Anti-Inflammatory Effects of Compounds from Cudrania tricuspidata in HaCaT Human Keratinocytes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Structures of 16 Compounds Isolated from C. tricuspidata
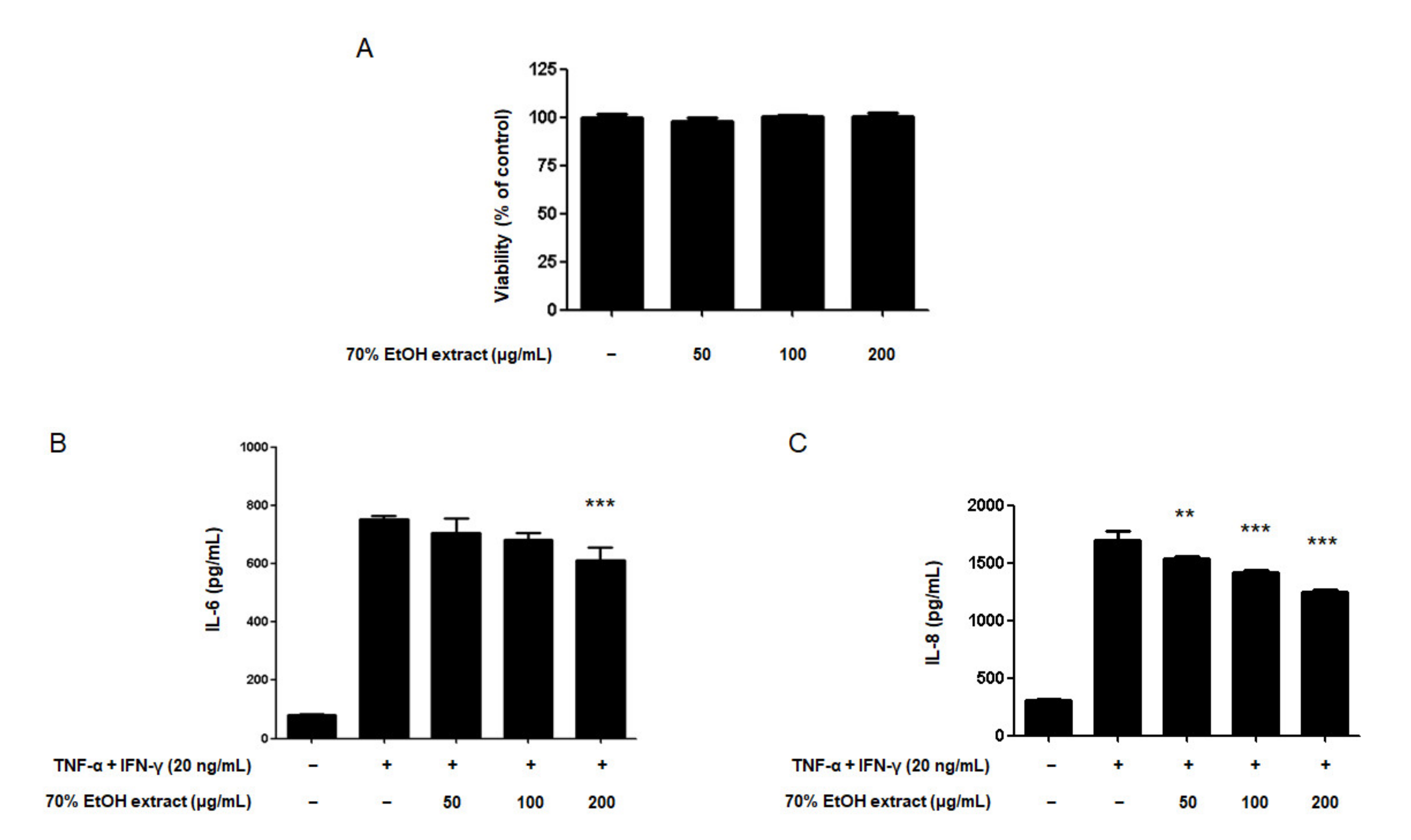
2.2. Effect of the 16 Compounds from C. tricuspidata on the Viability of HaCaT Cells
2.3. Effects of the 16 Compounds from C. tricuspidata on IL-6 Production in TNF-α + IFN-γ-Treated HaCaT Cells
2.4. Effects of the 16 Compounds from C. tricuspidata on IL-8 Production in TNF-α + IFN-γ-Treated HaCaT Cells
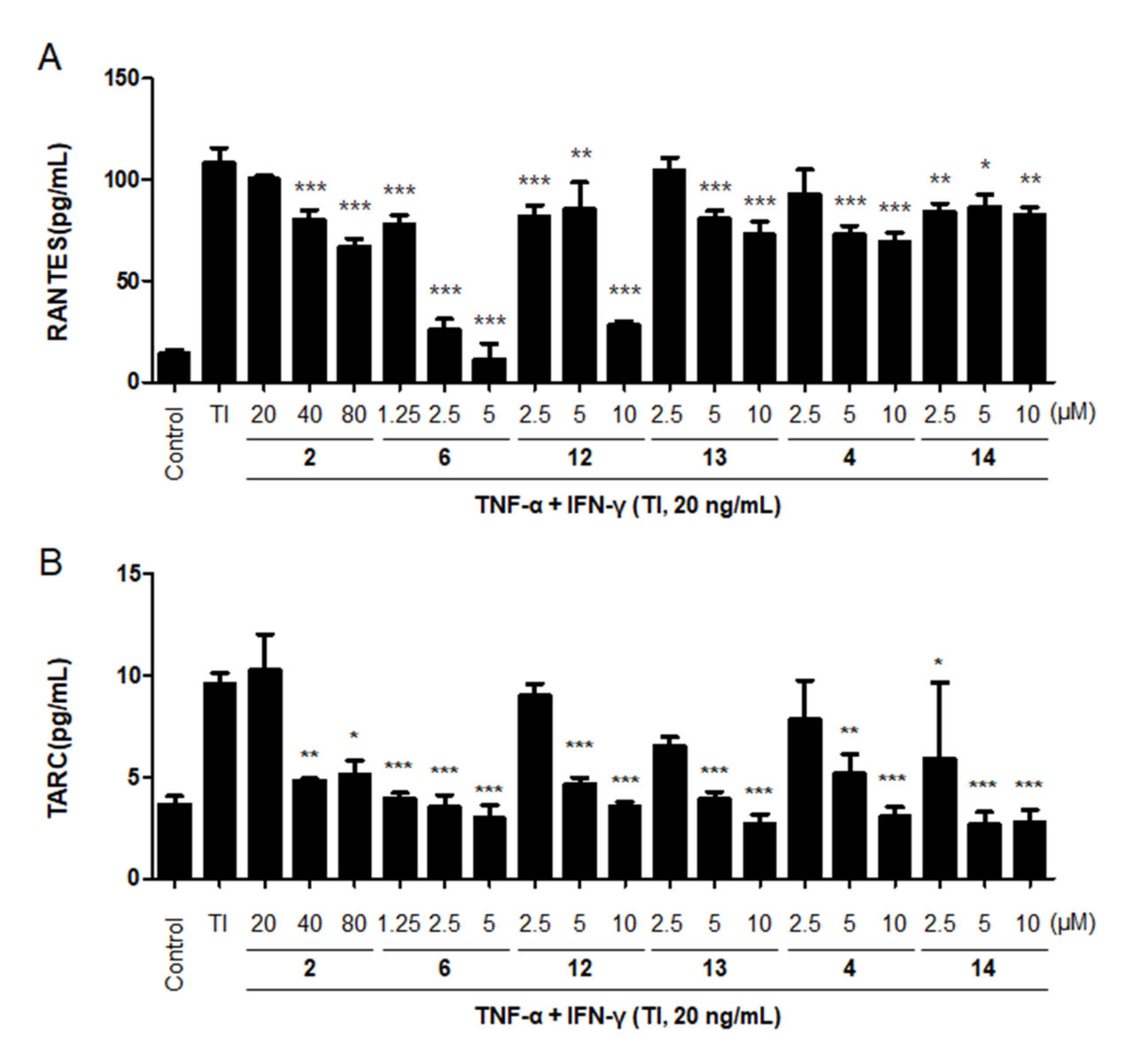
2.5. Effects of the Six Compounds from C. tricuspidata on RANTES and TARC Production by TNF-α + IFN-γ-Treated HaCaT Cells
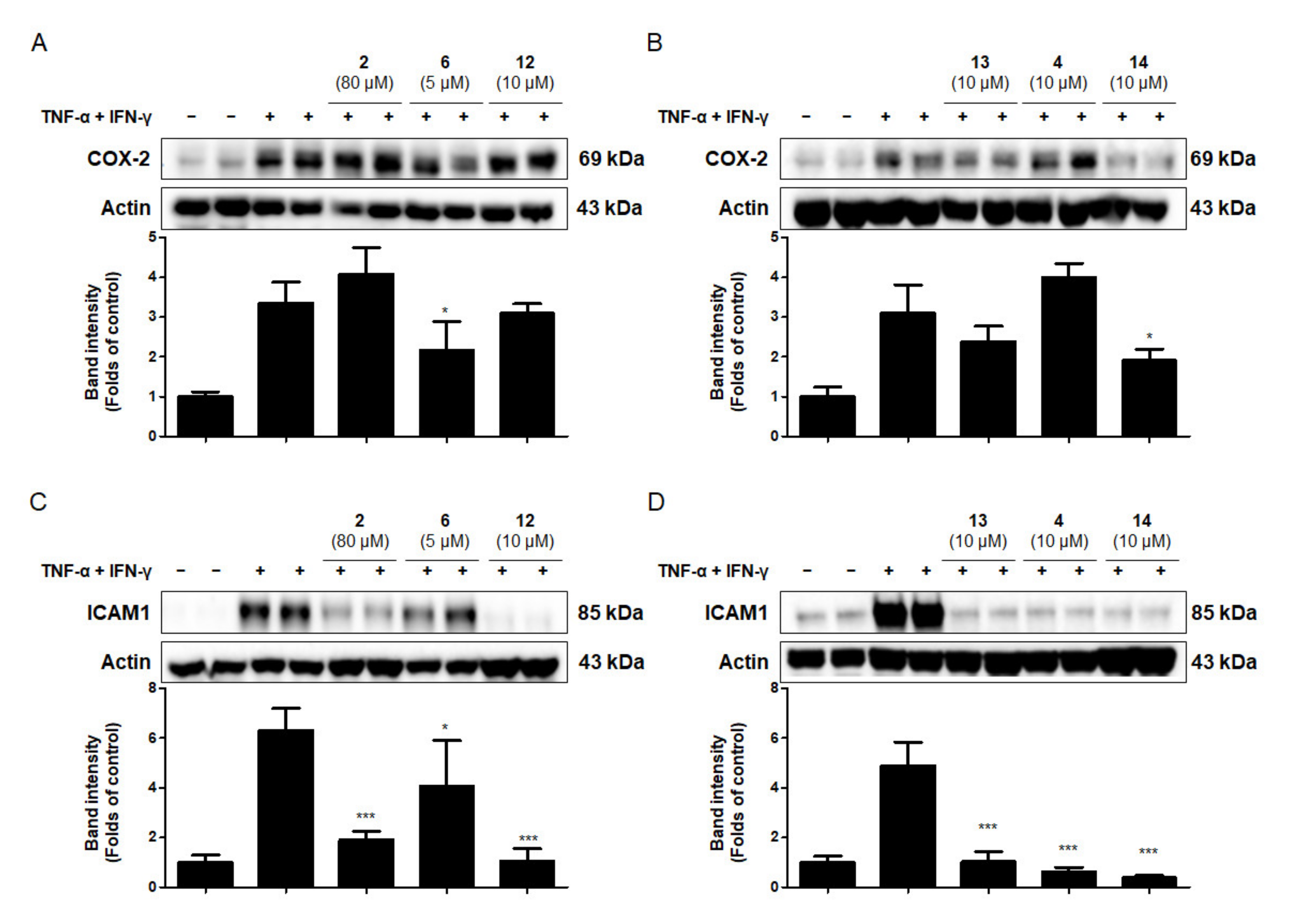
2.6. Effects of the Six Compounds from C. tricuspidata on COX-2 and ICAM-1 Protein Production by TNF-α + IFN-γ-Treated HaCaT Cells
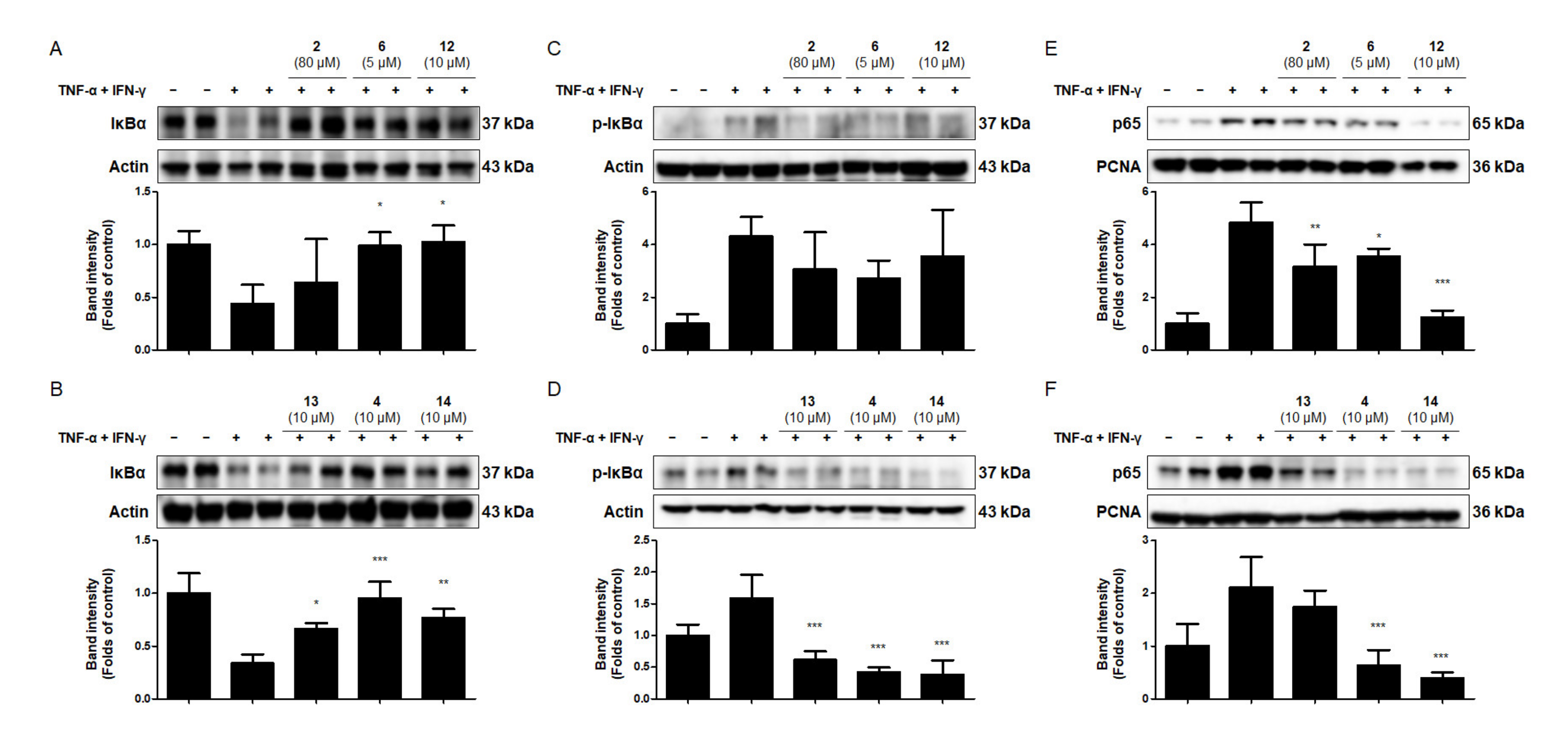
2.7. Effects of the Six Compounds from C. tricuspidata on NF-κB Activation in TNF-α + IFN-γ-Treated HaCaT Cells
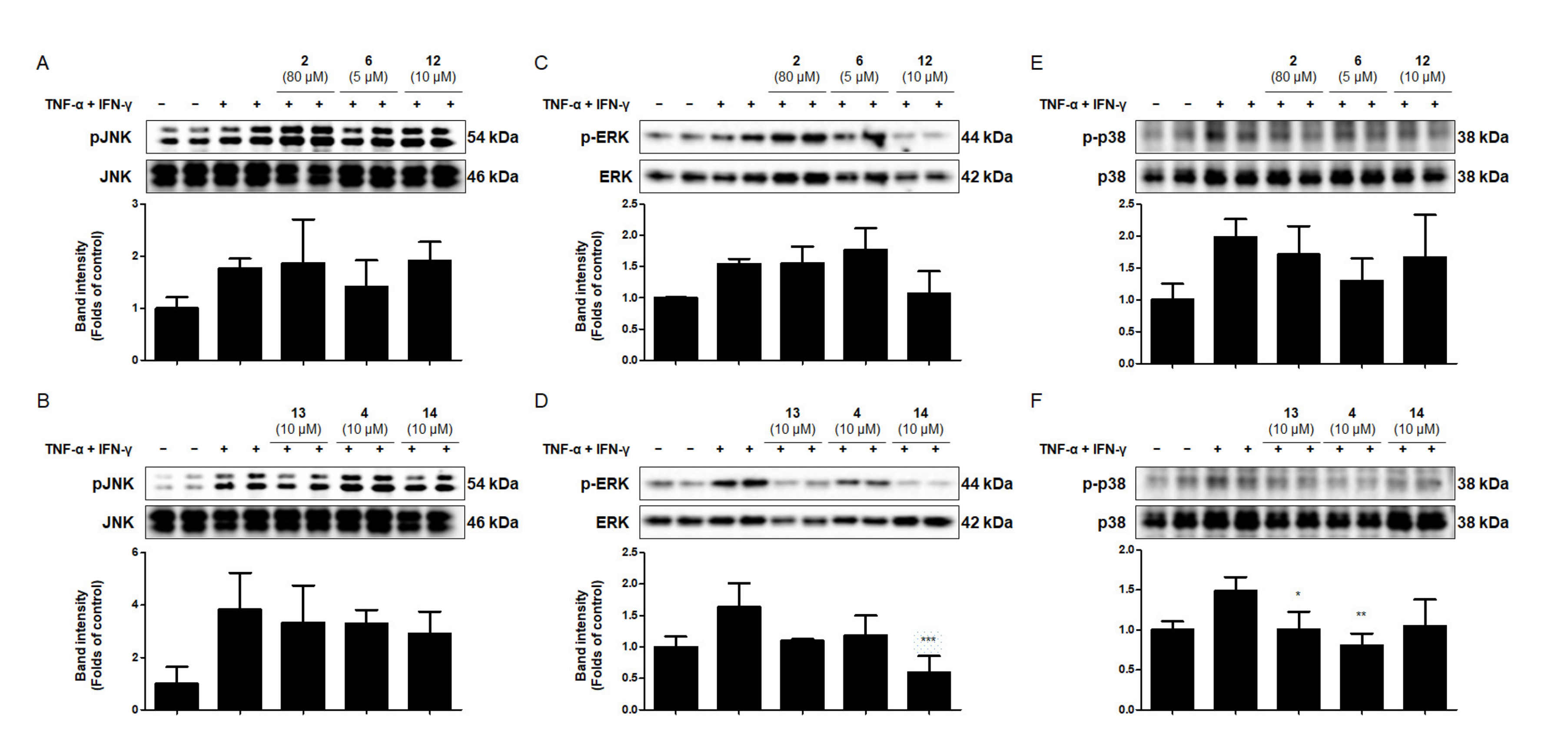
2.8. Effects of the Six Compounds from C. tricuspidata on MAPK Phosphorylation in TNF-α + IFN-γ-Treated HaCaT Cells
3. Materials and Methods
3.1. Chemicals and Reagents for Cell Culture
3.2. Cell Culture
3.3. CCK8 Assay
3.4. Enzyme-Linked Immunosorbent Assay (ELISA)
3.5. Western Blotting Analysis
3.6. Preparation of the Cytosolic and Nuclear Fractions
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoon, C.S.; Kim, D.C.; Quang, T.H.; Seo, J.; Kang, D.G.; Lee, H.S.; Oh, H.; Kim, Y.C. A prenylated xanthone, cudratricusxan-thone a, isolated from Cudrania tricuspidata inhibits lipopolysaccharide-induced neuroinflammation through inhibition of NF-κB and p38 MAPK pathways in BV2 microglia. Molecules 2016, 21, 1240. [Google Scholar] [CrossRef] [PubMed]
- Song, S.-H.; Ki, S.H.; Park, D.-H.; Moon, H.-S.; Lee, C.-D.; Yoon, I.-S.; Cho, S.-S. Quantitative Analysis, Extraction Optimization, and Biological Evaluation of Cudrania tricuspidata Leaf and Fruit Extracts. Molecules 2017, 22, 1489. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Ha, H.; Lee, J.K.; Seo, C.-S.; Lee, N.-H.; Jung, D.-Y.; Park, S.-J.; Shin, H.K. The Fruits of Cudrania tricuspidata Suppress Development of Atopic Dermatitis in NC/Nga Mice. Phytother. Res. 2011, 26, 594–599. [Google Scholar] [CrossRef]
