Carnosine Protects against Cerebral Ischemic Injury by Inhibiting Matrix-Metalloproteinases
Abstract
:1. Introduction
2. Results
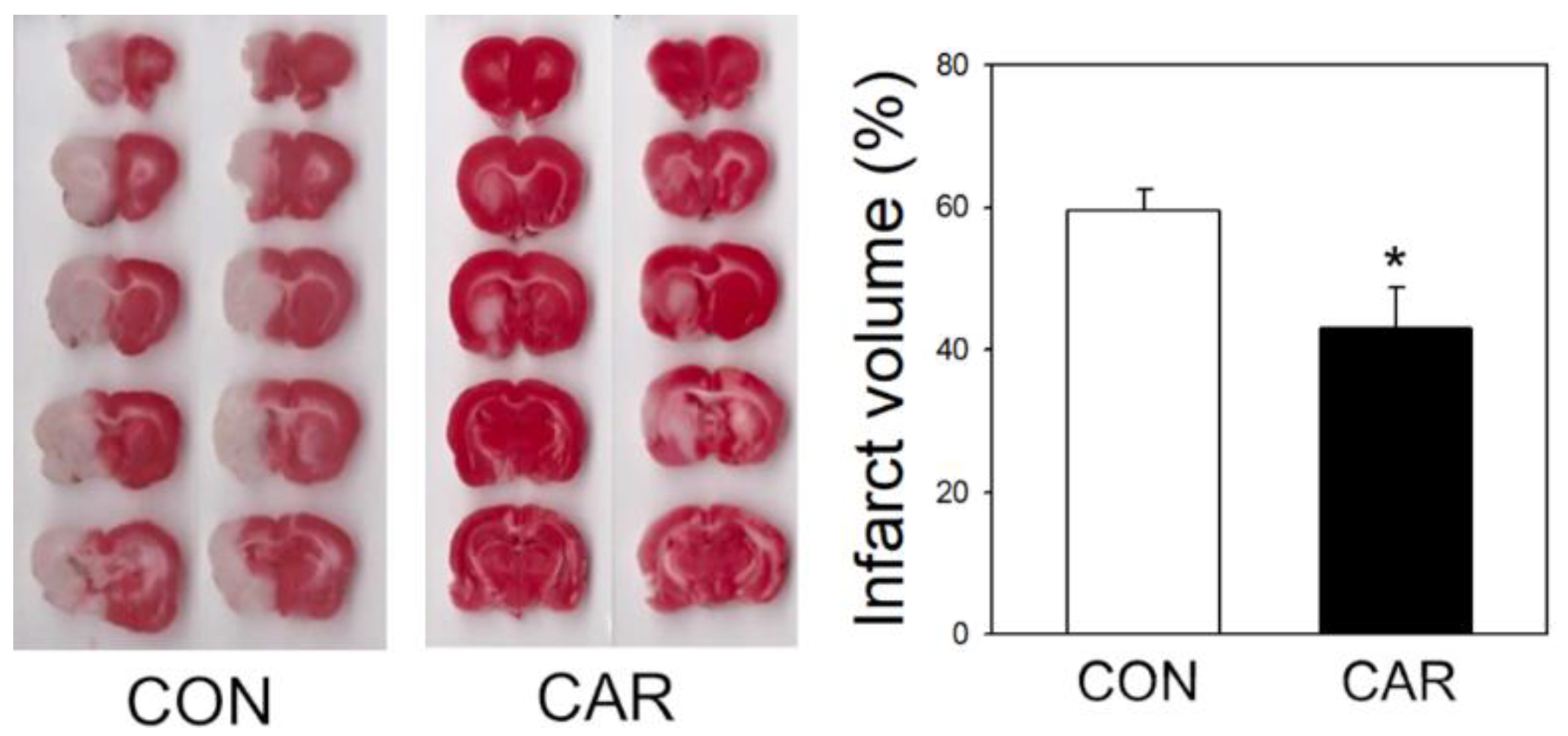
2.1. Reduction of Infarct Volume in Ischemic Brain
2.2. Reduction of Edema
2.3. Effect of Carnosine on Brain Water Content
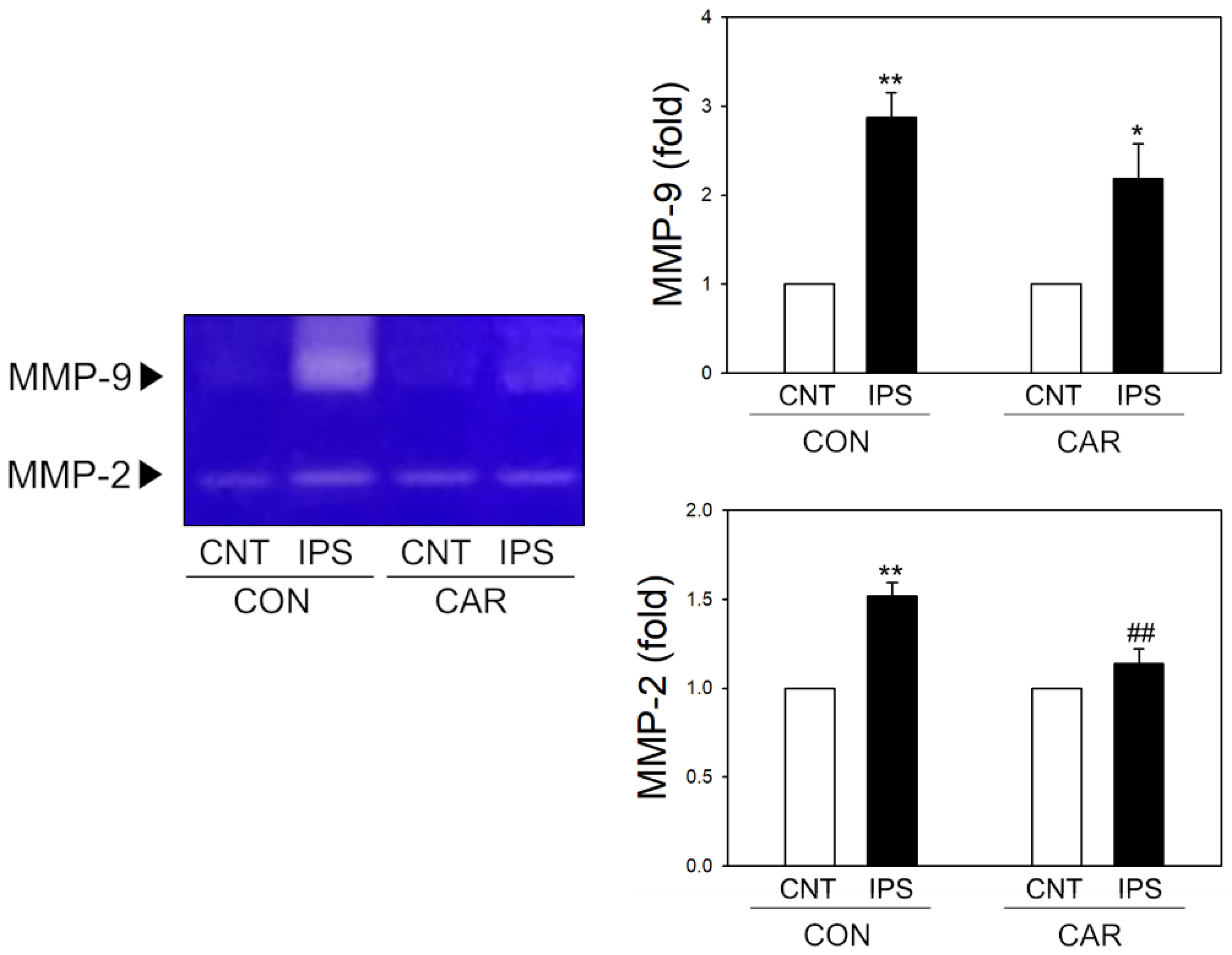
2.4. Suppression of MMP Activity by Carnosine in Ischemic Brain
2.5. Carnosine-Inhibition of MMPs in Enzymatic Assays
2.6. Inhibitory Effect of Carnosine on Zinc-Mediated MMP Activity
2.7. Protective Effect of TJ Proteins by Carnosine in OGD Exposure
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animal Treatment
4.3. Permanent Middle Cerebral Artery Occlusion (pMCAO) in Rat
4.4. Triphenyltetrazolium Chloride (TTC) Staining
4.5. Determination of Water Retention
4.6. Gelatin Zymography
4.7. In Vitro MMP Activity Assay
4.8. Cell Culture
4.9. Oxygen-Glucose Deprivation (OGD)
4.10. Immunofluorescence Staining
4.11. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, D.; Shi, J.; Elmadhoun, O.; Duan, Y.; An, H.; Zhang, J.; He, X.; Meng, R.; Liu, X.; Ji, X.; et al. Dihydrocapsaicin (DHC) enhances the hypothermia-induced neuroprotection following ischemic stroke via PI3K/Akt regulation in rat. Brain Res. 2017, 1671, 18–25. [Google Scholar] [CrossRef]
- Tran-Dinh, A.; Levoye, A.; Couret, D.; Galle-Treger, L.; Moreau, M.; Delbosc, S.; Hoteit, C.; Montravers, P.; Amarenco, P.; Huby, T.; et al. High-Density Lipoprotein Therapy in Stroke: Evaluation of Endothelial SR-BI-Dependent Neuroprotective Effects. Int. J. Mol. Sci. 2020, 22, 106. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Liu, Y.; Wang, S.; Zhang, H.; Fang, X.; Zhang, J.; Fan, L.; Zheng, B.; Roman, R.J.; Wang, Z.; et al. Novel Mechanistic Insights and Potential Therapeutic Impact of TRPC6 in Neurovascular Coupling and Ischemic Stroke. Int. J. Mol. Sci. 2021, 22, 2074. [Google Scholar] [CrossRef] [PubMed]
- Gravanis, I.; Tsirka, S.E. Tissue-type plasminogen activator as a therapeutic target in stroke. Expert Opin. Ther. Targets 2008, 12, 159–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anadani, M.; Ajinkya, S.; Alawieh, A.; Vargas, J.; Chatterjee, A.; Turk, A.; Spiotta, A.M. Intra-Arterial Tissue Plasminogen Activator Is a Safe Rescue Therapy with Mechanical Thrombectomy. World Neurosurg. 2019, 123, e604–e608. [Google Scholar] [CrossRef]
