Molecular Mechanism and Prevention Strategy of Chemotherapy- and Radiotherapy-Induced Ovarian Damage
Abstract
:1. Introduction
2. Regulation of the Ovarian Follicular Reserve
3. Cancer Treatment-Induced Ovarian Damage
3.1. Mechanism of Chemotherapy-Induced Ovarian Damage
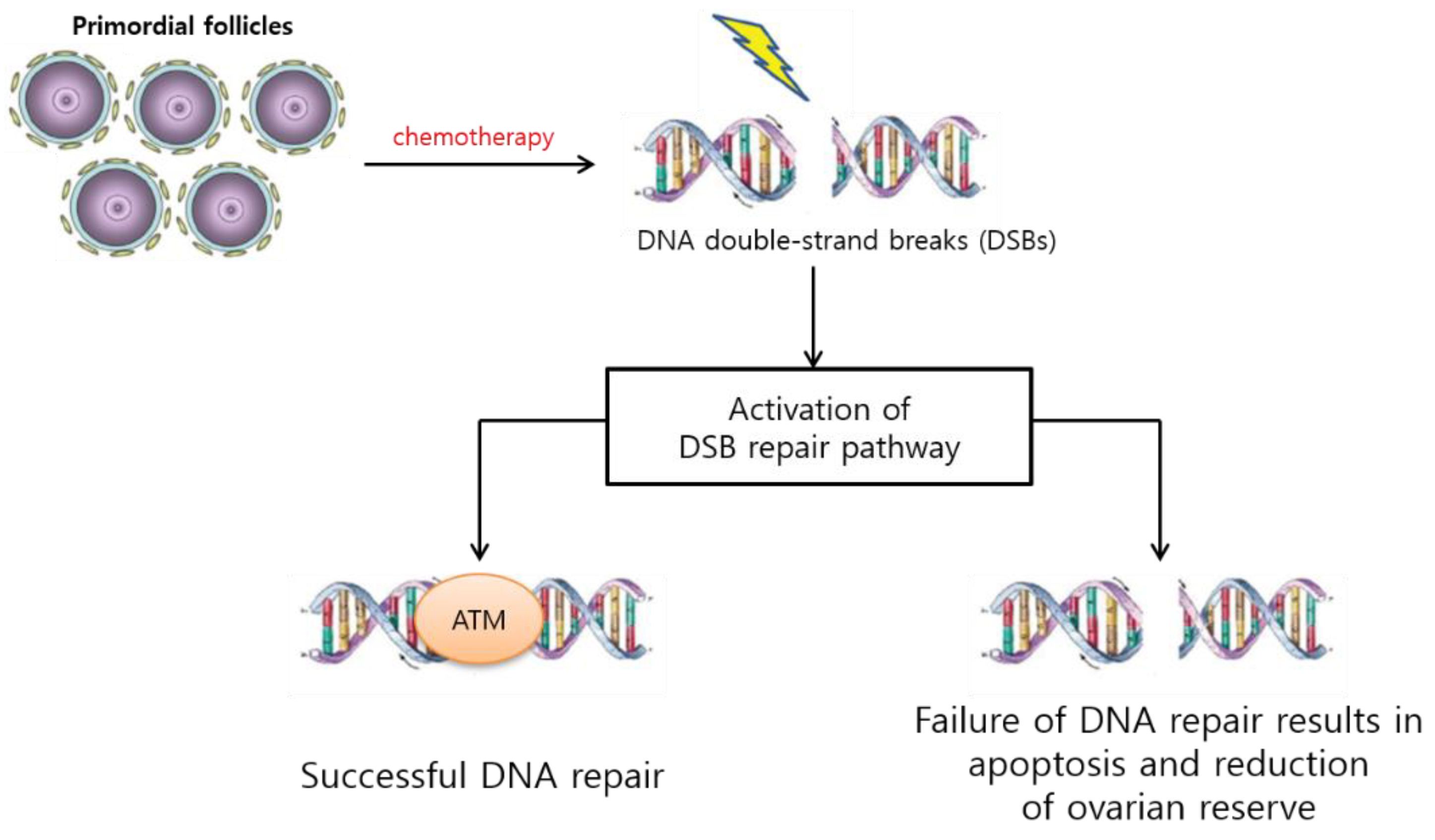
3.1.1. PF Loss via DNA Alteration, Follicular Atresia, and Apoptosis
3.1.2. Follicle Loss via Activation and “Burnout”
3.1.3. Stromal and Microvascular Damage
3.2. Radiation-Induced Ovarian Damage
3.2.1. Radiosensitivity of Oocytes
3.2.2. Linear Energy Transfer
3.2.3. Oxidative Stress Resulting in DNA Damage
4. Detection of Ovarian Damage
4.1. Clinical Considerations
4.2. Biochemical Markers for Ovarian Reserve
4.2.1. AMH
4.2.2. Basal Follicle-Stimulating Hormone and Estradiol
4.2.3. Inhibin-B
4.3. Ultrasonographic Markers
5. Prevention and Management of Ovarian Damage
5.1. Prevention of Chemotherapy-Induced Ovarian Damage
5.1.1. Gonadotropin-Releasing Hormone (GnRH) Agonists
5.1.2. AS101
5.1.3. Anti-Müllerian Hormone (AMH)
5.1.4. Imatinib
5.1.5. Sphingosine-1-Phosphate
5.1.6. Granulocyte Colony-Stimulating Factor
5.2. Prevention of Ovarian Damage before and during Radiation Treatment
5.2.1. Identifying the Location of the Ovaries
5.2.2. Alternative Techniques for Craniospinal Radiotherapy
5.2.3. Ovarian Transposition
5.3. Cryopreservation
5.3.1. Embryo Cryopreservation
5.3.2. Oocyte Cryopreservation
5.3.3. Ovarian Tissue Cryopreservation and Transplantation
6. Future Perspectives on FP
6.1. Whole Ovarian Transplantation
6.2. In Vitro Maturation
6.3. Artificial Ovaries
6.4. Ovarian Stem Cells
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Miller, K.D.; Benaoudia, M.F.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer statistics for adolescents and young adults. CA Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef]
- Birch, J.M.; Marsden, H.B.; Jones, P.H.; Pearson, D.; Blair, V. Improvements in survival from childhood cancer: Results of a population based survey over 30 years. Br. Med. J. (Clin. Res. Ed.) 1988, 296, 1372–1376. [Google Scholar] [CrossRef] [Green Version]
- Wingo, P.A.; Ries, L.A.; Parker, S.L.; Heath, C.W., Jr. Long-term cancer patient survival in the United States. Cancer Epidemiol. Biomark. Prev. 1998, 7, 271–282. [Google Scholar]
- Sklar, C.A.; Mertens, A.C.; Mitby, P.; Whitton, J.; Stovall, M.; Kasper, C.; Mulder, J.; Green, D.; Nicholson, H.S.; Yasui, Y.; et al. Premature menopause in survivors of childhood cancer: A report from the childhood cancer survivor study. J. Natl. Cancer Inst. 2006, 98, 890–896. [Google Scholar] [CrossRef]
- Wallace, W.H.B.; Thomson, A.B.; Saran, F.; Kelsey, T.W. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Vriens, I.J.H.; de Bie, A.J.R.; Aarts, M.J.B.; de Boer, M.; van Hellemond, I.E.G.; Roijen, J.H.E.; van Golde, R.J.T.; Voogd, A.C.; Heijnen, V.C.G.T. The correlation of age with chemotherapy-induced ovarian function failure in breast cancer patients. Oncotarget 2017, 8, 11372–11379. [Google Scholar] [CrossRef] [Green Version]
- Chemaitilly, W.; Li, Z.; Krasin, M.J.; Brooke, R.J.; Wilson, C.L.; Green, D.M.; Klosky, J.L.; Barnes, N.; Clark, K.L.; Farr, J.B.; et al. Premature Ovarian Insufficiency in Childhood Cancer Survivors: A Report From the St. Jude Lifetime Cohort. J. Clin. Endocrinol. Metab. 2017, 102, 2242–2250. [Google Scholar] [CrossRef] [Green Version]
- Ganz, P.A.; Greendale, G.A.; Petersen, L.; Kahn, B.; Bower, J.E. Breast cancer in younger women: Reproductive and late health effects of treatment. J. Clin. Oncol. 2003, 21, 4184–4193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muka, T.; Williams, C.O.; Kunutsor, S.; Laven, J.S.E.; Fauser, B.C.J.M.; Chowdhury, R.; Kavousi, M.; Franco, O.H. Association of Age at Onset of Menopause and Time Since Onset of Menopause With Cardiovascular Outcomes, Intermediate Vascular Traits, and All-Cause Mortality: A Systematic Review and Meta-analysis. JAMA Cardiol. 2016, 1, 767–776. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Cai, H.; Kallianpur, A.; Li, H.; Yang, G.; Gao, J.; Xiang, Y.B.; Ji, B.T.; Tang, Y.; Zheng, W.; et al. Impact of premature ovarian failure on mortality and morbidity among Chinese women. PLoS ONE 2014, 9, e89597. [Google Scholar] [CrossRef]
- Lee, S.; Heytens, E.; Moy, F.; Ozkavukcu, S.; Oktay, K. Determinants of access to fertility preservation in women with breast cancer. Fertil. Steril. 2011, 95, 1932–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Ozkavukcu, S.; Heytens, E.; Moy, F.; Oktay, K. Value of early referral to fertility preservation in young women with breast cancer. J. Clin. Oncol. 2010, 28, 4683–4686. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Oktay, K.; Gracia, C.; Lee, S.; Morse, C.; Mersereau, J.E. Which patients pursue fertility preservation treatments? A multicenter analysis of the predictors of fertility preservation in women with breast cancer. Fertil. Steril. 2012, 97, 671–676. [Google Scholar] [CrossRef] [Green Version]
- Oktay, K.; Harvey, B.E.; Partridge, A.H.; Quinn, G.P.; Reinecke, J.; Taylor, H.S.; Wallace, W.H.; Wang, E.T.; Loren, A.W. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1994–2001. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.K.; Hwang, K.J.; Kim, T.; Kim, S.H. Fertility preservation for patients with gynecologic malignancies: The Korean Society for Fertility Preservation clinical guidelines. Clin. Exp. Reprod. Med. 2017, 44, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Campbell, S.B.; Woodard, T.L. An update on fertility preservation strategies for women with cancer. Gynecol. Oncol. 2020, 156, 3–5. [Google Scholar] [CrossRef] [Green Version]
- Lambertini, M.; Peccatori, F.A.; Demeestere, I.; Amant, F.; Wyns, C.; Stukenborg, J.-B.; Paluch-Shimon, S.; Halaska, M.J.; Uzan, C.; Meissner, J.; et al. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines(dagger). Ann. Oncol. 2020, 31, 1664–1678. [Google Scholar] [CrossRef]
- Coccia, P.F.; Pappo, A.S.; Beaupin, L.; Borges, V.F.; Borinstein, S.C.; Chugh, R.; Dinner, S.; Folbrecht, J.; Frazier, A.L.; Goldsby, R.; et al. Adolescent and Young Adult Oncology, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2018, 16, 66–97. [Google Scholar]
- Letourneau, J.M.; Ebbel, E.E.; Katz, P.P.; Oktay, K.H.; McCulloch, C.E.; Ai, W.Z.; Chien, A.J.; Melisko, M.E.; Cedars, M.I.; Rosen, M.P. Acute ovarian failure underestimates age-specific reproductive impairment for young women undergoing chemotherapy for cancer. Cancer 2012, 118, 1933–1939. [Google Scholar] [CrossRef] [Green Version]
- Davies, C.; Pan, H.; Godwin, J.; Gray, R.; Arriagada, R.; Raina, V.; Abraham, M.; Alencar, V.H.M.; Badran, A.; Bonfill, X.; et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013, 381, 805–816. [Google Scholar] [CrossRef] [Green Version]
- Stupart, D.; Win, A.K.; Jenkins, M.; Winship, I.M. Female fertility and colorectal cancer. Int. J. Colorectal. Dis. 2008, 23, 735–743. [Google Scholar]
- Zapardiel, I.; Cruz, M.; Diestro, M.D.; Requena, A.; Velasco, J.A.G. Assisted reproductive techniques after fertility-sparing treatments in gynaecological cancers. Hum. Reprod. Update 2016, 22, 281–305. [Google Scholar] [CrossRef] [Green Version]
- Roness, H.; Kashi, O.; Meirow, D. Prevention of chemotherapy-induced ovarian damage. Fertil. Steril. 2016, 105, 20–29. [Google Scholar] [CrossRef]
- Sonigo, C.; Beau, I.; Binart, N.; Grynberg, M. The Impact of Chemotherapy on the Ovaries: Molecular Aspects and the Prevention of Ovarian Damage. Int. J. Mol. Sci. 2019, 20, 5342. [Google Scholar] [CrossRef] [Green Version]
- Szymanska, K.J.; Tan, X.; Oktay, K. Unraveling the mechanisms of chemotherapy-induced damage to human primordial follicle reserve: Road to developing therapeutics for fertility preservation and reversing ovarian aging. Mol. Hum. Reprod. 2020, 26, 553–566. [Google Scholar] [CrossRef]
- Cosgrove, C.M.; Salani, R. Ovarian effects of radiation and cytotoxic chemotherapy damage. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 55, 37–48. [Google Scholar] [CrossRef]
- Marci, R.; Mallozzi, M.; di Benedetto, L.; Schimberni, M.; Mossa, S.; Soave, I.; Palomba, S.; Caserta, D. Radiations and female fertility. Reprod. Biol. Endocrinol. 2018, 16, 112. [Google Scholar] [CrossRef]
- Bedoschi, G.; Navarro, P.A.; Oktay, K. Chemotherapy-induced damage to ovary: Mechanisms and clinical impact. Future Oncol. 2016, 12, 2333–2344. [Google Scholar] [CrossRef] [Green Version]
- Wallace, W.H.; Kelsey, T.W. Human ovarian reserve from conception to the menopause. PLoS ONE 2010, 5, e8772. [Google Scholar] [CrossRef] [Green Version]
- Reddy, P.; Zheng, W.; Liu, K. Mechanisms maintaining the dormancy and survival of mammalian primordial follicles. Trends Endocrinol. Metab. 2010, 21, 96–103. [Google Scholar] [CrossRef]
- Adhikari, D.; Liu, K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr. Rev. 2009, 30, 438–464. [Google Scholar] [CrossRef]
