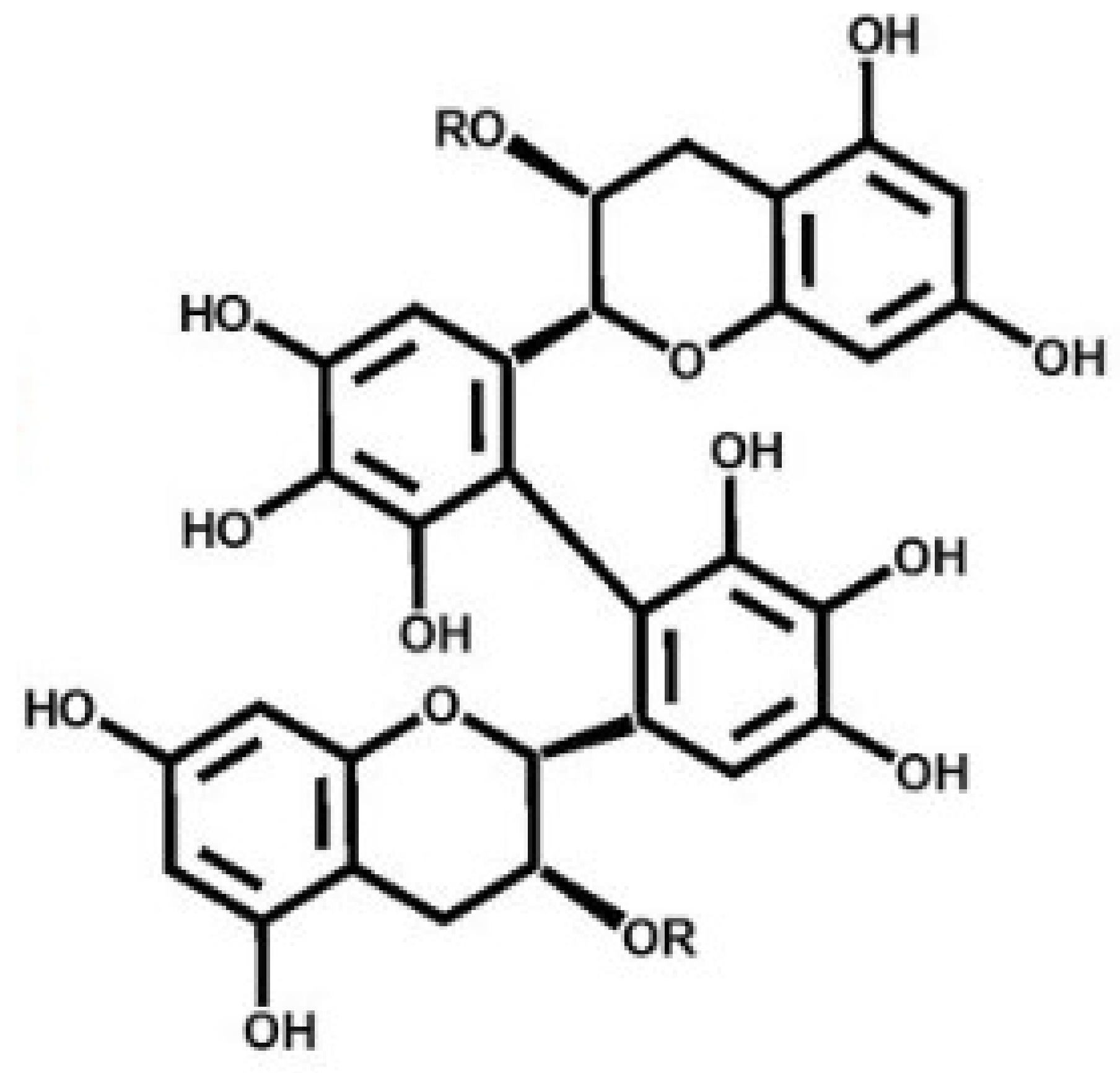
Antimelanogenesis Effects of Theasinensin A
Abstract
:1. Introduction
2. Results
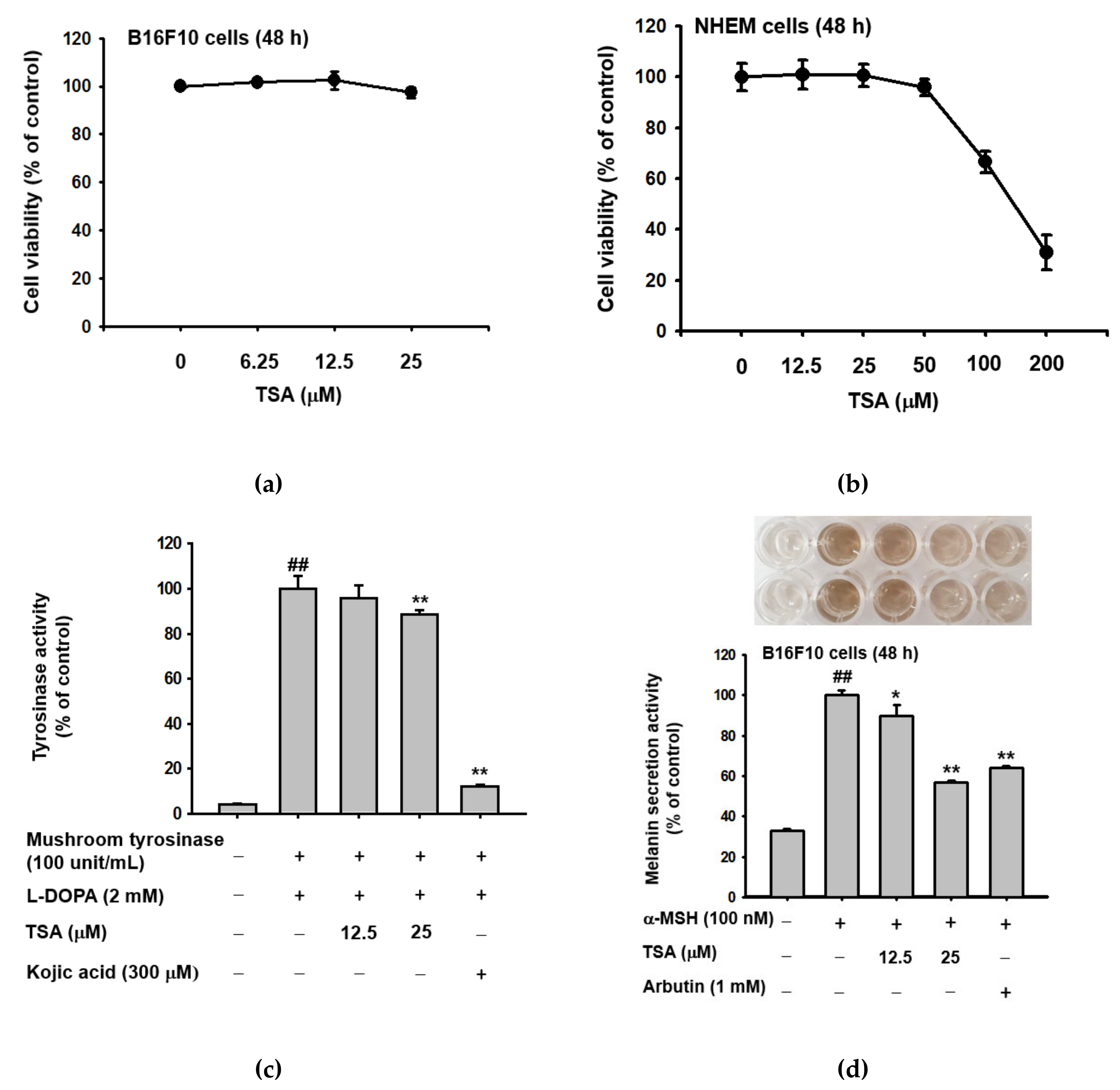
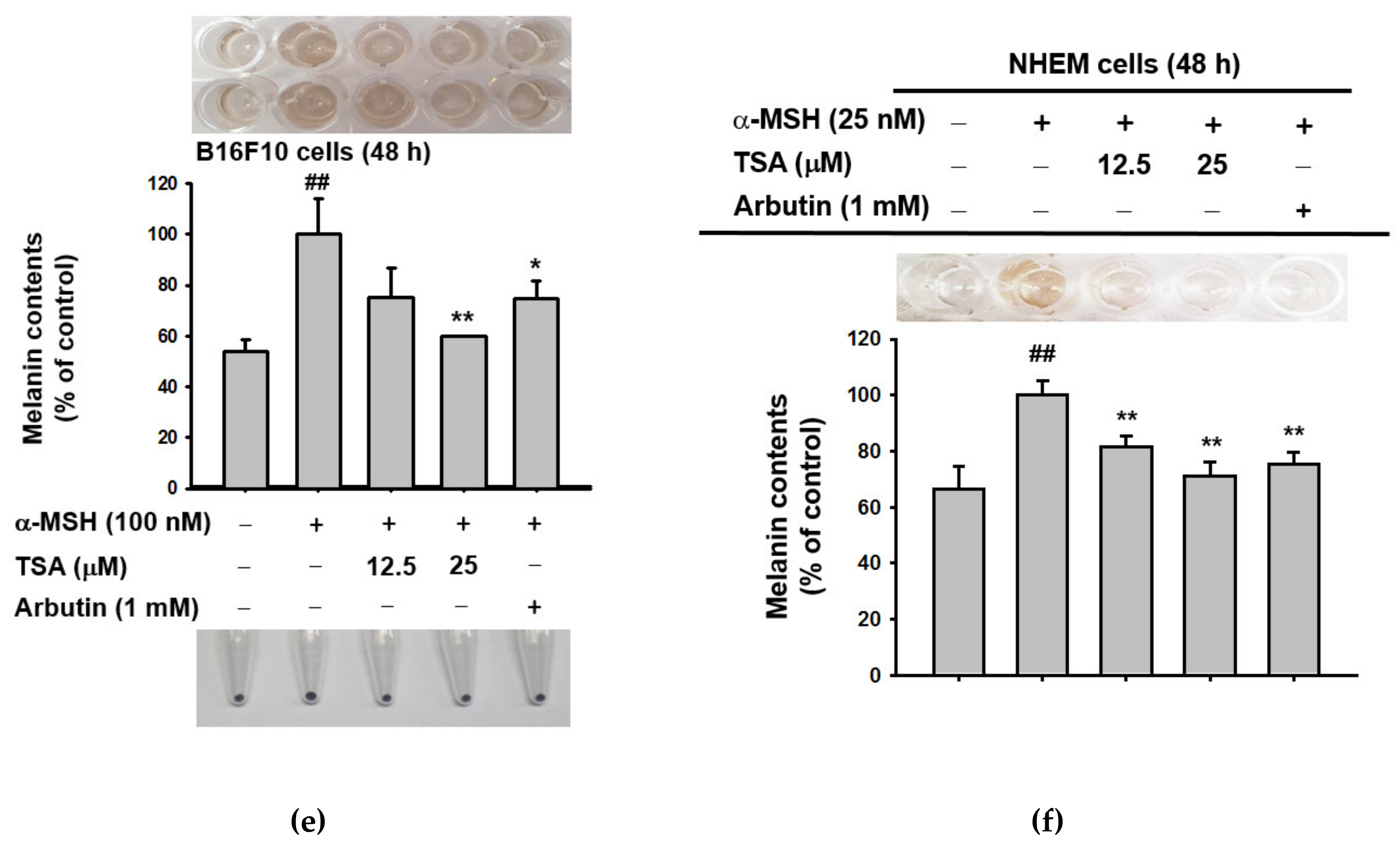
2.1. Effect of Theasinensin A on Melanin Produced by B16F10 Melanoma Cells and Human Epidermal Melanocytes
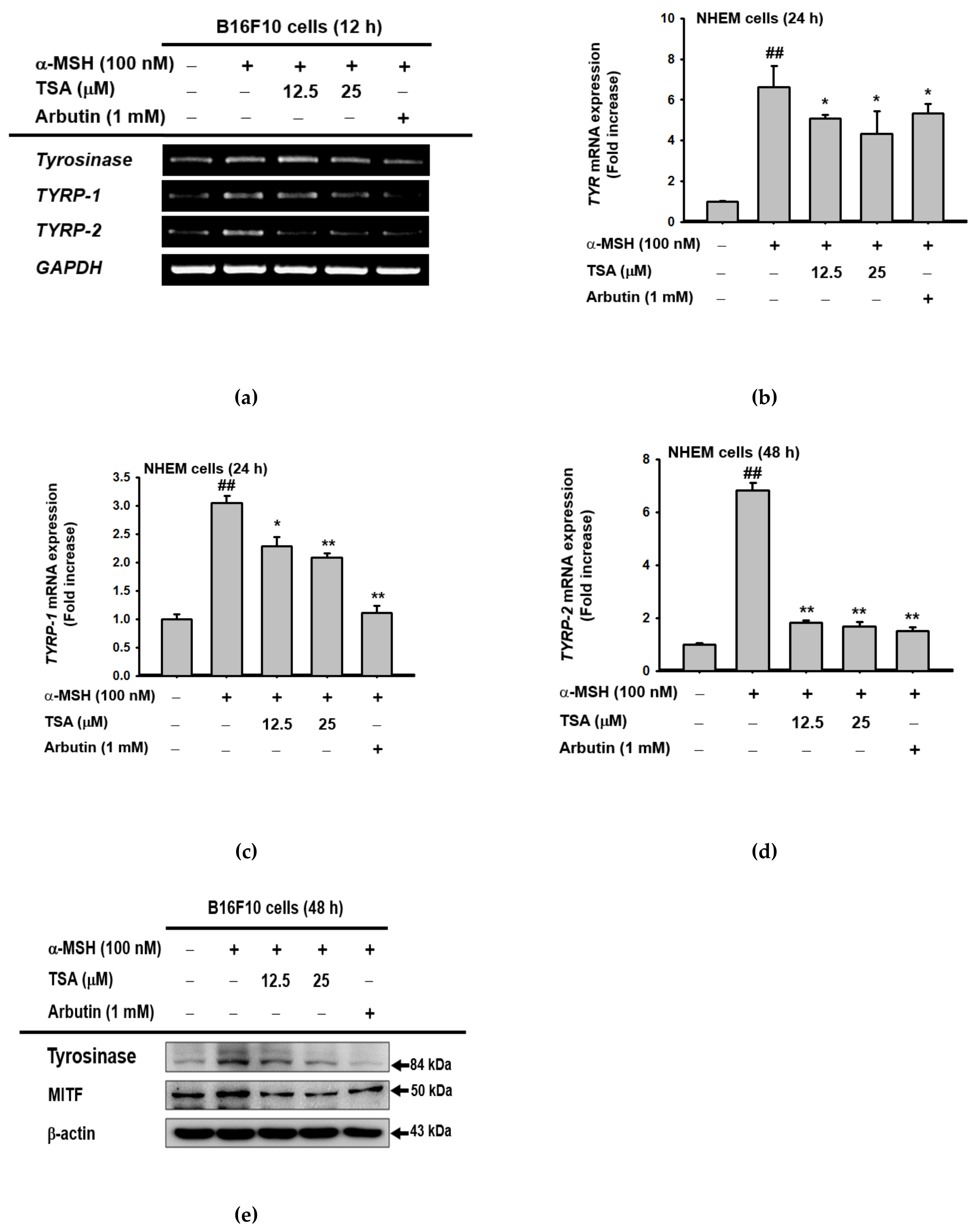
2.2. Effect of TSA on the Expression of Genes Related to Melanogenesis
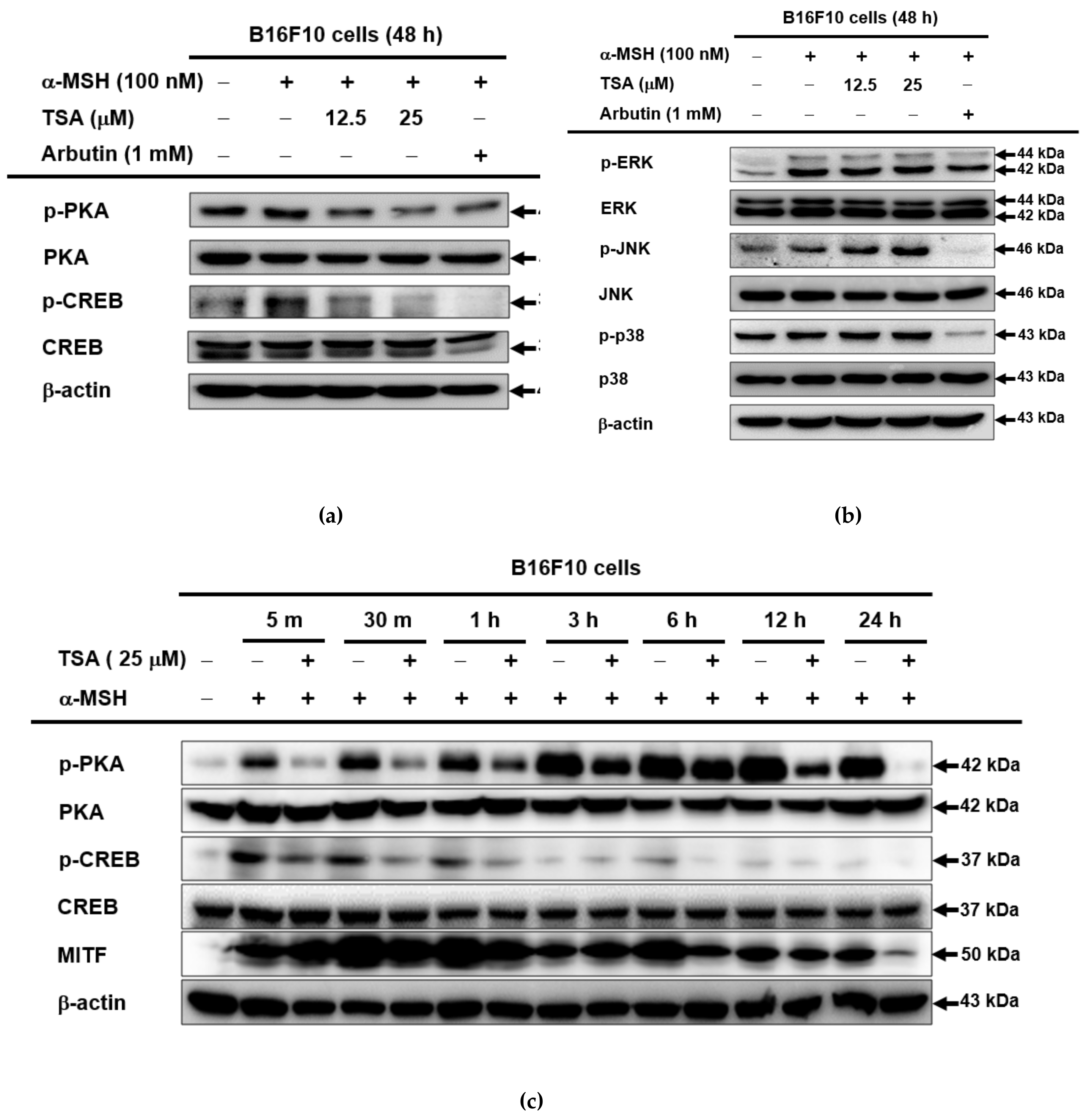
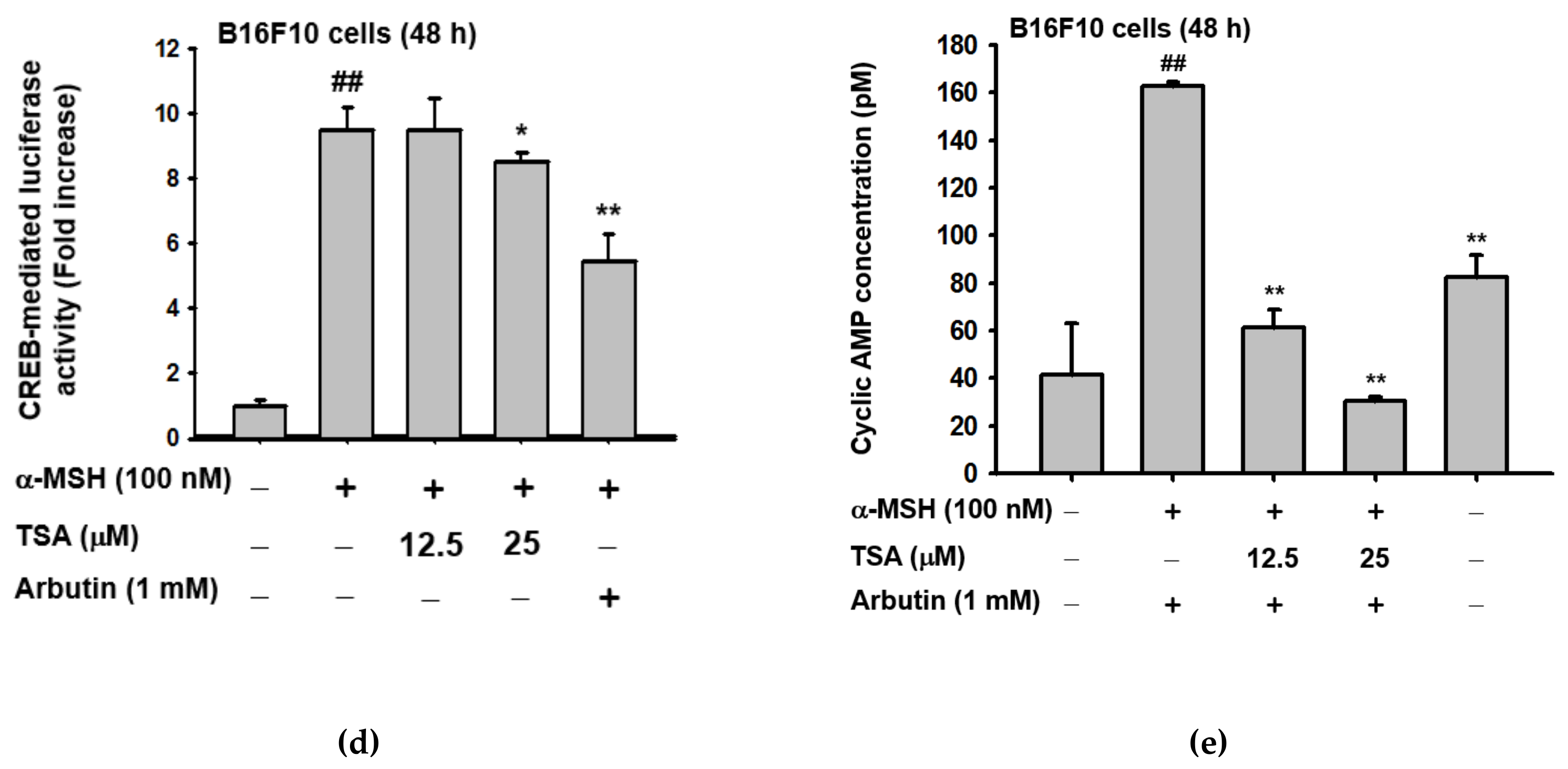
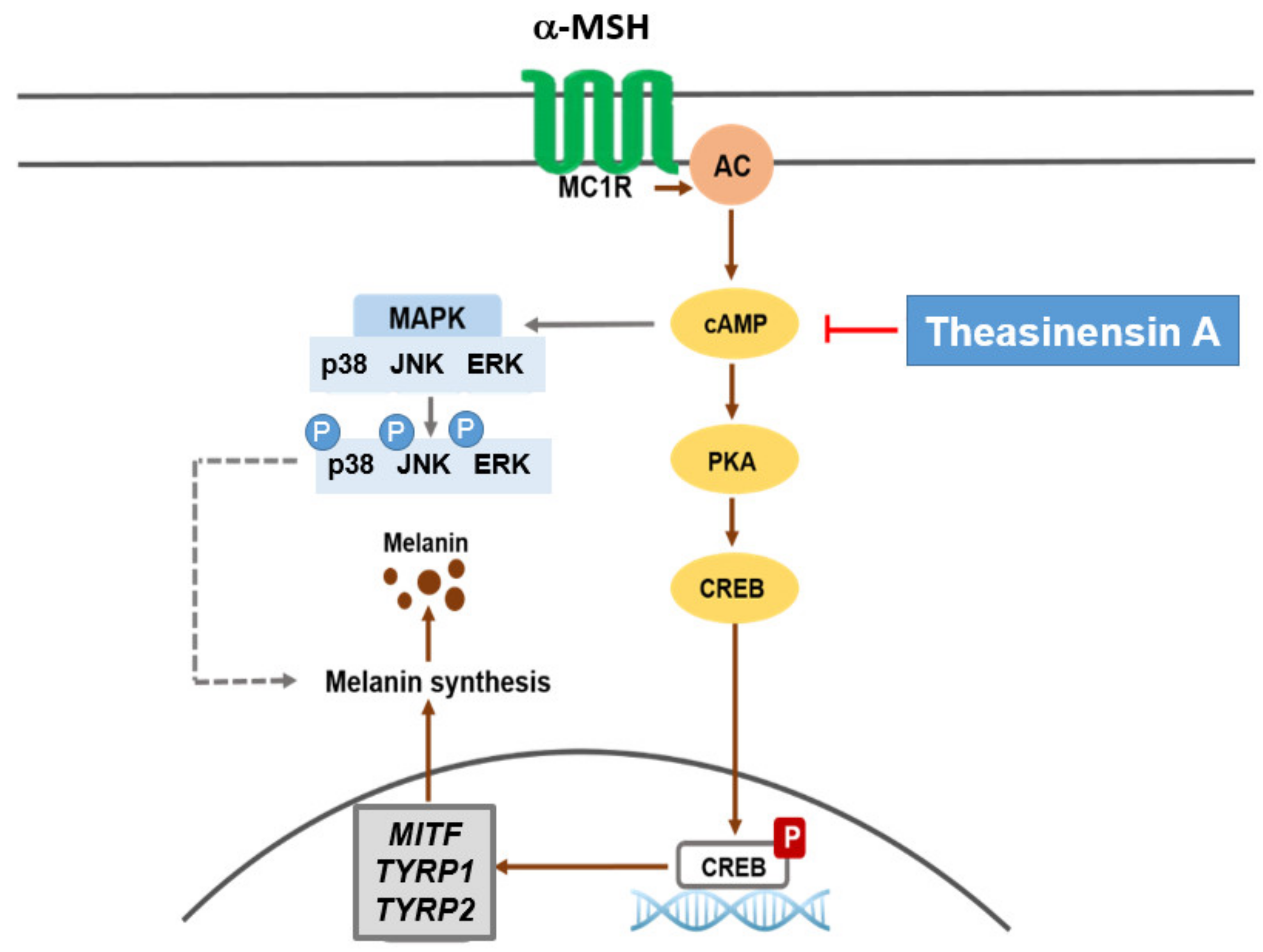
2.3. Effect of TSA on the cAMP/CREB Signaling Pathway through Regulation of Melanogenesis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Compound Preparation and Treatment
4.3. Cell Culture
4.4. MTT Assay
4.5. Tyrosinase Activity Assay
4.6. Melanin Secretion and Content Assay
4.7. Reverse Transcription Polymerase Chain Reaction (RT-PCR) and Real-Time PCR
4.8. Preparation of Total Cell and Lysates
4.9. Western Blotting Analysis
4.10. Luciferase Assay
4.11. Enzyme-Linked Immunosorbent Assay (ELISA)
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TSA | Theasinensin A |
| AP-1 | Activator protein 1 |
| Tyrp1 | Tyrosinase-related protein 1 |
| Tyrp2 | Tyrosinase-related protein 2 |
| CREB | cAMP response element binding |
| PKA | Protein kinase A |
| MITF | Microphthalmia-associated transcription factor |
| MC1R | Melanocortin 1 receptor |
| α-MSH | α-Melanocyte-stimulating hormone |
| NHEM | Normal human epidermal melanocytes |
References
- Qomaladewi, N.P.; Kim, M.Y.; Cho, J.Y. Rottlerin Reduces cAMP/CREB-Mediated Melanogenesis via Regulation of Autophagy. Int. J. Mol. Sci. 2019, 20, 2081. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Lu, Y.; Yu, W.G.; Zhao, X.; Lu, Y.H. Anti-photoageing and anti-melanogenesis activities of chrysin. Pharm. Biol. 2016, 54, 2692–2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabrowska, A.K.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D.; Rossi, R.M. The relationship between skin function, barrier properties, and body-dependent factors. Skin Res. Technol. 2018, 24, 165–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gravitz, L. Skin. Nature 2018, 563, S83. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.O.; Kim, E.; Kim, J.H.; Hong, Y.H.; Kim, H.G.; Jeong, D.; Kim, J.; Kim, S.H.; Park, C.; Seo, D.B.; et al. Antimelanogenesis and skin-protective activities of Panax ginseng calyx ethanol extract. J. Ginseng Res. 2018, 42, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chery, S.; Lazerson, A.; Altman, N.H.; Jackson, R.; Holt, G.; Campos, M.; Schally, A.V.; Mirsaeidi, M. Anti-inflammatory effects of alpha-MSH through p-CREB expression in sarcoidosis like granuloma model. Sci. Rep. 2020, 10, 7277. [Google Scholar] [CrossRef]
- Ravnskjaer, K.; Madiraju, A.; Montminy, M. Role of the cAMP Pathway in Glucose and Lipid Metabolism. Handb. Exp. Pharmacol. 2016, 233, 29–49. [Google Scholar] [CrossRef]
- Ukiya, M.; Sato, D.; Kimura, H.; Hirai, Y.; Nishina, A. Tokoronin Contained in Dioscorea tokoro Makino ex Miyabe Suppressed alpha-MSH-Induced Melanogenesis in B16 Cells via Suppression of Classical MAPK Pathway Activation. Chem. Biodivers. 2020. [CrossRef]
- Herraiz, C.; Garcia-Borron, J.C.; Jimenez-Cervantes, C.; Olivares, C. MC1R signaling. Intracellular partners and pathophysiological implications. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2448–2461. [Google Scholar] [CrossRef]
- Cheng, M.C.; Lee, T.H.; Chu, Y.T.; Syu, L.L.; Hsu, S.J.; Cheng, C.H.; Wu, J.; Lee, C.K. Melanogenesis Inhibitors from the Rhizoma of Ligusticum Sinense in B16-F10 Melanoma Cells In Vitro and Zebrafish In Vivo. Int. J. Mol. Sci. 2018, 19, 3994. [Google Scholar] [CrossRef] [Green Version]
- Haraszti, S.; Ezaldein, H.; Delost, G.R. Eruptive melanocytic nevi in a patient with Parkinson disease treated by carbidopa-levodopa. JAAD Case Rep. 2019, 5, 21–23. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Wong, Z.C.; Wang, C.; Fung, A.H.; Wong, E.O.; Chan, G.K.; Dong, T.T.; Chen, Y.; Tsim, K.W. Isoorientin derived from Gentiana veitchiorum Hemsl. flowers inhibits melanogenesis by down-regulating MITF-induced tyrosinase expression. Phytomedicine Int. J. Phytother. Phytopharm. 2019, 57, 129–136. [Google Scholar] [CrossRef]
- Jin, J.; Nguyen, T.T.H.; Kim, C.; Kim, D. Antimelanogenesis Effects of Fungal Exopolysaccharides Prepared from Submerged Culture of Fomitopsis castanea Mycelia. J. Microbiol. Biotechnol. 2019, 29, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, S.; Ryu, J.H.; Yoon, K.D.; Shin, S.S. Effect of Aurea Helianthus stem extract on anti-melanogenesis. Biosci. Biotechnol. Biochem. 2018, 82, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.N. Methodology for evaluation of melanin content and production of pigment cells in vitro. Photochem. Photobiol. 2008, 84, 645–649. [Google Scholar] [CrossRef]