- Lansberg, M.G.; Fields, J.D.; Albers, G.W.; Jayaraman, M.V.; Do, H.M.; Marks, M.P. Mechanical thrombectomy following intravenous thrombolysis in the treatment of acute stroke. Arch. Neurol. 2005, 62, 1763–1765. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Hu, K. Tissue Plasminogen Activator: Side Effects and Signaling. J. Drug Des. Res. 2014, 1, 1. [Google Scholar]
- Zhang, J.; Yang, Y.; Sun, H.; Xing, Y. Hemorrhagic transformation after cerebral infarction: Current concepts and challenges. Ann. Transl. Med. 2014, 2, 81. [Google Scholar] [PubMed]
- Wardlaw, J.M.; Murray, V.; Berge, E.; del Zoppo, G.; Sandercock, P.; Lindley, R.L.; Cohen, G. Recombinant tissue plasminogen activator for acute ischaemic stroke: An updated systematic review and meta-analysis. Lancet 2012, 379, 2364–2372. [Google Scholar] [CrossRef] [Green Version]
- Cord, B.J.; Kodali, S.; Strander, S.; Silverman, A.; Wang, A.; Chouairi, F.; Koo, A.B.; Nguyen, C.K.; Peshwe, K.; Kimmel, A.; et al. Direct carotid puncture for mechanical thrombectomy in acute ischemic stroke patients with prohibitive vascular access. J. Neurosurg. 2020, 135, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Schoknecht, K.; David, Y.; Heinemann, U. The blood-brain barrier-gatekeeper to neuronal homeostasis: Clinical implications in the setting of stroke. Semin. Cell Dev. Biol. 2015, 38, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Balami, J.S.; Chen, R.L.; Grunwald, I.Q.; Buchan, A.M. Neurological complications of acute ischaemic stroke. Lancet Neurol. 2011, 10, 357–371. [Google Scholar] [CrossRef]
- Simard, J.M.; Kent, T.A.; Chen, M.; Tarasov, K.V.; Gerzanich, V. Brain oedema in focal ischaemia: Molecular pathophysiology and theoretical implications. Lancet Neurol. 2007, 6, 258–268. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Yuan, R.; Wang, Y.; Wei, C.; Zhang, S.; Yang, X.; Wu, B.; Liu, M. Early Prediction of Malignant Brain Edema After Ischemic Stroke. Stroke 2018, 49, 2918–2927. [Google Scholar] [CrossRef] [PubMed]
- Bardutzky, J.; Schwab, S. Antiedema therapy in ischemic stroke. Stroke 2007, 38, 3084–3094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makin, S.D.; Doubal, F.N.; Dennis, M.S.; Wardlaw, J.M. Clinically Confirmed Stroke With Negative Diffusion-Weighted Imaging Magnetic Resonance Imaging: Longitudinal Study of Clinical Outcomes, Stroke Recurrence, and Systematic Review. Stroke 2015, 46, 3142–3148. [Google Scholar] [CrossRef] [PubMed]
- Hofmeijer, J.; van Putten, M.J. Ischemic cerebral damage: An appraisal of synaptic failure. Stroke 2012, 43, 607–615. [Google Scholar] [CrossRef] [Green Version]