- Chon, I.J.; Lee, S.W.; Cha, J.H.; Han, J.H.; Whang, W.K. Anti-oxidant compounds of Cudrania tricuspidata leaves. Yakhak Hoeji 2005, 49, 416–421. [Google Scholar]
- Lee, T.; Kwon, J.; Lee, D.; Mar, W.G. Effects of Cudrania tricuspidata fruit extract and its active compound, 5,7,3’,4’-tetrahydroxy-6,8-diprenylisoflavone, on the high-affinity IgE receptor-mediated activation of syk in mast cells. J. Agric. Food Chem. 2015, 22, 5459–5467. [Google Scholar] [CrossRef] [PubMed]
- Han, X.H.; Hong, S.S.; Hwang, J.S.; Jeong, S.H.; Hwang, J.H.; Lee, M.H.; Lee, M.K.; Lee, D.; Ro, J.S.; Hwang, B.Y. Monoamine oxidase inhibitory constituents from the fruits of Cudrania tricuspidata. Arch. Pharm. Res. 2005, 28, 1324–1327. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, B.; Alghamdi, S.A. The neuroprotective role of melatonin in neurological disorders. J. Neurosci. Res. 2017, 96, 1136–1149. [Google Scholar] [CrossRef]
- Uttarkar, S.; Brembilla, N.C.; Boehncke, W.-H. Regulatory cells in the skin: Pathophysiologic role and potential targets for anti-inflammatory therapies. J. Allergy Clin. Immunol. 2019, 143, 1302–1310. [Google Scholar] [CrossRef]
- Buchholz, T.; Melzig, M.F. Polyphenolic compounds as pancreatic lipase inhibitors. Planta Med. 2015, 81, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Afrin, L.B.; Khoruts, A. Mast cell activation disease and microbiotic interactions. Clin. Ther. 2015, 37, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, R.R.; Tipton, K.F. Assessment of enzyme inhibition: A review with examples from the development of monoamine oxidase and cholinesterase inhibitory drugs. Molecules 2017, 22, 1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, O.K.; Ho, J.N.; Nam, D.E.; Jun, W.J.; Lee, J.M. Anti-wrinkle activity of a Curdrania tricuspidata extract on ultravio-let-induced photoaging. J. Korean Soc. Food Sci. Nutr. 2013, 42, 608–614. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Mei, N.; Guo, X.; Ren, Z.; Kobayashi, D.; Wada, K.; Guo, L. Review of ginkgo biloba-induced toxicity, from experimental stud-ies to human case reports. