N-Acetylcysteine Added to Local Anesthesia Reduces Scar Area and Width in Early Wound Healing—An Animal Model Study
Abstract
:1. Introduction
2. Results
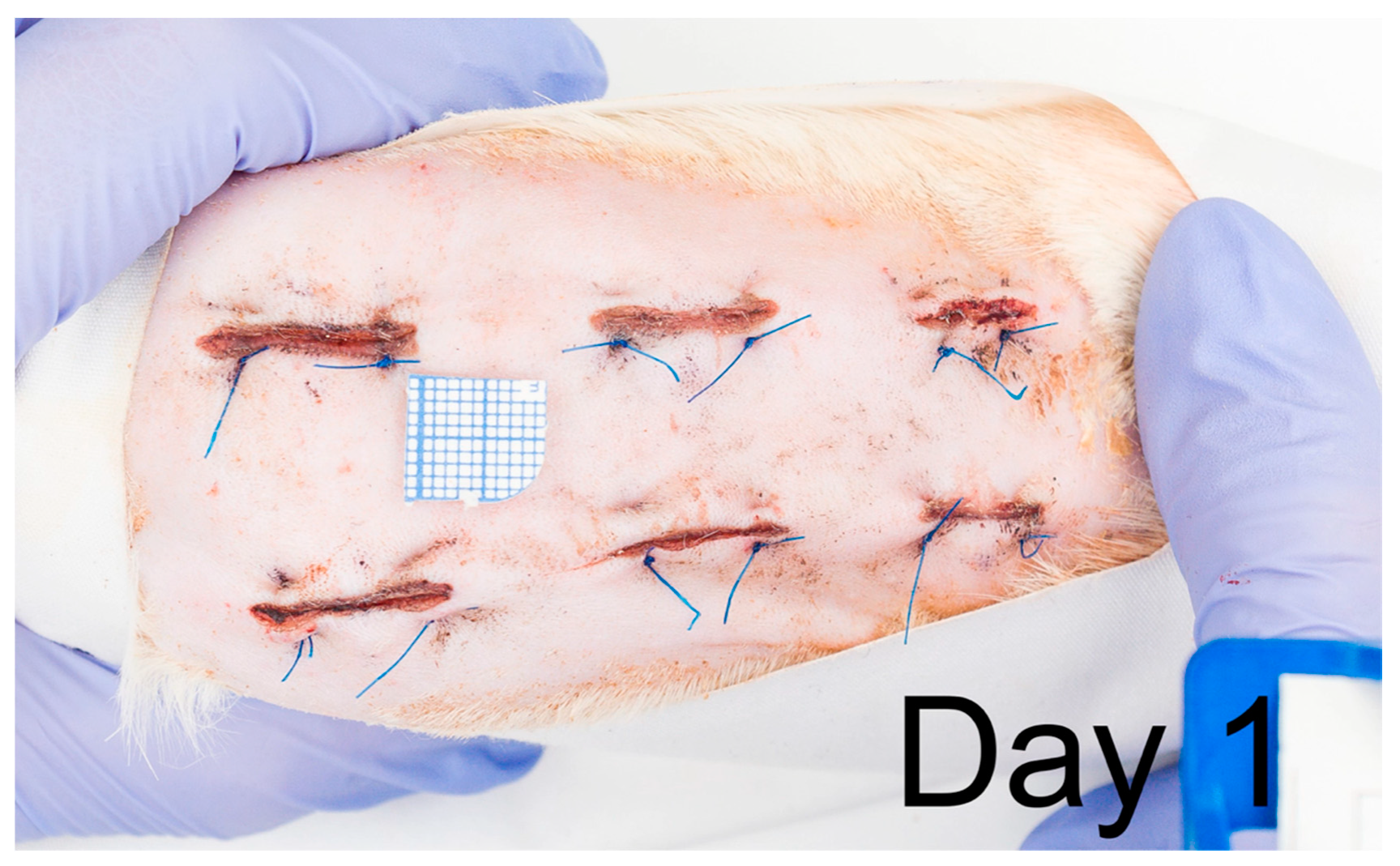
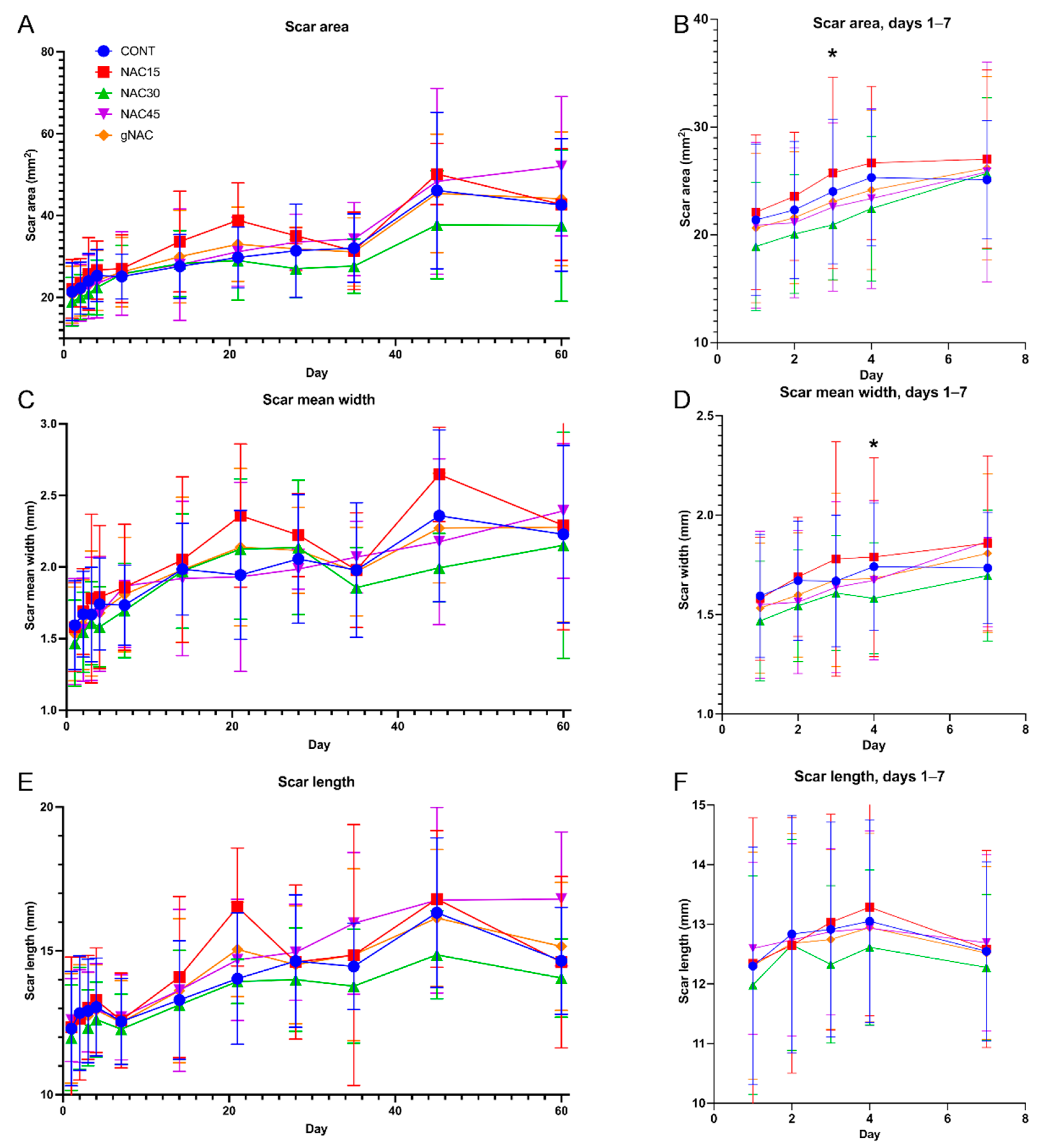
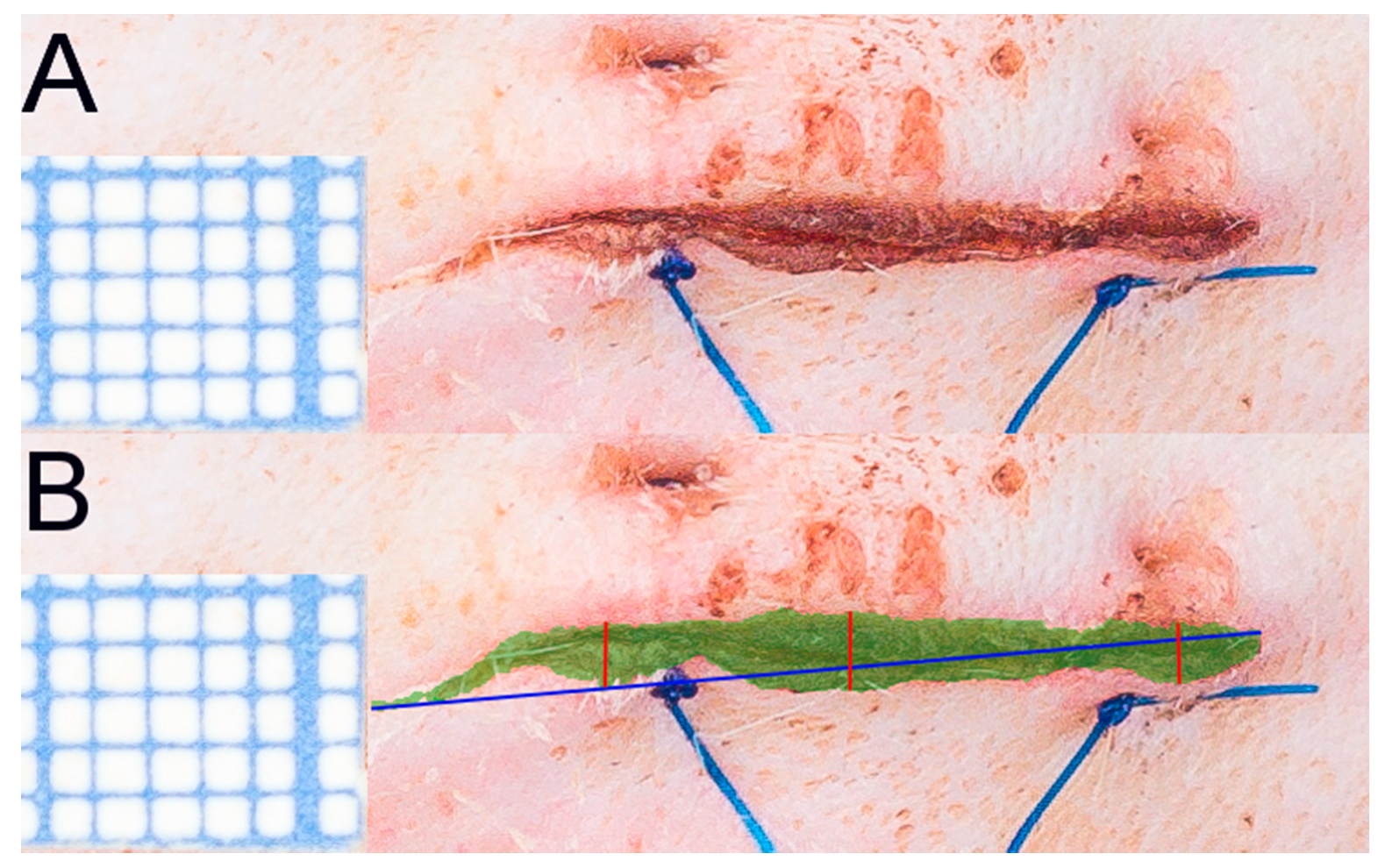