- Lee, A.; Kim, J.Y.; Heo, J.; Cho, D.H.; Kim, H.S.; An, I.S.; An, S.; Bae, S. The Inhibition of Melanogenesis Via the PKA and ERK Signaling Pathways by Chlamydomonas reinhardtii Extract in B16F10 Melanoma Cells and Artificial Human Skin Equivalents. J. Microbiol. Biotechnol. 2018, 28, 2121–2132. [Google Scholar] [CrossRef]
- Makbal, R.; Villareal, M.O.; Gadhi, C.; Hafidi, A.; Isoda, H. Argania Spinosa Fruit Shell Extract-Induced Melanogenesis via cAMP Signaling Pathway Activation. Int. J. Mol. Sci. 2020, 21, 2539. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, Y.; Ananthaswamy, H.N. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol. 2004, 195, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Mun, G.-I.; Kim, S.; Choi, E.; Kim, C.S.; Lee, Y.-S. Pharmacology of natural radioprotectors. Arch. Pharmacal Res. 2018, 41, 1033–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garmyn, M.; Young, A.R.; Miller, S.A. Mechanisms of and variables affecting UVR photoadaptation in human skin. Photochem. Photobiol. Sci. 2018, 17, 1932–1940. [Google Scholar] [CrossRef]
- Choudhary, R.; Sharma, A.; Kumar, S.; Upadhyay, R.C.; Singh, S.V.; Mohanty, A. Role of alpha-melanocyte stimulating hormone (alpha-MSH) in modulating the molecular mechanism adopted by melanocytes of Bos indicus under UVR stress. Mol. Cell. Biochem. 2020, 465, 141–153. [Google Scholar] [CrossRef]
- Reinehr, C.P.H.; Bakos, R.M. Actinic keratoses: Review of clinical, dermoscopic, and therapeutic aspects. Bras. Derm. 2019, 94, 637–657. [Google Scholar] [CrossRef]
- Rastrelli, M.; Tropea, S.; Rossi, C.R.; Alaibac, M. Melanoma: Epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo 2014, 28, 1005–1011. [Google Scholar]
- Vanhaecke, T.; Papeleu, P.; Elaut, G.; Rogiers, V. Trichostatin A-like hydroxamate histone deacetylase inhibitors as therapeutic agents: Toxicological point of view. Curr. Med. Chem. 2004, 11, 1629–1643. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Steegborn, C. Structural Basis of Sirtuin 6 Inhibition by the Hydroxamate Trichostatin A: Implications for Protein Deacylase Drug Development. J. Med. Chem. 2018, 61, 10922–10928. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Srivastava, R.K. Histone deacetylase inhibitors: Mechanisms and clinical significance in cancer: HDAC inhibitor-induced apoptosis. Adv. Exp. Med. Biol. 2008, 615, 261–298. [Google Scholar] [CrossRef]