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2017, 35, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Shadboorestan, A. Oxidative stress and cancer; the role of hesperidin, a citrus natural bioflavonoid, as a cancer chemoprotective agent. Nutr. Cancer 2016, 68, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Clementina, S.M.; Freitas, M.; Fernandes, E. A comprehensive review on xanthone derivatives as α-glucosidase inhibitors. Eur. J. Med. Chem. 2018, 5, 1460–1479. [Google Scholar]
- Fujimoto, T.; Hano, Y.; Nomura, T.; Uzawa, J. Components of Root Bark of Cudrania tricuspidata 2. Structures of Two New Isoprenylated Flavones, Cudraflavones A and B. Planta Med. 1984, 50, 161–163. [Google Scholar] [CrossRef]
- Choi, H.J.; Kim, T.K.; Do, Y.; Rang, J. Physiological activities of Curdrania tricuspidata extracts on the skin. J. Korean Oil Chem. Soc. 2015, 32, 260–274. [Google Scholar] [CrossRef]
- Habtemariam, S. The Nrf2/HO-1 Axis as Targets for Flavanones: Neuroprotection by Pinocembrin, Naringenin, and Eriodictyol. Oxidative Med. Cell. Longev. 2019, 2019, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.K.; Song, G.S.; Kim, C.J.; Kim, H.M.; Oh, G.T.; Yue, Y.D. Tumor cell growth inhibition of cancer cell growth and antiox-idant activities of flavonoid compounds isolated from Curdrania tricuspidata. Appl. Biol. Chem. 1994, 37, 105–109. [Google Scholar]
- Pang, W.; Qi, X.; Cao, C.; Zhang, S. Inhibitory effects of TGP on KGF-induced hyperproliferation of HaCaT cells via sup-pression of the p38 MAPK/NF-κB p65 pathway. Mol. Med. Rep. 2018, 18, 2207–2215. [Google Scholar] [PubMed] [Green Version]
- Sikandan, A.; Shinomiya, T.; Nagahara, Y. Ashwagandha root extract exerts anti-inflammatory effects in HaCaT cells by inhibiting the MAPK/NF-κB pathways and by regulating cytokines. Int. J. Mol. Med. 2018, 42, 425–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.J.; Lee, J.H.; Jung, Y.S. (+)-Nootkatone inhibits tumor necrosis factor α /interferon I-induced production of chemo-kines in HaCaT cells. Biochem. Biophys. Res. Commun. 2014, 447, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-H.; Hwang, Y.-H.; Gu, M.-J.; Cho, W.-K.; Ma, J.Y. Ethanol extracts of Sanguisorba officinalis L. suppress TNF-α/IFN-γ-induced pro-inflammatory chemokine production in HaCaT cells. Phytomedicine 2015, 22, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Liu, J.; Wang, J.; Luo, Q.; Zhang, H.; Liu, B.; Xu, F.; Pang, Q.; Liu, Y.; Dong, J. Icariin inhibits TNF-α/IFN-γ induced inflammatory response via inhibition of the substance P and p38-MAPK signaling pathway in human keratinocytes. Int. Immunopharmacol. 2015, 29, 401–407. [Google Scholar] [CrossRef]