2.1. Wound and Scar Planimetric Analysis Based on Photographic Documentation
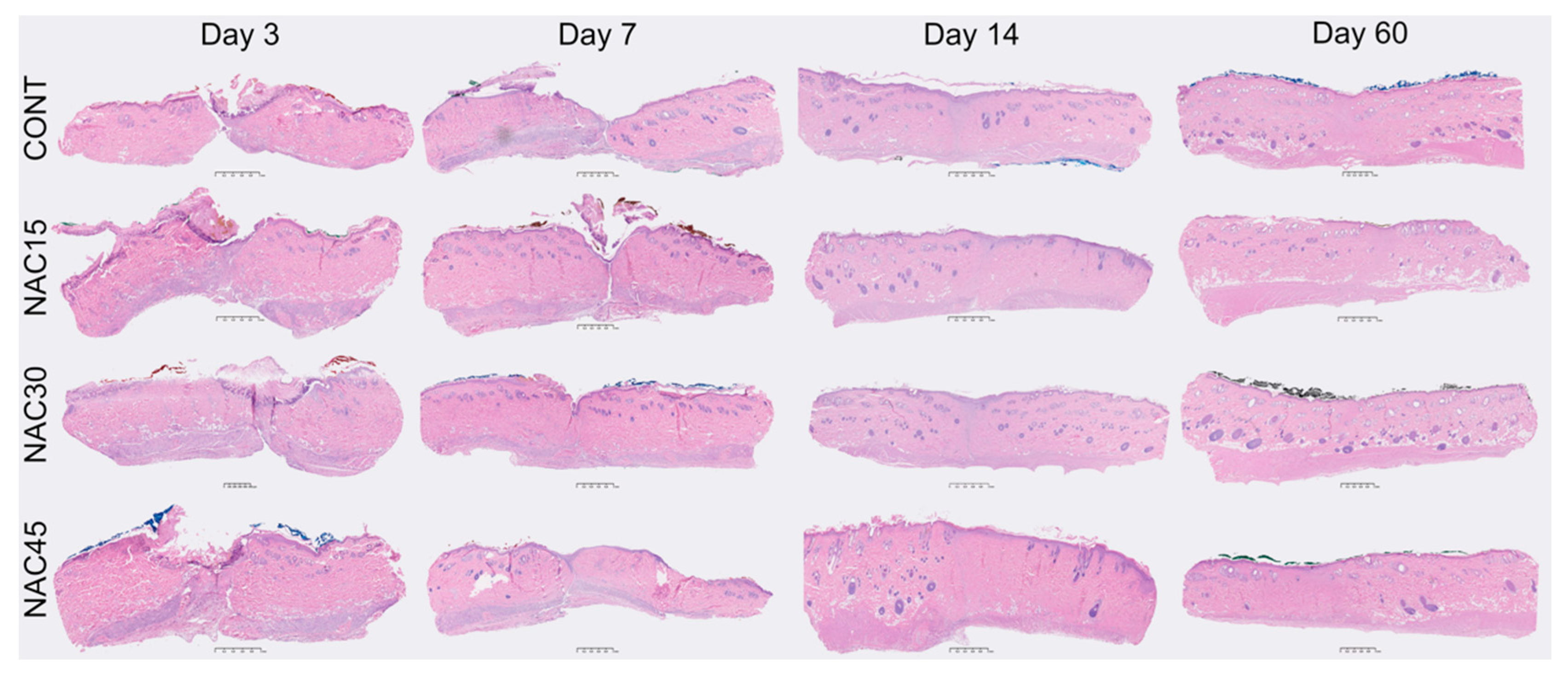