- Hou, D.X.; Masuzaki, S.; Tanigawa, S.; Hashimoto, F.; Chen, J.; Sogo, T.; Fujii, M. Oolong tea theasinensins attenuate cyclooxygenase-2 expression in lipopolysaccharide (LPS)-activated mouse macrophages: Structure-activity relationship and molecular mechanisms. J. Agric. Food Chem. 2010, 58, 12735–12743. [Google Scholar] [CrossRef] [PubMed]
- Hisanaga, A.; Ishida, H.; Sakao, K.; Sogo, T.; Kumamoto, T.; Hashimoto, F.; Hou, D.X. Anti-inflammatory activity and molecular mechanism of Oolong tea theasinensin. Food Funct. 2014, 5, 1891–1897. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.M.; Sathisha, U.V.; Dharmesh, S.; Rao, A.G.; Singh, S.A. Interaction of sesamol (3,4-methylenedioxyphenol) with tyrosinase and its effect on melanin synthesis. Biochimie 2011, 93, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Mendes, E.; Perry Mde, J.; Francisco, A.P. Design and discovery of mushroom tyrosinase inhibitors and their therapeutic applications. Expert Opin. Drug Discov. 2014, 9, 533–554. [Google Scholar] [CrossRef]
- Lee, J.O.; Hwang, S.H.; Shen, T.; Kim, J.H.; You, L.; Hu, W.; Cho, J.Y. Enhancement of skin barrier and hydration-related molecules by protopanaxatriol in human keratinocytes. J. Ginseng Res. 2021, 45, 354–360. [Google Scholar] [CrossRef]
- Saba, E.; Kim, S.H.; Lee, Y.Y.; Park, C.K.; Oh, J.W.; Kim, T.H.; Kim, H.K.; Roh, S.S.; Rhee, M.H. Korean Red Ginseng extract ameliorates melanogenesis in humans and induces antiphotoaging effects in ultraviolet B-irradiated hairless mice. J. Ginseng Res. 2020, 44, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Schioth, H.B.; Mutulis, F.; Muceniece, R.; Prusis, P.; Wikberg, J.E. Discovery of novel melanocortin4 receptor selective MSH analogues. Br. J. Pharmacol. 1998, 124, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.Y.; Lee, J.; Min, D.; Kim, J.; Kim, H.J.; No, K.T. Tyrosinase-Targeting Gallacetophenone Inhibits Melanogenesis in Melanocytes and Human Skin-Equivalents. Int. J. Mol. Sci. 2020, 21, 3144. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.H.; Jin, S.H.; Kang, H.Y. LPS induces melanogenesis through p38 MAPK activation in human melanocytes. Arch. Dermatol. Res. 2008, 300, 325–329. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Oh, S.W.; Park, S.-H.; Lee, J.; Yoo, J.A.; Kwon, K.; Park, S.J.; Kim, J.; Yu, E.; Cho, J.Y. Melanogenic Effects of Maclurin Are Mediated through the Activation of cAMP/PKA/CREB and p38 MAPK/CREB Signaling Pathways. Oxidative Med. Cell. Longev. 2019, 2019, 9827519. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.; Park, S.-H.; Oh, S.W.; Lee, S.E.; Yoo, J.A.; Nho, Y.H.; Lee, S.; Han, B.S.; Cho, J.Y.; Lee, J. Anti-melanogenic effects of resorcinol are mediated by suppression of cAMP signaling and activation of p38 MAPK signaling. Biosci. Biotechnol. Biochem. 2018, 82, 1188–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellbrock, C.; Arozarena, I. Microphthalmia-associated transcription factor in melanoma development and MAP-kinase pathway targeted therapy. Pigment Cell Melanoma Res. 2015, 28, 390–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellei, B.; Maresca, V.; Flori, E.; Pitisci, A.; Larue, L.; Picardo, M. p38 regulates pigmentation via proteasomal degradation of tyrosinase. J. Biol. Chem. 2010, 285, 7288–7299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, M.B.; Bajpai, V.K.; Lee, J.; Zhao, P.; Byeon, J.-H.; Ra, J.-S.; Majumder, R.; Lee, J.S.; Yoon, J.-I.; Rather, I.A. Inhibition of melanogenesis by jineol from Scolopendra subspinipes mutilans via MAP-Kinase mediated MITF downregulation and the proteasomal degradation of tyrosinase. Sci. Rep. 2017, 7, 45858. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-C.; Liao, C.-C.; Peng, C.-C.; Lim, J.-M.; Siao, J.-H.; Wei, C.-M.; Chen, C.-C.; Wu, C.-S.; Chang, T.-M. Dihydromyricetin from Ampelopsis grossedentata inhibits melanogenesis through down-regulation of MAPK, PKA and PKC signaling pathways. Chem. Biol. Interact. 2016, 258, 166–174. [Google Scholar] [CrossRef]
- Lee, S.E.; Park, S.-H.; Oh, S.W.; Yoo, J.A.; Kwon, K.; Park, S.J.; Kim, J.; Lee, H.S.; Cho, J.Y.; Lee, J. Beauvericin inhibits melanogenesis by regulating cAMP/PKA/CREB and LXR-α/p38 MAPK–mediated pathways. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-O.; Park, S.J.; Park, S.-H.; Oh, S.W.; Lee, S.E.; Yoo, J.A.; Kwon, K.; Kim, J.; Kim, M.H.; Cho, J.Y. Ethanolic extract of Melia azedarach L. induces melanogenesis through the cAMP-PKA-CREB signaling pathway. Mol. Cell. Toxicol. 2019, 15, 75–83. [Google Scholar] [CrossRef]
