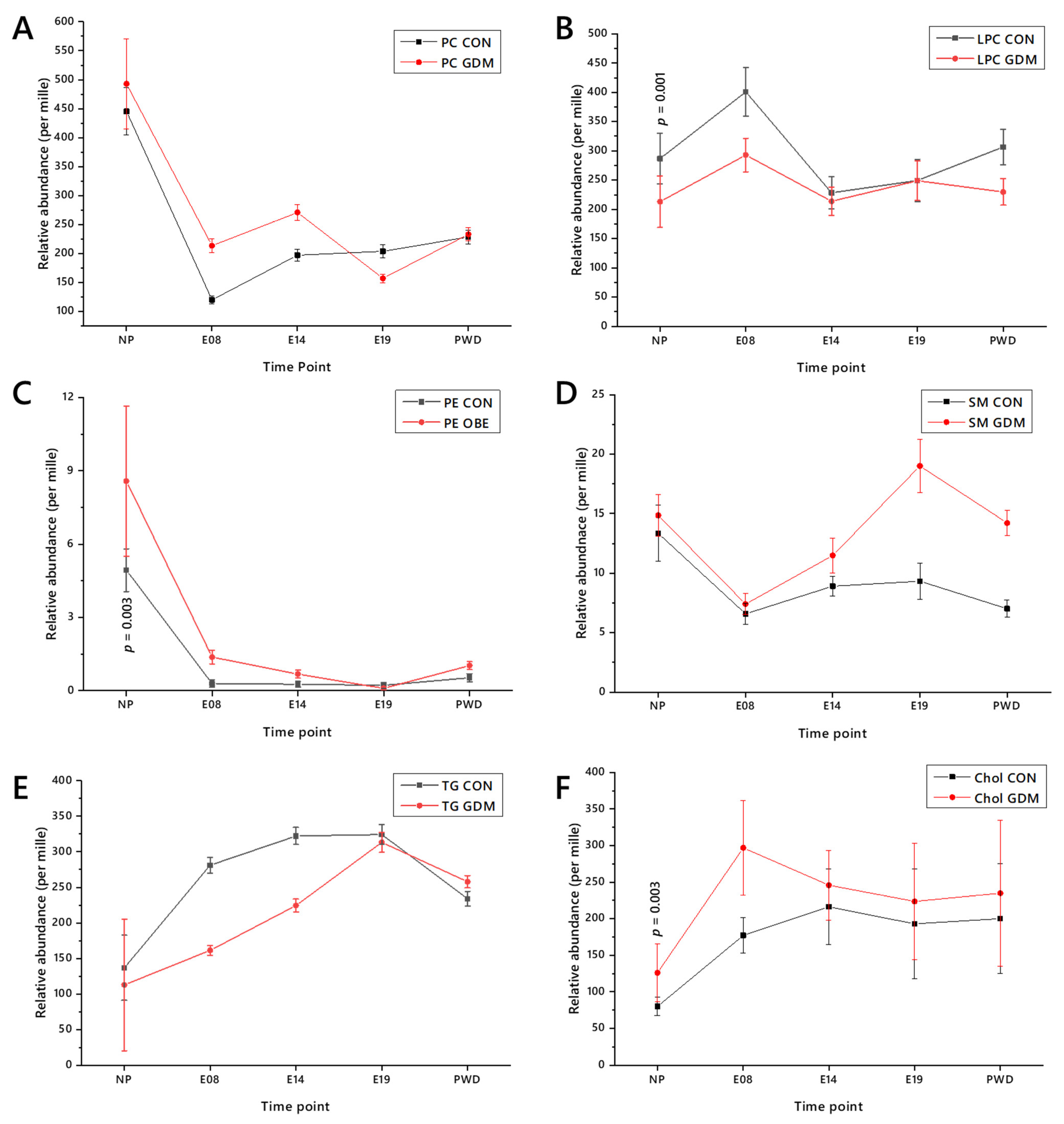
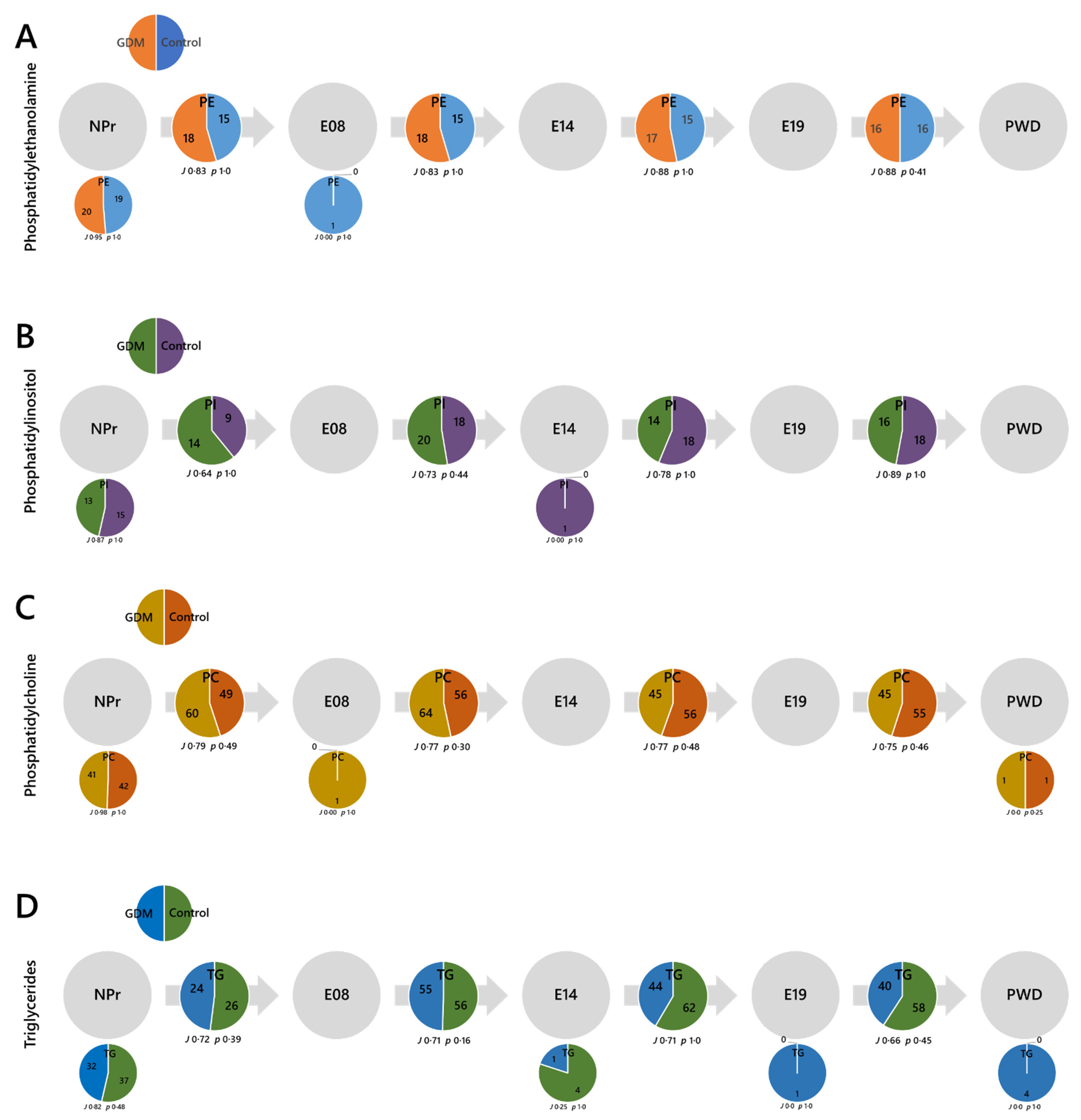
2.3. Lipid Abundances by Head Group/Class
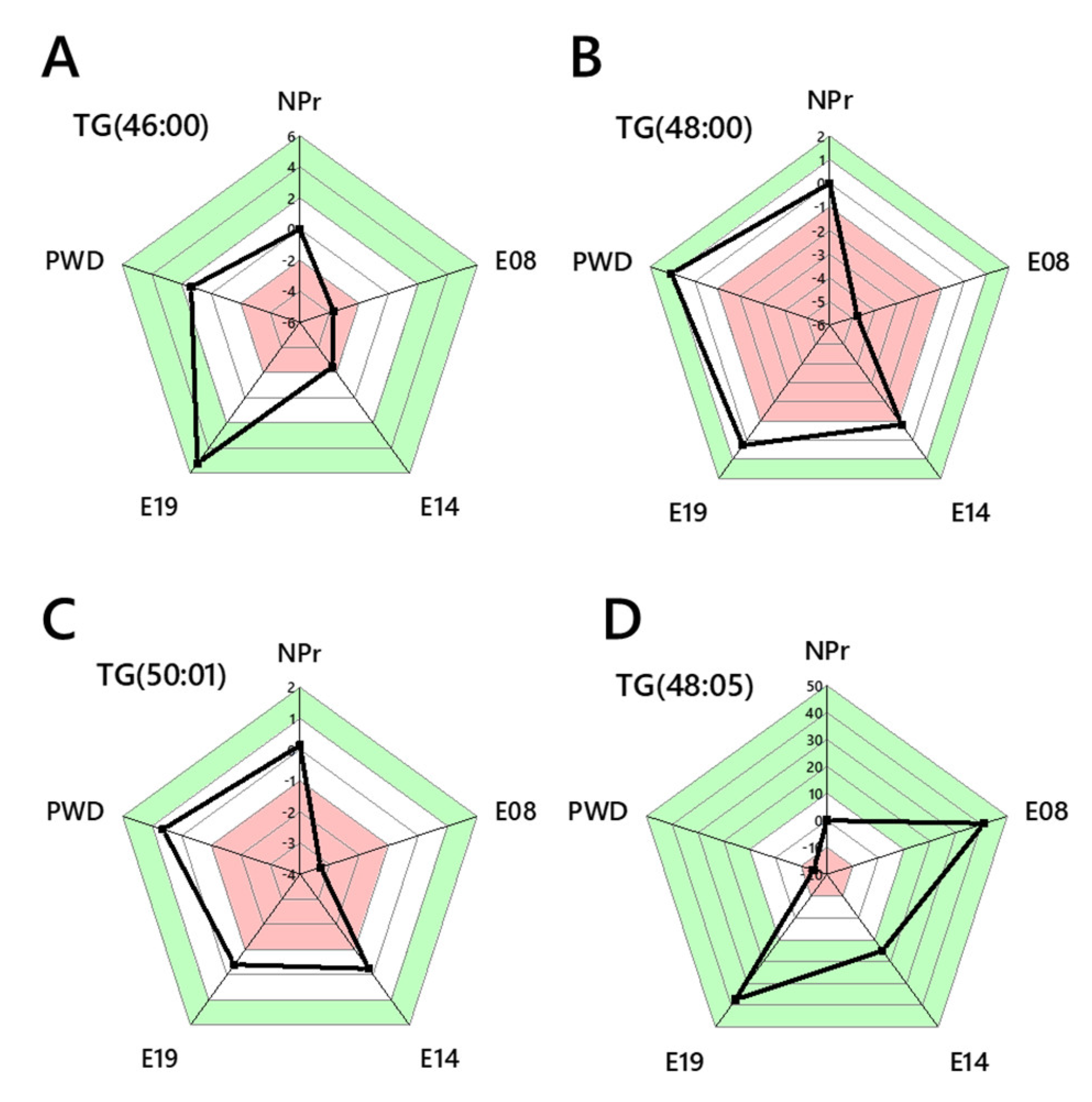
2.4. Lipid Traffic Analysis
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. Blood Glucose Measurement
4.3. Lipidomics
4.3.1. Isolation of the Organically Soluble Fraction for MS
4.3.2. Mass Spectrometry
4.3.3. Data Processing
4.3.4. Lipid Traffic Analysis
4.4. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Primers 2019, 5, 47. [Google Scholar] [CrossRef]
- Poston, L.; Bell, R.; Croker, H.; Flynn, A.C.; Godfrey, K.M.; Goff, L.; Hayes, L.; Khazaezadeh, N.; Nelson, S.M.; Oteng-Ntim, E.; et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): A multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015, 3, 767–777. [Google Scholar] [CrossRef]
- Briley, A.L.; Barr, S.; Badger, S.; Bell, R.; Croker, H.; Godfrey, K.M.; Holmes, B.; Kinnunen, T.I.; Nelson, S.M.; Oteng-Ntim, E.; et al. A complex intervention to improve pregnancy outcome in obese women; the UPBEAT randomised controlled trial. BMC Pregnancy Childbirth 2014, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; England, L.; Wilson, H.G.; Bish, C.; Satten, G.A.; Dietz, P. Percentage of gestational diabetes mellitus attributable to overweight and obesity. Am. J. Public Health 2010, 100, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Alfaradhi, M.Z.; Fernandez-Twinn, D.S.; Martin-Gronert, M.S.; Musial, B.; Fowden, A.; Ozanne, S.E. Oxidative stress and altered lipid homeostasis in the programming of offspring fatty liver by maternal obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R26–R34. [Google Scholar] [CrossRef]
- Fernandez-Twinn, D.S.; Gascoin, G.; Musial, B.; Carr, S.; Duque-Guimaraes, D.; Blackmore, H.L.; Alfaradhi, M.Z.; Loche, E.; Sferruzzi-Perri, A.N.; Fowden, A.L.; et al. Exercise rescues obese mothers’ insulin sensitivity, placental hypoxia and male offspring insulin sensitivity. Sci. Rep. 2017, 7, 44650. [Google Scholar] [CrossRef]