- Bolay, H.; Gursoy-Ozdemir, Y.; Sara, Y.; Onur, R.; Can, A.; Dalkara, T. Persistent defect in transmitter release and synapsin phosphorylation in cerebral cortex after transient moderate ischemic injury. Stroke 2002, 33, 1369–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatashita, S.; Hoff, J.T.; Salamat, S.M. Ischemic brain edema and the osmotic gradient between blood and brain. J. Cereb. Blood Flow Metab. 1988, 8, 552–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahle, K.T.; Simard, J.M.; Staley, K.J.; Nahed, B.V.; Jones, P.S.; Sun, D. Molecular mechanisms of ischemic cerebral edema: Role of electroneutral ion transport. Physiology 2009, 24, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Zhang, Y.; Liao, X.; Yang, R.; Lei, Y.; Luo, J. Potential Therapies for Cerebral Edema After Ischemic Stroke: A Mini Review. Front. Aging Neurosci. 2020, 12, 618819. [Google Scholar] [CrossRef]
- Krueger, M.; Mages, B.; Hobusch, C.; Michalski, D. Endothelial edema precedes blood-brain barrier breakdown in early time points after experimental focal cerebral ischemia. Acta Neuropathol. Commun. 2019, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Payen, J.F.; Fauvage, B.; Falcon, D.; Lavagne, P. Brain oedema following blood-brain barrier disruption: Mechanisms and diagnosis. Ann. Fr. Anesth. Reanim. 2003, 22, 220–225. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Byun, H.M.; Chung, E.C.; Chung, H.Y.; Bae, O.N. Loss of Integrity: Impairment of the Blood-brain Barrier in Heavy Metal-associated Ischemic Stroke. Toxicol. Res. 2013, 29, 157–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.A.; Kim, D.; Kim, J.H.; Shin, Y.J.; Kim, E.S.; Akram, M.; Kim, E.H.; Majid, A.; Baek, S.H.; Bae, O.N. Autophagy-mediated occludin degradation contributes to blood-brain barrier disruption during ischemia in bEnd.3 brain endothelial cells and rat ischemic stroke models. Fluids Barriers CNS 2020, 17, 21. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.A.; Shin, D.; Kim, J.H.; Shin, Y.J.; Rajanikant, G.K.; Majid, A.; Baek, S.H.; Bae, O.N. Role of Autophagy in Endothelial Damage and Blood-Brain Barrier Disruption in Ischemic Stroke. Stroke 2018, 49, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, W.; Tripathi, D.; Ronaldson, P.T. Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. Am. J. Physiol. Cell Physiol. 2018, 315, C343–C356. [Google Scholar] [CrossRef] [PubMed]
