Diagnostic Tools and Biomarkers for Severe Drug Eruptions
Abstract
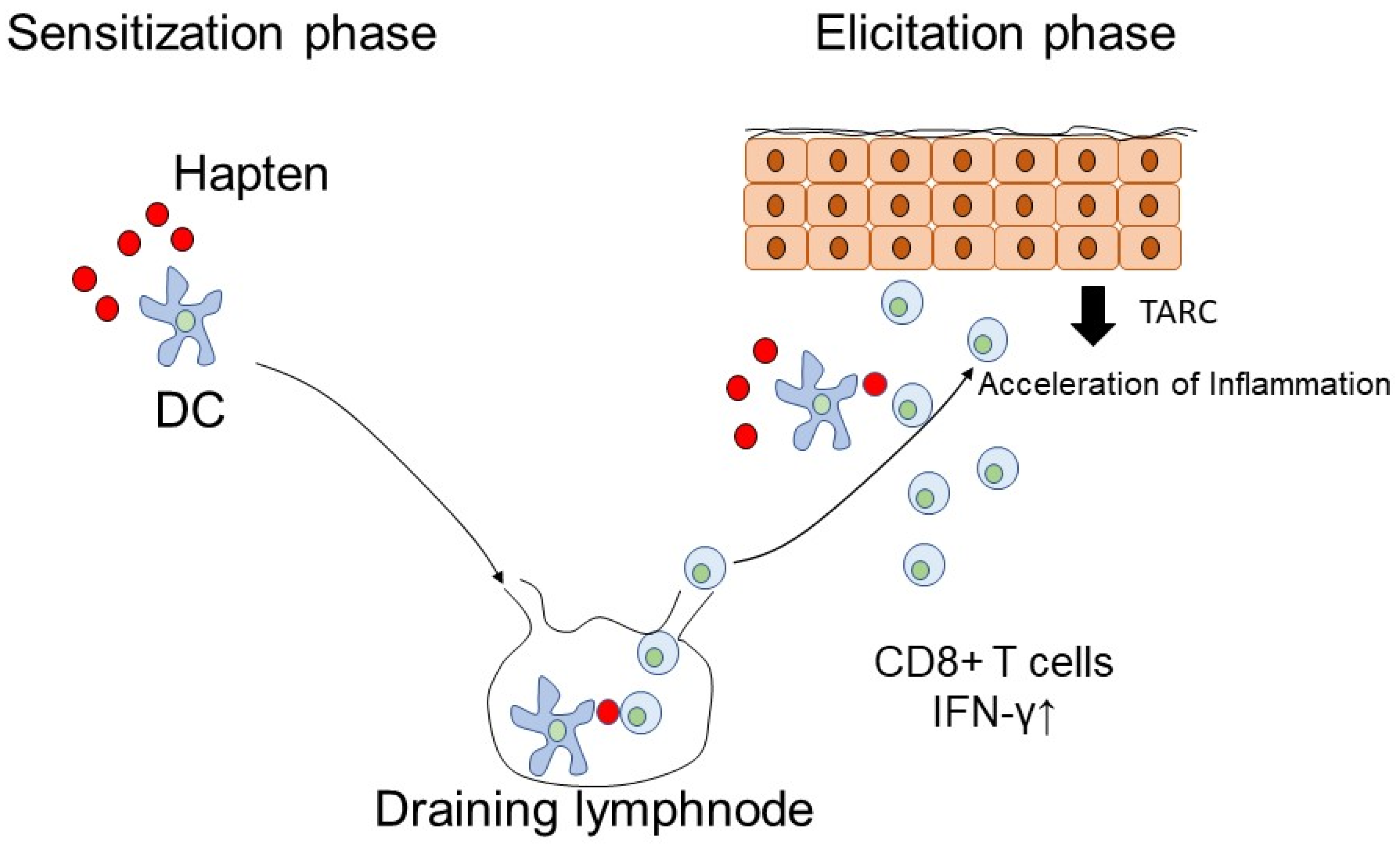
:1. Introduction
2. Pathogenesis of Drug Eruption
3. Clinical Sign and Biomarker for Drug Eruption
3.1. Intertriginous Eruption as a Clinical Sign for Acute Generalized Exanthematous Pustulosis (AGEP)
3.2. Peripheral Blood Eosinophil Count
3.3. Serum Eosinophilic Cationic Protein
3.4. The Presence of CD4+CD25+ Cell in the Epidermis as a Sign of Desensitization in Fixed Drug Eruption
3.5. miR-18a-5p, miR-124, and miR-214
3.6. Perforin and Granzyme B
3.7. Annexin A1 and RIP3
3.8. Soluble Fas Ligand
3.9. Granulysin
3.10. High Mobility Group Box 1 Protein (HMGB1)
3.11. Thymus and Activation-Regulated Chemokine (TARC)
3.12. Th17
3.13. Serum Galectin-7
3.14. S100A2
3.15. The Difference of Generalized Bullous Fixed Drug Eruption Compared with SJS and TEN
3.16. T Cell Receptor-Cβ1 (TCR-Cβ1) Gene Rearrangement for Pseudolymphoma CD30+ Large Cell Transformation
3.17. Metalloproteinase
3.18. Prognostic Biomarkers for SJS and TEN
4. The Summary of Biomarkers Depending on the Time Course of SJS and TEN
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Reen, F.J.; Romano, S.; Dobson, A.D.; O’Gara, F. The Sound of Silence: Activating Silent Biosynthetic Gene Clusters in Marine Microorganisms. Mar. Drugs 2015, 13, 4754–4783. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y.; Wanibuchi, S.; Sato, A.; Kasahara, T.; Fujita, M. Precipitation of test chemicals in reaction solutions used in the amino acid derivative reactivity assay and the direct peptide reactivity assay. J. Pharm. Toxicol. Methods 2019, 100, 106624. [Google Scholar] [CrossRef]
- White, K.D.; Abe, R.; Ardern-Jones, M.; Beachkofsky, T.; Bouchard, C.; Carleton, B.; Chodosh, J.; Cibotti, R.; Davis, R.; Denny, J.C.; et al. SJS/TEN 2017: Building Multidisciplinary Networks to Drive Science and Translation. J. Allergy Clin. Immunol. Pract. 2018, 6, 38–69. [Google Scholar] [CrossRef] [PubMed]
- Sunaga, Y.; Kurosawa, M.; Ochiai, H.; Watanabe, H.; Sueki, H.; Azukizawa, H.; Asada, H.; Watanabe, Y.; Yamaguchi, Y.; Aihara, M.; et al. The nationwide epidemiological survey of Stevens-Johnson syndrome and toxic epidermal necrolysis in Japan, 2016–2018. J. Dermatol. Sci. 2020, 100, 175–182. [Google Scholar] [CrossRef]
- Watanabe, T.; Go, H.; Saigusa, Y.; Takamura, N.; Watanabe, Y.; Yamane, Y.; Totsuka, M.; Ishikawa, H.; Nakamura, K.; Matsukura, S.; et al. Mortality and risk factors on admission in toxic epidermal necrolysis: A cohort study of 59 patients. Allergol. Int. 2020, 70, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Dainichi, T.; Kitoh, A.; Otsuka, A.; Nakajima, S.; Nomura, T.; Kaplan, D.H.; Kabashima, K. The epithelial immune microenvironment (EIME) in atopic dermatitis and psoriasis. Nat. Immunol. 2018, 19, 1286–1298. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Gallo, R.L. Role of Epigenetics in the Regulation of Immune Functions of the Skin. J. Investig. Dermatol. 2020, 141, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Egawa, G.; Grabbe, S.; Kabashima, K. Update of immune events in the murine contact hypersensitivity model: Toward the understanding of allergic contact dermatitis. J. Investig. Dermatol. 2013, 133, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Johnstone, D.F. Fatal case of Stevens-Johnson syndrome. Lancet 1947, 2, 276. [Google Scholar] [CrossRef]
- Silvestri, M.; Cristaudo, A.; Morrone, A.; Messina, C.; Bennardo, L.; Nisticò, S.P.; Mariano, M.; Cameli, N. Emerging Skin Toxicities in Patients with Breast Cancer Treated with New Cyclin-Dependent Kinase 4/6 Inhibitors: A Systematic Review. Drug Saf. 2021, 44, 725–732. [Google Scholar] [CrossRef]
- Roberti, R.; Iannone, L.F.; Palleria, C.; De Sarro, C.; Spagnuolo, R.; Barbieri, M.A.; Vero, A.; Manti, A.; Pisana, V.; Fries, W.; et al. Safety profiles of biologic agents for inflammatory bowel diseases: A prospective pharmacovigilance study in Southern Italy. Curr. Med. Res. Opin. 2020, 36, 1457–1463. [Google Scholar] [CrossRef]
- Thong, B.Y.; Tan, T.C. Epidemiology and risk factors for drug allergy. Br. J. Clin. Pharm. 2011, 71, 684–700. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Kabashima-Kubo, R.; Hino, R.; Nakamura, M. Fatal case of toxic epidermal necrolysis caused by cefozopran and associated with psoriasis. Acta Derm.-Venereol. 2014, 94, 341–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawada, Y.; Kawakami, C.; Nakamura, M.; Tokura, Y.; Yoshiki, R. Toxic epidermal necrosis-like dermatosis induced by the first course of methotrexate. Eur. J. Dermatol. 2009, 19, 397–398. [Google Scholar] [CrossRef]
- Sawada, Y.; Nakamura, M.; Tokura, Y. Generalized fixed drug eruption caused by pazufloxacin. Acta Derm.-Venereol. 2011, 91, 600–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, R.; Sawada, Y.; Nakamura, M. Two cases of eczematous drug eruption caused by oral tacrolimus administration. Contact Dermat. 2017, 77, 128–130. [Google Scholar] [CrossRef]
- Sawada, Y.; Honda, T.; Hanakawa, S.; Nakamizo, S.; Murata, T.; Ueharaguchi-Tanada, Y.; Ono, S.; Amano, W.; Nakajima, S.; Egawa, G.; et al. Resolvin E1 inhibits dendritic cell migration in the skin and attenuates contact hypersensitivity responses. J. Exp. Med. 2015, 212, 1921–1930. [Google Scholar] [CrossRef] [Green Version]
- Sawada, Y.; Sugita, K.; Fukamachi, S.; Bito, T.; Nakamura, M.; Tokura, Y. Doripenem-induced intertriginous drug eruption as a mild form of AGEP. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 974–976. [Google Scholar] [CrossRef]
