Biomarkers of Genotoxicity in Medical Workers Exposed to Low-Dose Ionizing Radiation: Systematic Review and Meta-Analyses
Abstract
1. Introduction
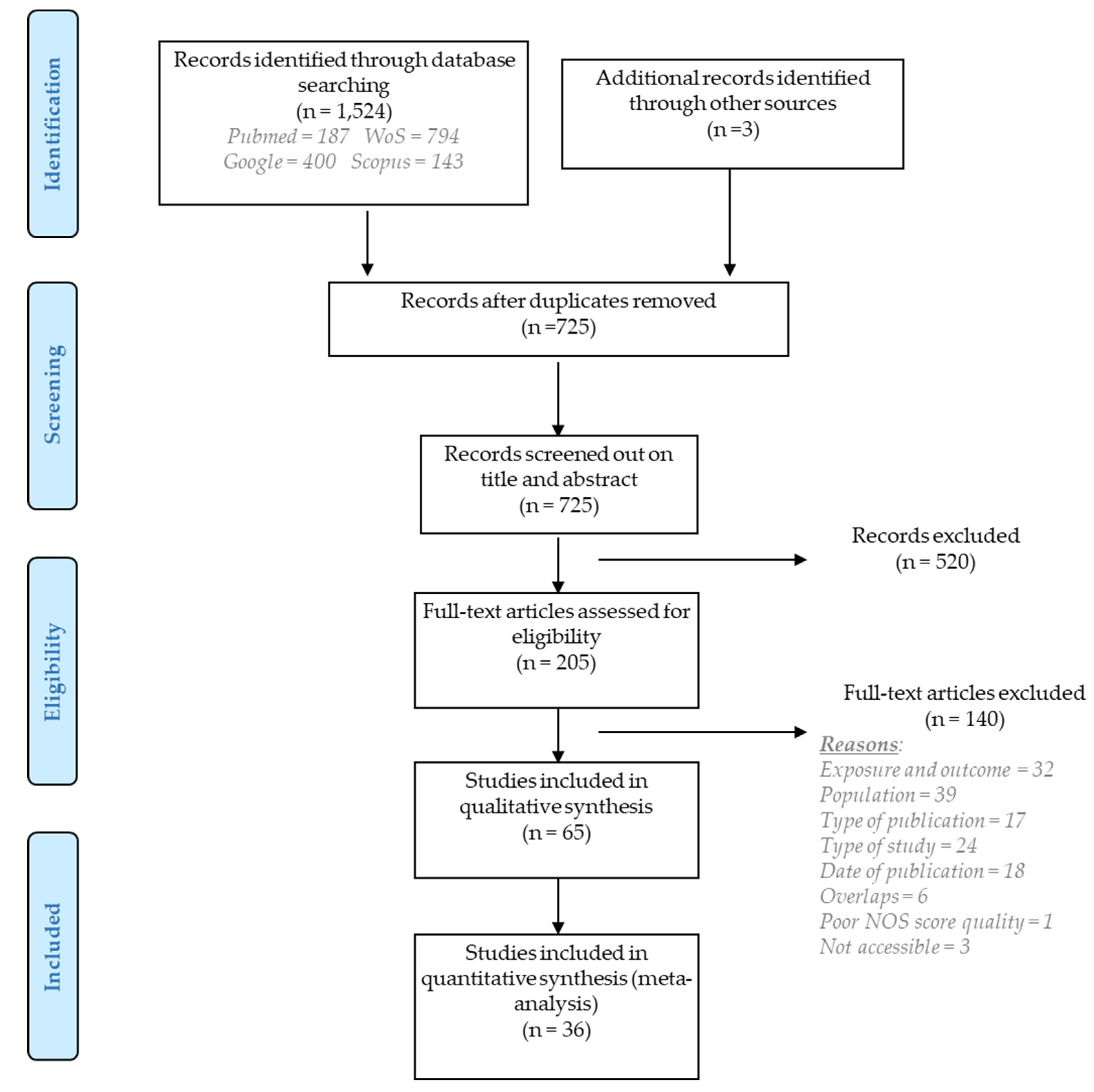
2. Methods
2.1. Online Searches
2.2. Selection Criteria
2.3. Quality Assessment
2.4. Statistical Analysis
3. Results
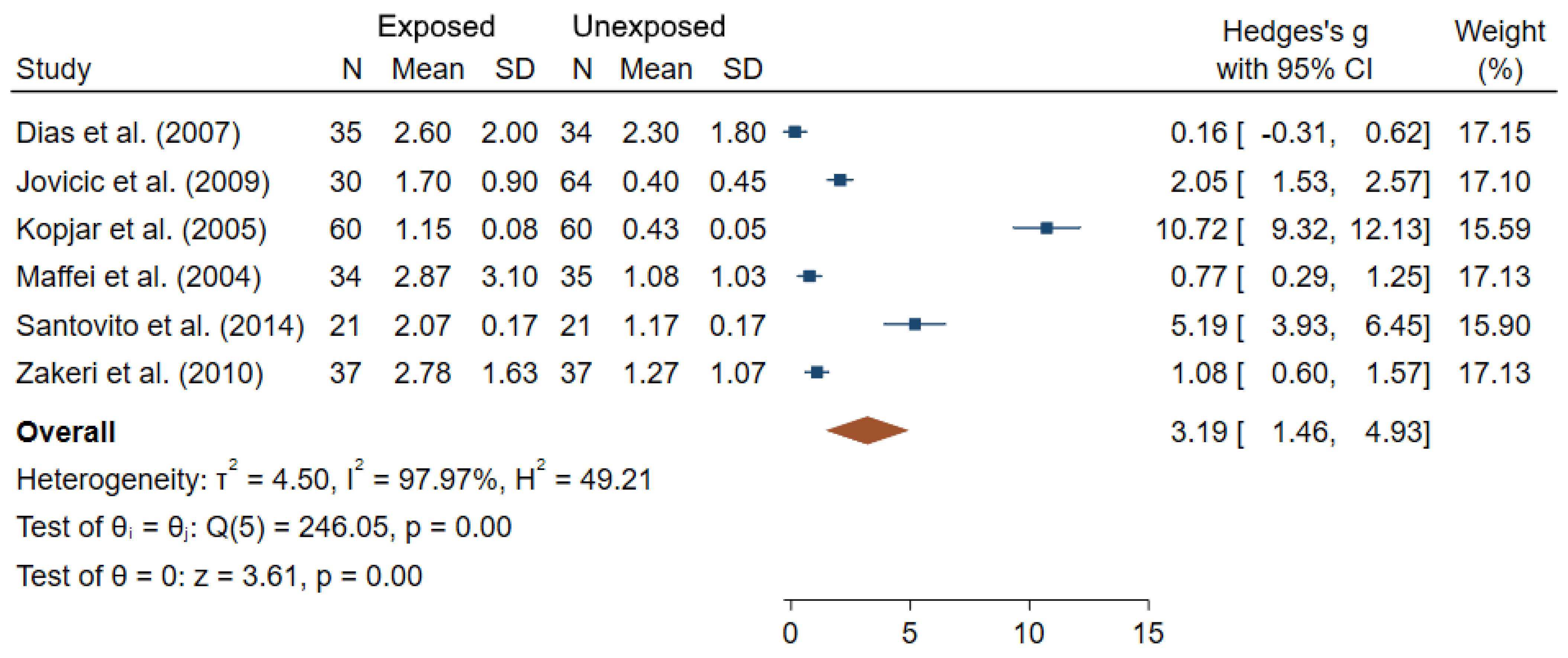
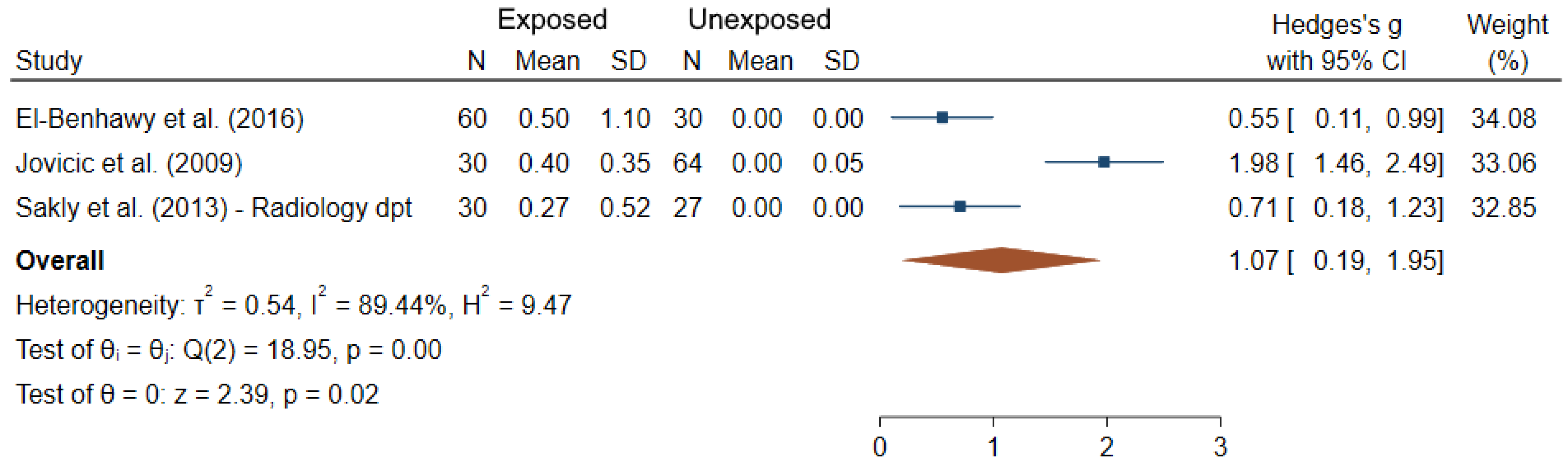
3.1. Cytogenetic Biomarkers
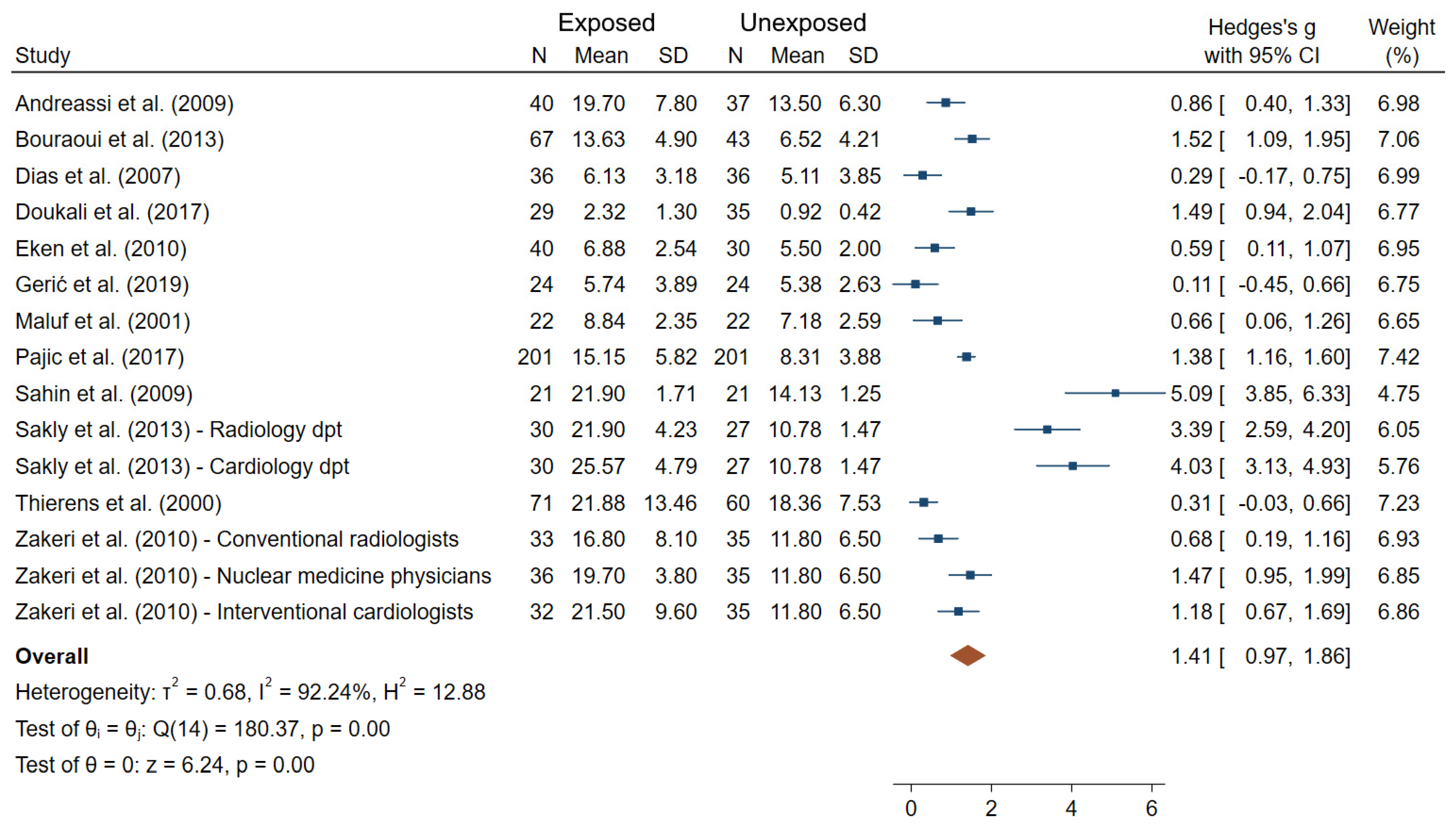
3.2. DNA Integrity Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fazel, R.; Krumholz, H.M.; Wang, Y.; Ross, J.S.; Chen, J.; Ting, H.H.; Shah, N.D.; Nasir, K.; Einstein, A.J.; Nallamothu, B.K. Exposure to Low-Dose Ionizing Radiation from Medical Imaging Procedures in the United States. N. Engl. J. Med. 2009, 361, 849–857. [Google Scholar] [CrossRef]
- Brenner, D.J.; Hall, E.J. Computed Tomography—An Increasing Source of Radiation Exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Tafreshi, N.K.; Doligalski, M.L.; Tichacek, C.J.; Pandya, D.N.; Budzevich, M.M.; El-Haddad, G.; Khushalani, N.I.; Moros, E.G.; McLaughlin, M.L.; Wadas, T.J.; et al. Development of Targeted Alpha Particle Therapy for Solid Tumors. Molecules 2019, 24, 4314. [Google Scholar] [CrossRef]
- UNSCEAR. Sources and Effects of Ionizing Radiation, Report to the General Assembly with Scientific Annexes; UNSCEAR: New York, NY, USA, 2008. [Google Scholar]
- Grosse, Y.; Baan, R.; Straif, K.; Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Galichet, L.; Cogliano, V.; et al. A Review of Human Carcinogens--Part A: Pharmaceuticals. Lancet Oncol. 2009, 10, 13–14. [Google Scholar] [CrossRef]
- Kreuzer, M.; Auvinen, A.; Cardis, E.; Durante, M.; Harms-Ringdahl, M.; Jourdain, J.R.; Madas, B.G.; Ottolenghi, A.; Pazzaglia, S.; Prise, K.M.; et al. Multidisciplinary European Low Dose Initiative (MELODI): Strategic Research Agenda for Low Dose Radiation Risk Research. Radiat. Environ. Biophys. 2018, 57, 5–15. [Google Scholar] [CrossRef]
- Hall, J.; Jeggo, P.A.; West, C.; Gomolka, M.; Quintens, R.; Badie, C.; Laurent, O.; Aerts, A.; Anastasov, N.; Azimzadeh, O.; et al. Ionizing Radiation Biomarkers in Epidemiological Studies—An Update. Mutat. Res. 2017, 771, 59–84. [Google Scholar] [CrossRef] [PubMed]
- Little, J.B. Radiation Carcinogenesis. Carcinogenesis 2000, 21, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Morton, L.M.; Karyadi, D.M.; Stewart, C.; Bogdanova, T.I.; Dawson, E.T.; Steinberg, M.K.; Dai, J.; Hartley, S.W.; Schonfeld, S.J.; Sampson, J.N.; et al. Radiation-Related Genomic Profile of Papillary Thyroid Cancer after the Chernobyl Accident. Science 2021. [Google Scholar] [CrossRef]
- Natarajan, A.T.; Boei, J.J.W.A. Formation of Chromosome Aberrations: Insights from FISH. Mutat. Res./Rev. Mutat. Res. 2003, 544, 299–304. [Google Scholar] [CrossRef]
- Boffetta, P.; van der Hel, O.; Norppa, H.; Fabianova, E.; Fucic, A.; Gundy, S.; Lazutka, J.; Cebulska-Wasilewska, A.; Puskailerova, D.; Znaor, A.; et al. Chromosomal Aberrations and Cancer Risk: Results of a Cohort Study from Central Europe. Am. J. Epidemiol. 2007, 165, 36–43. [Google Scholar] [CrossRef]
- Bonassi, S.; Norppa, H.; Ceppi, M.; Strömberg, U.; Vermeulen, R.; Znaor, A.; Cebulska-Wasilewska, A.; Fabianova, E.; Fucic, A.; Gundy, S.; et al. Chromosomal Aberration Frequency in Lymphocytes Predicts the Risk of Cancer: Results from a Pooled Cohort Study of 22 358 Subjects in 11 Countries. Carcinogenesis 2008, 29, 1178–1183. [Google Scholar] [CrossRef]
- Hagmar, L.; Strömberg, U.; Bonassi, S.; Hansteen, I.-L.; Knudsen, L.E.; Lindholm, C.; Norppa, H. Impact of Types of Lymphocyte Chromosomal Aberrations on Human Cancer Risk: Results from Nordic and Italian Cohorts. Cancer Res. 2004, 64, 2258–2263. [Google Scholar] [CrossRef] [PubMed]
- International Atomic Energy Agency (IAEA). Cytogenetic Dosimetry: Applications in Preparedness for and Response to Radiation Emergencies; International Atomic Energy Agency: Vienna, Austria, 2011. [Google Scholar]
- Fučić, A.; Želježić, D.; Kašuba, V.; Kopjar, N.; Rozgaj, R.; Lasan, R.; Mijić, A.; Hitrec, V.; Lucas, J.N. Stable and Unstable Chromosome Aberrations Measured after Occupational Exposure to Ionizing Radiation and Ultrasound. Croat. Med. J. 2007, 48, 371–377. [Google Scholar]
- De Pascalis, I.; Pilato, B.; Mazzotta, A.; Dell’Endice, T.S.; Rubini, V.; Simone, G.; Paradiso, A.; Aiello, V.; Mangia, A. Sister Chromatid Exchange: A Possible Approach to Characterize Familial Breast Cancer Patients. Oncol. Rep. 2015, 33, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Perry, P.; Evans, H.J. Cytological Detection of Mutagen-Carcinogen Exposure by Sister Chromatid Exchange. Nature 1975, 258, 121–125. [Google Scholar] [CrossRef]
- Lazutka, J.R.; Lekevicius, R.; Dedonyte, V.; Maciuleviciute-Gervers, L.; Mierauskiene, J.; Rudaitiene, S.; Slapsyte, G. Chromosomal Aberrations and Sister-Chromatid Exchanges in Lithuanian Populations: Effects of Occupational and Environmental Exposures. Mutat. Res. 1999, 445, 225–239. [Google Scholar] [CrossRef]
- Gutiérrez-Enríquez, S.; Ramón y Cajal, T.; Alonso, C.; Corral, A.; Carrasco, P.; Cornet, M.; Sanz, J.; Ribas, M.; Baiget, M.; Diez, O. Ionizing Radiation or Mitomycin-Induced Micronuclei in Lymphocytes of BRCA1 or BRCA2 Mutation Carriers. Breast Cancer Res. Treat. 2011, 127, 611–622. [Google Scholar] [CrossRef]
- Blackburn, E.H. Switching and Signaling at the Telomere. Cell 2001, 106, 661–673. [Google Scholar] [CrossRef]
- Reste, J.; Zvigule, G.; Zvagule, T.; Kurjane, N.; Eglite, M.; Gabruseva, N.; Berzina, D.; Plonis, J.; Miklasevics, E. Telomere Length in Chernobyl Accident Recovery Workers in the Late Period after the Disaster. J. Radiat. Res. 2014, 55, 1089–1100. [Google Scholar] [CrossRef][Green Version]
- Pernot, E.; Hall, J.; Baatout, S.; Benotmane, M.A.; Blanchardon, E.; Bouffler, S.; El Saghire, H.; Gomolka, M.; Guertler, A.; Harms-Ringdahl, M.; et al. Ionizing Radiation Biomarkers for Potential Use in Epidemiological Studies. Mutat. Res./Rev. Mutat. Res. 2012, 751, 258–286. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Dong, X.; Liu, T.; Zhang, L.; Ao, L. Antioxidant Status and Cytogenetic Damage in Hospital Workers Occupationally Exposed to Low Dose Ionizing Radiation. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis 2020, 850–851, 503152. [Google Scholar] [CrossRef] [PubMed]
- Gharibdousty Low Levels of Ionizing Radiation Exposure and Cytogenetic Effects in Radiopharmacists. BBRC 2017. [CrossRef]
- Hedges, L.V. Distribution Theory for Glass’s Estimator of Effect Size and Related Estimators. J. Educ. Stat. 1981, 6, 107–128. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Andreassi, M.G.; Foffa, I.; Manfredi, S.; Botto, N.; Cioppa, A.; Picano, E. Genetic Polymorphisms in XRCC1, OGG1, APE1 and XRCC3 DNA Repair Genes, Ionizing Radiation Exposure and Chromosomal DNA Damage in Interventional Cardiologists. Mutat. Res. 2009, 666, 57–63. [Google Scholar] [CrossRef]
- Andreassi, M.G.; Piccaluga, E.; Gargani, L.; Sabatino, L.; Borghini, A.; Faita, F.; Bruno, R.M.; Padovani, R.; Guagliumi, G.; Picano, E. Subclinical Carotid Atherosclerosis and Early Vascular Aging from Long-Term Low-Dose Ionizing Radiation Exposure: A Genetic, Telomere, and Vascular Ultrasound Study in Cardiac Catheterization Laboratory Staff. JACC Cardiovasc. Interv. 2015, 8, 616–627. [Google Scholar] [CrossRef]