- McLaughlin, M.; Kinnell, H.L.; Anderson, R.A.; Telfer, E.E. Inhibition of phosphatase and tensin homologue (PTEN) in human ovary in vitro results in increased activation of primordial follicles but compromises development of growing follicles. Mol. Hum. Reprod. 2014, 20, 736–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Kawamura, K.; Cheng, Y.; Liu, S.; Klein, C.; Liu, S.; Duan, E.; Hsueh, A.J.W. Activation of dormant ovarian follicles to generate mature eggs. Proc. Natl. Acad. Sci. USA 2010, 107, 10280–10284. [Google Scholar] [CrossRef] [Green Version]
- Hsueh, A.J.W.; Kawamura, K.; Cheng, Y.; Fauser, B.C.J.M. Intraovarian control of early folliculogenesis. Endocr. Rev. 2015, 36, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Gawriluk, T.R.; Hale, A.N.; Flaws, J.A.; Dillon, C.P.; Green, D.R.; Rucker, E.B., 3rd. Autophagy is a cell survival program for female germ cells in the murine ovary. Reproduction 2011, 141, 759–765. [Google Scholar]
- Kuma, A.; Hatano, M.; Matsui, M.; Yamamoto, A.; Nakaya, H.; Yoshimori, T.; Ohsumi, Y.; Tokuhisa, T.; Mizushima, N. The role of autophagy during the early neonatal starvation period. Nature 2004, 432, 1032–1036. [Google Scholar] [CrossRef] [PubMed]
- De Vos, M.; Devroey, P.; Fauser, B.C. Primary ovarian insufficiency. Lancet 2010, 376, 911–921. [Google Scholar] [CrossRef]
- Chapman, R.M. Effect of cytotoxic therapy on sexuality and gonadal function. Semin. Oncol. 1982, 9, 84–94. [Google Scholar] [PubMed]
- Miller, J.J., 3rd; Williams, G.F.; Leissring, J.C. Multiple late complications of therapy with cyclophosphamide, including ovarian destruction. Am. J. Med. 1971, 50, 530–535. [Google Scholar] [CrossRef]
- Rose, D.P.; Davis, T.E. Ovarian function in patients receiving adjuvant chemotherapy for breast cancer. Lancet 1977, 1, 1174–1176. [Google Scholar] [CrossRef]
- Shalet, S.M. Effects of cancer chemotherapy on gonadal function of patients. Cancer Treat. Rev. 1980, 7, 141–152. [Google Scholar] [CrossRef]
- Koyama, H.; Wada, T.; Nishizawa, Y.; Iwanaga, T.; Aoki, Y. Cyclophosphamide-induced ovarian failure and its therapeutic significance in patients with breast cancer. Cancer 1977, 39, 1403–1409. [Google Scholar] [CrossRef]
- Andrieu, J.M.; Ochoa-Molina, M.E. Menstrual cycle, pregnancies and offspring before and after MOPP therapy for Hodgkin’s disease. Cancer 1983, 52, 435–438. [Google Scholar] [CrossRef]
- Cho, H.W.; Lee, S.; Min, K.J.; Hong, J.H.; Song, J.Y.; Lee, J.K.; Lee, N.W.; Kim, T. Advances in the Treatment and Prevention of Chemotherapy-Induced Ovarian Toxicity. Int. J. Mol. Sci. 2020, 21, 7792. [Google Scholar] [CrossRef]
- Thomson, A.B.; Critchley, H.O.; Wallace, W.H. Fertility and progeny. Eur. J. Cancer 2002, 38, 1634–1644, discussion 1645–1646. [Google Scholar] [CrossRef]
- Morgan, S.; Anderson, R.A.; Gourley, C.; Wallace, W.H.; Spears, N. How do chemotherapeutic agents damage the ovary? Hum. Reprod. Update 2012, 18, 525–535. [Google Scholar] [CrossRef] [Green Version]
- Winship, A.L.; Stringer, J.M.; Liew, S.H.; Hutt, K.J. The importance of DNA repair for maintaining oocyte quality in response to anti-cancer treatments, environmental toxins and maternal ageing. Hum. Reprod. Update 2018, 24, 119–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Utsunomiya, T.; Tanaka, T.; Utsunomiya, H.; Umesaki, N. A novel molecular mechanism for anticancer drug-induced ovarian failure: Irinotecan HCl, an anticancer topoisomerase I inhibitor, induces specific FasL expression in granulosa cells of large ovarian follicles to enhance follicular apoptosis. Int. J. Oncol. 2008, 32, 991–1000. [Google Scholar] [CrossRef] [Green Version]
- Kerr, J.B.; Hutt, K.J.; Michalak, E.M.; Cook, M.; Vandenberg, C.J.; Liew, S.H.; Bouillet, P.; Mills, A.; Scott, C.L.; Findlay, J.K.; et al. DNA damage-induced primordial follicle oocyte apoptosis and loss of fertility require TAp63-mediated induction of Puma and Noxa. Mol. Cell 2012, 48, 343–352. [Google Scholar] [CrossRef] [Green Version]
- Oktem, O.; Oktay, K. A novel ovarian xenografting model to characterize the impact of chemotherapy agents on human primordial follicle reserve. Cancer Res. 2007, 67, 10159–10162. [Google Scholar] [CrossRef] [Green Version]
- Petrillo, S.K.; Desmeules, P.; Truong, T.Q.; Devine, P.J. Detection of DNA damage in oocytes of small ovarian follicles following phosphoramide mustard exposures of cultured rodent ovaries in vitro. Toxicol. Appl. Pharmacol. 2011, 253, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Keating, A.F. The ovarian DNA damage repair response is induced prior to phosphoramide mustard-induced follicle depletion, and ataxia telangiectasia mutated inhibition prevents PM-induced follicle depletion. Toxicol. Appl. Pharmacol. 2016, 292, 65–74. [Google Scholar] [CrossRef]
- Nguyen, Q.N.; Zerafa, N.; Liew, S.H.; Findlay, J.K.; Hickey, M.; Hutt, K.J. Cisplatin- and cyclophosphamide-induced primordial follicle depletion is caused by direct damage to oocytes. Mol. Hum. Reprod. 2019, 25, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, R.; Heytens, E.; Darzynkiewicz, Z.; Oktay, K. Mechanisms of chemotherapy-induced human ovarian aging: Double strand DNA breaks and microvascular compromise. Aging 2011, 3, 782–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roness, H.; Gavish, Z.; Cohen, Y.; Meirow, D. Ovarian follicle burnout: A universal phenomenon? Cell Cycle 2013, 12, 3245–3246. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.Y.; Xia, H.X.; Guan, H.Y.; Li, B.; Zhang, W. Follicle Loss and Apoptosis in Cyclophosphamide-Treated Mice: What’s the Matter? Int. J. Mol. Sci. 2016, 17, 836. [Google Scholar] [CrossRef] [Green Version]
- Sonigo, C.; Beau, I.; Grynberg, M.; Binart, N. AMH prevents primordial ovarian follicle loss and fertility alteration in cyclophosphamide-treated mice. FASEB J. 2019, 33, 1278–1287. [Google Scholar] [CrossRef]
- Sobinoff, A.P.; Nixon, B.; Roman, S.D.; McLaughlin, E.A. Staying alive: PI3K pathway promotes primordial follicle activation and survival in response to 3MC-induced ovotoxicity. Toxicol. Sci. 2012, 128, 258–271. [Google Scholar] [CrossRef] [Green Version]
- Chang, E.M.; Lim, E.; Yoon, S.; Jeong, K.; Bae, S.; Lee, D.R.; Yoon, T.K.; Choi, Y.; Lee, W.S. Cisplatin Induces Overactivation of the Dormant Primordial Follicle through PTEN/AKT/FOXO3a Pathway which Leads to Loss of Ovarian Reserve in Mice. PLoS ONE 2015, 10, e0144245. [Google Scholar] [CrossRef]
- Jang, H.; Na, Y.; Hong, K.; Lee, S.; Moon, S.; Cho, M.; Park, M.; Lee, O.H.; Chang, E.M.; Lee, D.R. Synergistic effect of melatonin and ghrelin in preventing cisplatin-induced ovarian damage via regulation of FOXO3a phosphorylation and binding to the p27(Kip1) promoter in primordial follicles. J. Pineal. Res. 2017, 63. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Dolmans, M.M.; Donnez, O.; Masuzaki, H.; Soares, M.; Donnez, J. Enhanced follicular recruitment and atresia in cortex derived from ovaries with endometriomas. Fertil. Steril. 2014, 101, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Marcello, M.F.; Nuciforo, G.; Romeo, R.; di Dino, G.; Russo, I.; Russo, A.; Palumbo, G.; Schilirò, G. Structural and ultrastructural study of the ovary in childhood leukemia after successful treatment. Cancer 1990, 66, 2099–2104. [Google Scholar] [CrossRef]
- Doll, D.C.; Ringenberg, Q.S.; Yarbro, J.W. Vascular toxicity associated with antineoplastic agents. J. Clin. Oncol. 1986, 4, 1405–1417. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, S.V.; Matus-Ridley, M.; Meadows, A.T. Gonadal effects of cancer therapy in girls. Cancer 1985, 55, 2364–2372. [Google Scholar] [CrossRef]
- Aharon, I.B.; Meizner, I.; Granot, T.; Uri, S.; Hasky, N.; Rizel, S.; Yerushalmi, R.; Sulkes, A.; Stemmer, S.M. Chemotherapy-induced ovarian failure as a prototype for acute vascular toxicity. Oncologist 2012, 17, 1386–1393. [Google Scholar] [CrossRef] [Green Version]
- Meirow, D.; Dor, J.; Kaufman, B.; Shrim, A.; Rabinovici, J.; Schiff, E.; Raanani, H.; Levron, J.; Fridman, E. Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum. Reprod. 2007, 22, 1626–1633. [Google Scholar] [CrossRef] [Green Version]
- Oktay, K.; Oktem, O.; Reh, A.; Vahdat, L. Measuring the impact of chemotherapy on fertility in women with breast cancer. J. Clin. Oncol. 2006, 24, 4044–4046. [Google Scholar] [CrossRef]
- Soleimani, R.; Heytens, E.; Oktay, K. Enhancement of neoangiogenesis and follicle survival by sphingosine-1-phosphate in human ovarian tissue xenotransplants. PLoS ONE 2011, 6, e19475. [Google Scholar] [CrossRef] [Green Version]
- Howell, S.; Shalet, S. Gonadal damage from chemotherapy and radiotherapy. Endocrinol. Metab. Clin. N. Am. 1998, 27, 927–943. [Google Scholar] [CrossRef]