- Totonchy, M.B.; McNiff, J.M.; Bunick, C.G. Koebnerization of Hailey-Hailey disease into a cutaneous drug eruption of acute generalized exanthematous pustulosis associated with systemic symptoms. J. Cutan. Pathol. 2016, 43, 1031–1035. [Google Scholar] [CrossRef]
- Yang, J.; Yang, X.; Li, M. Peripheral blood eosinophil counts predict the prognosis of drug eruptions. J. Investig. Allergol. Clin. Immunol. 2013, 23, 248–255. [Google Scholar]
- Dhar, S.; Malakar, R.; Chattopadhyay, S.; Dhar, S.; Banerjee, R.; Ghosh, A. Correlation of the severity of atopic dermatitis with absolute eosinophil counts in peripheral blood and serum IgE levels. Indian J. Dermatol. Venereol. Leprol. 2005, 71, 246–249. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, S.; Liu, P.; Mu, Z.; Zhang, J. Clinical relevance of eosinophils, basophils, serum total IgE level, allergen-specific IgE, and clinical features in atopic dermatitis. J. Clin. Lab. Anal. 2020, 34, e23214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.Y.; Park, H.J.; Kim, C.W. Eosinophil cationic protein (ECP) level and its correlation with eosinophil number or IgE level of peripheral blood in patients with various skin diseases. J. Dermatol. Sci. 1997, 15, 89–94. [Google Scholar] [CrossRef]
- Nielsen, P.N.; Skov, P.S.; Poulsen, L.K.; Schmelz, M.; Petersen, L.J. Cetirizine inhibits skin reactions but not mediator release in immediate and developing late-phase allergic cutaneous reactions. A double-blind, placebo-controlled study. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2001, 31, 1378–1384. [Google Scholar] [CrossRef]
- Juhlin, L.; Venge, P. Eosinophilic cationic protein (ECP) in skin disorders. Acta Derm.-Venereol. 1991, 71, 495–501. [Google Scholar] [PubMed]
- Teraki, Y.; Shiohara, T. Successful desensitization to fixed drug eruption: The presence of CD25+CD4+ T cells in the epidermis of fixed drug eruption lesions may be involved in the induction of desensitization. Dermatology 2004, 209, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Ibrahim, S.; Patel, K.; Luthra, R.; Duvic, M.; Medeiros, L.J. Degree of CD25 expression in T-cell lymphoma is dependent on tissue site: Implications for targeted therapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004, 10, 5587–5594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabian, M.R.; Sonenberg, N. The mechanics of miRNA-mediated gene silencing: A look under the hood of miRISC. Nat. Struct. Mol. Biol. 2012, 19, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.K.; Wong, S.H.D.; Bian, L. Long-Term Detection of Oncogenic MicroRNA in Living Human Cancer Cells by Gold@ Polydopamine-Shell Nanoprobe. ACS Biomater. Sci. Eng. 2020, 6, 3778–3783. [Google Scholar] [CrossRef]
- Evangelista, A.F.; Oliveira, R.J.; VA, O.S.; RA, D.C.V.; Reis, R.M.; MM, C.M. Integrated analysis of mRNA and miRNA profiles revealed the role of miR-193 and miR-210 as potential regulatory biomarkers in different molecular subtypes of breast cancer. BMC Cancer 2021, 21, 76. [Google Scholar] [CrossRef]
- Tang, S.; Li, S.; Liu, T.; He, Y.; Hu, H.; Zhu, Y.; Tang, S.; Zhou, H. MicroRNAs: Emerging oncogenic and tumor-suppressive regulators, biomarkers and therapeutic targets in lung cancer. Cancer Lett. 2021, 502, 71–83. [Google Scholar] [CrossRef]
- Ichihara, A.; Wang, Z.; Jinnin, M.; Izuno, Y.; Shimozono, N.; Yamane, K.; Fujisawa, A.; Moriya, C.; Fukushima, S.; Inoue, Y.; et al. Upregulation of miR-18a-5p contributes to epidermal necrolysis in severe drug eruptions. J. Allergy Clin. Immunol. 2014, 133, 1065–1074. [Google Scholar] [CrossRef]
- Sato, S.; Ichihara, A.; Jinnin, M.; Izuno, Y.; Fukushima, S.; Ihn, H. Serum miR-124 up-regulation as a disease marker of toxic epidermal necrolysis. Eur. J. Dermatol. 2015, 25, 457–462. [Google Scholar] [CrossRef]
- Sawada, Y.; Nakatsuji, T.; Dokoshi, T.; Kulkarni, N.N.; Liggins, M.C.; Sen, G.; Gallo, R.L. Cutaneous innate immune tolerance is mediated by epigenetic control of MAP2K3 by HDAC8/9. Sci. Immunol. 2021, 6, eabe1935. [Google Scholar] [CrossRef]
- Chuang, J.C.; Jones, P.A. Epigenetics and microRNAs. Pediatr. Res. 2007, 61 Pt 2, 24–29. [Google Scholar] [CrossRef]
- Arif, K.M.T.; Elliott, E.K.; Haupt, L.M.; Griffiths, L.R. Regulatory Mechanisms of Epigenetic miRNA Relationships in Human Cancer and Potential as Therapeutic Targets. Cancers 2020, 12, 2922. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, H.; Liu, Y.; Su, P.; Zhang, J.; Wang, X.; Sun, M.; Chen, B.; Zhao, W.; Wang, L.; et al. SREBP1, targeted by miR-18a-5p, modulates epithelial-mesenchymal transition in breast cancer via forming a co-repressor complex with Snail and HDAC1/2. Cell Death Differ. 2019, 26, 843–859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Laux, A.; Stenmark, K.R.; Hu, C.J. Mechanisms Contributing to the Dysregulation of miRNA-124 in Pulmonary Hypertension. Int. J. Mol. Sci. 2021, 22, 3852. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Li, C.; McRae, G.; Lykken, E.; Sevilla, J.; Liu, S.Q.; Wan, Y.; Li, Q.J. MeCP2 reinforces STAT3 signaling and the generation of effector CD4+ T cells by promoting miR-124-mediated suppression of SOCS5. Sci. Signal. 2014, 7, ra25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, F.; Liao, Y.J.; Cai, M.Y.; Liu, Y.H.; Liu, T.H.; Chen, S.P.; Bian, X.W.; Guan, X.Y.; Lin, M.C.; Zeng, Y.X.; et al. The putative tumour suppressor microRNA-124 modulates hepatocellular carcinoma cell aggressiveness by repressing ROCK2 and EZH2. Gut 2012, 61, 278–289. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, Z.; Tan, Z.; He, R.; Zeng, X.; Xie, Y.; Li, S.; Tang, G.; Tang, H.; He, X. MicroRNA-124 inhibits proliferation and induces apoptosis by directly repressing EZH2 in gastric cancer. Mol. Cell. Biochem. 2014, 392, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Juan, A.H.; Kumar, R.M.; Marx, J.G.; Young, R.A.; Sartorelli, V. Mir-214-dependent regulation of the polycomb protein Ezh2 in skeletal muscle and embryonic stem cells. Mol. Cell 2009, 36, 61–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.C.; Walsh, C.M.; Young, J.D. Perforin: Structure and function. Immunol. Today 1995, 16, 194–201. [Google Scholar] [CrossRef]