- Andreassi, M.G.; Borghini, A.; Vecoli, C.; Piccaluga, E.; Guagliumi, G.; Del Greco, M.; Gaita, F.; Picano, E. Reproductive Outcomes and Y Chromosome Instability in Radiation-Exposed Male Workers in Cardiac Catheterization Laboratory. Environ. Mol. Mutagen. 2020, 61, 361–368. [Google Scholar] [CrossRef]
- Angelini, S.; Kumar, R.; Carbone, F.; Maffei, F.; Forti, G.C.; Violante, F.S.; Lodi, V.; Curti, S.; Hemminki, K.; Hrelia, P. Micronuclei in Humans Induced by Exposure to Low Level of Ionizing Radiation: Influence of Polymorphisms in DNA Repair Genes. Mutat. Res. 2005, 570, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, P.; Preston, D.L.; Doody, M.M.; Hauptmann, M.; Kampa, D.; Alexander, B.H.; Petibone, D.; Simon, S.L.; Weinstock, R.M.; Bouville, A.; et al. Retrospective Biodosimetry among United States Radiologic Technologists. Radiat. Res. 2007, 167, 727–734. [Google Scholar] [CrossRef]
- Bouraoui, S.; Mougou, S.; Drira, A.; Tabka, F.; Bouali, N.; Mrizek, N.; Elghezal, H.; Saad, A. A Cytogenetic Approach to the Effects of Low Levels of Ionizing Radiation (IR) on the Exposed Tunisian Hospital Workers. Int. J. Occup. Med. Environ. Health 2013, 26, 144–154. [Google Scholar] [CrossRef]
- Caradonna, F. Nucleoplasmic Bridges and Acrocentric Chromosome Associations as Early Markers of Exposure to Low Levels of Ionising Radiation in Occupationally Exposed Hospital Workers. Mutagenesis 2015, 30, 269–275. [Google Scholar] [CrossRef]
- Cigarrán, S.; Barquinero, J.F.; Barrios, L.; Ribas, M.; Egozcue, J.; Caballín, M.R. Cytogenetic Analyses by Fluorescence In Situ Hybridization (FISH) in Hospital Workers Occupationally Exposed to Low Levels of Ionizing Radiation. Radiat. Res. 2001, 155, 417–423. [Google Scholar] [CrossRef]
- Dias, F.L.; Antunes, L.M.G.; Rezende, P.A.; Carvalho, F.E.S.; Silva, C.M.D.; Matheus, J.M.; Oliveira, J.V.; Lopes, G.P.; Pereira, G.A.; Balarin, M.A.S. Cytogenetic Analysis in Lymphocytes from Workers Occupationally Exposed to Low Levels of Ionizing Radiation. Environ. Toxicol. Pharmacol. 2007, 23, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Djokovic-Davidovic, J.; Milovanovic, A.; Milovanovic, J.; Antic, V.; Gajic, M. Analysis of Chromosomal Aberrations Frequency, Haematological Parameters and Received Doses by Nuclear Medicine Professionals. J. BUON 2016, 21, 1307–1315. [Google Scholar]
- Dobrzyńska, M.M.; Pachocki, K.A.; Gajowik, A.; Radzikowska, J.; Sackiewicz, A. The Effect Occupational Exposure to Ionizing Radiation on the DNA Damage in Peripheral Blood Leukocytes of Nuclear Medicine Personnel. J. Occup. Health 2014, 56, 379–386. [Google Scholar] [CrossRef]
- Doukali, H.; Ben Salah, G.; Ben Rhouma, B.; Hajjaji, M.; Jaouadi, A.; Belguith-Mahfouth, N.; Masmoudi, M.-L.; Ammar-Keskes, L.; Kamoun, H. Cytogenetic Monitoring of Hospital Staff Exposed to Ionizing Radiation: Optimize Protocol Considering DNA Repair Genes Variability. Int. J. Radiat. Biol. 2017, 93, 1283–1288. [Google Scholar] [CrossRef]
- Eken, A.; Aydin, A.; Erdem, O.; Akay, C.; Sanal, H.T.; Soykut, B.; Sayal, A.; Somuncu, I. Cytogenetic Analysis of Peripheral Blood Lymphocytes of Hospital Staff Occupationally Exposed to Low Doses of Ionizing Radiation. Toxicol. Ind. Health 2010, 26, 273–280. [Google Scholar] [CrossRef] [PubMed]
- El-Benhawy, S.A.; Sadek, N.A.; Behery, A.K.; Issa, N.M.; Ali, O.K. Chromosomal Aberrations and Oxidative DNA Adduct 8-Hydroxy-2-Deoxyguanosine as Biomarkers of Radiotoxicity in Radiation Workers. J. Radiat. Res. Appl. Sci. 2016, 9, 249–258. [Google Scholar] [CrossRef]
- Engin, A.B.; Ergun, M.A.; Yurtcu, E.; Kan, D.; Sahin, G. Effect of Ionizing Radiation on the Pteridine Metabolic Pathway and Evaluation of Its Cytotoxicity in Exposed Hospital Staff. Mutat. Res. 2005, 585, 184–192. [Google Scholar] [CrossRef]
- Fang, L.; Li, J.; Li, W.; Mao, X.; Ma, Y.; Hou, D.; Zhu, W.; Jia, X.; Qiao, J. Assessment of Genomic Instability in Medical Workers Exposed to Chronic Low-Dose X-Rays in Northern China. Dose Response 2019, 17, 1559325819891378. [Google Scholar] [CrossRef]
- Gaetani, S.; Monaco, F.; Bracci, M.; Ciarapica, V.; Impollonia, G.; Valentino, M.; Tomasetti, M.; Santarelli, L.; Amati, M. DNA Damage Response in Workers Exposed to Low-Dose Ionising Radiation. Occup. Environ. Med. 2018, 75, 724–729. [Google Scholar] [CrossRef]
- Garaj-Vrhovac, V.; Kopjar, N. The Alkaline Comet Assay as Biomarker in Assessment of DNA Damage in Medical Personnel Occupationally Exposed to Ionizing Radiation. Mutagenesis 2003, 18, 265–271. [Google Scholar] [CrossRef]
- Gerić, M.; Popić, J.; Gajski, G.; Garaj-Vrhovac, V. Cytogenetic Status of Interventional Radiology Unit Workers Occupationally Exposed to Low-Dose Ionising Radiation: A Pilot Study. Mutat. Res. 2019, 843, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Yoo, K.-Y.; Cho, S.-H. Glycophorin A Mutant Frequency in Radiation Workers at the Nuclear Power Plants and a Hospital. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 2002, 501, 45–56. [Google Scholar] [CrossRef]
- Joseph, L.J.; Patwardhan, U.N.; Samuel, A.M. Frequency of Micronuclei in Peripheral Blood Lymphocytes from Subjects Occupationally Exposed to Low Levels of Ionizing Radiation. Mutat. Res. 2004, 564, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Jovicic, D.; Milacic, S.; Milic, N.; Bukvic, N.; Vukov, T.D. Chromosomal Aberrations in Subjects Exposed to Ionizing Radiation. J. Environ. Pathol. Toxicol. Oncol. 2009, 28, 75–82. [Google Scholar] [CrossRef]
- Jovicić, D.; Milacić, S.; Vukov, T.D.; Rakić, B.; Stevanović, M.; Drakulić, D.; Rakić, R.; Bukvić, N. Detection of Premature Segregation of Centromeres in Persons Exposed to Ionizing Radiation. Health Phys. 2010, 98, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Kasuba, V.; Rozgaj, R.; Jazbec, A. Chromosome Aberrations in Peripheral Blood Lymphocytes of Croatian Hospital Staff Occupationally Exposed to Low Levels of Ionising Radiation. Arh. Hig. Rada Toksikol. 2008, 59, 251–259. [Google Scholar] [CrossRef]
- Khisroon, M.; Khan, A.; Naseem, M.; Ali, N.; Khan, S.; Rasheed, S.B. Evaluation of DNA Damage in Lymphocytes of Radiology Personnel by Comet Assay. J. Occup. Health 2015, 57, 268–274. [Google Scholar] [CrossRef]
- Kopjar, N.; Garaj-Vrhovac, V. Assessment of DNA Damage in Nuclear Medicine Personnel—Comparative Study with the Alkaline Comet Assay and the Chromosome Aberration Test. Int. J. Hyg. Environ. Health 2005, 208, 179–191. [Google Scholar] [CrossRef]
- Kumar, D.; Kumari, S.; Salian, S.R.; Uppangala, S.; Kalthur, G.; Challapalli, S.; Chandraguthi, S.G.; Kumar, P.; Adiga, S.K. Genetic Instability in Lymphocytes Is Associated With Blood Plasma Antioxidant Levels in Health Care Workers Occupationally Exposed to Ionizing Radiation. Int. J. Toxicol. 2016, 35, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Lalić, H.; Lekić, A.; Radosević-Stasić, B. Comparison of Chromosome Aberrations in Peripheral Blood Lymphocytes from People Occupationally Exposed to Ionizing and Radiofrequency Radiation. Acta Med. Okayama 2001, 55, 117–127. [Google Scholar] [CrossRef]