- Madsen, B.L.; Giudice, L.; Donaldson, S.S. Radiation-induced premature menopause: A misconception. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 1461–1464. [Google Scholar] [CrossRef]
- Beerendonk, C.C.M.; Braat, D.D.M. Present and future options for the preservation of fertility in female adolescents with cancer. Endocr. Dev. 2005, 8, 166–175. [Google Scholar] [PubMed]
- Stroud, J.S.; Mutch, D.; Rader, J.; Powell, M.; Thaker, P.H.; Grigsby, P.W. Effects of cancer treatment on ovarian function. Fertil. Steril. 2009, 92, 417–427. [Google Scholar] [CrossRef]
- Wallace, W.H.; Thomson, A.B.; Kelsey, T.W. The radiosensitivity of the human oocyte. Hum. Reprod. 2003, 18, 117–121. [Google Scholar] [CrossRef] [Green Version]
- Lushbaugh, C.C.; Casarett, G.W. The effects of gonadal irradiation in clinical radiation therapy: A review. Cancer 1976, 37 (Suppl. 2), 1111–1125. [Google Scholar] [CrossRef]
- Wo, J.Y.; Viswanathan, A.N. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1304–1312. [Google Scholar] [CrossRef] [Green Version]
- Hall, E.J. Cancer caused by x-rays—A random event? Lancet Oncol. 2007, 8, 369–370. [Google Scholar] [CrossRef]
- Maier, P.; Hartmann, L.; Wenz, F.; Herskind, C. Cellular Pathways in Response to Ionizing Radiation and Their Targetability for Tumor Radiosensitization. Int. J. Mol. Sci. 2016, 17, 102. [Google Scholar] [CrossRef] [Green Version]
- Alhumaidha, K.A.; Saleh, D.O.; el Fattah, M.A.A.; el Eraky, W.I.; Moawad, H. Cardiorenal protective effect of taurine against cyclophosphamide-induced toxicity in albino rats. Can. J. Physiol. Pharmacol. 2016, 94, 131–139. [Google Scholar] [CrossRef]
- Kumar, M.; Pathak, D.; Venkatesh, S.; Kriplani, A.; Ammini, A.C.; Dada, R. Chromosomal abnormalities & oxidative stress in women with premature ovarian failure (POF). Indian J. Med. Res. 2012, 135, 92–97. [Google Scholar] [PubMed]
- Devine, P.J.; Perreault, S.D.; Luderer, U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol. Reprod. 2012, 86, 27. [Google Scholar] [CrossRef]
- Pala, Ş.; Atilgan, R.; Kuloğlu, T.; Kara, M.; Başpinar, M.; Can, B.; Artaş, G. Protective effects of vitamin C and vitamin E against hysterosalpingography-induced epithelial degeneration and proliferation in rat endometrium. Drug Des. Devel. Ther. 2016, 10, 4079–4089. [Google Scholar] [CrossRef] [Green Version]
- Nambiar, D.; Rajamani, P.; Singh, R.P. Effects of phytochemicals on ionization radiation-mediated carcinogenesis and cancer therapy. Mutat. Res. 2011, 728, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M.; Hong, Y.; Lee, S.; Liu, P.; Lim, J.H.; Lee, Y.H.; Lee, T.H.; Chang, K.T.; Hong, Y. Therapeutic Implications for Overcoming Radiation Resistance in Cancer Therapy. Int. J. Mol. Sci. 2015, 16, 26880–26913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantawy, E.M.; Said, R.S.; Abdel-Aziz, A.K. Mechanistic approach of the inhibitory effect of chrysin on inflammatory and apoptotic events implicated in radiation-induced premature ovarian failure: Emphasis on TGF-beta/MAPKs signaling pathway. Biomed. Pharmacother. 2019, 109, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Jaroudi, S.; Kakourou, G.; Cawood, S.; Doshi, A.; Ranieri, D.M.; Serhal, P.; Harper, J.C.; SenGupta, S.B. Expression profiling of DNA repair genes in human oocytes and blastocysts using microarrays. Hum. Reprod. 2009, 24, 2649–2655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, F.E.; Kimler, B.F.; Briley, S.M. Combating radiation therapy-induced damage to the ovarian environment. Future Oncol. 2016, 12, 1687–1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrek, J.A.; Naughton, M.J.; Case, L.D.; Paskett, E.D.; Naftalis, E.Z.; Singletary, S.E.; Sukumvanich, P. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: A prospective study. J. Clin. Oncol. 2006, 24, 1045–1051. [Google Scholar] [CrossRef]
- Anderson, R.A.; Wallace, W.H. Antimullerian hormone, the assessment of the ovarian reserve, and the reproductive outcome of the young patient with cancer. Fertil. Steril. 2013, 99, 1469–1475. [Google Scholar] [CrossRef]
- Gracia, C.R.; Sammel, M.D.; Freeman, E.; Prewitt, M.; Carlson, C.; Ray, A.; Vance, A.; Ginsberg, J.P. Impact of cancer therapies on ovarian reserve. Fertil. Steril. 2012, 97, 134–140.e1. [Google Scholar] [CrossRef] [Green Version]
- Kevenaar, M.E.; Meerasahib, M.F.; Kramer, P.; Born, B.M.N.V.; de Jong, F.H.; Groome, N.P.; Themmen, A.P.N.; Visser, J.A. Serum anti-mullerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology 2006, 147, 3228–3234. [Google Scholar] [CrossRef] [Green Version]
- Peñarrubia, J.; Fábregues, F.; Manau, D.; Creus, M.; Casals, G.; Casamitjana, R.; Carmona, F.; Vanrell, J.A.; Balasch, J. Basal and stimulation day 5 anti-Mullerian hormone serum concentrations as predictors of ovarian response and pregnancy in assisted reproductive technology cycles stimulated with gonadotropin-releasing hormone agonist—Gonadotropin treatment. Hum. Reprod. 2005, 20, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Peigne, M.; Decanter, C. Serum AMH level as a marker of acute and long-term effects of chemotherapy on the ovarian follicular content: A systematic review. Reprod. Biol. Endocrinol. 2014, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- D’Avila, Â.M.; Biolchi, V.; Capp, E.; Corleta, H.V. Age, anti-mullerian hormone, antral follicles count to predict amenorrhea or oligomenorrhea after chemotherapy with cyclophosphamide. J. Ovarian. Res. 2015, 8, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anders, C.; Marcom, P.K.; Peterson, B.; Gu, L.; Unruhe, S.; Welch, R.; Lyons, P.; Behera, M.; Copland, S.; Kimmick, G.; et al. A pilot study of predictive markers of chemotherapy-related amenorrhea among premenopausal women with early stage breast cancer. Cancer Investig. 2008, 26, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Cameron, D.A. Pretreatment serum anti-mullerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J. Clin. Endocrinol. Metab. 2011, 96, 1336–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toner, J.P.; Seifer, D.B. Why we may abandon basal follicle-stimulating hormone testing: A sea change in determining ovarian reserve using antimullerian hormone. Fertil. Steril. 2013, 99, 1825–1830. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.M. Biomarkers of ovarian response: Current and future applications. Fertil. Steril. 2013, 99, 963–969. [Google Scholar] [CrossRef]
- Jung, M.; Shin, H.J.; Rha, S.Y.; Jeung, H.C.; Hong, S.; Moon, Y.W.; Kim, H.S.; Oh, K.J.; Yang, W.I.; Roh, J.K.; et al. The clinical outcome of chemotherapy-induced amenorrhea in premenopausal young patients with breast cancer with long-term follow-up. Ann. Surg. Oncol. 2010, 17, 3259–3268. [Google Scholar] [CrossRef]
- Broekmans, F.J.; Kwee, J.; Hendriks, D.J.; Mol, B.W.; Lambalk, C.B. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum. Reprod. Update 2006, 12, 685–718. [Google Scholar] [CrossRef] [Green Version]
- Knauff, E.A.H.; Eijkemans, M.J.C.; Lambalk, C.B.; Booij, M.J.t.K.; Hoek, A.; Beerendonk, C.C.M.; Laven, J.S.E.; Goverde, A.J.; Broekmans, F.J.M.; Themmen, A.P.N. Anti-Mullerian hormone, inhibin B, and antral follicle count in young women with ovarian failure. J. Clin. Endocrinol. Metab. 2009, 94, 786–792. [Google Scholar] [CrossRef] [Green Version]
- Muttukrishna, S.; McGarrigle, H.; Wakim, R.; Khadum, I.; Ranieri, D.M.; Serhal, P. Antral follicle count, anti-mullerian hormone and inhibin B: Predictors of ovarian response in assisted reproductive technology? BJOG 2005, 112, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Frattarelli, J.L.; Levi, A.J.; Miller, B.T.; Segars, J.H. A prospective assessment of the predictive value of basal antral follicles in in vitro fertilization cycles. Fertil. Steril. 2003, 80, 350–355. [Google Scholar] [CrossRef]
- Magon, N. Gonadotropin releasing hormone agonists: Expanding vistas. Indian J. Endocrinol. Metab. 2011, 15, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, Z.; Dann, E. GnRH agonist for the prevention of chemotherap.y-induced ovarian failure in lymphoma. J. Clin. Oncol. 2013, 31, 3721. [Google Scholar] [CrossRef]
- Blumenfeld, Z.; Avivi, I.; Linn, S.; Epelbaum, R.; Shahar, M.B.; Haim, N. Prevention of irreversible chemotherapy-induced ovarian damage in young women with lymphoma by a gonadotrophin-releasing hormone agonist in parallel to chemotherapy. Hum. Reprod. 1996, 11, 1620–1626. [Google Scholar] [CrossRef] [Green Version]
- Pacheco, B.P.; Ribas, J.M.M.; Milone, G.; Fernández, I.; Kvicala, R.; Mila, T.; di Noto, A.; Ortiz, O.C.; Pavlovsky, S. Use of GnRH analogs for functional protection of the ovary and preservation of fertility during cancer treatment in adolescents: A preliminary report. Gynecol. Oncol. 2001, 81, 391–397. [Google Scholar] [CrossRef]
- Recchia, F.; Sica, G.; de Filippis, S.; Saggio, G.; Rosselli, M.; Rea, S. Goserelin as ovarian protection in the adjuvant treatment of premenopausal breast cancer: A phase II pilot study. Anticancer Drugs 2002, 13, 417–424. [Google Scholar] [CrossRef]