- Loche, E.; Blackmore, H.L.; Carpenter, A.A.; Beeson, J.H.; Pinnock, A.; Ashmore, T.J.; Aiken, C.E.; de Almeida-Faria, J.; Schoonejans, J.M.; Giussani, D.A.; et al. Maternal diet-induced obesity programmes cardiac dysfunction in male mice independently of post-weaning diet. Cardiovasc. Res. 2018, 114, 1372–1384. [Google Scholar] [CrossRef]
- Alfaradhi, M.Z.; Kusinski, L.C.; Fernandez-Twinn, D.S.; Pantaleão, L.C.; Carr, S.K.; Ferland-McCollough, D.; Yeo, G.S.H.; Bushell, M.; Ozanne, S.E. Maternal Obesity in Pregnancy Developmentally Programs Adipose Tissue Inflammation in Young, Lean Male Mice Offspring. Endocrinology 2016, 157, 4246–4256. [Google Scholar] [CrossRef]
- Fernandez-Twinn, D.S.; Hjort, L.; Novakovic, B.; Ozanne, S.E.; Saffery, R. Intrauterine programming of obesity and type 2 diabetes. Diabetologia 2019, 62, 1789–1801. [Google Scholar] [CrossRef]
- Samuelsson, A.-M.; Matthews, P.A.; Argenton, M.; Christie, M.R.; McConnell, J.M.; Jansen, E.H.J.M.; Piersma, A.H.; Ozanne, S.E.; Fernandez-Twinn, D.; Remacle, C.; et al. Diet-Induced Obesity in Female Mice Leads to Offspring Hyperphagia, Adiposity, Hypertension, and Insulin Resistance. Hypertension 2008, 51, 383–392. [Google Scholar] [CrossRef]
- Shelley, P.; Martin-Gronert, M.S.; Rowlerson, A.; Poston, L.; Heales, S.J.R.; Hargreaves, I.P.; McConnell, J.M.; Ozanne, S.E.; Fernandez-Twinn, D.S. Altered skeletal muscle insulin signaling and mitochondrial complex II-III linked activity in adult offspring of obese mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R675–R681. [Google Scholar] [CrossRef]
- Damm, P. Future risk of diabetes in mother and child after gestational diabetes mellitus. Int. J. Gynecol. Obstet. 2009, 104, S25–S26. [Google Scholar] [CrossRef] [PubMed]
- Herath, H.; Herath, R.; Wickremasinghe, R. Gestational diabetes mellitus and risk of type 2 diabetes 10 years after the index pregnancy in Sri Lankan women-A community based retrospective cohort study. PLoS ONE 2017, 12, e0179647. [Google Scholar] [CrossRef] [PubMed]