- Little, M.P.; Kwon, D.; Doi, K.; Simon, S.L.; Preston, D.L.; Doody, M.M.; Lee, T.; Miller, J.S.; Kampa, D.M.; Bhatti, P.; et al. Association of Chromosome Translocation Rate with Low Dose Occupational Radiation Exposures in U.S. Radiologic Technologists. Radiat. Res. 2014, 182, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Maffei, F.; Angelini, S.; Forti, G.C.; Lodi, V.; Violante, F.S.; Mattioli, S.; Hrelia, P. Micronuclei Frequencies in Hospital Workers Occupationally Exposed to Low Levels of Ionizing Radiation: Influence of Smoking Status and Other Factors. Mutagenesis 2002, 17, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Maffei, F.; Angelini, S.; Forti, G.C.; Violante, F.S.; Lodi, V.; Mattioli, S.; Hrelia, P. Spectrum of Chromosomal Aberrations in Peripheral Lymphocytes of Hospital Workers Occupationally Exposed to Low Doses of Ionizing Radiation. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 2004, 547, 91–99. [Google Scholar] [CrossRef]
- Maluf, S.W.; Passos, D.F.; Bacelar, A.; Speit, G.; Erdtmann, B. Assessment of DNA Damage in Lymphocytes of Workers Exposed to X-Radiation Using the Micronucleus Test and the Comet Assay. Environ. Mol. Mutagen. 2001, 38, 311–315. [Google Scholar] [CrossRef]
- Martínez, A.; Coleman, M.; Romero-Talamás, C.A.; Frias, S. An Assessment of Immediate DNA Damage to Occupationally Exposed Workers to Low Dose Ionizing Radiation by Using the Comet Assay. Rev. Invest. Clin. 2010, 62, 23–30. [Google Scholar] [PubMed]
- Milacic, S. Frequency of Chromosomal Lesions and Damaged Lymphocytes of Workers Occupationally Exposed to x Rays. Health Phys. 2005, 88, 334–339. [Google Scholar] [CrossRef]
- Milić, M.; Rozgaj, R.; Kašuba, V.; Jazbec, A.-M.; Starčević, B.; Lyzbicki, B.; Ravegnini, G.; Zenesini, C.; Musti, M.; Hrelia, P.; et al. Polymorphisms in DNA Repair Genes: Link with Biomarkers of the CBMN Cytome Assay in Hospital Workers Chronically Exposed to Low Doses of Ionising Radiation. Arh. Hig. Rada Toksikol. 2015, 66, 109–120. [Google Scholar] [CrossRef]
- Movafagh, A.; Farajolah, A.M.; Fadaie, S.; Azargashb, E. Movafagh Persistent Unstable Chromosomal Aberrations in Lymphocytes Of Radiotherapy Workers After 1(St) Mitotic Division In Tehran, Iran. Pak. J. Med. Sci. 2007, 23, 254–258. [Google Scholar]
- Mrdjanović, J.; Jakimov, D.; Tursijan, S.; Bogdanović, G. Evaluation of Sister Chromatid Exchanges, Micronuclei, And Proliferating Rate Index In Hospital Workers Chronically Exposed To Ionizing Radiation. J. BUON 2005, 10, 99–103. [Google Scholar]
- Pajic, J.; Rakic, B.; Jovicic, D.; Milovanovic, A. A Cytogenetic Study of Hospital Workers Occupationally Exposed to Radionuclides in Serbia: Premature Centromere Division as Novel Biomarker of Exposure? Int. Arch. Occup. Environ. Health 2016, 89, 477–484. [Google Scholar] [CrossRef]
- Pajic, J.; Jovicic, D.; Ps Milovanovic, A. Micronuclei as a Marker for Medical Screening of Subjects Continuously Occupationally Exposed to Low Doses of Ionizing Radiation. Biomarkers 2017, 22, 439–445. [Google Scholar] [CrossRef]
- Pakniat, F.; Mozdarani, H.; Nasirian, B.; Faeghi, F. Radioadaptive Response in Peripheral Blood Leukocytes of Occupationally Exposed Medical Staff with Investigation of DNA Damage by the Use of Neutral Comet Assay. Int. J. Radiat. Res. 2013, 11, 91–97. [Google Scholar]
- Qian, Q.-Z.; Cao, X.-K.; Shen, F.-H.; Wang, Q. Effects of Ionising Radiation on Micronucleus Formation and Chromosomal Aberrations in Chinese Radiation Workers. Radiat. Prot. Dosim. 2016, 168, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Raavi, V.; Basheerudeen, S.A.S.; Jagannathan, V.; Joseph, S.; Chaudhury, N.K.; Venkatachalam, P. Frequency of Gamma H2AX Foci in Healthy Volunteers and Health Workers Occupationally Exposed to X-Irradiation and Its Relevance in Biological Dosimetry. Radiat. Environ. Biophys. 2016, 55, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Ropolo, M.; Balia, C.; Roggieri, P.; Lodi, V.; Nucci, M.C.; Violante, F.S.; Silingardi, P.; Colacci, A.; Bolognesi, C. The Micronucleus Assay as a Biological Dosimeter in Hospital Workers Exposed to Low Doses of Ionizing Radiation. Mutat. Res. 2012, 747, 7–13. [Google Scholar] [CrossRef]
- Sahin, A.; Tatar, A.; Oztas, S.; Seven, B.; Varoglu, E.; Yesilyurt, A.; Ayan, A.K. Evaluation of the Genotoxic Effects of Chronic Low-Dose Ionizing Radiation Exposure on Nuclear Medicine Workers. Nucl. Med. Biol. 2009, 36, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Sakly, A.; Gaspar, J.F.; Kerkeni, E.; Silva, S.; Teixeira, J.P.; Chaari, N.; Ben Cheikh, H. Genotoxic Damage in Hospital Workers Exposed to Ionizing Radiation and Metabolic Gene Polymorphisms. J. Toxicol. Environ. Health Part A 2012, 75, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Sakly, A.; Ayed, Y.; Chaari, N.; Akrout, M.; Bacha, H.; Cheikh, H.B. Assessment of Chromosomal Aberrations and Micronuclei in Peripheral Lymphocytes from Tunisian Hospital Workers Exposed to Ionizing Radiation. Genet. Test. Mol. Biomark. 2013, 17, 650–655. [Google Scholar] [CrossRef]
- Santovito, A.; Cervella, P.; Delpero, M. Increased Frequency of Chromosomal Aberrations and Sister Chromatid Exchanges in Peripheral Lymphocytes of Radiology Technicians Chronically Exposed to Low Levels of Ionizing Radiations. Environ. Toxicol. Pharmacol. 2014, 37, 396–403. [Google Scholar] [CrossRef]
- Sari-Minodier, I.; Orsière, T.; Auquier, P.; Martin, F.; Botta, A. Cytogenetic Monitoring by Use of the Micronucleus Assay among Hospital Workers Exposed to Low Doses of Ionizing Radiation. Mutat. Res. 2007, 629, 111–121. [Google Scholar] [CrossRef]
- Scarpato, R.; Antonelli, A.; Ballardin, M.; Cipollini, M.; Fallahi, P.; Tomei, A.; Traino, C.; Barale, R. Analysis of Chromosome Damage in Circulating Lymphocytes of Radiological Workers Affected by Thyroid Nodules. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis 2006, 606, 21–26. [Google Scholar] [CrossRef]
- Shafiee, M.; Borzoueisileh, S.; Rashidfar, R.; Dehghan, M.; Jaafarian Sisakht, Z. Chromosomal Aberrations in C-Arm Fluoroscopy, CT-Scan, Lithotripsy, and Digital Radiology Staff. Mutat. Res. 2020, 849, 503131. [Google Scholar] [CrossRef]
- Siama, Z.; Zosang-Zuali, M.; Vanlalruati, A.; Jagetia, G.C.; Pau, K.S.; Kumar, N.S. Chronic Low Dose Exposure of Hospital Workers to Ionizing Radiation Leads to Increased Micronuclei Frequency and Reduced Antioxidants in Their Peripheral Blood Lymphocytes. Int. J. Radiat. Biol. 2019, 95, 697–709. [Google Scholar] [CrossRef]
- Silva, R.G.; Oliviera Barros Alencar, M.V.; Silva Teixeira, J.; Rodrigues e Silva, R.; Paz, M.F.C.J.; de Castro e Sousa, J.M.; de Aguiar, R.P.S.; de Carvalho, R.M.; Gomerz, A.L., Jr.; da Mata, A.M.O.F.; et al. Genotoxicity and DNA Repair Indicative in Blood Cells after Occupational Exposure to Ionizing Radiation. Int. Arch. Med. 2016, 9. [Google Scholar] [CrossRef]
- Surniyantoro, H.N.E.; Lusiyanti, Y.; Rahardjo, T.; Nurhayati, S.; Tetriana, D. Association between XRCC1 Exon 10 (Arg399Gln) Gene Polymorphism and Micronucleus as a Predictor of DNA Damage among Radiation Workers. Biodiversitas J. Biol. Divers. 2018, 19, 1676–1682. [Google Scholar] [CrossRef]