- Valverde, P.; Healy, E.; Jackson, I.; Rees, J.L.; Thody, A.J. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat. Genet. 1995, 11, 328–330. [Google Scholar] [CrossRef]
- Ozdeslik, R.N.; Olinski, L.E.; Trieu, M.M.; Oprian, D.D.; Oancea, E. Human nonvisual opsin 3 regulates pigmentation of epidermal melanocytes through functional interaction with melanocortin 1 receptor. Proc. Natl. Acad. Sci. USA 2019, 116, 11508–11517. [Google Scholar] [CrossRef] [Green Version]
- Seo, G.Y.; Ha, Y.; Park, A.H.; Kwon, O.W.; Kim, Y.J. Leathesia difformis Extract Inhibits α-MSH-Induced Melanogenesis in B16F10 Cells via Down-Regulation of CREB Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 536. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.-Y.; You, Y.-J.; Liu, Y.-J.; Hou, C.-W.; Wu, C.-S.; Wen, K.-C.; Lin, C.-Y.; Chiang, H.-M. Sesamol inhibited melanogenesis by regulating melanin-related signal transduction in B16F10 cells. Int. J. Mol. Sci. 2018, 19, 1108. [Google Scholar] [CrossRef] [Green Version]
- Chung, Y.C.; Kim, M.-J.; Kang, E.Y.; Kim, Y.B.; Kim, B.S.; Park, S.-M.; Hyun, C.-G. Anti-Melanogenic Effects of Hydroxyectoine via MITF Inhibition by JNK, p38, and AKT Pathways in B16F10 Melanoma Cells. Nat. Prod. Commun. 2019, 14, 1934578X19858523. [Google Scholar] [CrossRef]
- Kuo, Y.-H.; Chen, C.-C.; Wu, P.-Y.; Wu, C.-S.; Sung, P.-J.; Lin, C.-Y.; Chiang, H.-M. N-(4-methoxyphenyl) caffeamide-induced melanogenesis inhibition mechanisms. BMC Complementary Altern. Med. 2017, 17, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Niu, C.; Yin, L.; Aisa, H.A. Novel furocoumarin derivatives stimulate melanogenesis in B16 melanoma cells by up-regulation of MITF and TYR family via Akt/GSK3β/β-catenin signaling pathways. Int. J. Mol. Sci. 2018, 19, 746. [Google Scholar] [CrossRef] [Green Version]
- Jeong, D.; Qomaladewi, N.P.; Lee, J.; Park, S.H.; Cho, J.Y. The Role of Autophagy in Skin Fibroblasts, Keratinocytes, Melanocytes, and Epidermal Stem Cells. J. Investig. Dermatol. 2020, 140, 1691–1697. [Google Scholar] [CrossRef]