- Velotti, F.; Barchetta, I.; Cimini, F.A.; Cavallo, M.G. Granzyme B in Inflammatory Diseases: Apoptosis, Inflammation, Extracellular Matrix Remodeling, Epithelial-to-Mesenchymal Transition and Fibrosis. Front. Immunol. 2020, 11, 587581. [Google Scholar] [CrossRef]
- Lisi, P.; Pelliccia, S.; Bellini, V. Histopathological and immunohistochemical features of drug-induced exanthems. G Ital. Dermatol. Venereol. 2014, 149, 237–241. [Google Scholar]
- Posadas, S.J.; Padial, A.; Torres, M.J.; Mayorga, C.; Leyva, L.; Sanchez, E.; Alvarez, J.; Romano, A.; Juarez, C.; Blanca, M. Delayed reactions to drugs show levels of perforin, granzyme B, and Fas-L to be related to disease severity. J. Allergy Clin. Immunol. 2002, 109, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Baracco, E.E.; Petrazzuolo, A.; Kroemer, G. Assessment of annexin A1 release during immunogenic cell death. Methods Enzym. 2019, 629, 71–79. [Google Scholar]
- Saito, N.; Qiao, H.; Yanagi, T.; Shinkuma, S.; Nishimura, K.; Suto, A.; Fujita, Y.; Suzuki, S.; Nomura, T.; Nakamura, H.; et al. An annexin A1-FPR1 interaction contributes to necroptosis of keratinocytes in severe cutaneous adverse drug reactions. Sci. Transl. Med. 2014, 6, 245ra95. [Google Scholar] [CrossRef]
- Honda, T.; Yamamoto, O.; Sawada, Y.; Egawa, G.; Kitoh, A.; Otsuka, A.; Dainichi, T.; Nakajima, S.; Miyachi, Y.; Kabashima, K. Receptor-interacting protein kinase 3 controls keratinocyte activation in a necroptosis-independent manner and promotes psoriatic dermatitis in mice. J. Allergy Clin. Immunol. 2017, 140, 619–622.e6. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, A.; Shinkuma, S.; Hayashi, R.; Hama, N.; Watanabe, H.; Kinoshita, M.; Ogawa, Y.; Abe, R. RIP3 as a diagnostic and severity marker for Stevens-Johnson syndrome and toxic epidermal necrolysis. J. Allergy Clin. Immunol. Pract. 2020, 8, 1768–1771.e7. [Google Scholar] [CrossRef] [PubMed]
- Murata, J.; Abe, R.; Shimizu, H. Increased soluble Fas ligand levels in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis preceding skin detachment. J. Allergy Clin. Immunol. 2008, 122, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Krensky, A.M.; Clayberger, C. Biology and clinical relevance of granulysin. Tissue Antigens 2009, 73, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Yoshioka, N.; Abe, R.; Murata, J.; Hoshina, D.; Mae, H.; Shimizu, H. Rapid immunochromatographic test for serum granulysin is useful for the prediction of Stevens-Johnson syndrome and toxic epidermal necrolysis. J. Am. Acad. Dermatol. 2011, 65, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, S.A.; Wu, X.; Zhu, Q.; Luo, X. Overexpression of cytotoxic proteins correlates with liver function impairment in patients with drug reaction with eosinophilia and systemic symptoms (DRESS). Eur. J. Dermatol. 2018, 28, 13–25. [Google Scholar]
- Stros, M. HMGB proteins: Interactions with DNA and chromatin. Biochim. Biophys. Acta 2010, 1799, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Schmohl, J.; Guenther, T.; Sutanto, W.; Schuster, F.; Kroell, T.; Hartmann, A.; Salih, H.; Stoetzer, O.; Schmetzer, H. Expression profiles of HMGB1 on B-CLL related leukocytes contribute to prediction of relapse. Immunobiology 2020, 226, 152048. [Google Scholar] [CrossRef]
- Kader, M.; El Andaloussi, A.; Vorhaour, J.; Tamama, K.; Nieto, N.; Scott, M.J.; Ismail, N. Interferon Type I Regulates Inflammasome Activation and High Mobility Group Box 1 Translocation in Hepatocytes During Ehrlichia-Induced Acute Liver Injury. Hepatol Commun. 2021, 5, 33–51. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Q.; Wang, Y.; Liang, T.; Li, X.; Wang, D.; Wang, X.; Zhu, H.; Xiao, K. Necroptosis is active and contributes to intestinal injury in a piglet model with lipopolysaccharide challenge. Cell Death Dis. 2021, 12, 62. [Google Scholar] [CrossRef]
- de Oliveira Gomes, C.G.; de Andrade, M.V.M.; Guedes, L.R.; Rocha, H.C.; Guimarães, R.G.; Carvalho, F.A.C.; Vilela, E.G. Evaluation of the Biomarkers HMGB1 and IL-6 as Predictors of Mortality in Cirrhotic Patients with Acute Kidney Injury. Mediat. Inflamm. 2020, 2020, 2867241. [Google Scholar] [CrossRef]
- Watanabe, T.; Yamaguchi, Y.; Watanabe, Y.; Takamura, N.; Aihara, M. Increased level of high mobility group box 1 in the serum and skin in patients with generalized pustular psoriasis. J. Dermatol. 2020, 47, 1033–1036. [Google Scholar] [CrossRef]
- Nakajima, S.; Watanabe, H.; Tohyama, M.; Sugita, K.; Iijima, M.; Hashimoto, K.; Tokura, Y.; Nishimura, Y.; Doi, H.; Tanioka, M.; et al. High-mobility group box 1 protein (HMGB1) as a novel diagnostic tool for toxic epidermal necrolysis and Stevens-Johnson syndrome. Arch. Dermatol. 2011, 147, 1110–1112. [Google Scholar] [CrossRef] [Green Version]