- Thierens, H.; Vral, A.; Morthier, R.; Aousalah, B.; de Ridder, L. Cytogenetic Monitoring of Hospital Workers Occupationally Exposed to Ionizing Radiation Using the Micronucleus Centromere Assay. Mutagenesis 2000, 15, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Tug, E.; Kayhan, G.; Kan, D.; Guntekin, S.; Ergun, M.A. The Evaluation of Long-Term Effects of Ionizing Radiation through Measurement of Current Sister Chromatid Exchange (SCE) Rates in Radiology Technologists, Compared with Previous SCE Values. Mutat. Res. 2013, 757, 28–30. [Google Scholar] [CrossRef]
- Vellingiri, B.; Shanmugam, S.; Subramaniam, M.D.; Balasubramanian, B.; Meyyazhagan, A.; Alagamuthu, K.; Prakash, V.; Shafiahammedkhan, M.; Kathannan, S.; Pappuswamy, M.; et al. Cytogenetic Endpoints and Xenobiotic Gene Polymorphism in Lymphocytes of Hospital Workers Chronically Exposed to Ionizing Radiation in Cardiology, Radiology and Orthopedic Laboratories. Ecotoxicol. Environ. Saf. 2014, 100, 266–274. [Google Scholar] [CrossRef]
- Vral, A.; Decorte, V.; Depuydt, J.; Wambersie, A.; Thierens, H. A Semi-Automated FISH-Based Micronucleus-Centromere Assay for Biomonitoring of Hospital Workers Exposed to Low Doses of Ionizing Radiation. Mol. Med. Rep. 2016, 14, 103–110. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Q.; Liu, G.; Tian, Y.; Zhang, F.; Qu, J.; Lim, D.; Feng, Z. The Comparison of Health Status Between Male and Female Medical Radiation Workers in China. Radiat. Prot. Dosim. 2017, 175, 508–516. [Google Scholar] [CrossRef]
- Zakeri, F.; Assaei, R.; Varzegar, R. Chromosomal Aberrations in Workers Occupationally Exposed to Chronic Low-Level Ionizing Radiation. Occup. Environ. Med. 2003, 9, 33–38. [Google Scholar]
- Zakeri, F.; Assaei, R.G. Cytogenetic Monitoring of Personnel Working in Angiocardiography Laboratories in Iran Hospitals. Mutat. Res. 2004, 562, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, F.; Hirobe, T. A Cytogenetic Approach to the Effects of Low Levels of Ionizing Radiations on Occupationally Exposed Individuals. Eur. J. Radiol. 2010, 73, 191–195. [Google Scholar] [CrossRef]
- Zakeri, F.; Hirobe, T.; Akbari Noghabi, K. Biological Effects of Low-Dose Ionizing Radiation Exposure on Interventional Cardiologists. Occup. Med. 2010, 60, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.D.; Yao, L.; Guo, K.M.; Lu, C.W. Cytogenetic Evaluation of Cataract Patients Occupationally Exposed to Ionizing Radiation in Northeast China. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Bhatti, P.; Doody, M.M.; Preston, D.L.; Kampa, D.; Ron, E.; Weinstock, R.W.; Simon, S.; Edwards, A.A.; Sigurdson, A.J. Increased Frequency of Chromosome Translocations Associated with Diagnostic X-Ray Examinations. Radiat. Res. 2008, 170, 149–155. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oestreicher, U.; Samaga, D.; Ainsbury, E.; Antunes, A.C.; Baeyens, A.; Barrios, L.; Beinke, C.; Beukes, P.; Blakely, W.F.; Cucu, A.; et al. RENEB Intercomparisons Applying the Conventional Dicentric Chromosome Assay (DCA). Int. J. Radiat. Biol. 2017, 93, 20–29. [Google Scholar] [CrossRef]
- Silva-Barbosa, I.; Pereira-Magnata, S.; Amaral, A.; Sotero, G.; Melo, H.C. Dose Assessment by Quantification of Chromosome Aberrations and Micronuclei in Peripheral Blood Lymphocytes from Patients Exposed to Gamma Radiation. Genet. Mol. Biol. 2005, 28, 452–457. [Google Scholar] [CrossRef]
- Rothkamm, K.; Beinke, C.; Romm, H.; Badie, C.; Balagurunathan, Y.; Barnard, S.; Bernard, N.; Boulay-Greene, H.; Brengues, M.; de Amicis, A.; et al. Comparison of Established and Emerging Biodosimetry Assays. Radiat. Res. 2013, 180, 111–119. [Google Scholar] [CrossRef][Green Version]
- Iwasaki, T.; Takashima, Y.; Suzuki, T.; Yoshida, M.A.; Hayata, I. The Dose Response of Chromosome Aberrations in Human Lymphocytes Induced in Vitro by Very Low-Dose γ Rays. Radiat. Res. 2011, 175, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Miura, T.; Yoshida, M.A.; Ujiie, R.; Kurosu, Y.; Kato, N.; Katafuchi, A.; Tsuyama, N.; Ohba, T.; Inamasu, T.; et al. Increase in Dicentric Chromosome Formation after a Single CT Scan in Adults. Sci. Rep. 2015, 5, 13882. [Google Scholar] [CrossRef]
- Golfier, S.; Jost, G.; Pietsch, H.; Lengsfeld, P.; Eckardt-Schupp, F.; Schmid, E.; Voth, M. Dicentric Chromosomes and Gamma-H2AX Foci Formation in Lymphocytes of Human Blood Samples Exposed to a CT Scanner: A Direct Comparison of Dose Response Relationships. Radiat. Prot. Dosim. 2009, 134, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Fujioka, K.; Sakurai-Ozato, N.; Fukumoto, W.; Satoh, K.; Sun, J.; Awazu, A.; Tanaka, K.; Ishida, M.; Ishida, T.; et al. Chromosomal Abnormalities in Human Lymphocytes after Computed Tomography Scan Procedure. Radiat. Res. 2018, 190, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.D. Low-Dose Ionizing Radiation and Chromosome Translocations: A Review of the Major Considerations for Human Biological Dosimetry. Mutat. Res. 2008, 659, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Sommer, S.; Buraczewska, I.; Kruszewski, M. Micronucleus Assay: The State of Art, and Future Directions. Int. J. Mol. Sci. 2020, 21, 1534. [Google Scholar] [CrossRef] [PubMed]
- Aguiar Torres, L.; dos Santos Rodrigues, A.; Linhares, D.; Camarinho, R.; Nunes Páscoa Soares Rego, Z.M.; Ventura Garcia, P. Buccal Epithelial Cell Micronuclei: Sensitive, Non-Invasive Biomarkers of Occupational Exposure to Low Doses of Ionizing Radiation. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis 2019, 838, 54–58. [Google Scholar] [CrossRef]
- Padilla-Raygoza, N.; Del Rocio Adame Gutiérrez, M.; Martínez, I.Z.M.; Beltran-Campos, V.; Del Carmen Delgado-Sandoval, S.; de Lourdes Garcia-Campos, M.; Sosa-Aquino, M.A.; Cordova-Fraga, T.; Guzman-Cabrera, R. Evaluation of Micronuclei in Oral Mucosa of Individuals Exposed to Ionizing Radiation: A Pilot Study from Celaya, México. Cent. Asian J. Glob. Health 2019, 8, 331. [Google Scholar] [CrossRef]
- Vral, A.; Fenech, M.; Thierens, H. The Micronucleus Assay as a Biological Dosimeter of in Vivo Ionising Radiation Exposure. Mutagenesis 2011. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Schneider, E.L. In Vivo Sister-Chromatid Exchange: A Sensitive Measure of DNA Damage. Mutat. Res. 1979, 60, 329–337. [Google Scholar] [CrossRef]
- Lialiaris, T.S. Sister Chromatid Exchange. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Maloy, S., Hughes, K., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 454–457. ISBN 978-0-08-096156-9. [Google Scholar]
- Olive, P.L.; Banáth, J.P.; Durand, R.E. Heterogeneity in Radiation-Induced DNA Damage and Repair in Tumor and Normal Cells Measured Using the “Comet” Assay. Radiat. Res. 1990, 122, 86–94. [Google Scholar] [CrossRef]
- Møller, P.; Loft, S.; Ersson, C.; Koppen, G.; Dusinska, M.; Collins, A. On the Search for an Intelligible Comet Assay Descriptor. Front. Genet. 2014, 5. [Google Scholar] [CrossRef]
- Gedik, C.M.; Ewen, S.W.; Collins, A.R. Single-Cell Gel Electrophoresis Applied to the Analysis of UV-C Damage and Its Repair in Human Cells. Int. J. Radiat. Biol. 1992, 62, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R.; Dobson, V.; Dušinská, M.; Kennedy, G.; Štětina, R. The Comet Assay: What Can It Really Tell Us? Mutat. Res./Fundam. Mol. Mech. Mutagenesis 1997, 375, 183–193. [Google Scholar] [CrossRef]