- Henklova, P.; Vrzal, R.; Papouskova, B.; Bednar, P.; Jancova, P.; Anzenbacherova, E.; Ulrichova, J.; Maurel, P.; Pavek, P.; Dvorak, Z. SB203580, a pharmacological inhibitor of p38 MAP kinase transduction pathway activates ERK and JNK MAP kinases in primary cultures of human hepatocytes. Eur. J. Pharmacol. 2008, 593, 16–23. [Google Scholar] [CrossRef]
- Plotnikov, A.; Zehorai, E.; Procaccia, S.; Seger, R. The MAPK cascades: Signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim. Biophys. Acta (BBA) Bioenergy 2011, 1813, 1619–1633. [Google Scholar] [CrossRef] [Green Version]
- Sha, W.C. Regulation of immune responses by NF-kappa B/Rel transcription factor. J. Exp. Med. 1998, 187, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Barkett, M.; Gilmore, T.D. Control of apoptosis by Rel/NF-κB transcription factors. Oncogene 1999, 18, 6910–6924. [Google Scholar] [CrossRef] [Green Version]
- Quang, T.H.; Ngan, N.T.T.; Yoon, C.-S.; Richomme, P.; Kang, D.G.; Lee, H.S.; Kim, Y.-C.; Oh, H. Protein Tyrosine Phosphatase 1B Inhibitors from the Roots of Cudrania tricuspidata. Molecules 2015, 20, 11173–11183. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-L.; Wang, Y.-G.; Gao, G.-M.; Feng, L.; Guo, N.; Zhang, C.-X. Overexpression of lncRNA TCL6 promotes preeclampsia progression by regulating PTEN. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4066–4072. [Google Scholar] [PubMed]
- Su, B.-N.; Cuendet, M.; Hawthorne, M.E.; Kardono, L.B.S.; Riswan, S.; Fong, H.H.S.; Mehta, R.G.; Pezzuto, J.M.; Kinghorn, A.D. Constituents of the bark and twigs of Artocarpus dadah with cyclooxygenase inhibitory activity. J. Nat. Prod. 2002, 65, 163–169. [Google Scholar] [CrossRef]
- Zheng, Z.P.; Cheng, K.W.; To, J.T.; Li, H.; Wang, M. Isolation of tyrosinase inhibitors from Artocarpus heterophyllus and use of its extract as antibrowning agent. Mol. Nutr. Food. Res. 2008, 52, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-P.; Tan, H.-Y.; Chen, J.; Wang, M. Characterization of tyrosinase inhibitors in the twigs of Cudrania tricuspidata and their structure–activity relationship study. Fitoterapia 2013, 84, 242–247. [Google Scholar] [CrossRef]