- Carr, D.F.; Wang, C.W.; Bellón, T.; Ressel, L.; Nwikue, G.; Shrivastava, V.; Bergfeld, W.; Jorgensen, A.L.; Chung, W.H.; Pirmohamed, M. Serum and blister-fluid elevation and decreased epidermal content of high-mobility group box 1 protein in drug-induced Stevens-Johnson syndrome/toxic epidermal necrolysis. Br. J. Dermatol. 2019, 181, 166–174. [Google Scholar] [CrossRef]
- Thijs, J.; Krastev, T.; Weidinger, S.; Buckens, C.F.; de Bruin-Weller, M.; Bruijnzeel-Koomen, C.; Flohr, C.; Hijnen, D. Biomarkers for atopic dermatitis: A systematic review and meta-analysis. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 453–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawada, Y.; Honda, T.; Nakamizo, S.; Nakajima, S.; Nonomura, Y.; Otsuka, A.; Egawa, G.; Yoshimoto, T.; Nakamura, M.; Narumiya, S.; et al. Prostaglandin E(2) (PGE(2))-EP2 signaling negatively regulates murine atopic dermatitis-like skin inflammation by suppressing thymic stromal lymphopoietin expression. J. Allergy Clin. Immunol. 2019, 144, 1265–1273.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iellem, A.; Mariani, M.; Lang, R.; Recalde, H.; Panina-Bordignon, P.; Sinigaglia, F.; D’Ambrosio, D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4+CD25+ regulatory T cells. J. Exp. Med. 2001, 194, 847–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komatsu-Fujii, T.; Chinuki, Y.; Niihara, H.; Hayashida, K.; Ohta, M.; Okazaki, R.; Kaneko, S.; Morita, E. The thymus and activation-regulated chemokine (TARC) level in serum at an early stage of a drug eruption is a prognostic biomarker of severity of systemic inflammation. Allergol. Int. 2018, 67, 90–95. [Google Scholar] [CrossRef]
- Vestergaard, C.; Bang, K.; Gesser, B.; Yoneyama, H.; Matsushima, K.; Larsen, C.G. A Th2 chemokine, TARC, produced by keratinocytes may recruit CLA+CCR4+ lymphocytes into lesional atopic dermatitis skin. J. Investig. Dermatol. 2000, 115, 640–646. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, Y.; Kamata, M.; Shimizu, T.; Nagata, M.; Fukaya, S.; Hayashi, K.; Fukuyasu, A.; Tanaka, T.; Ishikawa, T.; Ohnishi, T.; et al. Thymus and activation-regulated chemokine (TARC) in patients with psoriasis: Increased serum TARC levels in patients with generalized pustular psoriasis. J. Dermatol. 2020, 47, 1149–1156. [Google Scholar] [CrossRef]
- Mease, P.J.; McInnes, I.B.; Kirkham, B.; Kavanaugh, A.; Rahman, P.; van der Heijde, D.; Landewé, R.; Nash, P.; Pricop, L.; Yuan, J.; et al. Secukinumab Inhibition of Interleukin-17A in Patients with Psoriatic Arthritis. N. Engl. J. Med. 2015, 373, 1329–1339. [Google Scholar] [CrossRef] [Green Version]
- Yoshiki, R.; Kabashima, K.; Honda, T.; Nakamizo, S.; Sawada, Y.; Sugita, K.; Yoshioka, H.; Ohmori, S.; Malissen, B.; Tokura, Y.; et al. IL-23 from Langerhans cells is required for the development of imiquimod-induced psoriasis-like dermatitis by induction of IL-17A-producing γδ T cells. J. Investig. Dermatol. 2014, 134, 1912–1921. [Google Scholar] [CrossRef] [Green Version]
- Sawada, Y.; Honda, T.; Nakamizo, S.; Otsuka, A.; Ogawa, N.; Kobayashi, Y.; Nakamura, M.; Kabashima, K. Resolvin E1 attenuates murine psoriatic dermatitis. Sci. Rep. 2018, 8, 11873. [Google Scholar] [CrossRef] [Green Version]
- Ueharaguchi, Y.; Honda, T.; Kusuba, N.; Hanakawa, S.; Adachi, A.; Sawada, Y.; Otsuka, A.; Kitoh, A.; Dainichi, T.; Egawa, G.; et al. Thromboxane A(2) facilitates IL-17A production from Vγ4+ γδ T cells and promotes psoriatic dermatitis in mice. J. Allergy Clin. Immunol. 2018, 142, 680–683.e2. [Google Scholar] [CrossRef] [Green Version]
- Saito-Sasaki, N.; Sawada, Y.; Mashima, E.; Yamaguchi, T.; Ohmori, S.; Yoshioka, H.; Haruyama, S.; Okada, E.; Nakamura, M. Maresin-1 suppresses imiquimod-induced skin inflammation by regulating IL-23 receptor expression. Sci. Rep. 2018, 8, 5522. [Google Scholar] [CrossRef]
- Östling, J.; van Geest, M.; Schofield, J.P.R.; Jevnikar, Z.; Wilson, S.; Ward, J.; Lutter, R.; Shaw, D.E.; Bakke, P.S.; Caruso, M.; et al. IL-17-high asthma with features of a psoriasis immunophenotype. J. Allergy Clin. Immunol. 2019, 144, 1198–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; McAlees, J.W.; Bischoff, L.J.; Kaur, D.; Houshel, L.K.; Gray, J.; Hargis, J.; Davis, X.; Dudas, P.L.; Deshmukh, H.; et al. Combined administration of anti-IL-13 and anti-IL-17A at individually sub-therapeutic doses limits asthma-like symptoms in a mouse model of Th2/Th17 high asthma. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2019, 49, 317–330. [Google Scholar] [CrossRef]
- Busse, W.W. Asthma and psoriasis: What do they have in common? IL-17A! J. Allergy Clin. Immunol. 2019, 144, 1169–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, T.; Qiu, J.; Ji, Y.; Li, W.; Ding, Z.; Suo, C.; Chang, J.; Wang, J.; He, R.; Qian, Y.; et al. IL-17-producing ST2+ group 2 innate lymphoid cells play a pathogenic role in lung inflammation. J. Allergy Clin. Immunol. 2019, 143, 229–244.e9. [Google Scholar] [CrossRef] [PubMed]
- Lenberg, J.; Qian, Q.; Sun, Z.; Alam, R.; Gorska, M.M. Pre-pregnancy exposure to diesel exhaust predisposes offspring to asthma through IL-1β and IL-17A. J. Allergy Clin. Immunol. 2018, 141, 1118–1122.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koga, C.; Kabashima, K.; Shiraishi, N.; Kobayashi, M.; Tokura, Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J. Investig. Dermatol. 2008, 128, 2625–2630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topal, F.A.; Zuberbier, T.; Makris, M.P.; Hofmann, M. The role of IL-17, IL-23 and IL-31, IL-33 in allergic skin diseases. Curr Opin Allergy Clin. Immunol. 2020, 20, 367–373. [Google Scholar] [CrossRef]