- Hagmar, L.; Brøgger, A.; Hansteen, I.L.; Heim, S.; Högstedt, B.; Knudsen, L.; Lambert, B.; Linnainmaa, K.; Mitelman, F.; Nordenson, I. Cancer Risk in Humans Predicted by Increased Levels of Chromosomal Aberrations in Lymphocytes: Nordic Study Group on the Health Risk of Chromosome Damage. Cancer Res. 1994, 54, 2919–2922. [Google Scholar]
- Vodicka, P.; Polivkova, Z.; Sytarova, S.; Demova, H.; Kucerova, M.; Vodickova, L.; Polakova, V.; Naccarati, A.; Smerhovsky, Z.; Ambrus, M.; et al. Chromosomal Damage in Peripheral Blood Lymphocytes of Newly Diagnosed Cancer Patients and Healthy Controls. Carcinogenesis 2010, 31, 1238–1241. [Google Scholar] [CrossRef][Green Version]
- Fenech, M.; Knasmueller, S.; Bolognesi, C.; Holland, N.; Bonassi, S.; Kirsch-Volders, M. Micronuclei as Biomarkers of DNA Damage, Aneuploidy, Inducers of Chromosomal Hypermutation and as Sources of pro-Inflammatory DNA in Humans. Mutat. Res. 2020, 786, 108342. [Google Scholar] [CrossRef] [PubMed]
- Kirsch-Volders, M.; Bolognesi, C.; Ceppi, M.; Bruzzone, M.; Fenech, M. Micronuclei, Inflammation and Auto-Immune Disease. Mutat. Res. 2020, 786, 108335. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Pieper, D.; Buechter, R.; Jerinic, P.; Eikermann, M. Overviews of Reviews Often Have Limited Rigor: A Systematic Review. J. Clin. Epidemiol. 2012, 65, 1267–1273. [Google Scholar] [CrossRef]
- Jacob, S.; Scanff, P.; Bertrand, A.; Laurier, D.; Bernier, M.-O. Use of Personal Radiation Protection Tools and Individual Dosimetric Monitoring in a Sample of Interventional Cardiologists in France, 2005–2009. Radioprotection 2014, 49, 257–260. [Google Scholar] [CrossRef]
- Andreassi, M.G.; Cioppa, A.; Botto, N.; Joksic, G.; Manfredi, S.; Federici, C.; Ostojic, M.; Rubino, P.; Picano, E. Somatic DNA Damage in Interventional Cardiologists: A Case-Control Study. FASEB J. 2005, 19, 998–999. [Google Scholar] [CrossRef]
- Bauchinger, M. Quantification of Low-Level Radiation Exposure by Conventional Chromosome Aberration Analysis. Mutat. Res./Rev. Genet. Toxicol. 1995, 339, 177–189. [Google Scholar] [CrossRef]
- Olivieri, G.; Bodycote, J.; Wolff, S. Adaptive Response of Human Lymphocytes to Low Concentrations of Radioactive Thymidine. Science 1984, 223, 594–597. [Google Scholar] [CrossRef]
- Hou, J.; Wang, F.; Kong, P.; Yu, P.K.N.; Wang, H.; Han, W. Gene Profiling Characteristics of Radioadaptive Response in AG01522 Normal Human Fibroblasts. PLoS ONE 2015, 10, e0123316. [Google Scholar] [CrossRef]
- Ebrahimian, T.G.; Beugnies, L.; Surette, J.; Priest, N.; Gueguen, Y.; Gloaguen, C.; Benderitter, M.; Jourdain, J.R.; Tack, K. Chronic Exposure to External Low-Dose Gamma Radiation Induces an Increase in Anti-Inflammatory and Anti-Oxidative Parameters Resulting in Atherosclerotic Plaque Size Reduction in ApoE-/- Mice. Radiat. Res. 2018, 189, 187–196. [Google Scholar] [CrossRef]
- Frey, B.; Hehlgans, S.; Rödel, F.; Gaipl, U.S. Modulation of Inflammation by Low and High Doses of Ionizing Radiation: Implications for Benign and Malign Diseases. Cancer Lett. 2015, 368, 230–237. [Google Scholar] [CrossRef]
- Luzhna, L.; Kovalchuk, O. Low Dose Irradiation Profoundly Affects Transcriptome and MicroRNAme in Rat Mammary Gland Tissues. Oncoscience 2014, 1, 751–762. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rödel, F.; Frey, B.; Manda, K.; Hildebrandt, G.; Hehlgans, S.; Keilholz, L.; Seegenschmiedt, M.H.; Gaipl, U.S.; Rödel, C. Immunomodulatory Properties and Molecular Effects in Inflammatory Diseases of Low-Dose x-Irradiation. Front. Oncol. 2012, 2, 120. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.R.; Kovacs, J.J.; Whalen, E.J.; Rajagopal, S.; Strachan, R.T.; Grant, W.; Towers, A.J.; Williams, B.; Lam, C.M.; Xiao, K.; et al. A Stress Response Pathway Regulates DNA Damage through β 2-Adrenoreceptors and β-Arrestin-1. Nature 2011, 477, 349–353. [Google Scholar] [CrossRef]
- Huang, P.; Huang, B.; Weng, H.; Nakayama, K.; Morimoto, K. Effects of Lifestyle on Micronuclei Frequency in Human Lymphocytes in Japanese Hard-Metal Workers. Prev. Med. 2009, 48, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Flint, M.S.; Baum, A.; Chambers, W.H.; Jenkins, F.J. Induction of DNA Damage, Alteration of DNA Repair and Transcriptional Activation by Stress Hormones. Psychoneuroendocrinology 2007, 32, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Forlenza, M.J.; Latimer, J.J.; Baum, A. The Effects of Stress on DNA Repair Capacity. Psychol. Health 2000, 15, 881–891. [Google Scholar] [CrossRef]
- Antoni, M.H.; Lutgendorf, S.K.; Cole, S.W.; Dhabhar, F.S.; Sephton, S.E.; McDonald, P.G.; Stefanek, M.; Sood, A.K. The Influence of Bio-Behavioural Factors on Tumour Biology: Pathways and Mechanisms. Nat. Rev. Cancer 2006, 6, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Niazi, Y.; Thomsen, H.; Smolkova, B.; Vodickova, L.; Vodenkova, S.; Kroupa, M.; Vymetalkova, V.; Kazimirova, A.; Barancokova, M.; Volkovova, K.; et al. DNA Repair Gene Polymorphisms and Chromosomal Aberrations in Healthy, Nonsmoking Population. DNA Repair 2021, 101, 103079. [Google Scholar] [CrossRef]
- Scholten, B.; Vlaanderen, J.; Stierum, R.; Portengen, L.; Rothman, N.; Lan, Q.; Pronk, A.; Vermeulen, R. A Quantitative Meta-Analysis of the Relation between Occupational Benzene Exposure and Biomarkers of Cytogenetic Damage. Environ. Health Perspect. 2020, 128, 87004. [Google Scholar] [CrossRef] [PubMed]


| PECO elements | PECO Question Formulation: What genotoxicity biomarkers can be used in future similar reviews or future prospective epidemiological studies to examine their association with long-term health outcomes following IR-exposure? |
| Population | All studies involving medical workers, regardless of the profession and service |
| Exposure | Studies dealing with ionizing radiation from medical sources and containing dose estimates or surrogates |
| Comparator |
|
| Outcome | Frequencies of micronuclei or chromosome aberrations or sister chromatid exchanges, telomere length and DNA damage parameters |
| Study | Country Sample Size Design | Healthcare Services, Department, Units, Occupations of Hospital Workers Exposed to IR | Exposure Assessment (Mean of Exposure) a and/or Type of Exposure | Biomarker(s) | Major Results | NOS Score |
|---|---|---|---|---|---|---|
| Andreassi et al. (2009) [27] | Italy N = 77 40 exposed/ 37 unexposed | Cardiac catheterization laboratories (interventional cardiologists) | Badge doses (1.6 ± 2.4 mSv for the last 6 months) DOE to IR (12.0 ± 9.9 years) | MN | 19.7 ± 7.8 (E); 13.5 ± 6.3 (NE) R: 0.265 β = 0.34 (p = 0.004) | 6 |
| Andreassi et al. (2015) [28] | Italy N = 445 223 exposed/ 222 unexposed | Catheterization laboratories | Lifetime cumulative professional exposure reconstruction (21.1 ± 26.3 mSv) DOE to IR (12.2 ± 8.3 years) ORRS (18.5 ± 20) | Leukocyte telomere length | R: −0.319 β = −0.14 (p = 0.03) | 6 |
| Andreassi et al. (2020) [29] | Italy N = 130 83 exposed/ 47 unexposed | Catheterization laboratories (Cath lab workers) | DOE to IR (median = 6 years (IQR 1–25)) ORRS (median = 11 (IQR = 1–63)) | Copy number status (microdeletion and microduplication) in AZFc region for two markers | ORadjusted (SY1197) = 2.66 (95% CI: 1.09–6.31), p = 0.02 | 6 |