- Blumenfeld, Z. How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist cotreatment in addition to cryopreservation of embrya, oocytes, or ovaries. Oncologist 2007, 12, 1044–1054. [Google Scholar] [CrossRef]
- Blumenfeld, Z.; Eckman, A. Preservation of fertility and ovarian function and minimization of chemotherapy-induced gonadotoxicity in young women by GnRH-a. J. Natl. Cancer Inst. Monogr. 2005, 2005, 40–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambertini, M.; Horicks, F.; del Mastro, L.; Partridge, A.H.; Demeestere, I. Ovarian protection with gonadotropin-releasing hormone agonists during chemotherapy in cancer patients: From biological evidence to clinical application. Cancer Treat. Rev. 2019, 72, 65–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumenfeld, Z. Fertility preservation and GnRHa for chemotherapy: Debate. Cancer Manag. Res. 2014, 6, 313–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oktay, K.; Sönmezer, M.; Oktem, O.; Fox, K.; Emons, G.; Bang, H. Absence of conclusive evidence for the safety and efficacy of gonadotropin-releasing hormone analogue treatment in protecting against chemotherapy-induced gonadal injury. Oncologist 2007, 12, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Bedaiwy, M.A.; Abou-Setta, A.M.; Desai, N.; Hurd, W.; Starks, D.; El-Nashar, S.A.; al Inany, H.G.; Falcone, T. Gonadotropin-releasing hormone analog cotreatment for preservation of ovarian function during gonadotoxic chemotherapy: A systematic review and meta-analysis. Fertil. Steril. 2011, 95, 906–914. [Google Scholar] [CrossRef]
- Chen, H.; Xiao, L.; Li, J.; Cui, L.; Huang, W. Adjuvant gonadotropin-releasing hormone analogues for the prevention of chemotherapy-induced premature ovarian failure in premenopausal women. Cochrane Database Syst. Rev. 2019, 3, CD008018. [Google Scholar] [CrossRef]
- Clowse, M.E.B.; Behera, M.A.; Anders, C.K.; Copland, S.; Coffman, C.J.; Leppert, P.C.; Bastian, L.A. Ovarian preservation by GnRH agonists during chemotherapy: A meta-analysis. J. Womens Health 2009, 18, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Del Mastro, L.; Boni, L.; Michelotti, A.; Gamucci, T.; Olmeo, N.; Gori, S.; Giordano, M.; Garrone, O.; Pronzato, P.; Bighin, C.; et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: A randomized trial. JAMA 2011, 306, 269–276. [Google Scholar] [CrossRef] [Green Version]
- De Pedro, M.; Otero, B.; Martin, B. Fertility preservation and breast cancer: A review. Ecancermedicalscience 2015, 9, 503. [Google Scholar] [CrossRef] [Green Version]
- Peccatori, F.A.; Azim, H.A., Jr.; Orecchia, R.; Hoekstra, H.J.; Pavlidis, N.; Kesic, V.; Pentheroudakis, G.; ESMO Guidelines Working Group. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24 (Suppl. 6), vi160–vi170. [Google Scholar] [CrossRef]
- Woodruff, T.K. The Oncofertility Consortium—Addressing fertility in young people with cancer. Nat. Rev. Clin. Oncol. 2010, 7, 466–475. [Google Scholar] [CrossRef]
- Makarovsky, D.; Kalechman, Y.; Sonino, T.; Freidkin, I.; Teitz, S.; Albeck, M.; Weil, M.; Geffen-Aricha, R.; Yadid, G.; Sredni, B. Tellurium compound AS101 induces PC12 differentiation and rescues the neurons from apoptotic death. Ann. N. Y. Acad. Sci. 2003, 1010, 659–666. [Google Scholar] [CrossRef]
- Hayun, M.; Naor, Y.; Weil, M.; Albeck, M.; Peled, A.; Don, J.; Haran-Ghera, N.; Sredni, B. The immunomodulator AS101 induces growth arrest and apoptosis in multiple myeloma: Association with the Akt/survivin pathway. Biochem. Pharmacol. 2006, 72, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Philosoph, L.K.; Roness, H.; Carmely, A.; Fishel-Bartal, M.; Ligumsky, H.; Paglin, S.; Wolf, I.; Kanety, H.; Sredni, B.; Meirow, D. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci. Transl. Med. 2013, 5, 185ra62. [Google Scholar] [CrossRef] [PubMed]
- Kalechman, Y.; Shani, A.; Barkai, I.S.; Albeck, M.; Sredni, B. The protective role of ammonium trichloro(dioxoethylene-O,O’)tellurate in combination with several cytotoxic drugs acting by different mechanisms of action. Cancer Res. 1993, 53, 5962–5969. [Google Scholar] [PubMed]
- Kalechman, Y.; Barkai, I.S.; Albeck, M.; Sredni, B. Protection of bone marrow stromal cells from the toxic effects of cyclophosphamide in vivo and of ASTA-Z 7557 and etoposide in vitro by ammonium trichloro(dioxyethylene-O-O’)tellurate (AS101). Cancer Res. 1993, 53, 1838–1844. [Google Scholar]
- Sredni, B.; Albeck, M.; Kazimirsky, G.; Shalit, F. The immunomodulator AS101 administered orally as a chemoprotective and radioprotective agent. Int. J. Immunopharmacol. 1992, 14, 613–619. [Google Scholar] [CrossRef]
- Kalechman, Y.; Albeck, M.; Oron, M.; Sobelman, D.; Gurwith, M.; Horwith, G.; Kirsch, T.; Maida, B.; Sehgal, S.N.; Sredni, B. Protective and restorative role of AS101 in combination with chemotherapy. Cancer Res. 1991, 51, 1499–1503. [Google Scholar] [PubMed]
- Carlsson, I.B.; Scott, J.E.; Visser, J.A.; Ritvos, O.; Themmen, A.P.N.; Hovatta, O. Anti-Mullerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum. Reprod. 2006, 21, 2223–2237. [Google Scholar] [CrossRef] [Green Version]
- Durlinger, A.L.L.; Gruijters, M.J.G.; Kramer, P.; Karels, B.; Ingraham, H.A.; Nachtigal, M.W.; Uilenbroek, J.J.; Grootegoed, J.A.; Themmen, A.P.N. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology 2002, 143, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Durlinger, A.L.; Kramer, P.; Karels, B.; de Jong, F.H.; Uilenbroek, J.T.; Grootegoed, J.A.; Themmen, A.P. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology 1999, 140, 5789–5796. [Google Scholar] [CrossRef]
- Druker, B.J.; Tamura, S.; Buchdunger, E.; Ohno, S.; Segal, G.M.; Fanning, S.; Zimmermann, J.; Lydon, N.B. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat. Med. 1996, 2, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; von Mehren, M.; Blanke, C.D.; van den Abbeele, A.D.; Eisenberg, B.; Roberts, P.J.; Heinrich, M.C.; Tuveson, D.A.; Singer, S.; Janicek, M.; et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 2002, 347, 472–480. [Google Scholar] [CrossRef] [Green Version]
- Jaeckle, K.A.; Anderson, S.K.; Twohy, E.L.; Dixon, J.G.; Giannini, C.; Jenkins, R.; Egorin, M.J.; Sarkaria, J.N.; Brown, P.D.; Flynn, P.J.; et al. Phase I-II trial of imatinib mesylate (Gleevec; STI571) in treatment of recurrent oligodendroglioma and mixed oligoastrocytoma. North central cancer treatment group study N0272 (ALLIANCE/NCCTG). J. Neurooncol. 2019, 143, 573–581. [Google Scholar] [CrossRef]
- Gonfloni, S.; di Tella, L.; Caldarola, S.; Cannata, S.M.; Klinger, F.G.; di Bartolomeo, C.; Mattei, M.; Candi, E.; de Felici, M.; Melino, G.; et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat. Med. 2009, 15, 1179–1185. [Google Scholar] [CrossRef]
- Kharbanda, S.; Pandey, P.; Morris, P.L.; Whang, Y.; Xu, Y.; Sawant, S.; Zhu, L.J.; Kumar, N.; Yuan, Z.M.; Weichselbaum, R.; et al. Functional role for the c-Abl tyrosine kinase in meiosis I. Oncogene 1998, 16, 1773–1777. [Google Scholar] [CrossRef]
- Kharbanda, S.; Yuan, Z.M.; Weichselbaum, R.; Kufe, D. Determination of cell fate by c-Abl activation in the response to DNA damage. Oncogene 1998, 17, 3309–3318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerr, J.B.; Hutt, K.J.; Cook, M.; Speed, T.P.; Strasser, A.; Findlay, J.K.; Scott, C.L. Cisplatin-induced primordial follicle oocyte killing and loss of fertility are not prevented by imatinib. Nat. Med. 2012, 18, 1170–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-Y.; Cordeiro, M.H.; Serna, V.A.; Ebbert, K.; Butler, L.M.; Sinha, S.; Mills, A.A.; Woodruff, T.K.; Kurita, T. Rescue of platinum-damaged oocytes from programmed cell death through inactivation of the p53 family signaling network. Cell Death Differ. 2013, 20, 987–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, S.; Lopes, F.; Gourley, C.; Anderson, R.A.; Spears, N. Cisplatin and doxorubicin induce distinct mechanisms of ovarian follicle loss; imatinib provides selective protection only against cisplatin. PLoS ONE 2013, 8, e70117. [Google Scholar] [CrossRef] [Green Version]
- Morita, Y.; Perez, G.I.; Paris, F.; Miranda, S.R.; Ehleiter, D.; Haimovitz-Friedman, A.; Fuks, Z.; Xie, Z.; Reed, J.C.; Schuchman, E.H.; et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat. Med. 2000, 6, 1109–1114. [Google Scholar] [CrossRef]
- Li, F.; Turan, V.; Lierman, S.; Cuvelier, C.; de Sutter, P.; Affiliations, K.O. Sphingosine-1-phosphate prevents chemotherapy-induced human primordial follicle death. Hum. Reprod. 2014, 29, 107–113. [Google Scholar] [CrossRef]
- Hancke, K.; Strauch, O.; Kissel, C.; Göbel, H.; Schäfer, W.; Denschlag, D. Sphingosine 1-phosphate protects ovaries from chemotherapy-induced damage in vivo. Fertil. Steril. 2007, 87, 172–177. [Google Scholar] [CrossRef]