- Nakajima, S.; Kitoh, A.; Egawa, G.; Natsuaki, Y.; Nakamizo, S.; Moniaga, C.S.; Otsuka, A.; Honda, T.; Hanakawa, S.; Amano, W.; et al. IL-17A as an inducer for Th2 immune responses in murine atopic dermatitis models. J. Investig. Dermatol. 2014, 134, 2122–2130. [Google Scholar] [CrossRef] [Green Version]
- Ryu, S.; Song, P.I.; Seo, C.H.; Cheong, H.; Park, Y. Colonization and infection of the skin by S. aureus: Immune system evasion and the response to cationic antimicrobial peptides. Int. J. Mol. Sci. 2014, 15, 8753–8772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyerich, K.; Pennino, D.; Scarponi, C.; Foerster, S.; Nasorri, F.; Behrendt, H.; Ring, J.; Traidl-Hoffmann, C.; Albanesi, C.; Cavani, A. IL-17 in atopic eczema: Linking allergen-specific adaptive and microbial-triggered innate immune response. J. Allergy Clin. Immunol. 2009, 123, 59–66.e4. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Nakamura, M.; Kabashima-Kubo, R.; Shimauchi, T.; Kobayashi, M.; Tokura, Y. Defective epidermal innate immunity and resultant superficial dermatophytosis in adult T-cell leukemia/lymphoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 3772–3779. [Google Scholar] [CrossRef] [Green Version]
- Sawada, Y.; Nakamura, M.; Kabashima-Kubo, R.; Shimauchi, T.; Kobayashi, M.; Tokura, Y. Defective epidermal induction of S100A7/psoriasin associated with low frequencies of skin-infiltrating Th17 cells in dermatophytosis-prone adult T cell leukemia/lymphoma. Clin. Immunol. 2013, 148, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Fujiyama, T.; Kawakami, C.; Sugita, K.; Kubo-Kabashima, R.; Sawada, Y.; Hino, R.; Nakamura, M.; Shimauchi, T.; Ito, T.; Kabashima, K.; et al. Increased frequencies of Th17 cells in drug eruptions. J. Dermatol. Sci. 2014, 73, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, N.; Suto, A.; Abe, R.; Saito, N.; Murata, J.; Hayashi-Ujiie, I.; Hoshina, D.; Fujita, Y.; Shimizu, H. Disturbed balance in three subpopulations of CD4+Foxp3+ regulatory T cells in Stevens-Johnson syndrome and toxic epidermal necrolysis patients. Clin. Immunol. 2013, 148, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, R.; Sugita, K.; Sawada, Y.; Hino, R.; Nakamura, M.; Tokura, Y. Increased circulating Th17 frequencies and serum IL-22 levels in patients with acute generalized exanthematous pustulosis. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 485–488. [Google Scholar] [CrossRef]
- Cohen, J.N.; Bowman, S.; Laszik, Z.G.; North, J.P. Clinicopathologic overlap of psoriasis, eczema, and psoriasiform dermatoses: A retrospective study of T helper type 2 and 17 subsets, interleukin 36, and β-defensin 2 in spongiotic psoriasiform dermatitis, sebopsoriasis, and tumor necrosis factor α inhibitor-associated dermatitis. J. Am. Acad. Dermatol. 2020, 82, 430–439. [Google Scholar]
- Saito-Sasaki, N.; Sawada, Y.; Ohmori, S.; Omoto, D.; Haruyama, S.; Yoshioka, M.; Nishio, D.; Okada, E.; Nakamura, M. A possible role of IL-23-producing cells in a patient with psoriasiform drug eruption due to tazobactam and piperacillin hydrate: A case study and literature review. Eur. J. Dermatol. 2017, 27, 88–89. [Google Scholar] [CrossRef]
- Chen, H.L.; Chiang, P.C.; Lo, C.H.; Lo, Y.H.; Hsu, D.K.; Chen, H.Y.; Liu, F.T. Galectin-7 Regulates Keratinocyte Proliferation and Differentiation through JNK-miR-203-p63 Signaling. J. Investig. Dermatol. 2016, 136, 182–191. [Google Scholar] [CrossRef] [Green Version]
- Takagi, D.; Hato, N.; Okada, M.; Hakuba, N.; Gyo, K.; Shigemoto, K.; Toda, T.; Ogasawara, M.; Kameda, K. Galectin-7 as a marker of cholesteatoma residue and its detection during surgery by an immunofluorescent method—A preliminary study. Otol. Neurotol. 2012, 33, 396–399. [Google Scholar] [CrossRef] [Green Version]
- Matsukawa, S.; Morita, K.; Negishi, A.; Harada, H.; Nakajima, Y.; Shimamoto, H.; Tomioka, H.; Tanaka, K.; Ono, M.; Yamada, T.; et al. Galectin-7 as a potential predictive marker of chemo- and/or radio-therapy resistance in oral squamous cell carcinoma. Cancer Med. 2014, 3, 349–361. [Google Scholar] [CrossRef]
- Mesquita, J.A.; Queiroz, L.M.; Silveira, É.J.; Gordon-Nunez, M.A.; Godoy, G.P.; Nonaka, C.F.; Alves, P.M. Association of immunoexpression of the galectins-3 and -7 with histopathological and clinical parameters in oral squamous cell carcinoma in young patients. Eur. Arch. Otorhinolaryngol. 2016, 273, 237–243. [Google Scholar] [CrossRef]
- Grosset, A.A.; Poirier, F.; Gaboury, L.; St-Pierre, Y. Galectin-7 Expression Potentiates HER-2-Positive Phenotype in Breast Cancer. PLoS ONE 2016, 11, e0166731. [Google Scholar] [CrossRef] [Green Version]
- Schulz, H.; Schmoeckel, E.; Kuhn, C.; Hofmann, S.; Mayr, D.; Mahner, S.; Jeschke, U. Galectins-1, -3, and -7 Are Prognostic Markers for Survival of Ovarian Cancer Patients. Int. J. Mol. Sci. 2017, 18, 1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niiyama, S.; Yoshino, T.; Yasuda, C.; Yu, X.; Izumi, R.; Ishiwatari, S.; Matsukuma, S.; Mukai, H. Galectin-7 in the stratum corneum: A biomarker of the skin barrier function. Int. J. Cosmet. Sci. 2016, 38, 487–495. [Google Scholar] [CrossRef]