| Angelini et al. (2005) [30] | Germany N = 42 21 exposed/ 21 unexposed | Units of Radiology Radiotherapy Cardiology (physicians and technicians) | Badge doses (40.6 ± 37.7 mSv) | MN | MN: 8.6 ± 2.8 (E); 6.7 ± 2.7 (NE) β = 0.004 (p = 0.941) | 6 |
| Bhatti et al. (2007) [31] | USA N = 152 Cohort | Radiologic technologists who began working before 1950 (USRT study) | Estimated cumulative occupational red bone marrow radiation dose score (1.9 ± 1.4 cGy) | FISH for translocations | ERR = 0.09/100 CE per mGy (95% CI −0.01–0.20, p = 0.07) | 7 |
| Bouraoui et al. (2013) [32] | Tunisia N = 110 67 exposed/ 43 unexposed | Nuclear medicine Radiology Orthopedic Radiotherapy Physiology Cardiology departments | DOE to IR (18.4 ± 9.3 years) X-ray, γ-ray,125I, 131I, 57CO, etc. | MN | 13.6 ± 4.9 (E); 6.5 ± 4.2 (NE) β = 0.7 (p = 0.04) | 6 |
| Caradonna (2015) [33] | Italy N = 110 56 exposed/ 54 unexposed | (clinicians, technicians, attendants) | - | NPB CA (Chromatid breaks, chromosomal breaks, dicentrics, radial configurations) | CA: 2.87 ± 0.17 (E); 1.15 ± 0.05 (NE) NPB: 5.18 ± 0.23 (E); 1.42 ± 0.15 (NE) | 3 |
| Cigarran et al. (2001) [34] | Spain N = 38 20 exposed 18 unexposed | Badge doses (38.1 ± 31.7 mSv) | CA (translocations, dicentrics) | Translocations: 1.04 ± 0.11 (E); 0.90 ± 0.12 (NE) Dicentrics: 0.09 ± 0.03 (E); 0.15 ± 0.04 (NE) | 4 | |
| Dias et al. (2007) [35] | Brazil N = 72 36 exposed/ 36 unexposed | Radiology Units (physicians, technicians) | DOE to IR (6.5 ± 5.0 years) | CA (chromatid breaks, chromosome breaks, exchange figure) MN | MN: 6.13 ± 3.18 (E); 5.11 ± 3.85 (NE) CA: 2.60 ± 2.00 (E); 2.30 ± 1.80 (NE) | 5 |
| Djokovic et al. (2016) [36] | Serbia N = 65 Cohort | Nuclear Medicine Centre | Badge doses | CA (dicentrics, acentrics, rings, chromatid lesions, isochromatid lesions) | No significant difference for dicentrics, rings, chromatid lesions between the initial and periodical medical examinations (during exposure), but significant increase of acentric fragments | 6 |
| Dobrzynska et al. (2014) [37] | Poland N = 86 46 exposed/ 40 unexposed | Nuclear Medicine Oncological Endocrinology (doctors, nurses, technicians, radiochemists and administrative staff) | Badge doses (0.3 ± 0.2 mSv/year) DOE to IR (8.5 ± 6.7 years) | TM % DNA | TM: 0.90 ± 1.09 (E); 0.30 ± 0.44 (NE) %DNA: 1.60 ± 1.50 (E); 0.78 ± 0.54 (NE) | 6 |
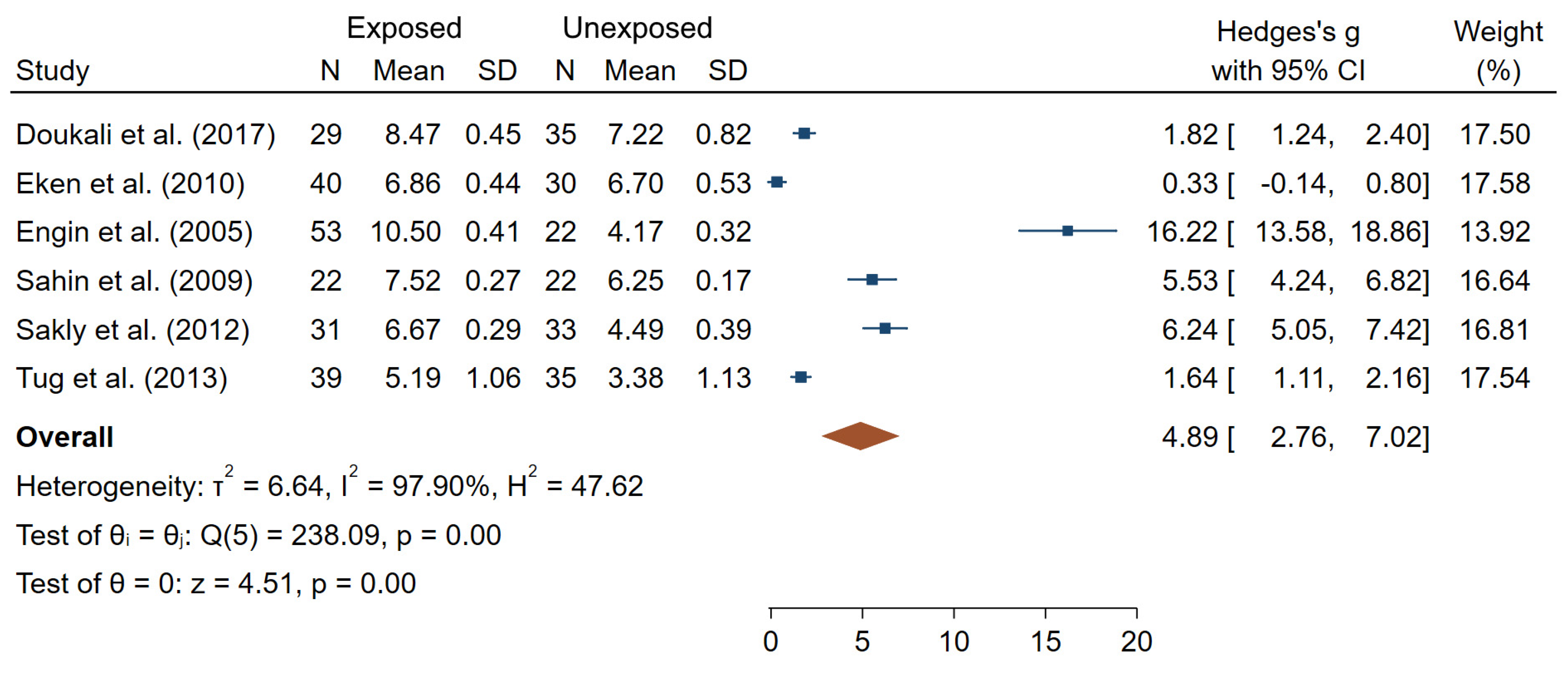
| Doukali et al. (2017) [38] | Tunisia N = 64 29 exposed/ 35 unexposed | Radiotherapy Radiology departments | DOE to IR (8.8 ± 4.1 years in Group I, 20.1 ± 4.7 years in Group II) | MN SCE | MN: 1.16 ± 0.65 (E); 0.46 ± 0.21 (NE) SCE: 8.47 ± 0.45 (E); 7.22 ± 0.82 (NE) | 4 |
| Eken et al. (2010) [39] | Turkey N = 70 40 exposed/ 30 unexposed | Radiology unit (physicians, technicians) | Badge doses (median = 0.17 (range 0.10–3.86 in the last 6 months) | MN SCE | MN: 6.88 ± 2.54 (E); 5.50 ± 2.00 (NE) SCE: 6.86 ± 0.44 (E); 6.70 ± 0.53 (NE) | 7 |
| El-Benhawy et al. (2016) [40] | Egypt N = 90 60 exposed/ 30 unexposed | Radiotherapy Diagnostic radiology Industrial radiographers | Badge doses (2.9 ± 1.9 mSv/year in radiologists, 3.1 ± 1.5 mSv/year in radiotherapists) | CA (gaps, breaks, fragments and dicentrics) | All types of CA in (E) significantly higher than in (NE) | 6 |
| Engin et al. (2005) [41] | Turkey N = 75 20 + 33 exposed/ 22 unexposed | Radiotherapy Radio-diagnostic | Badge doses DOE to IR (11.2 ± 0.8 years in X-ray group, 6.5 ± 0.9 in γ-rays group) γ-rays and X-rays | SCE | 10.50 ± 0.41 (E); 4.17 ± 0.32 (NE) | 4 |
| Fang et al. (2019) [42] | China N = 334 175 exposed/ 159 unexposed | Badge doses (38.4 ± 27.4 mSv) X-ray radiation | CA (dicentrics, ring, and acentric fragments) MN % DNA TM & Olive TM | MN, CA, %DNA, TM significantly greater for (E) compared to (NE) | 8 | |
| Gaetani et al. (2018) [43] | Italia N = 248 116 exposed/ 132 unexposed | Department of Nuclear Medicine Radiology Interventional Radiology Oncological Radiotherapy (doctors, nurses, technicians and radiochemists) | Badge doses (1.9 ± 1.6 mSv in group with accumulated IR dose <6 mSv; 34.0 ± 30.4 in group with accumulated IR dose >6 mSv) | DNA SBs | No difference in SBs frequencies between IR dose groups | 6 |
| Gao et al. (2020) [23] | China N = 336 218 exposed/ 118 unexposed | Diagnostic radiology Radiotherapy Interventional radiology Nuclear medicine (technicians, physicians and nurses) | Badge doses (median = 0.5 mSv (IQR = 0.4–0.7)) | MN | MN (median, IQR): 3 (1, 5) (E); 2 (0.75, 4) (NE)) | 7 |
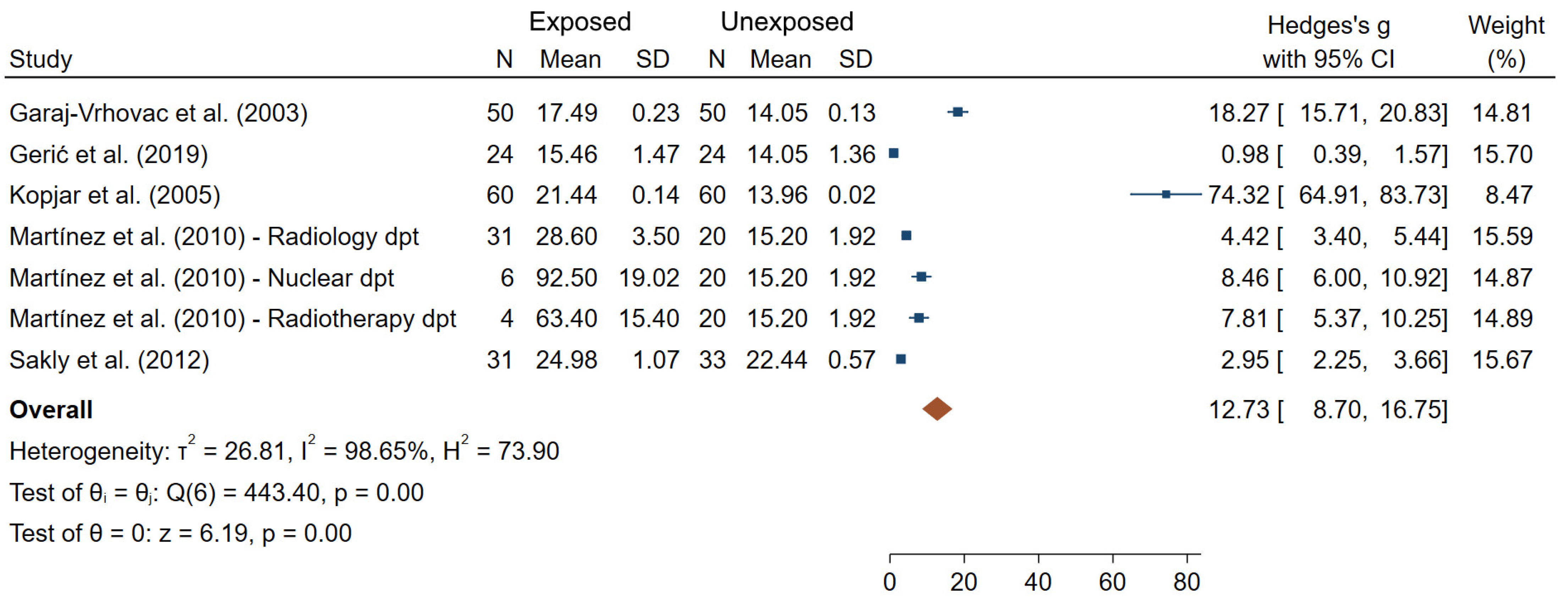
| Garaj-Vrhovac et al. (2003) [44] | Croatia N = 100 50 exposed/ 50 unexposed | Radiology Surgery | Badge doses (range 0–8548 μSv in the previous year) | TL TM | TL: 14.85 ± 0.21 (E); 11.46 ± 0.15 (NE) TM: 17.49 ± 0.23 (E); 14.05 ± 0.13 (NE) | 5 |
| Gerić et al. (2019) [45] | Croatia N = 48 24 exposed/ 24 unexposed | Badge doses (1.8 ± 3.6 mSv over the last year) X-rays | MN NPB TL %DNA | MN: 5.74 ± 3.89 (E); 5.38 ± 2.63 (NE) NPB: 1.61 ± 1.08 (E); 1.38 ± 1.65 (NE) TL: 15.46 ± 1.47 (E); 14.05 ± 1.36 (NE) %DNA: 1.57 ± 0.47 (E); 1.49 ± 0.89 (NE) | 6 | |
| Gharibdousty et al. (2017) [24] | Iran N = 70 35 exposed/ 35 unexposed | (Radiopharmacists) | Badge doses (6.6 ± 5.8 mSv in the last year) | MN NPB | MN: 25.82 ± 8.67 (E); 10.52 ± 6.83 (NE) NPB: 1.02 ± 0.02 (E); 0.85 ± 0.37 (NE) | 6 |
| Ha et al. (2002) [46] | Korea N = 176 Cross sectional | 144 workers in two nuclear power plants 32 workers in one university hospital | Badge doses (0.9 ± 1.3 cGy for hospital workers) | Glycophorin A mutant assay (NO or NN variants) | NO variant: β = 1.88 (p = 0.003) NN variant: β = 2.23 (p = 0.0001) | 3 |
| Joseph et al. (2004) [47] | India N = 73 46 exposed/ 27 unexposed | Nuclear Medicine | Badge doses (range 0.25–62.9 mSv) | MN | 9.80 ± 6.20 (E); 7.00 ± 3.80 (NE) | 6 |
| Jovicic et al. (2009) [48] | Serbia N = 94 30 exposed/ 64 unexposed | Badge doses DOE to IR (years) X-ray | CA (chromatid and chromosome breaks, acentrics, dicentrics and rings) | Aberrant cells: 3.40 ± 1.80 (E); 0.80 ± 0.90 (NE) | 6 | |
| Jovicic et al. (2010) [49] | Serbia N = 53 30 exposed/ 23 unexposed | Badge doses (13.3 mSv (range 4.81−24.76)) DOE to IR (12.7 ± 7.4 years) X-rays | CA (chromatid and chromosome breaks, acentrics, dicentrics and rings) PCD | CA and PCD significantly higher in (E) compared to (NE) (except rings) R Total life effective dose-PCD = 0.71 (p < 0.001) | 6 | |
| Kasuba et al. (2008) [50] | Croatia N = 785 765 exposed/ 200 unexposed | Anesthesiologists, anesthetic technicians, radiology technicians, operating room nurses, surgeons, nurses, radiologists, and urologists/gynecologists | DOE to IR (12.1 ± 8.40 to 15.8 ± 9.8 years) | CA (dicentrics and rings, acentric fragments, and tri- and tetra-radial exchanges) | CA significantly higher in (E) compared to (NE) (except rings) | 5 |
| Khisroon et al. (2015) [51] | Pakistan N = 144 74 exposed/ 70 unexposed | Radiology personnel | DOE to IR (7.8 ± 5.3 years) | CS | CS: 129.8 ± 17.2 (E); 53.0 ± 25.0 (NE) R DOE-CS = 0.62 (p < 0.001) | 6 |
| Kopjar et al. (2005) [52] | Croatia N = 120 60 exposed/ 60 unexposed | Nuclear medicine physicians, technical experts, engineers, nurses, cleaners | Badge dose (196 µSv (range 0–1401) Radionuclides (dominantly 131I and 99mtc) | TL CA (number of sister chromatids and breakage events) | TL: 21.44 ± 0.14μm (E); 13.96 ± 0.02μm (NE) CA mean: 2.37 ± 0.16 (E); 0.85 ± 0.09 (NE) Aberrant cells: 1.15 ± 0.08; 0.23 ± 0.06 (NE) | 6 |