- Kaya, H.; Desdicioglu, R.; Sezik, M.; Ulukaya, E.; Ozkaya, O.; Yilmaztepe, A.; Demirci, M. Does sphingosine-1-phosphate have a protective effect on cyclophosphamide- and irradiation-induced ovarian damage in the rat model? Fertil. Steril. 2008, 89, 732–735. [Google Scholar] [CrossRef]
- Wikiel, M.E.S.; McGuire, M.M.; Sukhwani, M.; Donohue, J.; Chu, T.; Krivak, T.C.; Rajkovic, A.; Orwig, K.E. Granulocyte colony-stimulating factor with or without stem cell factor extends time to premature ovarian insufficiency in female mice treated with alkylating chemotherapy. Fertil. Steril. 2013, 99, 2045–2054.e3. [Google Scholar] [CrossRef] [Green Version]
- Solaroglu, I.; Cahill, J.; Jadhav, V.; Zhang, J.H. A novel neuroprotectant granulocyte-colony stimulating factor. Stroke 2006, 37, 1123–1128. [Google Scholar] [CrossRef]
- Rigsby, C.K.; Siegel, M.J. CT appearance of pediatric ovaries and uterus. J. Comput. Assist. Tomogr. 1994, 18, 72–76. [Google Scholar] [CrossRef]
- Nicholson, R.; Coucher, J.; Thornton, A.; Connor, F. Effect of a full and empty bladder on radiation dose to the uterus, ovaries and bladder from lumbar spine CT and X-ray examinations. Br. J. Radiol. 2000, 73, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, S.; Hong, J.H.; Park, Y.J.; Song, J.Y.; Lee, J.K.; Lee, N.W. Comparing efficacy of high-dose rate brachytherapy versus helical tomotherapy in the treatment of cervical cancer. J. Gynecol. Oncol. 2020, 31, e42. [Google Scholar] [CrossRef] [PubMed]
- Hamre, M.R.; Robison, L.L.; Nesbit, M.E.; Sather, H.N.; Meadows, A.T.; Ortega, J.A.; D’Angio, G.J.; Hammond, G.D. Effects of radiation on ovarian function in long-term survivors of childhood acute lymphoblastic leukemia: A report from the Childrens Cancer Study Group. J. Clin. Oncol. 1987, 5, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Livesey, E.A.; Brook, C.G. Gonadal dysfunction after treatment of intracranial tumours. Arch. Dis. Child. 1988, 63, 495–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harden, S.V.; Twyman, N.; Lomas, D.J.; Williams, D.; Burnet, N.G.; Williams, M.V. A method for reducing ovarian doses in whole neuro-axis irradiation for medulloblastoma. Radiother. Oncol. 2003, 69, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Bilton, S.D.; Famiglietti, R.M.; Riley, B.A.; Mahajan, A.; Chang, E.L.; Maor, M.H.; Woo, S.Y.; Cox, J.D.; Smith, A.R. Treatment planning with protons for pediatric retinoblastoma, medulloblastoma, and pelvic sarcoma: How do protons compare with other conformal techniques? Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Miralbell, R.; Lomax, A.; Bortfeld, T.; Rouzaud, M.; Carrie, C. Potential role of proton therapy in the treatment of pediatric medulloblastoma/primitive neuroectodermal tumors: Reduction of the supratentorial target volume. Int. J. Radiat. Oncol. Biol. Phys. 1997, 38, 477–484. [Google Scholar] [CrossRef]
- Yuh, G.E.; Loredo, L.N.; Yonemoto, L.T.; Bush, D.A.; Shahnazi, K.; Preston, W.; Slater, J.M.; Slater, J.D. Reducing toxicity from craniospinal irradiation: Using proton beams to treat medulloblastoma in young children. Cancer J. 2004, 10, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Hadar, H.; Loven, D.; Herskovitz, P.; Bairey, O.; Yagoda, A.; Levavi, H. An evaluation of lateral and medial transposition of the ovaries out of radiation fields. Cancer 1994, 74, 774–779. [Google Scholar] [CrossRef]
- Le Floch, O.; Donaldson, S.S.; Kaplan, H.S. Pregnancy following oophoropexy and total nodal irradiation in women with Hodgkin’s disease. Cancer 1976, 38, 2263–2268. [Google Scholar] [CrossRef]
- Thibaud, E.; Ramirez, M.; Brauner, R.; Flamant, F.; Zucker, J.M.; Fékété, C.; Rappaport, R. Preservation of ovarian function by ovarian transposition performed before pelvic irradiation during childhood. J. Pediatr. 1992, 121, 880–884. [Google Scholar] [CrossRef]
- Loren, A.W.; Mangu, P.B.; Beck, L.N.; Brennan, L.; Magdalinski, A.J.; Partridge, A.H.; Quinn, G.; Wallace, W.H.; Oktay, K.; American Society of Clinical Oncology. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 2013, 31, 2500–2510. [Google Scholar] [CrossRef]
- Morice, P.; Ba, R.T.; Castaigne, D.; Haie-Meder, C.; Gerbaulet, A.; Pautier, P.; Duvillard, P.; Michel, G. Fertility results after ovarian transposition for pelvic malignancies treated by external irradiation or brachytherapy. Hum. Reprod. 1998, 13, 660–663. [Google Scholar] [CrossRef] [Green Version]
- Al-Asari, S.; Abduljabbar, A. Laparoscopic ovarian transposition before pelvic radiation in rectal cancer patient: Safety and feasibility. Ann. Surg. Innov. Res. 2012, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Dursun, P.; Ayhan, A.; Yanik, F.B.; Kuşçu, E. Ovarian transposition for the preservation of ovarian function in young patients with cervical carcinoma. Eur. J. Gynaecol. Oncol. 2009, 30, 13–15. [Google Scholar]
- Morice, P.; Juncker, L.; Rey, A.; El-Hassan, J.; Haie-Meder, C.; Castaigne, D. Ovarian transposition for patients with cervical carcinoma treated by radiosurgical combination. Fertil. Steril. 2000, 74, 743–748. [Google Scholar] [CrossRef]
- Han, S.S.; Kim, Y.H.; Lee, S.H.; Kim, G.J.; Kim, H.J.; Kim, J.W.; Park, N.H.; Song, Y.S.; Kang, S.B. Underuse of ovarian transposition in reproductive-aged cancer patients treated by primary or adjuvant pelvic irradiation. J. Obstet. Gynaecol. Res. 2011, 37, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.M.; Schlaff, W. Laparoscopic medial oophoropexy prior to radiation therapy in an adolescent with Hodgkin’s disease. J. Pediatr. Adolesc. Gynecol. 2005, 18, 355–357. [Google Scholar] [CrossRef]
- Williams, R.S.; Littell, R.D.; Mendenhall, N.P. Laparoscopic oophoropexy and ovarian function in the treatment of Hodgkin disease. Cancer 1999, 86, 2138–2142. [Google Scholar] [CrossRef]
- Bisharah, M.; Tulandi, T. Laparoscopic preservation of ovarian function: An underused procedure. Am. J. Obstet. Gynecol. 2003, 188, 367–370. [Google Scholar] [CrossRef]
- Wallace, W.H.; Anderson, R.A.; Irvine, D.S. Fertility preservation for young patients with cancer: Who is at risk and what can be offered? Lancet Oncol. 2005, 6, 209–218. [Google Scholar] [CrossRef]
- Gook, D.A.; Edgar, D.H. Human oocyte cryopreservation. Hum. Reprod. Update 2007, 13, 591–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Vos, M.; Smitz, J.; Woodruff, T.K. Fertility preservation in women with cancer. Clin. Exp. Reprod. Med. 2012, 39, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, H.; Rosen, M.P. Ovarian stimulation in cancer patients. Fertil. Steril. 2013, 99, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Devroey, P.; Polyzos, N.P.; Blockeel, C. An OHSS-Free Clinic by segmentation of IVF treatment. Hum. Reprod. 2011, 26, 2593–2597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roque, M.; Lattes, K.; Serra, S.; Solà, I.; Geber, S.; Carreras, R.; Checa, M.A. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: A systematic review and meta-analysis. Fertil. Steril. 2013, 99, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Rienzi, L.; Gracia, C.; Maggiulli, R.; LaBarbera, A.R.; Kaser, D.J.; Ubaldi, F.M.; Vanderpoel, S.; Racowsky, C. Oocyte, embryo and blastocyst cryopreservation in ART: Systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum. Reprod. Update 2017, 23, 139–155. [Google Scholar] [CrossRef]
- AbdelHafez, F.F.; Desai, N.; Setta, A.M.A.; Falcone, T.; Goldfarb, J. Slow freezing, vitrification and ultra-rapid freezing of human embryos: A systematic review and meta-analysis. Reprod. Biomed. Online 2010, 20, 209–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debrock, S.; Peeraer, K.; Gallardo, E.F.; de Neubourg, D.; Spiessens, C.; D’Hooghe, T.M. Vitrification of cleavage stage day 3 embryos results in higher live birth rates than conventional slow freezing: A RCT. Hum. Reprod. 2015, 30, 1820–1830. [Google Scholar] [CrossRef]
- Lee, S.; Oktay, K. Does higher starting dose of FSH stimulation with letrozole improve fertility preservation outcomes in women with breast cancer? Fertil. Steril. 2012, 98, 961–964.e1. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.; Donnez, J.; Ginsburg, E.; Meirow, D. Emergency IVF versus ovarian tissue cryopreservation: Decision making in fertility preservation for female cancer patients. Fertil. Steril. 2013, 99, 1534–1542. [Google Scholar] [CrossRef]
- Courbiere, B.; Decanter, C.; Deutsch, S.B.; Rives, N.; Mirallié, S.; Pech, J.C.; de Ziegler, D.; Pigeon, F.C.; Panloup, P.M.; Sifer, C.; et al. Emergency IVF for embryo freezing to preserve female fertility: A French multicentre cohort study. Hum. Reprod. 2013, 28, 2381–2388. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, H.; Katz, A.; Cedars, M.I.; Rosen, M.P. Effective method for emergency fertility preservation: Random-start controlled ovarian stimulation. Fertil. Steril. 2013, 100, 1673–1680. [Google Scholar] [CrossRef]