- Bae, O.N.; Serfozo, K.; Baek, S.H.; Lee, K.Y.; Dorrance, A.; Rumbeiha, W.; Fitzgerald, S.D.; Farooq, M.U.; Naravelta, B.; Bhatt, A.; et al. Safety and efficacy evaluation of carnosine, an endogenous neuroprotective agent for ischemic stroke. Stroke 2013, 44, 205–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nan, D.; Jin, H.; Deng, J.; Yu, W.; Liu, R.; Sun, W.; Huang, Y. Cilostazol ameliorates ischemia/reperfusion-induced tight junction disruption in brain endothelial cells by inhibiting endoplasmic reticulum stress. FASEB J. 2019, 33, 10152–10164. [Google Scholar] [CrossRef] [Green Version]
- Rajanikant, G.K.; Zemke, D.; Senut, M.C.; Frenkel, M.B.; Chen, A.F.; Gupta, R.; Majid, A. Carnosine is neuroprotective against permanent focal cerebral ischemia in mice. Stroke 2007, 38, 3023–3031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Planas, A.M.; Sole, S.; Justicia, C.; Farre, E.R. Estimation of gelatinase content in rat brain: Effect of focal ischemia. Biochem. Biophys. Res. Commun. 2000, 278, 803–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, A.W.; Krekoski, C.A.; Bou, S.S.; Chapman, K.R.; Edwards, D.R. Increased gelatinase A (MMP-2) and gelatinase B (MMP-9) activities in human brain after focal ischemia. Neurosci. Lett. 1997, 238, 53–56. [Google Scholar] [CrossRef]
- Rosenberg, G.A. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 2009, 8, 205–216. [Google Scholar] [CrossRef]
- Snoek-van Beurden, P.A.; Von den Hoff, J.W. Zymographic techniques for the analysis of matrix metalloproteinases and their inhibitors. Biotechniques 2005, 38, 73–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parks, W.C. Matrix metalloproteinases in repair. Wound Repair Regen. 1999, 7, 423–432. [Google Scholar] [CrossRef]
- Quilty, F.; Byrne, A.M.; Aird, J.; El Mashad, S.; Parra-Blanco, A.; Long, A.; Gilmer, J.F.; Medina, C. Impact of Deoxycholic Acid on Oesophageal Adenocarcinoma Invasion: Effect on Matrix Metalloproteinases. Int. J. Mol. Sci. 2020, 21, 8042. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, G.A. Ischemic brain edema. Prog. Cardiovasc. Dis. 1999, 42, 209–216. [Google Scholar] [CrossRef]
- Castellanos, M.; Leira, R.; Serena, J.; Pumar, J.M.; Lizasoain, I.; Castillo, J.; Davalos, A. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke 2003, 34, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Jian Liu, K.; Rosenberg, G.A. Matrix metalloproteinases and free radicals in cerebral ischemia. Free Radic. Biol. Med. 2005, 39, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.H.; Li, J.; Rafols, J.A.; Ding, Y. Reduced brain edema and matrix metalloproteinase (MMP) expression by pre-reperfusion infusion into ischemic territory in rat. Neurosci. Lett. 2004, 372, 35–39. [Google Scholar] [CrossRef]