| Kumar et al. (2016) [53] | India N = 134 83 exposed/ 51 unexposed | Diagnostic or therapeutic radiation facilities | Badge doses DOE to IR (6.5 ± 0.7 years) | MN CA (aneuploidy, acentric fragments) | CA and MN frequencies significantly higher in (E) compared to (NE) | 5 |
| Lalic et al. (2001) [54] | Croatia N = 45 25 IR-exposed/ 20 non-IR exposed | (Medical radiology, X-rays technicians, nurses) | Badge doses | CA (chromatid and chromosome breaks, acentric fragments, dicentrics) | Total number of CA: 4.08 ± 0.37 (E); 4.35 ± 0.50 (NIR) R with 6-years exposure dose = 0.62 | 4 |
| Little et al. (2014) [55] | USA N = 238 Cohort | USRT study CTS-I: inclusions in 1994–1995 CTS-II: inclusions in 2003 | Estimated cumulative personal-diagnostic-medical Occupational red bone marrow radiation dose scores | FISH for translocations | Translocation rates in relationship to occupational and personal-diagnostic-medical (PDM) doses = 7.0 (95% CI 1.2, 12.9) × 10−2 translocations Gy−1 | 7 |
| Maffei et al. (2002) [56] | Italy N = 74 37 exposed/ 37 unexposed | (Physicians and technicians) | Badge doses (35.1 ± 40.8 mSv) X and γ-rays | MN | MN: 6.78 ± 4.92 (E); 5.54 ± 2.99 (NE) | 6 |
| Maffei et al. (2004) [57] | Italy N = 69 34 exposed/ 35 unexposed | (Physicians and technicians) | Badge doses (35.8 ± 38.9 mSv) X and γ-rays IR-exposure for at least 3 years | CA (chromatid breaks, chromatid exchanges, chromosome breaks and chromosome exchanges) | Aberrant cells: 2.87 ± 3.10 (E); 1.08 ± 1.03 (NE) | 6 |
| Maluf et al. (2001) [58] | Brazil N = 44 22 exposed/ 22 unexposed | Badge doses (range 0.2–121.8 mSv) X-rays | MN NPB CS | MN: 8.84 ± 2.35 (E); 7.18 ± 2.59 (NE) NPB: 2.98 ± 1.57 (E); 1.96 ± 1.04 (NE) CS: 17.73 ± 10.51 (E); 8.54 ± 7.11 (NE) | 6 | |
| Martínez et al. (2010) [59] | Mexico N = 61 41 exposed/ 20 unexposed | Nuclear Medicine Radiotherapy Radiology | Badge doses (0.21 ± 0.02 mSv/month, 0.4 ± 0.2 mSv/month, 0.17 ± 0.02 in Nuclear Medicine, radiotherapy, and radiology departments respectively) | TL | Radiology: 28.6 ± 3.50 (E); 15.2 ± 1.92 (NE) Nuclear: 92.5 ± 19.02 (E); 15.2 ± 1.92 (NE) Radiotherapy: 63.4 ± 15.4 (E); 15.2 ± 1.92 (NE) | 4 |
| Milacic et al. (2005) [60] | Serbia and Montenegro N = 68 46 exposed/ 22 unexposed | Badge doses (7.9 ± 5.0 mSv) DOE to IR (10.6 ± 6.4 years) X-rays IR-exposure for at least 3 years | CA (dicentrics, rings and acentric fragment, breaks, exchanges) | CA frequencies correlate with absorbed doses. During breaks of exposure, number of damaged cells decreased Time necessary for aberrations to disappear not in relation with former frequency of aberrations or DOE and absorbed dose | 5 | |
| Milic et al. (2015) [61] | Croatia N = 147 77 exposed/ 70 unexposed | DOE to IR (13.7 ± 8.9 years) | MN NPB | MN: 16.20 ± 10.40 (E); 11.50 ± 9.40 (NE) β = 0.403 (p = 0.003) NPB: 0.90 ± 1.50 (E); 1.70 ± 4.00 (NE) β = 0.024 (p = 0.230) | 6 | |
| Movafagh et al. (2007) [62] | Iran N = 93 50 exposed/ 43 unexposed | Radiotherapy | Badge doses X-rays IR-exposure for at least 5 years | CA (Dicentrics, Fragments and Rings) | Total CA: 3.40 ± 1.18 (E); 2.00 ± 0.82 (NE) | 6 |
| Mrdjanovic et al. (2005) [63] | Serbia-Montenegro N = 45 30 exposed/ 15 unexposed | Radiotherapy Cardiology | DOE to IR (11.9 ± 9.04 years) | SCE MN | MN for Radiology group: 15.00 ± 9.39 (E); 9.06 ± 3.23 (NE) SCE: no significant difference between (E) and (NE) | 5 |
| Pajic et al. (2016) [64] | Serbia N = 90 50 exposed/ 40 unexposed | Badge doses (9.9 ± 6.8 mSv in last 5 years) DOE to IR (18.0 ± 8.1 years) Radionuclides (Y90 and I131) | CA (chromatid and isochromatid breaks, acentrics, dicentrics and rings) MN PCD | MN (ratio per number of analyzed cells): 1/48.26 (E); 1/117.3 (NE) Dicentrics (ratio): 1/1600 (E); 1/303.03 (NE) Acentrics (ratio): 1/533 (E); 1/75.75 (NE) Chromatid breaks (ratio): 1/615 (E); 1/294 (NE) Isochromatid breaks (ratio): 1/1143 (E); 1/400 (NE) PCD (ratio): 1/800 (E); 1/94.33 (NE) R for DOE-aberrant cells = 0.77 R for DOE-MN = 0.82 R for DOE-PCD = 0.65 | 5 | |
| Pajic et al. (2017) [65] | Serbia N = 402 201 exposed/ 201 unexposed | Radiology | DOE to IR (15.1 ± 7.4 years) X-rays | MN NPB | MN: 15.15 ± 5.82 (E); 8.31 ± 3.88 (NE) NPB: 0.75 ± 0.85 (E); 0.23 ± 0.47 (NE) | 7 |
| Pakniat et al. (2016) [66] | Iran N = 40 20 exposed/ 20 unexposed | Radiology CT scan | Badge doses | CS | No significant difference between (E) and (NE) at baseline After irradiation by 4mGy, DNA damage frequencies significantly lower in (E) compared to (NE) | 4 |
| Qian et al. (2016) [67] | China N = 1535 1392 exposed/ 143 unexposed | Radiodiagnostic Radiotherapy | Badge doses (13.7 mSv (range 0.2–19.8)) | MN CA (dicentric, centric ring, and acentric fragment, translocation, inversion, insertion, and deletion) with FISH | Frequencies of CA and MN rates in (E) significantly higher than (NE) (0.68 vs. 0.22%, and 2.44 vs. 1.72‰ respectively) | 6 |
| Raavi et al. (2016) [68] | India N = 150 20 exposed/ 130 unexposed | Radiology (physicians, staff) | Badge doses (range 0.02–0.40) | γ-H2AX foci | Mean γ-H2AX foci: 0.066 ± 0.005 (E); 0.042 ± 0.001 (NE) | 5 |
| Ropolo et al. (2012) [69] | Italy N = 60 30 exposed/ 30 unexposed | Badge doses (19.5 ± 37.59 mSv) DOE to IR (12.5 ± 9.5 years) X- and gamma-radiation | MN NPB | MN: 3.87 ± 2.14 (E); 3.66 ± 1.68 (NE) NPB (median (range)): 0.50 (0–2.75) (E); 0.75 (0–2.25) (NE) | 5 | |
| Sahin et al. (2009) [70] | Turkey N = 21 “auto-controls” | Nuclear medicine | Badge doses (4.0 ± 10.2 mSv in last year) Occupational radiation exposure between two vacations and after 1 month of vacation either following or before occupational exposure | MN SCE | MN: 21.90 ± 1.71 (AE); 14.13 ± 1.25 (BE) SCE: 7.52 ± 0.27 (AE); 6.25 ± 0.17 (BE) | 5 |
| Sakly et al. (2012) [71] | Tunisia N = 64 31 exposed/ 33 unexposed | Radiology | DOE to IR (13.7 ± 9.4 years) | TL | 24.98 ± 1.07 µm (E); 22.44 ± 0.57 µm (NE) | 6 |
| Sakly et al. (2013) [72] | Tunisia N = 87 60 exposed/ 27 unexposed | Radiology Cardiology | DOE to IR (16.5 ± 10.2 years, 12.3 ± 9.4 years in radiology and cardiology departments respectively) | MN CA (gaps, simple-strand breaks and double-strand breaks, reciprocal translocations, rings, and dicentrics) | MN in Radiology: 21.90 ± 4.23 (E); 10.78 ± 1.47 (NE) MN in Cardiology: 25.57 ± 4.79 (E); 10.78 ± 1.47 (NE) CA in Radiology: 33.63 ± 4.40 (E); 14.26 ± 3.40 (NE) CA in Cardiology: 35.37 ± 5.19 (E); 14.26 ± 3.40 (NE) | 5 |
| Santovito et al. (2014) [73] | Italy N = 42 21 exposed/ 21 unexposed | Radiology | - | CA (chromatid breaks, chromosome breaks, dicentrics, acentric fragments, and Tri- or Tetra-radials, Gaps) SCE | Aberrant cells: 2.07 ± 0.17 (E); 1.17 ± 0.17 (NE) β = −0.08 (95% CI −2.22;3.76) per years of employment SCE: 6.67 ± 0.29 (E); 4.49 ± 0.39 (NE) β = 0.26 (95% CI −8.07;10.32) per years of employment | 5 |
| Sari-Minodier et al. (2007) [74] | France N = 201 132 exposed/ 69 controls | Radiotherapy Nuclear medicine Cardiology Radiology Pediatric operating room | Badge doses (0.17 ± 0.47 mSv in the last year) + Estimated medical radiation dose as a patient | MN | 14.90 ± 8.10 (E); 11.80 ± 6.50 (NE) β = 2.55 (95% CI 0.57;4.53, p = 0.012) (increase for (E) people vs. (NE)) | 6 |