- Oktay, K.; Cil, A.P.; Bang, H. Efficiency of oocyte cryopreservation: A meta-analysis. Fertil. Steril. 2006, 86, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.M.; de Ouderaen, S.H.; Demylle, D.; Pirard, C. Utilization rates and results of long-term embryo cryopreservation before gonadotoxic treatment. J. Assist. Reprod. Genet. 2015, 32, 1233–1237. [Google Scholar] [CrossRef] [Green Version]
- Mayeur, A.; Puy, V.; Windal, V.; Hesters, L.; Gallot, V.; Benoit, A.; Grynberg, M.; Sonigo, C.; Frydman, N. Live birth rate after use of cryopreserved oocytes or embryos at the time of cancer diagnosis in female survivors: A retrospective study of ten years of experience. J. Assist. Reprod. Genet. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, Y.; Lee, S.; Kim, T. Ovarian tissue cryopreservation and transplantation in patients with cancer. Obstet. Gynecol. Sci. 2018, 61, 431–442. [Google Scholar] [CrossRef]
- Cobo, A.; Velasco, J.A.G.; Domingo, J.; Remohí, J.; Pellicer, A. Is vitrification of oocytes useful for fertility preservation for age-related fertility decline and in cancer patients? Fertil. Steril. 2013, 99, 1485–1495. [Google Scholar] [CrossRef]
- Cobo, A.; Diaz, C. Clinical application of oocyte vitrification: A systematic review and meta-analysis of randomized controlled trials. Fertil. Steril. 2011, 96, 277–285. [Google Scholar] [CrossRef]
- Parmegiani, L.; Cognigni, G.E.; Bernardi, S.; Cuomo, S.; Ciampaglia, W.; Infante, F.E.; de Fatis, C.T.; Arnone, A.; Maccarini, A.M.; Filicori, M. Efficiency of aseptic open vitrification and hermetical cryostorage of human oocytes. Reprod. Biomed. Online 2011, 23, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Cobo, A.; Kuwayama, M.; Pérez, S.; Ruiz, A.; Pellicer, A.; Remohí, J. Comparison of concomitant outcome achieved with fresh and cryopreserved donor oocytes vitrified by the Cryotop method. Fertil. Steril. 2008, 89, 1657–1664. [Google Scholar] [CrossRef]
- Cobo, A.; Meseguer, M.; Remohí, J.; Pellicer, A. Use of cryo-banked oocytes in an ovum donation programme: A prospective, randomized, controlled, clinical trial. Hum. Reprod. 2010, 25, 2239–2246. [Google Scholar] [CrossRef] [Green Version]
- Rienzi, L.; Romano, S.; Albricci, L.; Maggiulli, R.; Capalbo, A.; Baroni, E.; Colamaria, S.; Sapienza, F.; Ubaldi, F. Embryo development of fresh ‘versus’ vitrified metaphase II oocytes after ICSI: A prospective randomized sibling-oocyte study. Hum. Reprod. 2010, 25, 66–73. [Google Scholar] [CrossRef]
- Ubaldi, F.; Anniballo, R.; Romano, S.; Baroni, E.; Albricci, L.; Colamaria, S.; Capalbo, A.; Sapienza, F.; Vajta, G.; Rienzi, L. Cumulative ongoing pregnancy rate achieved with oocyte vitrification and cleavage stage transfer without embryo selection in a standard infertility program. Hum. Reprod. 2010, 25, 1199–1205. [Google Scholar] [CrossRef]
- National Collaborating Centre for Women’s and Children’s Health. National Collaborating Centre for Women’s and Children’s Health. National Institute for Health and Clinical Excellence: Guidance. In Fertility: Assessment and Treatment for People with Fertility Problems; Royal College of Obstetricians & Gynaecologists: London, UK, 2013. [Google Scholar]
- Practice Committees of the American Society for Reproductive Medicine; The Society for Assisted Reproductive Technology. Intracytoplasmic sperm injection (ICSI) for non-male factor indications: A committee opinion. Fertil. Steril. 2020, 114, 239–245. [Google Scholar] [CrossRef]
- Dittrich, R.; Lotz, L.; Mueller, A.; Hoffmann, I.; Wachter, D.L.; Amann, K.U.; Beckmann, M.W.; Hildebrandt, T. Oncofertility: Combination of ovarian stimulation with subsequent ovarian tissue extraction on the day of oocyte retrieval. Reprod. Biol. Endocrinol. 2013, 11, 19. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, M.; Maestre, E.N.; Teruel, J.; Ortiz, E.; Pellicer, A. The Valencia Programme for Fertility Preservation. Clin. Transl. Oncol. 2008, 10, 433–438. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M.; Pellicer, A.; Garcia, C.D.; Serrano, M.S.; Schmidt, K.T.; Ernst, E.; Luyckx, V.; Andersen, C.Y. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: A review of 60 cases of reimplantation. Fertil. Steril. 2013, 99, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N. Ovarian tissue cryopreservation in young cancer patients for fertility preservation. Reprod. Med. Biol. 2015, 14, 1–4. [Google Scholar] [CrossRef]
- Corkum, K.S.; Laronda, M.M.; Rowell, E.E. A review of reported surgical techniques in fertility preservation for prepubertal and adolescent females facing a fertility threatening diagnosis or treatment. Am. J. Surg. 2017, 214, 695–700. [Google Scholar] [CrossRef]
- Lee, S.; Ryu, K.J.; Kim, B.; Kang, D.; Kim, Y.Y.; Kim, T. Comparison between Slow Freezing and Vitrification for Human Ovarian Tissue Cryopreservation and Xenotransplantation. Int. J. Mol. Sci. 2019, 20, 3346. [Google Scholar] [CrossRef] [Green Version]
- Isachenko, V.; Isachenko, E.; Weiss, J.M. Human ovarian tissue: Vitrification versus conventional freezing. Hum. Reprod. 2009, 24, 1767–1768. [Google Scholar] [CrossRef] [Green Version]
- Keros, V.; Xella, S.; Hultenby, K.; Pettersson, K.; Sheikhi, M.; Volpe, A.; Hreinsson, J.; Hovatta, O. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum. Reprod. 2009, 24, 1670–1683. [Google Scholar] [CrossRef] [Green Version]
- Klocke, S.; Bündgen, N.; Köster, F.; Eichenlaub-Ritter, U.; Griesinger, G. Slow-freezing versus vitrification for human ovarian tissue cryopreservation. Arch. Gynecol. Obstet. 2015, 291, 419–426. [Google Scholar] [CrossRef]
- Dolmans, M.M.; Luyckx, V.; Donnez, J.; Andersen, C.Y.; Greve, T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil. Steril. 2013, 99, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Loren, A.W.; Senapati, S. Fertility preservation in patients with hematologic malignancies and recipients of hematopoietic cell transplants. Blood 2019, 134, 746–760. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, M.; Andersen, M.T.; Ralfkiær, E.; Kjeldsen, L.; Andersen, M.K.; Andersen, C.Y. Evidence of residual disease in cryopreserved ovarian cortex from female patients with leukemia. Fertil. Steril. 2010, 94, 2186–2190. [Google Scholar] [CrossRef]
- Kim, B.; Ryu, K.J.; Lee, S.; Kim, T. Changes in telomere length and senescence markers during human ovarian tissue cryopreservation. Sci. Rep. 2021, 11, 2238. [Google Scholar] [CrossRef] [PubMed]
- Bedaiwy, M.A.; Falcone, T. Ovarian tissue banking for cancer patients: Reduction of post-transplantation ischaemic injury: Intact ovary freezing and transplantation. Hum. Reprod. 2004, 19, 1242–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madrid, B.M.; Dolmans, M.M.; van Langendonckt, A.; Defrère, S.; Donnez, J. Freeze-thawing intact human ovary with its vascular pedicle with a passive cooling device. Fertil. Steril. 2004, 82, 1390–1394. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wang, X.; Kim, S.S.; Chen, H.; Tan, S.L.; Gosden, R.G. Transplantation of intact rat gonads using vascular anastomosis: Effects of cryopreservation, ischaemia and genotype. Hum. Reprod. 2003, 18, 1165–1172. [Google Scholar] [CrossRef]
- Zhang, J.M.; Sheng, Y.; Cao, Y.Z.; Wang, H.Y.; Chen, Z.J. Cryopreservation of whole ovaries with vascular pedicles: Vitrification or conventional freezing? J. Assist. Reprod. Genet. 2011, 28, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Yao, H.; Liu, Y.; Ren, L.; Xiang, D.; Wang, Y. Hypothermic machine perfusion after static cold storage improves ovarian function in rat ovarian tissue transplantation. J. Assist. Reprod. Genet. 2020, 37, 1745–1753. [Google Scholar] [CrossRef]
- Hossay, C.; Donnez, J.; Dolmans, M.M. Whole Ovary Cryopreservation and Transplantation: A Systematic Review of Challenges and Research Developments in Animal Experiments and Humans. J. Clin. Med. 2020, 9, 3196. [Google Scholar] [CrossRef]
- Soleimani, R.; Heytens, E.; van den Broecke, R.; Rottiers, I.; Dhont, M.; Cuvelier, C.A.; de Sutter, P. Xenotransplantation of cryopreserved human ovarian tissue into murine back muscle. Hum. Reprod. 2010, 25, 1458–1470. [Google Scholar] [CrossRef] [Green Version]
- Gook, D.A.; Edgar, D.H.; Borg, J.; Archer, J.; Lutjen, P.J.; McBain, J.C. Oocyte maturation, follicle rupture and luteinization in human cryopreserved ovarian tissue following xenografting. Hum. Reprod. 2003, 18, 1772–1781. [Google Scholar] [CrossRef] [Green Version]