- Yang, Y.; Thompson, J.F.; Taheri, S.; Salayandia, V.M.; McAvoy, T.A.; Hill, J.W.; Yang, Y.; Estrada, E.Y.; Rosenberg, G.A. Early inhibition of MMP activity in ischemic rat brain promotes expression of tight junction proteins and angiogenesis during recovery. J. Cereb. Blood Flow Metab. 2013, 33, 1104–1114. [Google Scholar] [CrossRef] [Green Version]
- Bauer, A.T.; Burgers, H.F.; Rabie, T.; Marti, H.H. Matrix metalloproteinase-9 mediates hypoxia-induced vascular leakage in the brain via tight junction rearrangement. J. Cereb. Blood Flow Metab. 2010, 30, 837–848. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Estrada, E.Y.; Thompson, J.F.; Liu, W.; Rosenberg, G.A. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J. Cereb. Blood Flow Metab. 2007, 27, 697–709. [Google Scholar] [CrossRef]
- Mollica, A.; Costante, R.; Stefanucci, A.; Pinnen, F.; Lucente, G.; Fidanza, S.; Pieretti, S. Antinociceptive profile of potent opioid peptide AM94, a fluorinated analogue of biphalin with non-hydrazine linker. J. Pept. Sci. 2013, 19, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Feliciani, F.; Pinnen, F.; Stefanucci, A.; Costante, R.; Cacciatore, I.; Lucente, G.; Mollica, A. Structure-activity relationships of biphalin analogs and their biological evaluation on opioid receptors. Mini Rev. Med. Chem. 2013, 13, 11–33. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, H.; Shah, K.; Karamyan, V.T.; Abruscato, T.J. Opioid receptor agonists reduce brain edema in stroke. Brain Res. 2011, 1383, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Albekairi, T.H.; Vaidya, B.; Patel, R.; Nozohouri, S.; Villalba, H.; Zhang, Y.; Lee, Y.S.; Al-Ahmad, A.; Abbruscato, T.J. Brain Delivery of a Potent Opioid Receptor Agonist, Biphalin during Ischemic Stroke: Role of Organic Anion Transporting Polypeptide (OATP). Pharmaceutics 2019, 11, 467. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Shah, K.; Wang, H.; Karamyan, V.T.; Abbruscato, T.J. Characterization of neuroprotective effects of biphalin, an opioid receptor agonist, in a model of focal brain ischemia. J. Pharmacol. Exp. Ther. 2011, 339, 499–508. [Google Scholar] [CrossRef] [Green Version]
- Bae, O.N.; Majid, A. Role of histidine/histamine in carnosine-induced neuroprotection during ischemic brain damage. Brain Res. 2013, 1527, 246–254. [Google Scholar] [CrossRef]