| Scarpato et al. (2006) [75] | Italy N = 92 Cross-sectional | Orthopedic Radiology Cardiology | Badge doses (3.3 ± 5.6 mSv in the last 3 years) IR-exposure for at least 3 years | CA (breaks or fragments, quadri-radial and triradial, translocations, dicentrics and rings) | Total CA: 1.79 ± 0.23 (HE); 1.37 ± 0.24 (ME); 1.32 ± 0.19 (LE) | 5 |
| Shafiee et al. (2020) [76] | Iran N = 81 46 exposed/ 35 unexposed | Lithotripsy CT scan Digital radiology | Badge doses (range 0–2.99 mSv in the last year) | MN CA (acentric fragments, gap, rings, and dicentrics) | MN: 6.89 ± 2.25 (E); 5.17 ± 1.70 (NE) R with cumulative radiation dose = 0.98 (p = 0.02) Significantly higher frequencies of CA in (E) compared to (NE) (except dicentrics and rings). R with cumulative radiation dose = 0.97 (p = 0.02) | 6 |
| Siama et al. (2019) [77] | India N = 66 33 exposed/ 33 unexposed | Radiology | Badge doses (40.9 ± 39.9 mSv) DOE to IR (10.3 ± 7.1 years) | MN | Significant rise in MN frequency in (E) compared to (NE) β = 0.42 (p = 0.02) per years of employment | 6 |
| Silva et al. (2016) [78] | Brazil N = 90 45 exposed/ 45 unexposed | (Radiologists, technologists and technicians) | X-rays | CS | Significantly higher damages (minimum to maximum levels) in (E) compared to (NE) Significantly lower cells with no damage in (E) compared to (NE) R with time of work = 0.637 (p = 0.001) | 5 |
| Surniyantoro et al. (2018) [79] | Indonesia N = 101 81 exposed/ 20 unexposed | Radiology Radiotherapy (doctors, radiologists, radiotherapists, and nurses) | Badge doses (0.2 ± 0.2 mSv/year) DOE to IR (20.8 ± 7.5 years) | MN | 15.38 ± 7.72 (E); 9.00 ± 5.49 (NE) β = 0.05 (p = 0.69) | 5 |
| Thierens et al. (2000) [80] | Belgium N = 131 71 exposed/ 60 unexposed | Radiology Radiotherapy Nuclear Medicine Cardiology Urology Gastroenterology (doctors, nurses or technicians) | Badge doses (20.8 ± 7.5 mSv) | MN | 21.88 ± 13.46 (E); 18.36 ± 7.53 (NE) | 6 |
| Tug et al. (2013) [81] | Turkey N = 74 39 exposed/ 35 unexposed | (Radiology technologists) | - | SCE | 5.19 ± 1.06 (E); 3.38 ± 1.13 (NE) | 4 |
| Vellingiri et al. (2014) [82] | India N = 112 56 exposed/ 56 unexposed | Radiology Cardiology Orthopedic (nurses, technicians, physicians) | Badge doses (range 1.3–24.5 mSv) DOE to IR (years) | CA (dicentrics or unusual karyotypes and structural CA) MN TL TM | Significantly higher CA and MN frequencies, TL and TM in (E) compared to (NE) | 7 |
| Vral et al. (2016) [83] | Belgium N = 38 29 exposed/ 19 unexposed | Nuclear medicine Interventional radiation | Badge doses (Hp(10) 4.95 ± 2.00 mSv over the last year in Nuclear Medicine department) | MN | No significant difference between (E) and (NE) | 6 |
| Wang et al. (2017) [84] | Japan N = 530 Cross sectional | Badge doses (means from 0.4 to 1.7 mSv/year) | CA (Chromosome breaks, fragments and dicentrics) MN | No significant difference in CA and MN between years of service groups, except significantly higher CA in female with >20 years compared to lower classes | 4 | |
| Zakeri et al. (2003) [85] | Iran N = 508 450 exposed/ 58 unexposed | (Industrial radiographers, nuclear research center, nuclear medicine workers, medical X-ray diagnostic workers) | - | CA (dicentrics, rings and acentrics) | Acentrics and dicentrics significantly higher in the different job-groups of (E) compared to (NE) | 5 |
| Zakeri et al. (2004) [86] | Iran N = 107 71 exposed/ 36 unexposed | Cardiovascular laboratory (cardiologist, nurses and technicians) | Badge doses (range 0.25–15 mSv/year) DOE to IR (11 ± 7 years) X-rays | CA (dicentrics, and acentrics) MN | MN: 38.91 ± 15.58 (E); 11.05 ± 4.51 (NE) CA: 6.73 ± 2.23 (E); 1.0 ± 0.5 (NE) | 6 |
| Zakeri et al. (2010) [87] | Iran N = 136 101 exposed/ 35 unexposed | (Interventional cardiologist, nuclear medicine physicians, conventional radiologists) | Badges doses (range 0.25–48 during the previous year) | CA (gap, isogap, break, minute, fragment, dicentric) MN | MN: 21.5 ± 9.6 (IC); 19.7 ± 3.8 (NM); 16.8 ± 8.1 (CR); 11.8 ± 6.5 (NE) %acentrics: 3.23 ± 2.60 (IC); 2.87 ± 1.40 (NM); 2.18 ± 0.90 (CR); 1.28 ± 0.50 (NE) %dicentrics: 0.21 (IC); 0.14 (NM); 0.13 (CR); 0.04 (NE) | 6 |
| Zakeri et al. (2010) [88] | Iran N = 74 37 exposed/ 37 unexposed | (Interventional cardiologists, clinical physicians) | Badges doses (8.1 ± 7.8 mSv/year; 30.5 ± 24.3 over the last 5 years) | CA (Chromatid and chromosome breaks, gaps, dicentrics and centric rings) | Aberrant cells: 2.78 ± 1.63 (E); 1.27 ± 1.07 (NE) | 5 |
| Zhou et al. (2016) [89] | China N = 127 52 exposed/ 75 unexposed | Radiology Cardiology (radiologic technologist, radiologist, and interventional cardiologist) Participants with cataract | DOE to IR (9.3 ± 2.8 years) | CA (dicentrics, tricentrics, structural) | 1.77 ± 0.92 (E); 0.63 ± 0.51 (NE) | 4 |
| Endpoints | Number of Studies Carried Out on These Endpoints | Is This Biomarker Recommended for Use in Future Prospective Epidemiological Studies to Examine Their Association with Long-Term Health Outcomes Following IR-Exposure? |
|---|---|---|
| Dicentrics | 24 | Yes |
| Acentric fragments | 14 | Yes |
| Micronucleus | 32 | Yes |
| Rings | 14 | No |
| Nucleoplasmic bridges | 7 | No |
| Sister chromatid exchanges | 7 | ≈Yes |
| Translocations | 6 | ≈Yes |
| Comet tail length | 6 | ≈Yes |
| Comet tail moment | 4 | ≈Yes |
| Comet score (DNA damage extent) | 4 | ≈Yes |
| Premature centromere divisions | 2 | ? (Yes) |
| Glycophorin A (GPA) mutant | 1 | ? (Yes) |
| Leukocyte telomere length | 1 | ? (Yes) |
| Copy number variation in AZFc region | 1 | ? (Yes) |
| %DNA in the tail | 3 | ? (Yes) |
| γ-H2AX foci | 1 | ? (Yes) |
| Endpoints | Sensitivity | Specificity to IR |
|---|---|---|
| Dicentrics | 50–100 mGy | High |
| Translocations | 200–300 mGy | Good |
| Micronuclei | 100–200 mGy | Good |
| Comet assay | 50–100 mGy | Low |
| γ-H2AX foci | 10 mGy | Good |
| Leukocyte telomere length | unknown | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baudin, C.; Bernier, M.-O.; Klokov, D.; Andreassi, M.G. Biomarkers of Genotoxicity in Medical Workers Exposed to Low-Dose Ionizing Radiation: Systematic Review and Meta-Analyses. Int. J. Mol. Sci. 2021, 22, 7504. https://doi.org/10.3390/ijms22147504
Baudin C, Bernier M-O, Klokov D, Andreassi MG. Biomarkers of Genotoxicity in Medical Workers Exposed to Low-Dose Ionizing Radiation: Systematic Review and Meta-Analyses. International Journal of Molecular Sciences. 2021; 22(14):7504. https://doi.org/10.3390/ijms22147504
Chicago/Turabian StyleBaudin, Clémence, Marie-Odile Bernier, Dmitry Klokov, and Maria Grazia Andreassi. 2021. "Biomarkers of Genotoxicity in Medical Workers Exposed to Low-Dose Ionizing Radiation: Systematic Review and Meta-Analyses" International Journal of Molecular Sciences 22, no. 14: 7504. https://doi.org/10.3390/ijms22147504
APA StyleBaudin, C., Bernier, M.-O., Klokov, D., & Andreassi, M. G. (2021). Biomarkers of Genotoxicity in Medical Workers Exposed to Low-Dose Ionizing Radiation: Systematic Review and Meta-Analyses. International Journal of Molecular Sciences, 22(14), 7504. https://doi.org/10.3390/ijms22147504