- Kindl, G.H.; D’Orazio, J.A. Pharmacologic manipulation of skin pigmentation. Pigment Cell Melanoma Res. 2021. [CrossRef]
- Gado, F.; Digiacomo, M.; Salsano, J.E.; Macchia, M.; Manera, C. Phenolic Compounds in Prevention and Treatment of Skin Cancers: A review. Curr. Med. Chem. 2021. [CrossRef]
- Dybdahl, M.; Selesko, D.B.; Mikkelsen, U.R. Safety evaluation of whey derived beta-lactoglobulin, Lacprodan(R) BLG. Toxicol. Rep. 2021, 8, 617–626. [Google Scholar] [CrossRef]
- Lorz, L.R.; Yoo, B.C.; Kim, M.Y.; Cho, J.Y. Anti-Wrinkling and Anti-Melanogenic Effect of Pradosia mutisii Methanol Extract. Int. J. Mol. Sci. 2019, 20, 1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, H.Y.; Jeong, D.; Park, S.H.; Shin, K.K.; Hong, Y.H.; Kim, E.; Yu, Y.G.; Kim, T.R.; Kim, H.; Lee, J.; et al. Antiwrinkle and Antimelanogenesis Effects of Tyndallized Lactobacillus acidophilus KCCM12625P. Int. J. Mol. Sci. 2020, 21, 1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.H.; Yi, Y.-S.; Kim, M.-Y.; Cho, J.Y. Antioxidative and antimelanogenesis effect of momordica charantia methanol extract. Evid. -Based Complementary Altern. Med. 2019, 2019, 5091534. [Google Scholar] [CrossRef] [Green Version]
- Shin, K.K.; Park, J.G.; Hong, Y.H.; Aziz, N.; Park, S.H.; Kim, S.; Kim, E.; Cho, J.Y. Anti-Inflammatory Effects of Licania macrocarpa Cuatrec Methanol Extract Target Src-and TAK1-Mediated Pathways. Evid. Based Complementary Altern. Med. 2019, 2019, 4873870. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.G.; Choi, S.; Lee, J.; Hong, Y.H.; Jeong, D.; Yoon, K.; Yoon, D.H.; Sung, G.-H.; Lee, S.; Hong, S. Src is a prime target inhibited by celtis choseniana methanol extract in its anti-inflammatory action. Evid. Based Complementary Altern. Med. 2018, 2018, 3909038. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.J. Cnidium officinale Makino Extracts Inhibit α-MSH-induced Melanogenesis in B16F10 Mouse Melanoma Cells. Asian J. Beauty Cosmetol. 2018, 16, 122–130. [Google Scholar] [CrossRef]
- Qomaladewi, N.P.; Aziz, N.; Kim, M.-Y.; Cho, J.Y. Piper cubeba L. methanol extract has anti-inflammatory activity targeting Src/Syk via NF-B inhibition. Evid. Based Complementary Altern. Med. 2019, 2019, 1548125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Xu, X.; Wang, X.; Feng, W.; Shen, X.; Zhang, J.; Liu, H.; Xie, C.; Wu, Q.; Miao, X.; et al. beta-elemene promotes the senescence of glioma cells through regulating YAP-CDK6 signaling. Am. J. Cancer Res. 2021, 11, 370–388. [Google Scholar] [PubMed]