- Damm, P.; Houshmand-Oeregaard, A.; Kelstrup, L.; Lauenborg, J.; Mathiesen, E.R.; Clausen, T.D. Gestational diabetes mellitus and long-term consequences for mother and offspring: A view from Denmark. Diabetologia 2016, 59, 1396–1399. [Google Scholar] [CrossRef]
- Coustan, D.R. Recurrent GDM and the development of type 2 diabetes have similar risk factors. Endocrine 2016, 53, 624–625. [Google Scholar] [CrossRef][Green Version]
- Chen, L.-W.; Soh, S.E.; Tint, M.-T.; Loy, S.L.; Yap, F.; Tan, K.H.; Lee, Y.S.; Shek, L.P.-C.; Godfrey, K.M.; Gluckman, P.D.; et al. Combined analysis of gestational diabetes and maternal weight status from pre-pregnancy through post-delivery in future development of type 2 diabetes. Sci. Rep. 2021, 11, 5021. [Google Scholar] [CrossRef]
- Kramer, C.K.; Campbell, S.; Retnakaran, R. Gestational diabetes and the risk of cardiovascular disease in women: A systematic review and meta-analysis. Diabetologia 2019, 62, 905–914. [Google Scholar] [CrossRef]
- Rodrigo, N.; Glastras, S.J. The Emerging Role of Biomarkers in the Diagnosis of Gestational Diabetes Mellitus. J. Clin. Med. 2018, 7, 120. [Google Scholar] [CrossRef]
- Kumru, P.; Arisoy, R.; Erdogdu, E.; Demirci, O.; Kavrut, M.; Ardıc, C.; Aslaner, N.; Ozkoral, A.; Ertekin, A. Prediction of gestational diabetes mellitus at first trimester in low-risk pregnancies. Taiwan. J. Obstet. Gynecol. 2016, 55, 815–820. [Google Scholar] [CrossRef]
- Lamain-de Ruiter, M.; Kwee, A.; Naaktgeboren, C.A.; Franx, A.; Moons, K.G.M.; Koster, M.P.H. Prediction models for the risk of gestational diabetes: A systematic review. Diagn. Progn. Res. 2017, 1, 3. [Google Scholar] [CrossRef]
- HAPO Study Cooperative Research Group. Hyperglycemia and Adverse Pregnancy Outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar]
- IADPSG; Metzger, B.E. International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef]
- Powe, C.E.; Nodzenski, M.; Talbot, O.; Allard, C.; Briggs, C.; Leya, M.V.; Perron, P.; Bouchard, L.; Florez, J.C.; Scholtens, D.M.; et al. Genetic Determinants of Glycemic Traits and the Risk of Gestational Diabetes Mellitus. Diabetes 2018, 67, 2703–2709. [Google Scholar] [CrossRef]