- Hama, N.; Nishimura, K.; Hasegawa, A.; Yuki, A.; Kume, H.; Adachi, J.; Kinoshita, M.; Ogawa, Y.; Nakajima, S.; Nomura, T.; et al. Galectin-7 as a potential biomarker of Stevens-Johnson syndrome/toxic epidermal necrolysis: Identification by targeted proteomics using causative drug-exposed peripheral blood cells. J. Allergy Clin. Immunol. Pract. 2019, 7, 2894–2897.e7. [Google Scholar] [CrossRef] [PubMed]
- Umayahara, T.; Shimauchi, T.; Iwasaki, M.; Sakabe, J.I.; Aoshima, M.; Nakazawa, S.; Yatagai, T.; Yamaguchi, H.; Phadungsaksawasdi, P.; Kurihara, K.; et al. Protective role of Galectin-7 for skin barrier impairment in atopic dermatitis. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2020, 50, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Hiromasa, K.; Kabashima-Kubo, R.; Yoshioka, M.; Nakamura, M. Galectin-7, induced by cis-urocanic acid and ultraviolet B irradiation, down-modulates cytokine production by T lymphocytes. Exp. Dermatol. 2013, 22, 840–842. [Google Scholar] [CrossRef]
- Chen, H.L.; Lo, C.H.; Huang, C.C.; Lu, M.P.; Hu, P.Y.; Chen, C.S.; Chueh, D.Y.; Chen, P.; Lin, T.N.; Lo, Y.H.; et al. Galectin-7 downregulation in lesional keratinocytes contributes to enhanced IL-17A signaling and skin pathology in psoriasis. J. Clin. Investig. 2021, 131, 131. [Google Scholar] [CrossRef] [PubMed]
- Hountis, P.; Matthaios, D.; Froudarakis, M.; Bouros, D.; Kakolyris, S. S100A2 protein and non-small cell lung cancer. The dual role concept. Tumour Biol. 2014, 35, 7327–7333. [Google Scholar] [CrossRef] [PubMed]
- Wicki, R.; Franz, C.; Scholl, F.A.; Heizmann, C.W.; Schafer, B.W. Repression of the candidate tumor suppressor gene S100A2 in breast cancer is mediated by site-specific hypermethylation. Cell Calcium 1997, 22, 243–254. [Google Scholar] [CrossRef]
- Nagy, N.; Hoyaux, D.; Gielen, I.; Schafer, B.W.; Pochet, R.; Heizmann, C.W.; Kiss, R.; Salmon, I.; Decaestecker, C. The Ca2+-binding S100A2 protein is differentially expressed in epithelial tissue of glandular or squamous origin. Histol. Histopathol. 2002, 17, 123–130. [Google Scholar]
- Bachet, J.B.; Maréchal, R.; Demetter, P.; Bonnetain, F.; Cros, J.; Svrcek, M.; Bardier-Dupas, A.; Hammel, P.; Sauvanet, A.; Louvet, C.; et al. S100A2 is a predictive biomarker of adjuvant therapy benefit in pancreatic adenocarcinoma. Eur. J. Cancer 2013, 49, 2643–2653. [Google Scholar] [CrossRef]
- Wang, T.; Wang, N.; Zhang, L.; Liu, Y.; Thakur, A. S100A2: A potential biomarker to differentiate malignant from tuberculous pleural effusion. Indian J. Cancer 2021, 58, 241–247. [Google Scholar] [PubMed]
- Gupta, S.; Hussain, T.; MacLennan, G.T.; Fu, P.; Patel, J.; Mukhtar, H. Differential expression of S100A2 and S100A4 during progression of human prostate adenocarcinoma. J. Clin. Oncol. 2003, 21, 106–112. [Google Scholar] [CrossRef]
- Lauriola, L.; Michetti, F.; Maggiano, N.; Galli, J.; Cadoni, G.; Schäfer, B.W.; Heizmann, C.W.; Ranelletti, F.O. Prognostic significance of the Ca2+ binding protein S100A2 in laryngeal squamous-cell carcinoma. Int. J. Cancer 2000, 89, 345–349. [Google Scholar] [CrossRef]
- Luo, J.; Zhu, Y.; Yang, G.; Gong, L.; Wang, B.; Liu, H. Loss of Reprimo and S100A2 expression in human gastric adenocarcinoma. Diagn. Cytopathol. 2011, 39, 752–757. [Google Scholar] [CrossRef]
- Yoshioka, M.; Sawada, Y.; Saito-Sasaki, N.; Yoshioka, H.; Hama, K.; Omoto, D.; Ohmori, S.; Okada, E.; Nakamura, M. High S100A2 expression in keratinocytes in patients with drug eruption. Sci. Rep. 2021, 11, 5493. [Google Scholar] [CrossRef]
- Cho, Y.T.; Lin, J.W.; Chen, Y.C.; Chang, C.Y.; Hsiao, C.H.; Chung, W.H.; Chu, C.Y. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J. Am. Acad. Dermatol. 2014, 70, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Kobata, K.; Kimura, S.; Mihashi, Y.; Iwasaki, H.; Nonaka, S.; Matsumoto, S.; Takamatsu, Y.; Choi, I.; Kawauchi, S.; Ishitsuka, K.; et al. Clinical and cytopathological characteristics of HTLV-1+ hodgkin lymphoma. Cancer Med. 2020, 9, 5788–5797. [Google Scholar] [CrossRef] [PubMed]
- Piris, M.A.; Rodriguez-Pinilla, S.M.; Santonja, C.; Betancor, I.; Alonso-Alonso, R.; Gru, A.A.; Rodriguez, M. Update on peripheral T-cell lymphomas with T-helper phenotype: Are there too many subtypes? Semin. Diagn. Pathol. 2020, 37, 24–31. [Google Scholar] [CrossRef]
- Sawada, Y.; Sugita, K.; Kabashima, R.; Hino, R.; Nakamura, M.; Koga, C.; Tokura, Y. CD8+ CD56+ mycosis fungoides with an indolent clinical behaviour: Case report and literature review. Acta Derm.-Venereol. 2010, 90, 525–526. [Google Scholar] [CrossRef] [Green Version]
- Tian, Z.; Shiyu, Z.; Tao, W.; Li, L.; Yuehua, L.; Hongzhong, J. Lymphoma or pseudolymphoma: A report of six cases and review of the literature. Derm. Ther. 2019, 32, e12807. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Kabashima, K.; Tokura, Y. Pseudolymphomatous folliculitis presenting with multiple nodules. Eur. J. Dermatol. 2009, 19, 263–264. [Google Scholar] [CrossRef]
- Sawada, Y.; Hino, R.; Hama, K.; Ohmori, S.; Fueki, H.; Yamada, S.; Fukamachi, S.; Tajiri, M.; Kubo, R.; Yoshioka, M.; et al. Type of skin eruption is an independent prognostic indicator for adult T-cell leukemia/lymphoma. Blood 2011, 117, 3961–3967. [Google Scholar] [CrossRef] [PubMed]
- Tokura, Y.; Sawada, Y.; Shimauchi, T. Skin manifestations of adult T-cell leukemia/lymphoma: Clinical, cytological and immunological features. J. Dermatol. 2014, 41, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Sawada, Y.; Yoshiki, R.; Kawakami, C.; Fukamachi, S.; Sugita, K.; Nakamura, M.; Tokura, Y. Valsartan-induced drug eruption followed by CD30+ pseudolymphomatous eruption. Acta Derm.-Venereol. 2010, 90, 521–522. [Google Scholar] [CrossRef] [Green Version]