- Gook, D.A.; Edgar, D.H.; Borg, J.; Archer, J.; McBain, J.C. Diagnostic assessment of the developmental potential of human cryopreserved ovarian tissue from multiple patients using xenografting. Hum. Reprod. 2005, 20, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Lotz, L.; Schneider, H.; Hackl, J.; Wachter, D.; Hoffmann, I.; Jurgons, R.; Beckmann, M.W.; Dittrich, R. Does stimulation with human gonadotropins and gonadotropin-releasing hormone agonist enhance and accelerate the developmental capacity of oocytes in human ovarian tissue xenografted into severe combined immunodeficient mice? Fertil. Steril. 2014, 101, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Kang, H.G.; Kim, N.H.; Lee, H.C.; Lee, H.H. Assessment of the integrity of human oocytes retrieved from cryopreserved ovarian tissue after xenotransplantation. Hum. Reprod. 2005, 20, 2502–2508. [Google Scholar]
- Ayuandari, S.; Crepaz, K.W.; Paulitsch, M.; Wagner, C.; Zavadil, C.; Manzl, C.; Ziehr, S.C.; Wildt, L.; Tollinger, S.H. Follicular growth after xenotransplantation of cryopreserved/thawed human ovarian tissue in SCID mice: Dynamics and molecular aspects. J. Assist. Reprod. Genet. 2016, 33, 1585–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terada, Y.; Sato, Y.T.; Yoshimoto, T.K.; Hasegawa, H.; Ugajin, T.; Koyanagi, Y.; Ito, M.; Murakami, T.; Sasano, H.; Yaegashi, N.; et al. Development of human Graafian follicles following transplantation of human ovarian tissue into NOD/SCID/gammac null mice. Am. J. Reprod. Immunol. 2008, 60, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.M.; Yuan, W.Y.; Camboni, A.; Torre, A.; van Langendonckt, A.; Madrid, B.M.; Donnez, J. Development of antral follicles after xenografting of isolated small human preantral follicles. Reprod. Biomed. Online 2008, 16, 705–711. [Google Scholar] [CrossRef]
- Oktay, K.; Newton, H.; Mullan, J.; Gosden, R.G. Development of human primordial follicles to antral stages in SCID/hpg mice stimulated with follicle stimulating hormone. Hum. Reprod. 1998, 13, 1133–1138. [Google Scholar] [CrossRef] [Green Version]
- David, A.; Dolmans, M.M.; van Langendonckt, A.; Donnez, J.; Amorim, C.A. Immunohistochemical localization of growth factors after cryopreservation and 3 weeks’ xenotransplantation of human ovarian tissue. Fertil. Steril. 2011, 95, 1241–1246. [Google Scholar] [CrossRef]
- Weissman, A.; Gotlieb, L.; Colgan, T.; Jurisicova, A.; Greenblatt, E.M.; Casper, R.F. Preliminary experience with subcutaneous human ovarian cortex transplantation in the NOD-SCID mouse. Biol. Reprod. 1999, 60, 1462–1467. [Google Scholar] [CrossRef] [Green Version]
- Dath, C.; van Eyck, A.S.; Dolmans, M.M.; Romeu, L.; Vigne, L.D.; Donnez, J.; van Langendonckt, A. Xenotransplantation of human ovarian tissue to nude mice: Comparison between four grafting sites. Hum. Reprod. 2010, 25, 1734–1743. [Google Scholar] [CrossRef] [Green Version]
- Paulini, F.; Vilela, J.M.V.; Chiti, M.C.; Donnez, J.; Jadoul, P.; Dolmans, M.M.; Amorim, C.A. Survival and growth of human preantral follicles after cryopreservation of ovarian tissue, follicle isolation and short-term xenografting. Reprod. Biomed. Online 2016, 33, 425–432. [Google Scholar] [CrossRef] [Green Version]
- Amorim, C.A.; David, A.; Dolmans, M.M.; Camboni, A.; Donnez, J.; van Langendonckt, A. Impact of freezing and thawing of human ovarian tissue on follicular growth after long-term xenotransplantation. J. Assist. Reprod. Genet. 2011, 28, 1157–1165. [Google Scholar] [CrossRef] [Green Version]
- Jafarabadi, M.; Abdollahi, M.; Salehnia, M. Assessment of vitrification outcome by xenotransplantation of ovarian cortex pieces in gamma-irradiated mice: Morphological and molecular analyses of apoptosis. J. Assist. Reprod. Genet. 2015, 32, 195–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, P.H.A., Jr.; Alves, T.J.M.; Dias, M.T.; Assunçao, C.M.; Munk, M.; Mattos, M.S.; Kraemer, L.R.; Almeida, B.G.; Russo, R.C.; Barcelos, L.; et al. Ovarian Grafts 10 Days after Xenotransplantation: Folliculogenesis and Recovery of Viable Oocytes. PLoS ONE 2016, 11, e0158109. [Google Scholar]
- van Eyck, A.S.; Bouzin, C.; Feron, O.; Romeu, L.; van Langendonckt, A.; Donnez, J.; Dolmans, M.M. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertil. Steril. 2010, 93, 1676–1685. [Google Scholar] [CrossRef]
- Hormozi, M.; Talebi, S.; Zarnani, A.H.; Tehrani, M.J.; Gohari, L.H.; Soltanghoraei, H.; Jafarabadi, M.; Akhondi, M.M. 5’-(N-ethylcarboxamido) adenosine improves angiogenesis in transplanted human ovarian tissue. Fertil. Steril. 2011, 95, 2560–2563.e1–5. [Google Scholar] [CrossRef]
- Fonseca, H.H.; Bosch, P.; Sirisathien, S.; Wininger, J.D.; Massey, J.B.; Brackett, B.G. Effect of site of transplantation on follicular development of human ovarian tissue transplanted into intact or castrated immunodeficient mice. Fertil. Steril. 2004, 81 (Suppl. 1), 888–892. [Google Scholar] [CrossRef]
- Maltaris, T.; Koelbl, H.; Fischl, F.; Seufert, R.; Schmidt, M.; Kohl, J.; Beckmann, M.W.; Binder, H.; Hoffmann, I.; Mueller, A.; et al. Xenotransplantation of human ovarian tissue pieces in gonadotropin-stimulated SCID mice: The effect of ovariectomy. Anticancer Res. 2006, 26, 4171–4176. [Google Scholar]
- Friedman, O.; Orvieto, R.; Fisch, B.; Felz, C.; Freud, E.; Haroush, A.B.; Abir, R. Possible improvements in human ovarian grafting by various host and graft treatments. Hum. Reprod. 2012, 27, 474–482. [Google Scholar] [CrossRef] [Green Version]
- Fu, S.; Wang, J.; Sun, W.; Xu, Y.; Zhou, X.; Cheng, W. Preclinical humanized mouse model with ectopic ovarian tissues. Exp. Ther. Med. 2014, 8, 742–746. [Google Scholar] [CrossRef]
- van Langendonckt, A.; Romeu, L.; Ambroise, J.; Amorim, C.; Bearzatto, B.; Gala, J.L.; Donnez, J.; Dolmans, M.M. Gene expression in human ovarian tissue after xenografting. Mol. Hum. Reprod. 2014, 20, 514–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Ying, Y.F.; Ouyang, Y.L.; Wang, J.F.; Xu, J. VEGF and bFGF increase survival of xenografted human ovarian tissue in an experimental rabbit model. J. Assist. Reprod. Genet. 2013, 30, 1301–1311. [Google Scholar] [CrossRef] [Green Version]
- Ruan, X.; Cui, Y.; Du, J.; Jin, J.; Gu, M.; Chen, S.; Mueck, A.O. Randomized study to prove the quality of human ovarian tissue cryopreservation by xenotransplantation into mice. J. Ovarian Res. 2019, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, V.; Durant, J.F.; Camboni, A.; Gilliaux, S.; Amorim, C.A.; van Langendonckt, A.; Irenge, L.M.; Gala, J.L.; Donnez, J.; Dolmans, M.M. Is transplantation of cryopreserved ovarian tissue from patients with advanced-stage breast cancer safe? A pilot study. J. Assist. Reprod. Genet. 2013, 30, 1289–1299. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.S.; Radford, J.; Harris, M.; Varley, J.; Rutherford, A.J.; Lieberman, B.; Shalet, S.; Gosden, R. Ovarian tissue harvested from lymphoma patients to preserve fertility may be safe for autotransplantation. Hum. Reprod. 2001, 16, 2056–2060. [Google Scholar] [CrossRef] [Green Version]
- Greve, T.; Wielenga, V.T.; Grauslund, M.; Sørensen, N.; Christiansen, D.B.; Rosendahl, M.; Andersen, C.Y. Ovarian tissue cryopreserved for fertility preservation from patients with Ewing or other sarcomas appear to have no tumour cell contamination. Eur. J. Cancer 2013, 49, 1932–1938. [Google Scholar] [CrossRef] [PubMed]
- Lotz, L.; Montag, M.; van der Ven, H.; von Wolff, M.; Mueller, A.; Hoffmann, I.; Wachter, D.; Beckmann, M.W.; Dittrich, R. Xenotransplantation of cryopreserved ovarian tissue from patients with ovarian tumors into SCID mice--no evidence of malignant cell contamination. Fertil. Steril. 2011, 95, 2612–2614.e1. [Google Scholar] [CrossRef]
- Dolmans, M.M.; Marinescu, C.; Saussoy, P.; van Langendonckt, A.; Amorim, C.; Donnez, J. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood 2010, 116, 2908–2914. [Google Scholar] [CrossRef]
- Lee, J.A.; Barritt, J.; Moschini, R.M.; Slifkin, R.E.; Copperman, A.B. Optimizing human oocyte cryopreservation for fertility preservation patients: Should we mature then freeze or freeze then mature? Fertil. Steril. 2013, 99, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Son, W.Y.; Henderson, S.; Cohen, Y.; Dahan, M.; Buckett, W. Immature Oocyte for Fertility Preservation. Front. Endocrinol. 2019, 10, 464. [Google Scholar] [CrossRef] [Green Version]
- Prasath, E.B.; Chan, M.L.H.; Wong, W.H.W.; Lim, C.J.W.; Tharmalingam, M.D.; Hendricks, M.; Loh, S.F.; Chia, Y.N. First pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Hum. Reprod. 2014, 29, 276–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uzelac, P.S.; Delaney, A.A.; Christensen, G.L.; Bohler, H.C.L.; Nakajima, S.T. Live birth following in vitro maturation of oocytes retrieved from extracorporeal ovarian tissue aspiration and embryo cryopreservation for 5 years. Fertil. Steril. 2015, 104, 1258–1260. [Google Scholar] [CrossRef]
- Tavana, S.; Azarnia, M.; Valojerdi, M.R.; Shahverdi, A. Hyaluronic acid-based hydrogel scaffold without angiogenic growth factors enhances ovarian tissue function after autotransplantation in rats. Biomed. Mater. 2016, 11, 055006. [Google Scholar] [CrossRef] [PubMed]