- Baek, S.H.; Noh, A.R.; Kim, K.A.; Akram, M.; Shin, Y.J.; Kim, E.S.; Yu, S.W.; Majid, A.; Bae, O.N. Modulation of mitochondrial function and autophagy mediates carnosine neuroprotection against ischemic brain damage. Stroke 2014, 45, 2438–2443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.S.; Kim, D.; Nyberg, S.; Poma, A.; Cecchin, D.; Jain, S.A.; Kim, K.A.; Shin, Y.J.; Kim, E.H.; Kim, M.; et al. LRP-1 functionalized polymersomes enhance the efficacy of carnosine in experimental stroke. Sci. Rep. 2020, 10, 699. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Manias, J.L.; Stewart, D.J. Pathology and new players in the pathogenesis of brain edema. Acta Neuropathol. 2009, 118, 197–217. [Google Scholar] [CrossRef]
- Verma, R.P.; Hansch, C. Matrix metalloproteinases (MMPs): Chemical-biological functions and (Q)SARs. Bioorg. Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef]
- Horning, M.S.; Blakemore, L.J.; Trombley, P.Q. Endogenous mechanisms of neuroprotection: Role of zinc, copper, and carnosine. Brain Res. 2000, 852, 56–61. [Google Scholar] [CrossRef]
- Jiang, X.; Andjelkovic, A.V.; Zhu, L.; Yang, T.; Bennett, M.V.L.; Chen, J.; Keep, R.F.; Shi, Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog. Neurobiol. 2018, 163–164, 144–171. [Google Scholar] [CrossRef]
- Dostovic, Z.; Dostovic, E.; Smajlovic, D.; Ibrahimagic, O.C.; Avdic, L. Brain Edema After Ischaemic Stroke. Med. Arch. 2016, 70, 339–341. [Google Scholar] [CrossRef] [Green Version]
- Kurzepa, J.; Kurzepa, J.; Golab, P.; Czerska, S.; Bielewicz, J. The significance of matrix metalloproteinase (MMP)-2 and MMP-9 in the ischemic stroke. Int. J. Neurosci. 2014, 124, 707–716. [Google Scholar] [CrossRef]
- Machado, L.S.; Kozak, A.; Ergul, A.; Hess, D.C.; Borlongan, C.V.; Fagan, S.C. Delayed minocycline inhibits ischemia-activated matrix metalloproteinases 2 and 9 after experimental stroke. BMC Neurosci. 2006, 7, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and foe for ischemic stroke. J. Neuroinflamm. 2019, 16, 142. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Rosenberg, G.A. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 2011, 42, 3323–3328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Rosenberg, G.A. Matrix metalloproteinases as therapeutic targets for stroke. Brain Res. 2015, 1623, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Sullivan, S.; Medina, C.; Ledwidge, M.; Radomski, M.W.; Gilmer, J.F. Nitric oxide-matrix metaloproteinase-9 interactions: Biological and pharmacological significance—NO and MMP-9 interactions. Biochim. Biophys. Acta 2014, 1843, 603–617. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, M.; Tanaka, K.I.; Kato-Negishi, M. Zinc, Carnosine, and Neurodegenerative Diseases. Nutrients 2018, 10, 147. [Google Scholar] [CrossRef] [Green Version]