- Jin, Y.J.; Lin, C.C.; Lu, T.M.; Li, J.H.; Chen, I.S.; Kuo, Y.H.; Ko, H.H. Chemical constituents derived from Artocarpus xantho-carpus as inhibitors of melanin biosynthesis. Phytochemistry 2015, 117, 424–435. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Nguyen, N.T.; Nguyen, M.H.; Le, T.H.; Van Do, T.N.; Hung, T.M.; Nguyen, M.T. Tyrosinase inhibitory activi-ty of flavonoids from Artocarpus heterophyllous. Chem. Cent. J. 2016, 10, 2. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Tao, G.; Chen, J.; Zheng, Z.-P. Characterization of a New Flavone and Tyrosinase Inhibition Constituents from the Twigs of Morus alba L. Molecules 2016, 21, 1130. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.C.; Quang, T.H.; Oh, H.; Kim, Y.C. Steppogenin Isolated from Cudrania tricuspidata shows antineuroinflammatory effects via NF-kappaB and MAPK pathways in LPS-Stimulated BV2 and primary rat microglial cells. Molecules 2017, 22, 2130. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-W.; Yen, F.-L.; Ko, H.-H.; Li, S.-Y.; Chiang, Y.-C.; Lee, M.-H.; Tsai, M.-H.; Hsu, L.-F. Cudraflavone C Induces Apoptosis of A375.S2 Melanoma Cells through Mitochondrial ROS Production and MAPK Activation. Int. J. Mol. Sci. 2017, 18, 1508. [Google Scholar] [CrossRef] [Green Version]
- Soo, H.-C.; Chung, F.F.-L.; Lim, K.-H.; Yap, V.A.; Bradshaw, T.D.; Hii, L.-W.; Tan, S.-H.; See, S.-J.; Tan, Y.-F.; Leong, C.-O.; et al. Cudraflavone C Induces Tumor-Specific Apoptosis in Colorectal Cancer Cells through Inhibition of the Phosphoinositide 3-Kinase (PI3K)-AKT Pathway. PLoS ONE 2017, 12, e0170551. [Google Scholar] [CrossRef] [Green Version]
- Groweiss, A.; Cardellina, J.H.; Boyd, M.R. HIV-Inhibitory prenylated xanthones and flavones from Maclura tinctoria. J. Nat. Prod. 2000, 63, 1537–1539. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.H.; Kim, H.C.; Cui, J.M.; Kim, Y.C. Hepatoprotective constituents of Cudrania tricuspidata. Arch. Pharm. Res. 2005, 28, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.W.; Gal, S.W.; Park, K.-M. Cytotoxic Xanthones from Cudrania tricuspidata. J. Nat. Prod. 2005, 68, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.-S.; Hou, A.-J.; Zhu, G.