2.2. Histological Assessment of Scars
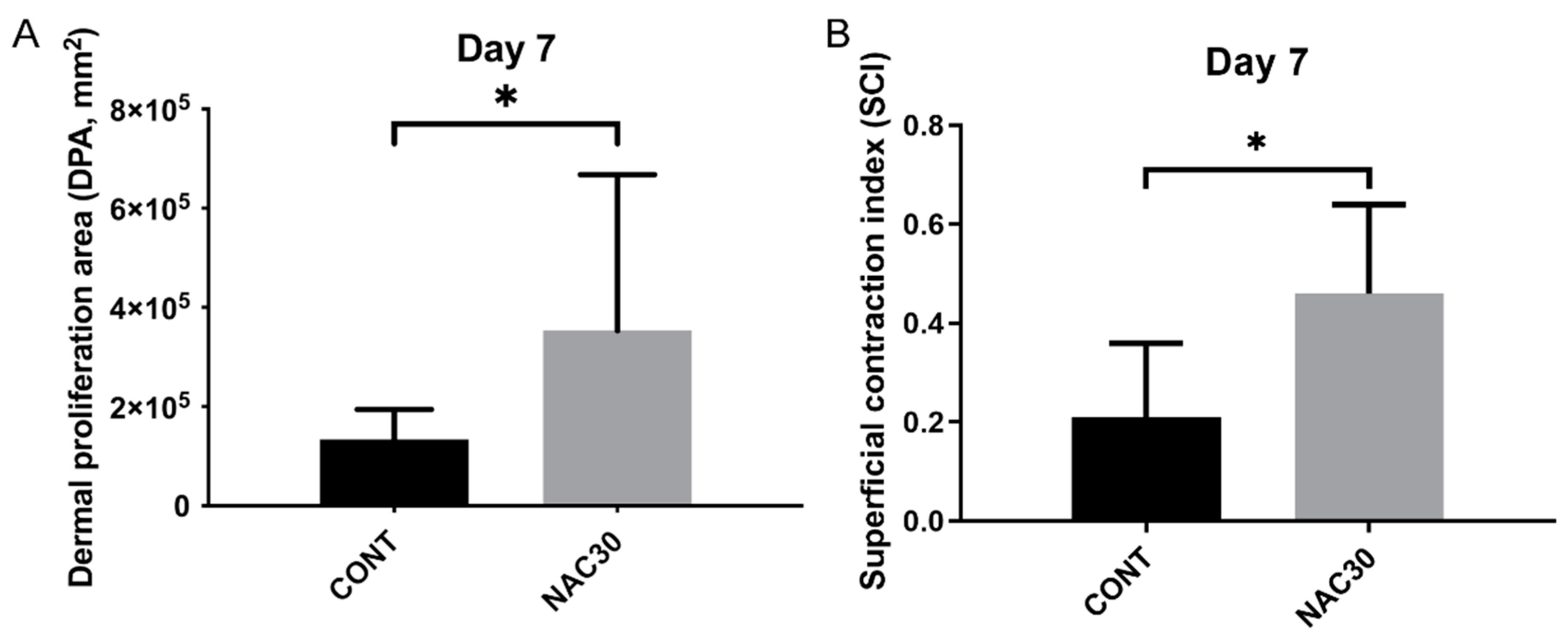
2.3. Histomorphometry
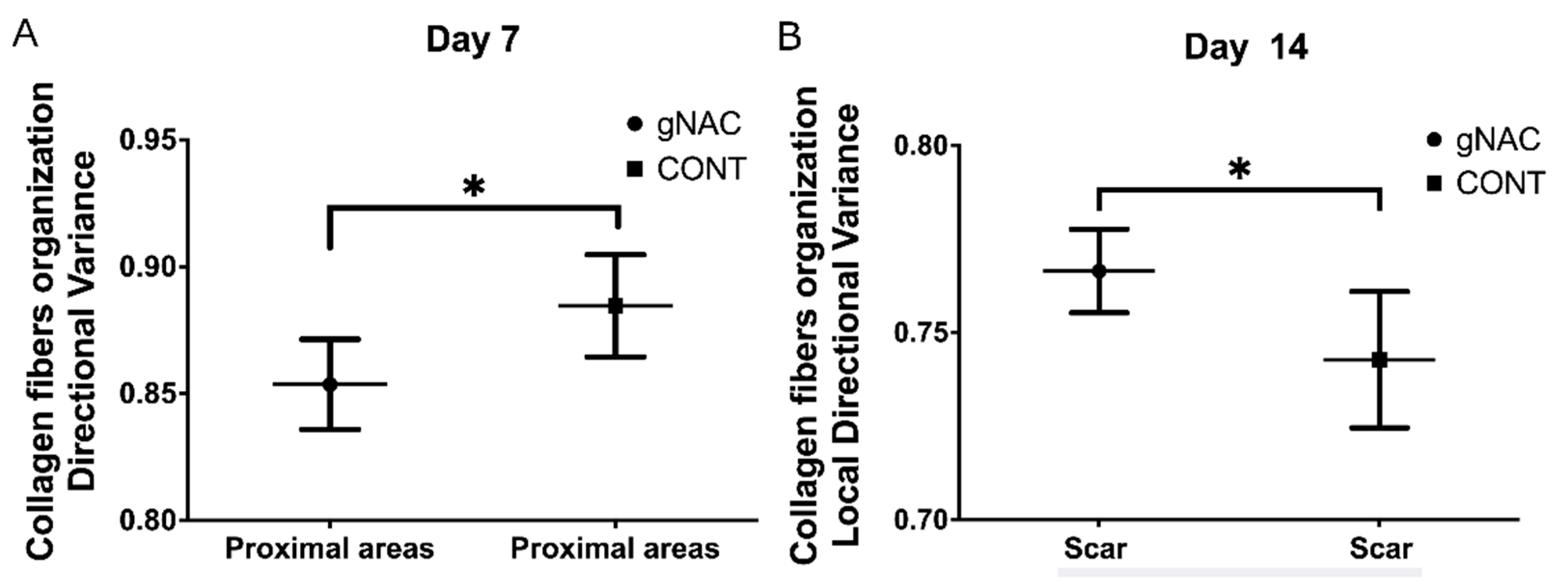