- Trombley, P.Q.; Horning, M.S.; Blakemore, L.J. Interactions between carnosine and zinc and copper: Implications for neuromodulation and neuroprotection. Biochemistry 2000, 65, 807–816. [Google Scholar]
- Ku, J.M.; Taher, M.; Chin, K.Y.; Grace, M.; McIntyre, P.; Miller, A.A. Characterisation of a mouse cerebral microvascular endothelial cell line (bEnd.3) after oxygen glucose deprivation and reoxygenation. Clin. Exp. Pharmacol. Physiol. 2016, 43, 777–786. [Google Scholar] [CrossRef]
- Kim, D.; Kim, K.A.; Kim, J.H.; Kim, E.H.; Bae, O.N. Methylglyoxal-Induced Dysfunction in Brain Endothelial Cells via the Suppression of Akt/HIF-1alpha Pathway and Activation of Mitophagy Associated with Increased Reactive Oxygen Species. Antioxidants 2020, 9, 820. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, E.H.; Choi, S.; Lim, K.M.; Tie, L.; Majid, A.; Bae, O.N. A Commonly Used Biocide 2-N-octyl-4-isothiazolin-3-oneInduces Blood-Brain Barrier Dysfunction via Cellular Thiol Modification and Mitochondrial Damage. Int. J. Mol. Sci. 2021, 22, 2563. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, K.E.; Witt, K.A. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol. Dis. 2008, 32, 200–219. [Google Scholar] [CrossRef]
- Szarka, N.; Toth, L.; Czigler, A.; Kellermayer, Z.; Ungvari, Z.; Amrein, K.; Czeiter, E.; Bali, Z.K.; Tadepalli, S.A.; Wahr, M.; et al. Single Mild Traumatic Brain Injury Induces Persistent Disruption of the Blood-Brain Barrier, Neuroinflammation and Cognitive Decline in Hypertensive Rats. Int. J. Mol. Sci. 2019, 20, 3223. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.-H.; Kim, E.-S.; Shin, D.; Kim, D.; Choi, S.; Shin, Y.-J.; Kim, K.-A.; Noh, D.; Caglayan, A.B.; Rajanikant, G.K.; et al. Carnosine Protects against Cerebral Ischemic Injury by Inhibiting Matrix-Metalloproteinases. Int. J. Mol. Sci. 2021, 22, 7495. https://doi.org/10.3390/ijms22147495
Kim E-H, Kim E-S, Shin D, Kim D, Choi S, Shin Y-J, Kim K-A, Noh D, Caglayan AB, Rajanikant GK, et al. Carnosine Protects against Cerebral Ischemic Injury by Inhibiting Matrix-Metalloproteinases. International Journal of Molecular Sciences. 2021; 22(14):7495. https://doi.org/10.3390/ijms22147495
Chicago/Turabian StyleKim, Eun-Hye, Eun-Sun Kim, Donggeun Shin, Donghyun Kim, Sungbin Choi, Young-Jun Shin, Kyeong-A Kim, Dabi Noh, Ahmet B. Caglayan, G.K. Rajanikant, and et al. 2021. "Carnosine Protects against Cerebral Ischemic Injury by Inhibiting Matrix-Metalloproteinases" International Journal of Molecular Sciences 22, no. 14: 7495. https://doi.org/10.3390/ijms22147495