- Nanni, V.; Canuti, L.; Gismondi, A.; Canini, A. Hydroalcoholic extract of Spartium junceum L. flowers inhibits growth and melanogenesis in B16-F10 cells by inducing senescence. Phytomedicine Int. J. Phytother. Phytopharm. 2018, 46, 1–10. [Google Scholar] [CrossRef]
| Name | Direction | Sequence (5′ to 3′) | Anneal Temp. (°C) |
|---|---|---|---|
| Mouse | |||
| Tyr | Forward | GTCCACTCACAGGGATAGCAG | 56 |
| Reverse | AGAGTCTCTGTTATGGCCGA | ||
| Tyrp1 | Forward Reverse | ATGGAACGGGAGGACAAACC TCCTGACCTGGCCATTGAAC | 57 |
| Tyrp2 | Forward Reverse | CAGTTTCCCCGAGTCTGCAT GTCTAAGGCGCCCAAGAACT | 58 |
| Gapdh | Forward Reverse | ACCACAGTCCATGCCATCAC CCACCACCCTGTTGCTGTAG | 58 |
| Human | |||
| Tyr | Forward Reverse | AACAAGCGAGTCGGATCTGG GACGACACAGCAAGCTCACA | 58 |
| Tyrp1 | Forward Reverse | GTGCCACTGTTGAGGCTTTG ATGGGGATACTGAGGGCTGT | 58 |
| Tyrp2 | Forward Reverse | CTGGCCCCTATTGGTCACAA TGGCAGATCGATGGCATAGC | 58 |
| Gapdh | Forward Reverse | TCAAGGCTGAGAACGGGAAG TCGCCCCACTTGATTTTGGA | 57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, H.Y.; Kim, E.; Park, S.H.; Hwang, K.H.; Kim, D.; Jung, Y.-J.; Kopalli, S.R.; Hong, Y.D.; Sung, G.-H.; Cho, J.Y. Antimelanogenesis Effects of Theasinensin A. Int. J. Mol. Sci. 2021, 22, 7453. https://doi.org/10.3390/ijms22147453
Lim HY, Kim E, Park SH, Hwang KH, Kim D, Jung Y-J, Kopalli SR, Hong YD, Sung G-H, Cho JY. Antimelanogenesis Effects of Theasinensin A. International Journal of Molecular Sciences. 2021; 22(14):7453. https://doi.org/10.3390/ijms22147453
Chicago/Turabian StyleLim, Hye Yeon, Eunji Kim, Sang Hee Park, Kyung Hwan Hwang, Donghyun Kim, You-Jung Jung, Spandana Rajendra Kopalli, Yong Deog Hong, Gi-Ho Sung, and Jae Youl Cho. 2021. "Antimelanogenesis Effects of Theasinensin A" International Journal of Molecular Sciences 22, no. 14: 7453. https://doi.org/10.3390/ijms22147453
APA StyleLim, H. Y., Kim, E., Park, S. H., Hwang, K. H., Kim, D., Jung, Y.-J., Kopalli, S. R., Hong, Y. D., Sung, G.-H., & Cho, J. Y. (2021). Antimelanogenesis Effects of Theasinensin A. International Journal of Molecular Sciences, 22(14), 7453. https://doi.org/10.3390/ijms22147453