- Inoue, A.; Sawada, Y.; Ohmori, S.; Omoto, D.; Haruyama, S.; Yoshioka, M.; Nishio, D.; Nakamura, M. CD30-positive Cutaneous Pseudolymphoma Caused by Tocilizumab in a Patient with Rheumatoid Arthritis: Case Report and Literature Review. Acta Derm.-Venereol. 2016, 96, 570–571. [Google Scholar] [CrossRef] [Green Version]
- Fukamachi, S.; Sugita, K.; Sawada, Y.; Bito, T.; Nakamura, M.; Tokura, Y. Drug-induced CD30+ T cell pseudolymphoma. Eur. J. Dermatol. 2009, 19, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Faveeuw, C.; Preece, G.; Ager, A. Transendothelial migration of lymphocytes across high endothelial venules into lymph nodes is affected by metalloproteinases. Blood 2001, 98, 688–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paquet, P.; Nusgens, B.V.; Piérard, G.E.; Lapière, C.M. Gelatinases in drug-induced toxic epidermal necrolysis. Eur. J. Clin. Investig. 1998, 28, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Gaultier, F.; Ejeil, A.L.; Igondjo-Tchen, S.; Dohan, D.; Dridi, S.M.; Maman, L.; Wierzba, C.B.; Stania, D.; Pellat, B.; Lafont, A.; et al. Possible involvement of gelatinase A (MMP2) and gelatinase B (MMP9) in toxic epidermal necrolysis or Stevens-Johnson syndrome. Arch. Dermatol. Res. 2004, 296, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Caproni, M.; Torchia, D.; Volpi, W.; Frezzolini, A.; Schena, D.; Marzano, A.; Quaglino, P.; De Simone, C.; Parodi, A.; Fabbri, P. Expression of matrix metalloproteinases 2, 9 and 11 in erythema multiforme: Immunohistochemical comparison with Stevens-Johnson syndrome/toxic epidermal necrolysis. Br. J. Dermatol. 2008, 158, 1163–1166. [Google Scholar] [CrossRef]
- Fleischmajer, R.; Kuroda, K.; Hazan, R.; Gordon, R.E.; Lebwohl, M.G.; Sapadin, A.N.; Unda, F.; Iehara, N.; Yamada, Y. Basement membrane alterations in psoriasis are accompanied by epidermal overexpression of MMP-2 and its inhibitor TIMP-2. J. Investig. Dermatol. 2000, 115, 771–777. [Google Scholar] [CrossRef] [Green Version]
- Devillers, A.C.; van Toorenenbergen, A.W.; Klein Heerenbrink, G.J.; Muldert, P.G.; Oranje, A.P. Elevated levels of plasma matrix metalloproteinase-9 in patients with atopic dermatitis: A pilot study. Clin. Exp. Dermatol. 2007, 32, 311–313. [Google Scholar] [CrossRef]
- Buisson-Legendre, N.; Emonard, H.; Bernard, P.; Hornebeck, W. Relationship between cell-associated matrix metalloproteinase 9 and psoriatic keratinocyte growth. J. Investig. Dermatol. 2000, 115, 213–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antiga, E.; Volpi, W.; Del Bianco, E.; Fabbri, P.; Caproni, M. Plasma levels of metalloproteinase-9 are elevated in patients with chronic autoimmune urticaria. Br. J. Dermatol. 2009, 161, 712–714. [Google Scholar] [CrossRef] [PubMed]
- Bastuji-Garin, S.; Fouchard, N.; Bertocchi, M.; Roujeau, J.C.; Revuz, J.; Wolkenstein, P. SCORTEN: A severity-of-illness score for toxic epidermal necrolysis. J. Investig. Dermatol. 2000, 115, 149–153. [Google Scholar]
- Chung, W.H.; Chang, W.C.; Stocker, S.L.; Juo, C.G.; Graham, G.G.; Lee, M.H.; Williams, K.M.; Tian, Y.C.; Juan, K.C.; Jan Wu, Y.J.; et al. Insights into the poor prognosis of allopurinol-induced severe cutaneous adverse reactions: The impact of renal insufficiency, high plasma levels of oxypurinol and granulysin. Ann. Rheum. Dis. 2015, 74, 2157–2164. [Google Scholar] [CrossRef]
- Yeong, E.K.; Lee, C.H.; Hu, F.C.; Wu, M. Z Serum bicarbonate as a marker to predict mortality in toxic epidermal necrolysis. J. Intensive Care Med. 2011, 26, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, J.; Meakins, J.L. Complications of povidone-iodine absorption in topically treated burn patients. Lancet 1976, 307, 280–282. [Google Scholar] [CrossRef]
- Syed, D.; Iqbal, O.; Mosier, M.; Mitchell, R.; Hoppensteadt, D.; Bouchard, C.; Fareed, J.; Gamelli, R. Elevated endocan levels and its association with clinical severity in Stevens-Johnson Syndrome and toxic epidermal necrolysis. Int. Angiol. J. Int. Union Angiol. 2015, 34, 483–488. [Google Scholar]
- Suzuki, H.; Miyagaki, T.; Otobe, S.; Nakajima, R.; Oka, T.; Takahashi, N.; Kabasawa, M.; Suga, H.; Yoshizaki, A.; Asano, Y.; et al. Increased endocan expression in lesional skin and decreased endocan expression in sera in atopic dermatitis. J. Dermatol. 2017, 44, 1392–1395. [Google Scholar] [CrossRef]
- Balta, I.; Balta, S.; Demirkol, S.; Mikhailidis, D.P.; Celik, T.; Akhan, M.; Kurt, O.; Kurt, Y.G.; Aydin, I.; Kilic, S. Elevated serum levels of endocan in patients with psoriasis vulgaris: Correlations with cardiovascular risk and activity of disease. Br. J. Dermatol. 2013, 169, 1066–1070. [Google Scholar] [CrossRef]
- Demirturk, M.; Akpinar, T.S.; Kose, M.; Gelincik, A.; Colakoğlu, B.; Buyukozturk, S. Endocan: A Novel Marker of Endothelial Dysfunction in C1-Inhibitor-Deficient Hereditary Angioedema. Int. Arch. Allergy Immunol. 2017, 174, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Caproni, M.; Torchia, D.; Schincaglia, E.; Volpi, W.; Frezzolini, A.; Schena, D.; Marzano, A.; Quaglino, P.; De Simone, C.; Parodi, A.; et al. Expression of cytokines and chemokine receptors in the cutaneous lesions of erythema multiforme and Stevens-Johnson syndrome/toxic epidermal necrolysis. Br. J. Dermatol. 2006, 155, 722–728. [Google Scholar] [CrossRef]