- Bartha, J.L.; Martinez-Del-Fresno, P.; Comino-Delgado, R. Early diagnosis of gestational diabetes mellitus and prevention of diabetes-related complications. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 109, 41–44. [Google Scholar] [CrossRef]
- McGarry, J. What if Minkowski had been ageusic? An alternative angle on diabetes. Science 1992, 258, 766–770. [Google Scholar] [CrossRef]
- Boden, G.; Laakso, M. Lipids and glucose in type 2 diabetes: What is the cause and effect? Diabetes Care 2004, 27, 2253–2259. [Google Scholar]
- White, S.L.; Pasupathy, D.; Sattar, N.; Nelson, S.M.; Lawlor, D.A.; Briley, A.L.; Seed, P.T.; Welsh, P.; Poston, L. Metabolic profiling of gestational diabetes in obese women during pregnancy. Diabetologia 2017, 60, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Furse, S.; White, S.L.; Meek, C.L.; Jenkins, B.; Petry, C.J.; Vieira, M.C.; Ozanne, S.E.; Dunger, D.B.; Poston, L.; Koulman, A. Altered triglyceride and phospholipid metabolism predates the diagnosis of gestational diabetes in obese pregnancy. Mol. Omics 2019, 15, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Koulman, A.; Petry, C.J.; Jenkins, B.; Matthews, L.; Hughes, I.A.; Acerini, C.L.; Ong, K.K.; Dunger, D.B. An Unbiased Lipidomics Approach Identifies Early Second Trimester Lipids Predictive of Maternal Glycemic Traits and Gestational Diabetes Mellitus. Diabetes Care 2016, 39, 2232. [Google Scholar] [CrossRef]
- Furse, S.; Watkins, A.J.; Hojat, N.; Smith, J.; Williams, H.E.L.; Chiarugi, D.; Koulman, A. Lipid Traffic Analysis reveals the impact of high paternal carbohydrate intake on offsprings’ lipid metabolism. Commun. Biol. 2021, 4, 163. [Google Scholar] [CrossRef] [PubMed]
- Furse, S.; Watkins, A.J.; Williams, H.E.L.; Snowden, S.G.; Chiarugi, D.; Koulman, A. Paternal nutritional programming of lipid metabolism is propagated by sperm and seminal plasma. Cell Press 2021. [Google Scholar] [CrossRef]