- Tavana, S.; Valojerdi, M.R.; Azarnia, M.; Shahverdi, A. Restoration of ovarian tissue function and estrous cycle in rat after autotransplantation using hyaluronic acid hydrogel scaffold containing VEGF and bFGF. Growth Factors 2016, 34, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Manavella, D.D.; Cacciottola, L.; Pommé, S.; Desmet, C.M.; Jordan, B.F.; Donnez, J.; Amorim, C.A.; Dolmans, M.M. Two-step transplantation with adipose tissue-derived stem cells increases follicle survival by enhancing vascularization in xenografted frozen-thawed human ovarian tissue. Hum. Reprod. 2018, 33, 1107–1116. [Google Scholar] [CrossRef] [Green Version]
- Damous, L.L.; Nakamuta, J.S.; de Carvalho, A.E.T.S.; Carvalho, K.C.; José, M.S., Jr.; Simões, M.D.; Krieger, J.E.; Baracat, E.C. Scaffold-based delivery of adipose tissue-derived stem cells in rat frozen-thawed ovarian autografts: Preliminary studies in a rat model. J. Assist. Reprod. Genet. 2015, 32, 1285–1294. [Google Scholar] [CrossRef] [Green Version]
- Manavella, D.D.; Cacciottola, L.; Payen, V.L.; Amorim, C.A.; Donnez, J.; Dolmans, M.M. Adipose tissue-derived stem cells boost vascularization in grafted ovarian tissue by growth factor secretion and differentiation into endothelial cell lineages. Mol. Hum. Reprod. 2019, 25, 184–193. [Google Scholar] [CrossRef]
- Cho, E.; Kim, Y.Y.; Noh, K.; Ku, S.Y. A new possibility in fertility preservation: The artificial ovary. J. Tissue Eng. Regen. Med. 2019, 13, 1294–1315. [Google Scholar] [CrossRef]
- Luyckx, V.; Dolmans, M.M.; Vanacker, J.; Legat, C.; Moya, C.F.; Donnez, J.; Amorim, C.A. A new step toward the artificial ovary: Survival and proliferation of isolated murine follicles after autologous transplantation in a fibrin scaffold. Fertil. Steril. 2014, 101, 1149–1156. [Google Scholar] [CrossRef]
- Vanacker, J.; Luyckx, V.; Dolmans, M.M.; Rieux, A.D.; Jaeger, J.; van Langendonckt, A.; Donnez, J.; Amorim, C.A. Transplantation of an alginate-matrigel matrix containing isolated ovarian cells: First step in developing a biodegradable scaffold to transplant isolated preantral follicles and ovarian cells. Biomaterials 2012, 33, 6079–6085. [Google Scholar] [CrossRef]
- Johnson, J.; Canning, J.; Kaneko, T.; Pru, J.K.; Tilly, J.L. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 2004, 428, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Tilly, J.L.; Telfer, E.E. Purification of germline stem cells from adult mammalian ovaries: A step closer towards control of the female biological clock? Mol. Hum. Reprod. 2009, 15, 393–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutt, K.J.; Albertini, D.F. Clinical applications and limitations of current ovarian stem cell research: A review. J. Exp. Clin. Assist. Reprod. 2006, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Himelstein-Braw, R.; Peters, H.; Faber, M. Morphological study of the ovaries of leukaemic children. Br. J. Cancer 1978, 38, 82–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Familiari, G.; Caggiati, A.; Nottola, S.A.; Ermini, M.; di Benedetto, M.R.; Motta, P.M. Ultrastructure of human ovarian primordial follicles after combination chemotherapy for Hodgkin’s disease. Hum. Reprod. 1993, 8, 2080–2087. [Google Scholar] [CrossRef] [PubMed]




| Class | Agents | Type of Cancer | Mechanisms of Action | Damage Risk |
|---|---|---|---|---|
| Alkylating agents | Cyclophosphamide Ifosfamide Nitrosureas Chlorambucil Melphalan Busulphan Mechlorethamine | Leukemia, breast cancer, lung cancer, ovarian cancer, prostate cancer, lymphoma, myeloma, sarcoma, Hodgkin’s disease | Interference with cell division via intra-strand/inter-strand cross-linking of DNA. Induction of a reduction in mitochondrial transmembrane potential. Inhibition of the accumulation of cytochrome c in the cytosol. Induction of DSBs in oocytes and GCs. | High |
| Vinka alkaloids | Vinblastine Vincristine | Testicular cancer, lymphoma, Hodgkin’s disease, breast cancer, germ cell tumors, lung cancer, sarcoma, neuroblastoma | Inhibition of tubulin from forming into microtubules. Not gonadotoxic. | Low |
| Antimetabolites | Cytarabine Methotrexate 5-fluorouracil | Leukemia, breast cancer, ovarian cancer, gastrointestinal cancer | Inhibition of purine, pyrimidine becoming incorporated into DNA during S phase of cell cycle. Inhibition of RNA synthesis. Not gonadotoxic. | Low |
| Platinum agents | Cisplatin Carboplatin Oxaliplatin | Bladder cancer, colorectal cancer, head and neck cancer, lung cancer, ovarian cancer, testicular cancer | DNA damage by formation of inter-strand/intra-strand DNA adducts which interfere with cellular transcription and replication. Accumulation of abl and TAp63-alpha protein in the oocyte leading to oocyte death. | Moderate |
| Anthracycline antibiotics | Daunorubicin Bleomycin Doxorubicin | Lymphoma, leukemia, breast cancer, sarcoma | Intercalation with DNA and prevention of its replication and transcription via the inhibition of topoisomerase II. Upregulation of P53 protein which induces apoptosis in the presence of high levels of DNA damage. DNA DSBs leading to activation of ATM, which initiates apoptosis. GCs are usually targeted due to their mitotically and metabolically active characteristics. | Low/moderate |
| Others | Procarbazine | Hodgkin’s disease, brain cancer | Inhibition of DNA methylation, and RNA and protein synthesis. | High |
| Evaluation | Animal | Materials | Transplantation Site | Publication |
|---|---|---|---|---|
| Oocyte maturation after xenotransplantation | SCID mice | Ovarian tissue | Back muscle, kidney capsule | Soleimani et al. [212] |
| SCID mice | Ovarian tissue | Kidney capsule | Gook et al. [213,214] | |
| SCID mice | Ovarian tissue | Neck muscle | Lotz et al. [215] | |
| SCID mice | Ovarian tissue | Subcutaneous space | Kim et al. [216] | |
| Follicular development | SCID mice | Ovarian tissue | Intraperitoneum | Ayuandari et al. [217] |
| NOG mice | Ovarian cortex | Back skin, kidney capsule, ovarian bursa | Terada et al. [218] | |
| SCID mice | Clot containing preantral follicle | Ovarian bursa | Dolmans et al. [219] | |
| SCID/hpg mice | Ovarian tissue | Kidney capsule | Oktay et al. [220] | |
| Swiss nu/nu mice | Ovarian tissue | Intraperitoneum | David et al. [221] | |
| NOD/SCID mice | Ovarian tissue | Subcutaneous space | Weissman et al. [222] | |
| NMRI nu/nu | Ovarian tissue | Intraperitoneum, subcutaneous space, ovarian bursa, thigh muscle | Dath et al. [223] | |
| Swiss nu/nu mice | Clot containing preantral follicle | Intraperitoneum | Paulini et al. [224] | |
| SCID mice | Ovarian tissue | Intraperitoneum | Amorim et al. [225] | |
| NMRI nu/nu | Ovarian tissue | Thigh muscle | Jafarabadi et al. [226] | |
| NOD/SCID mice | Ovarian cortex | Subcutaneous space | Campos-Junior et al. [227] | |
| Optimization of grafting protocols | NMRI nu/nu | Ovarian tissue | Intraperitoneum | Van Eyck et al. [228] |
| SCID mice | Ovarian cortex | Back muscle | Soleimani et al. [69] | |
| B6cg nude mice | Ovarian tissue | Back skin, thigh muscle | Hormozi et al. [229] | |
| NOD/SCID mice | Ovarian cortex | Kidney capsule, subcutaneous space | Hernandez-Fonseca et al. [230] | |
| SCID mice | Ovarian tissue | Neck muscle | Maltaris et al. [231] | |
| Balb/C nu/nu | Ovarian tissue | Back muscle | Friedman et al. [232] | |
| SCID mice | Ovarian stroma | Subcutaneous space | Fu et al. [233] | |
| NMRI nu/nu | Ovarian tissue | Intraperitoneum | Van Langendonckt et al. [234] | |
| New Zealand rabbits | Ovarian tissue | Back muscle | Wang et al. [235] | |
| Balb/C nu/nu | Ovarian tissue | Ovarian bursa, subcutaneous space | Ruan et al. [236] | |
| Reimplantation of malignant cells | SCID mice | Ovarian cortex | Intraperitoneum | Luyckx et al. [237] |
| NOD/SCID mice | Ovarian tissue | Subcutaneous space | Kim et al. [238] | |
| NMRI nu/nu | Ovarian tissue | Subcutaneous space | Greve et al. [239] | |
| SCID mice | Ovarian tissue | Neck muscle | Lotz et al. [240] | |
| SCID mice | Ovarian tissue | Intraperitoneum | Dolmans et al. [241] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Kim, S.-W.; Han, S.-J.; Lee, S.; Park, H.-T.; Song, J.-Y.; Kim, T. Molecular Mechanism and Prevention Strategy of Chemotherapy- and Radiotherapy-Induced Ovarian Damage. Int. J. Mol. Sci. 2021, 22, 7484. https://doi.org/10.3390/ijms22147484
Kim S, Kim S-W, Han S-J, Lee S, Park H-T, Song J-Y, Kim T. Molecular Mechanism and Prevention Strategy of Chemotherapy- and Radiotherapy-Induced Ovarian Damage. International Journal of Molecular Sciences. 2021; 22(14):7484. https://doi.org/10.3390/ijms22147484
Chicago/Turabian StyleKim, Seongmin, Sung-Woo Kim, Soo-Jin Han, Sanghoon Lee, Hyun-Tae Park, Jae-Yun Song, and Tak Kim. 2021. "Molecular Mechanism and Prevention Strategy of Chemotherapy- and Radiotherapy-Induced Ovarian Damage" International Journal of Molecular Sciences 22, no. 14: 7484. https://doi.org/10.3390/ijms22147484