-F. Isoprenylated Xanthones and Flavonoids from Cudrania tricuspidata. Chem. Biodivers. 2005, 2, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.; Kim, K.W.; Quang, T.H.; Yoon, C.S.; Kim, N.; Lee, H.; Kim, S.C.; Woo, E.R.; Kim, Y.C.; Oh, H.; et al. Cudrafla-vanone B isolated from the root bark of Cudrania tricuspidata alleviates lipopolysaccharide-induced inflammatory responses by downregulating NF-κB and ERK MAPK signaling pathways in RAW264.7 macrophages and BV2 microglia. Inflammation 2021, 44, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Haraldsen, G.; Pan, J.; Rottman, J.; Qin, S.; Ponath, P.; Andrew, D.P.; Warnke, R.; Ruffing, N.; Kassam, N.; et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nat. Cell Biol. 1999, 400, 776–780. [Google Scholar] [CrossRef]
- Ogawa, E.; Sato, Y.; Minagawa, A.; Okuyama, R. Pathogenesis of psoriasis and development of treatment. J. Dermatol. 2018, 45, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Krueger, J.G. The Immunopathogenesis of Psoriasis. Dermatol. Clin. 2015, 33, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Ulzii, D.; Vu, Y.H.; Tsuji, G.; Kido-Nakahara, M.; Nakahara, T. Pathogenesis of atopic dermatitis: Current para-digm. Iran J. Immunol. 2019, 16, 97–107. [Google Scholar]
- Jeong, S.-J.; Lim, H.-S.; Seo, C.-S.; Jin, S.-E.; Yoo, S.-R.; Lee, N.; Shin, H.-K. Anti-inflammatory Actions of Herbal Formula Gyejibokryeong-Hwan Regulated by Inhibiting Chemokine Production and STAT1 Activation in HaCaT Cells. Biol. Pharm. Bull. 2015, 38, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Shimada, Y.; Takehara, K.; Sato, S. Both Th2 and Th1 chemokines (TARC/CCL17, MDC/CCL22, and Mig/CXCL9) are elevated in sera from patients with atopic dermatitis. J. Dermatol. Sci. 2004, 34, 201–208. [Google Scholar] [CrossRef]
- Vejlsgaard, G.L.; Ralfkiaer, E.; Avnstorp, C. Kinetics and characterization of intercellular adhesion molecule-l (ICAM-1) expression on keratinocytes in various inflammatory skin lesions and malignant lymphomas. J. Am. Acad. Dermatol. 1989, 20, 782–790. [Google Scholar] [CrossRef]
- Chen, C.-C. Signal Transduction Pathways of Inflammatory Gene Expressions and Therapeutic Implications. Curr. Pharm. Des. 2006, 12, 3497–3508. [Google Scholar] [CrossRef] [PubMed]