- Sanders, F.; Acharjee, A.; Walker, C.; Marney, L.; Roberts, L.; Imamura, F.; Jenkins, B.; Case, J.; Ray, S.; Virtue, S.; et al. Hepatic steatosis risk is partly driven by increased de novo lipogenesis following carbohydrate consumption. Genome Biol. 2018, 19, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.F. Carbohydrate and lipid metabolism in pregnancy: Normal compared with gestational diabetes mellitus. Am. J. Clin. Nutr. 2000, 71, 1256S–1261S. [Google Scholar] [CrossRef]
- Catalano, P.M.; Tyzbir, E.D.; Wolfe, R.R.; Calles, J.; Roman, N.M.; Amini, S.B.; Sims, E.A. Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. Am. J. Physiol. Endocrinol. Metab. 1993, 264, E60–E67. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Huston, L.; Amini, S.B.; Kalhan, S.C. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am. J. Obstet. Gynecol. 1999, 180, 903–916. [Google Scholar] [CrossRef]
- Barrett, H.L.; Dekker Nitert, M.; McIntyre, H.D.; Callaway, L.K. Normalizing Metabolism in Diabetic Pregnancy: Is It Time to Target Lipids? Diabetes Care 2014, 37, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Casteels, M.; Sniekers, M.; Fraccascia, P.; Mannaerts, G.P.; Van Veldhoven, P.P. The role of 2-hydroxyacyl-CoA lyase, a thiamin pyrophosphate-dependent enzyme, in the peroxisomal metabolism of 3-methyl-branched fatty acids and 2-hydroxy straight-chain fatty acids. Biochem. Soc. Trans. 2007, 35, 876–880. [Google Scholar] [CrossRef]
- Smedman, A.E.; Gustafsson, I.-B.; Berglund, L.G.; Vessby, B.O. Pentadecanoic acid in serum as a marker for intake of milk fat: Relations between intake of milk fat and metabolic risk factors. Am. J. Clin. Nutr. 1999, 69, 22–29. [Google Scholar] [CrossRef]
- Huang, L.; Lin, J.-S.; Aris, I.M.; Yang, G.; Chen, W.-Q.; Li, L.-J. Circulating Saturated Fatty Acids and Incident Type 2 Diabetes: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 998. [Google Scholar] [CrossRef]
- Koynova, R.; Caffrey, M. Phases and phase transitions of the hydrated phosphatidylethanolamines. Chem. Phys. Lipids 1994, 69, 1–34. [Google Scholar] [CrossRef]
- Koynova, R.; Caffrey, M. Phases and phase transitions of the phosphatidylcholines. Biochim. Et Biophys. Acta 1998, 1376, 91–145. [Google Scholar] [CrossRef]