- Sadek, M.; Iqbal, O.; Siddiqui, F.; Till, S.; Mazariegos, M.; Campbell, E.; Mudaliar, K.; Speiser, J.; Bontekoe, E.; Kouta, A.; et al. The Role of IL-13, IL-15 and Granulysin in the Pathogenesis of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. Clin. Appl. Thromb. Hemost. Off. J. Int. Acad. Clin. Appl. Thromb. Hemost. 2021, 27, 1076029620950831. [Google Scholar]
- Su, S.C.; Mockenhaupt, M.; Wolkenstein, P.; Dunant, A.; Le Gouvello, S.; Chen, C.B.; Chosidow, O.; Valeyrie-Allanore, L.; Bellon, T.; Sekula, P.; et al. Interleukin-15 Is Associated with Severity and Mortality in Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. J. Investig. Dermatol. 2017, 137, 1065–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamid, Q.; Naseer, T.; Minshall, E.M.; Song, Y.L.; Boguniewicz, M.; Leung, D.Y. In vivo expression of IL-12 and IL-13 in atopic dermatitis. J. Allergy Clin. Immunol. 1996, 98, 225–231. [Google Scholar] [CrossRef]
- D’Auria, L.; Bonifati, C.; Cordiali-Fei, P.; Leone, G.; Picardo, M.; Pietravalle, M.; Giacalone, B.; Ameglio, F. Increased serum interleukin-15 levels in bullous skin diseases: Correlation with disease intensity. Arch. Dermatol. Res. 1999, 291, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Asadullah, K.; Haeussler-Quade, A.; Gellrich, S.; Hanneken, S.; Hansen-Hagge, T.E.; Döcke, W.D.; Volk, H.D.; Sterry, W. IL-15 and IL-16 overexpression in cutaneous T-cell lymphomas: Stage-dependent increase in mycosis fungoides progression. Exp. Dermatol. 2000, 9, 248–251. [Google Scholar] [CrossRef]
- Lesiak, A.; Bednarski, I.; Pałczyńska, M.; Kumiszcza, E.; Kraska-Gacka, M.; Woźniacka, A.; Narbutt, J. Are interleukin-15 and -22 a new pathogenic factor in pustular palmoplantar psoriasis? Postep. Dermatol. Alergol. 2016, 33, 336–339. [Google Scholar] [CrossRef] [Green Version]
- Abe, R.; Yoshioka, N.; Murata, J.; Fujita, Y.; Shimizu, H. Granulysin as a marker for early diagnosis of the Stevens-Johnson syndrome. Ann. Intern. Med. 2009, 151, 514–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.; Guo, Z.P.; Li, L.; Wang, L.; Jia, R.Z.; Cao, N.; Qin, S.; Li, M.M. Increased HMGB1 serum levels and altered HMGB1 expression in patients with psoriasis vulgaris. Arch. Dermatol. Res. 2013, 305, 263–267. [Google Scholar] [CrossRef]
- Cuppari, C.; Manti, S.; Salpietro, A.; Valenti, S.; Capizzi, A.; Arrigo, T.; Salpietro, C.; Leonardi, S. HMGB1 levels in children with atopic eczema/dermatitis syndrome (AEDS). Pediatr. Allergy Immunol. 2016, 27, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Drozd, D.R.; Graham, S.M.; Crane, H.M.; Delaney, J.A.; Kitahata, M.M.; Liles, W.C. Short Communication: Effect of Antiretroviral Therapy on Circulating Damage-Associated Molecular Pattern Molecules and CD4 Immune Reconstitution in HIV-Infected Individuals. AIDS Res. Hum. Retrovir. 2016, 32, 876–878. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Min, H.J.; Shin, J.S. Increased levels of HMGB1 and pro-inflammatory cytokines in children with febrile seizures. J. Neuroinflamm. 2011, 8, 135. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Chen, G.; Kong, X. Serum level of high mobility group box protein-1 and prognosis of patients with end-stage renal disease on hemodialysis and peritoneal dialysis. Medicine 2021, 100, e24275. [Google Scholar] [CrossRef] [PubMed]

| Biomarker | Type of Drug Eruption | Mechanism | Direct or Indirect Action | Detection |
|---|---|---|---|---|
| Eosinophil count | General drug eruption | Immune activation | Indirect action | Peripheral blood |
| Eosinophilic cationic protein | General drug eruption | Unknown | Indirect action | Serum |
| CD4+CD25+ cell | A sign of desensitization of fixed drug eruption | Immune activation | Direct action | Skin |
| miR-18a-5p | TEN | Immune activation | Direct action | Skin |
| miR-124 | TEN | Unknown | Unknown | Serum |
| miR-214 | TEN | Unknown | Unknown | Skin |
| Perforin | SJS/TEN | Immune activation | Direct action | Serum |
| Granzyme B | SJS/TEN | Immune activation | Direct action | Serum |
| Fas ligand | SJS/TEN | Immune activation | Direct action | Serum, bullous fluid |
| Granulysin | SJS/TEN | Immune activation | Direct action | Serum |
| HMGB1 | SJS/TEN | Tissue damage | Indirect action | Serum, bullous fluid, skin |
| TARC | General drug eruption | Immune activation | Indirect action | Serum |
| Th17 | DIHS, AGEP, SJS/TEN | Immune activation | Indirect action | Peripheral blood |
| Galectin 7 | SJS/TEN | Tissue damage | Indirect action | Serum |
| S100A2 | SJS/TEN | Tissue damage | Indirect action | Skin |
| Annexin A/FPR1 | SJS/TEN | Immune activation | Direct action | Skin |
| RIP3 | SJS/TEN | Immune activation | Direct action | Serum, skin |
| IL-13/IL-15 | SJS/TEN | Immune activation | Direct | Serum, skin |
| MMP2, MMP9, MMP11 | SJS/TEN | Immune activation | Direct action | Skin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshioka, M.; Sawada, Y.; Nakamura, M. Diagnostic Tools and Biomarkers for Severe Drug Eruptions. Int. J. Mol. Sci. 2021, 22, 7527. https://doi.org/10.3390/ijms22147527
Yoshioka M, Sawada Y, Nakamura M. Diagnostic Tools and Biomarkers for Severe Drug Eruptions. International Journal of Molecular Sciences. 2021; 22(14):7527. https://doi.org/10.3390/ijms22147527
Chicago/Turabian StyleYoshioka, Manabu, Yu Sawada, and Motonobu Nakamura. 2021. "Diagnostic Tools and Biomarkers for Severe Drug Eruptions" International Journal of Molecular Sciences 22, no. 14: 7527. https://doi.org/10.3390/ijms22147527