- Shaha, M.K.K.; Sirata, H.M.; Jamil, S.; Jalil, J. Flavonoids from the Bark of Artocarpus integer var. silvestris and their Anti-inflammatory Properties. Nat. Prod. Commun. 2016, 11, 1275–1278. [Google Scholar] [PubMed]
- Robert, C.; Kupper, T.S. Inflammatory Skin Diseases, T Cells, and Immune Surveillance. New Engl. J. Med. 1999, 341, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.-Y.; Kim, Y.S.; Kim, H.K. Illicium verum extract inhibits TNF-α- and IFN-γ-induced expression of chemokines and cytokines in human keratinocytes. J. Ethnopharmacol. 2012, 144, 182–189. [Google Scholar] [CrossRef]
- Kim, S.Y.; Sohn, E.J.; Kim, D.W.; Jeong, H.J.; Kim, M.J.; Kang, H.W.; Shin, M.J.; Ahn, E.H.; Kwon, S.W.; Kim, Y.N.; et al. Transduced PEP-1-FK506BP Ameliorates Atopic Dermatitis in NC/Nga Mice. J. Investig. Dermatol. 2011, 131, 1477–1485. [Google Scholar] [CrossRef] [Green Version]
- Funakoshi, M.; Tago, K.; Sonoda, Y.; Tominaga, S.; Kasahara, T. A MEK inhibitor, PD98059 enhances IL-1-induced NF-kappaB activation by the enhanced and sustained degradation of IkappaBalpha. Biochem. Biophys. Res. Commun. 2001, 283, 248–254. [Google Scholar] [CrossRef]
- Xu, J.; Xiong, H.; Zhao, Z.; Luo, M.; Ju, Y.; Yang, G.; Mei, Z. Genistein suppresses allergic contact dermatitis through regulating the MAP2K2/ERK pathway. Food Funct. 2021, 12, 4556–4569. [Google Scholar] [CrossRef]
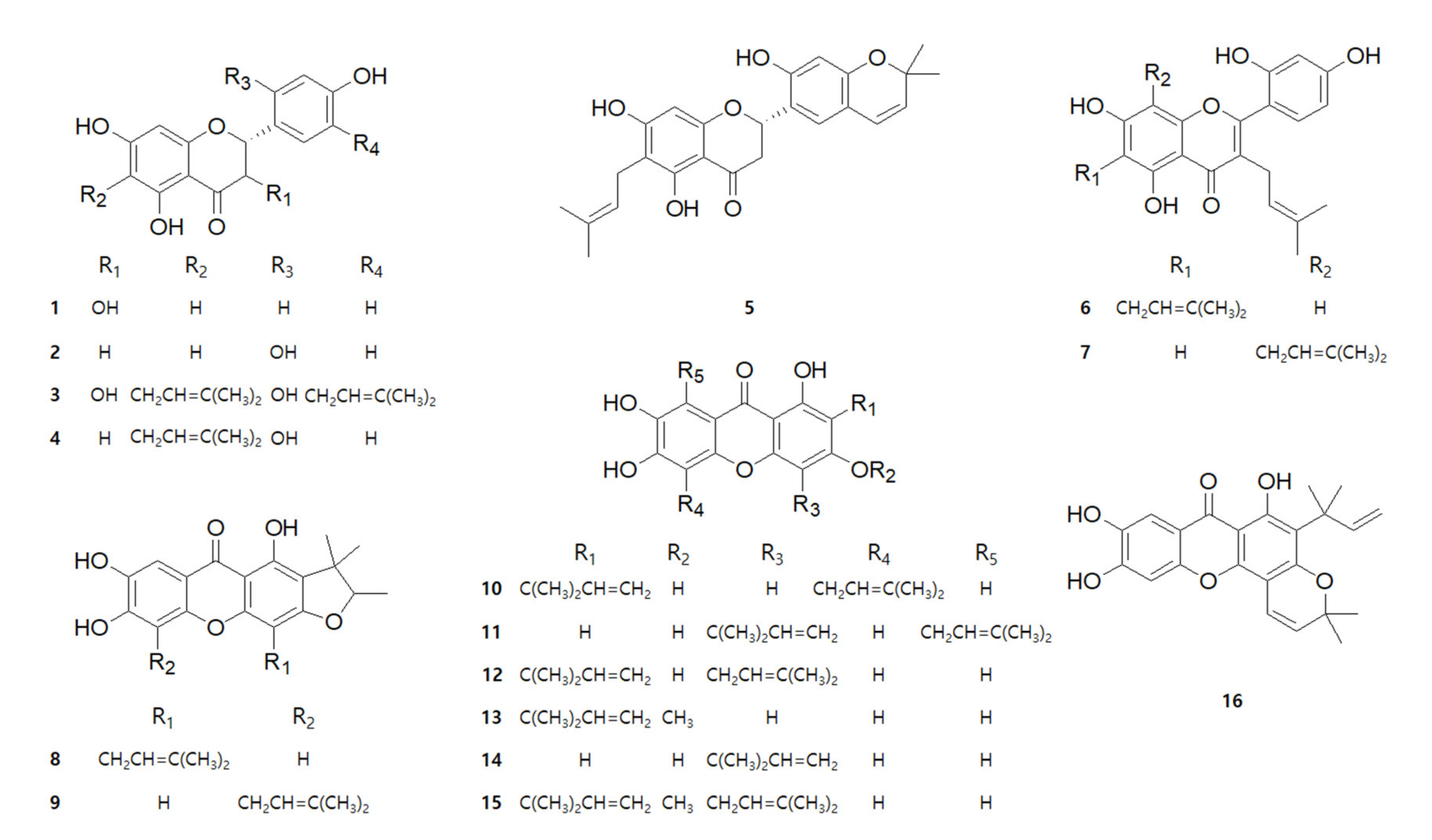
| Compound | Name |
|---|---|
| 1 | dihydrokaempferol |
| 2 | steppogenin |
| 3 | cudraflavanone D |
| 4 | cudraflavanone B |
| 5 | cudraflavanone A |
| 6 | cudraflavone C |
| 7 | kuwanon C |
| 8 | cudraxanthone M |
| 9 | cudraxanthone D |
| 10 | cudraxanthone L |
| 11 | cudratricusxanthone A |
| 12 | macluraxanthone B |
| 13 | 1,6,7-trihydroxy-2-(1,1-dimethyl-2-propenyl)-3-methoxyxanthone |
| 14 | cudratricusxanthone L |
| 15 | cudracuspixanthone A |
| 16 | cudratricusxanthone N |
| Compounds | Name | Cytotoxicity (μM) | |
|---|---|---|---|
| 1 | dihydrokaempferol | >80 | >80 |
| 2 | steppogenin | >80 | >80 |
| 3 | cudraflavanone D | >10 | >10 |
| 4 | cudraflavanone B | >20 | >20 |
| 5 | cudraflavanone A | >40 | >40 |
| 6 | cudraflavone C | >5 | >5 |
| 7 | kuwanon C | >40 | >40 |
| 8 | cudraxanthone M | >10 | >10 |
| 9 | cudraxanthone D | >5 | >5 |
| 10 | cudraxanthone L | >2 | >2 |
| 11 | cudratricusxanthone A | >5 | >5 |