- Furse, S.; de Kroon, A.I.P.M. Phosphatidylcholine’s functions beyond that of a membrane brick. Mol. Membr. Biol. 2015, 32, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Ferchaud-Roucher, V.; Kramer, A.; Silva, E.; Pantham, P.; Weintraub, S.T.; Jansson, T.; Powell, T.L. A potential role for lysophosphatidylcholine in the delivery of long chain polyunsaturated fatty acids to the fetal circulation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 394–402. [Google Scholar] [CrossRef]
- Calder, P.C.; Dangour, A.D.; Diekman, C.; Eilander, A.; Koletzko, B.; Meijer, G.W.; Mozaffarian, D.; Niinikoski, H.; Osendarp, S.J.M.; Pietinen, P.; et al. Essential fats for future health. Proceedings of the 9th Unilever Nutrition Symposium, 26–27 May 2010. Eur. J. Clin. Nutr. 2010, 64, S1–S13. [Google Scholar] [CrossRef]
- LaBarre, J.L.; Puttabyatappa, M.; Song, P.X.K.; Goodrich, J.M.; Zhou, L.; Rajendiran, T.M.; Soni, T.; Domino, S.E.; Treadwell, M.C.; Dolinoy, D.C.; et al. Maternal lipid levels across pregnancy impact the umbilical cord blood lipidome and infant birth weight. Sci. Rep. 2020, 10, 14209. [Google Scholar] [CrossRef]
- de Barros Mucci, D.; Kusinski, L.C.; Wilsmore, P.; Loche, E.; Pantaleão, L.C.; Ashmore, T.J.; Blackmore, H.L.; Fernandez-Twinn, D.S.; Carmo, M.d.G.T.d.; Ozanne, S.E. Impact of maternal obesity on placental transcriptome and morphology associated with fetal growth restriction in mice. Int. J. Obes. 2020, 44, 1087–1096. [Google Scholar] [CrossRef]
- Zore, T.; Palafox, M.; Reue, K. Sex differences in obesity, lipid metabolism, and inflammation—A role for the sex chromosomes? Mol. Metab. 2018, 15, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Furse, S.; Fernandez-Twinn, D.; Jenkins, B.; Meek, C.L.; Williams, H.E.; Smith, G.C.S.; Charnock-Jones, D.S.; Ozanne, S.E.; Koulman, A. A high throughput platform for detailed lipidomic analysis of a range of mouse and human tissues. Anal. Bioanal. Chem. 2020, 412, 2851–2862. [Google Scholar] [CrossRef]
- Furse, S.; Koulman, A. The Lipid and Glyceride Profiles of Infant Formula Differ by Manufacturer, Region and Date Sold. Nutrients 2019, 11, 1122. [Google Scholar] [CrossRef]
- Harshfield, E.L.; Koulman, A.; Ziemek, D.; Marney, L.; Fauman, E.B.; Paul, D.S.; Stacey, D.; Rasheed, A.; Lee, J.-J.; Shah, N.; et al. An Unbiased Lipid Phenotyping Approach To Study the Genetic Determinants of Lipids and Their Association with Coronary Heart Disease Risk Factors. J. Proteome Res. 2019, 18, 2397–2410. [Google Scholar] [CrossRef]

| PND2 | Control | Obese |
|---|---|---|
| Litter size | 7.8 ± 0.5 | 5.5 ± 0.4 ** |
| Pup weights (grams) | 1.8 (1.65, 1.92) | 1.45 (1.29, 1.6) ** |
| E18 | Control | Obese |
|---|---|---|
| FFA (μM) | 831 ± 266 | 713 ± 125 |
| TG (mM) | 1.1 ± 0.1 | 0.7 ± 0.05 * |
| Cholesterol (mM) | 1.3 ± 0.1 | 1.5 ± 0.1 * |
| Insulin (pM) | 121.8 ± 34.8 | 278.4 ± 52.2 * |
| Leptin (pg/mL) | 2171 ± 188 | 7778 ± 1390 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furse, S.; Fernandez-Twinn, D.S.; Chiarugi, D.; Koulman, A.; Ozanne, S.E. Lipid Metabolism Is Dysregulated before, during and after Pregnancy in a Mouse Model of Gestational Diabetes. Int. J. Mol. Sci. 2021, 22, 7452. https://doi.org/10.3390/ijms22147452
Furse S, Fernandez-Twinn DS, Chiarugi D, Koulman A, Ozanne SE. Lipid Metabolism Is Dysregulated before, during and after Pregnancy in a Mouse Model of Gestational Diabetes. International Journal of Molecular Sciences. 2021; 22(14):7452. https://doi.org/10.3390/ijms22147452
Chicago/Turabian StyleFurse, Samuel, Denise S. Fernandez-Twinn, Davide Chiarugi, Albert Koulman, and Susan E. Ozanne. 2021. "Lipid Metabolism Is Dysregulated before, during and after Pregnancy in a Mouse Model of Gestational Diabetes" International Journal of Molecular Sciences 22, no. 14: 7452. https://doi.org/10.3390/ijms22147452
APA StyleFurse, S., Fernandez-Twinn, D. S., Chiarugi, D., Koulman, A., & Ozanne, S. E. (2021). Lipid Metabolism Is Dysregulated before, during and after Pregnancy in a Mouse Model of Gestational Diabetes. International Journal of Molecular Sciences, 22(14), 7452. https://doi.org/10.3390/ijms22147452