| 12 | macluraxanthone B | >5 | >5 |
| 13 | 1,6,7-trihydroxy-2-(1,1-dimethyl-2-propenyl)-3-methoxyxanthone | >40 | >40 |
| 14 | cudratricusxanthone L | >20 | >20 |
| 15 | cudracuspixanthone A | >2 | >2 |
| 16 | cudratricusxanthone N | >2 | >2 |
| Compounds | Name | IC50 (μM) | |
|---|---|---|---|
| IL-6 | IL-8 | ||
| 1 | dihydrokaempferol | >80 a | >80 a |
| 2b | steppogenin | >80 a | 62.17 ± 16.46 a |
| 3 | cudraflavanone D | >10 a | 4.20 ± 1.03 a |
| 4b | cudraflavanone B | >20 a | 9.88 ± 3.22 a |
| 5 | cudraflavanone A | >40 | >40 a |
| 6b | cudraflavone C | 2.78 ± 0.75 a | 2.16 ± 0.50 a |
| 7 | kuwanon C | 25.75 ± 5.16 a | >40 a |
| 8 | cudraxanthone M | >10 | >10 a |
| 9 | cudraxanthone D | >5 a | >5 a |
| 10 | cudraxanthone L | >2 a | >2 a |
| 11 | cudratricusxanthone A | >5 | >5 |
| 12b | macluraxanthone B | >5 a | 7.88 ± 3.09 a |
| 13b | 1,6,7-trihydroxy-2-(1,1-dimethyl-2-propenyl)-3-methoxyxanthone | 9.69 ± 2.15 a | 3.87 ± 0.96 a |
| 14b | cudratricusxanthone L | 9.86 ± 0.92 a | 3.70 ± 0.70 a |
| 15 | cudracuspixanthone A | >2 a | 1.54 ± 0.53 a |
| 16 | cudratricusxanthone N | >2 | >2 a |
| Positive control | curcumin | 11.96 ± 3.13 | 10.29 ± 2.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, W.; Kim, N.; Lee, H.; Woo, E.-R.; Kim, Y.-C.; Oh, H.; Lee, D.-S. Anti-Inflammatory Effects of Compounds from Cudrania tricuspidata in HaCaT Human Keratinocytes. Int. J. Mol. Sci. 2021, 22, 7472. https://doi.org/10.3390/ijms22147472
Ko W, Kim N, Lee H, Woo E-R, Kim Y-C, Oh H, Lee D-S. Anti-Inflammatory Effects of Compounds from Cudrania tricuspidata in HaCaT Human Keratinocytes. International Journal of Molecular Sciences. 2021; 22(14):7472. https://doi.org/10.3390/ijms22147472
Chicago/Turabian StyleKo, Wonmin, Nayeon Kim, Hwan Lee, Eun-Rhan Woo, Youn-Chul Kim, Hyuncheol Oh, and Dong-Sung Lee. 2021. "Anti-Inflammatory Effects of Compounds from Cudrania tricuspidata in HaCaT Human Keratinocytes" International Journal of Molecular Sciences 22, no. 14: 7472. https://doi.org/10.3390/ijms22147472
APA StyleKo, W., Kim, N., Lee, H., Woo, E.-R., Kim, Y.-C., Oh, H., & Lee, D.-S. (2021). Anti-Inflammatory Effects of Compounds from Cudrania tricuspidata in HaCaT Human Keratinocytes. International Journal of Molecular Sciences, 22(14), 7472. https://doi.org/10.3390/ijms22147472