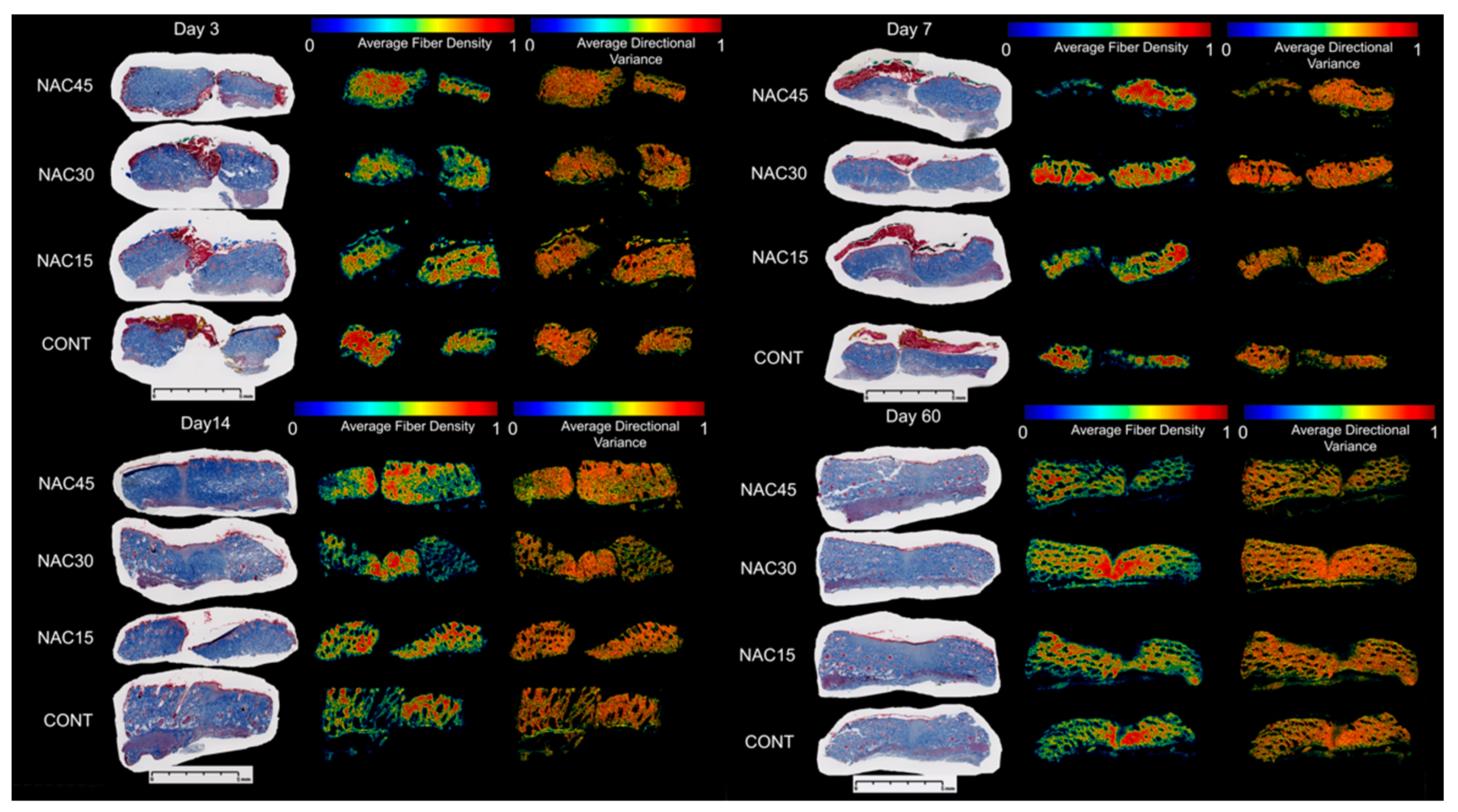
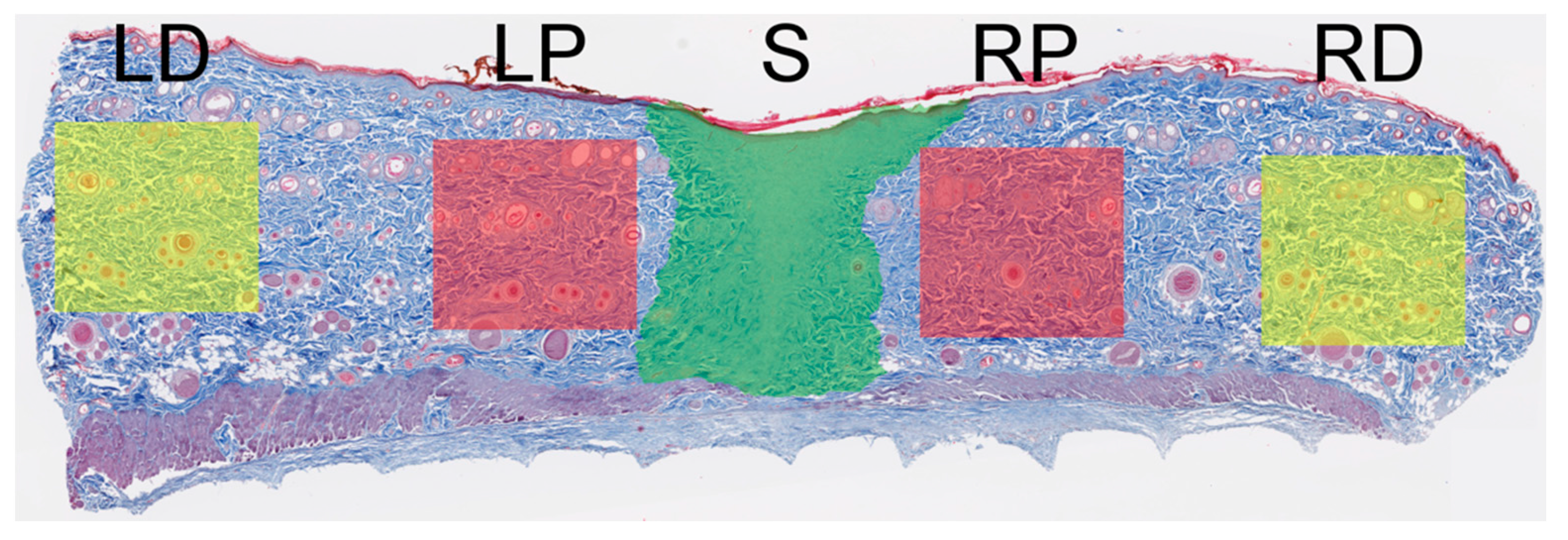
2.4. Collagen Fiber Organization
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Surgical Procedure
4.3. Evaluation of Macroscopic Wound Healing in Time; Photographic Documentation and Scar Area Quantification
4.4. Scar Tissue Collection and Analysis
4.5. Scar Histology Analysis
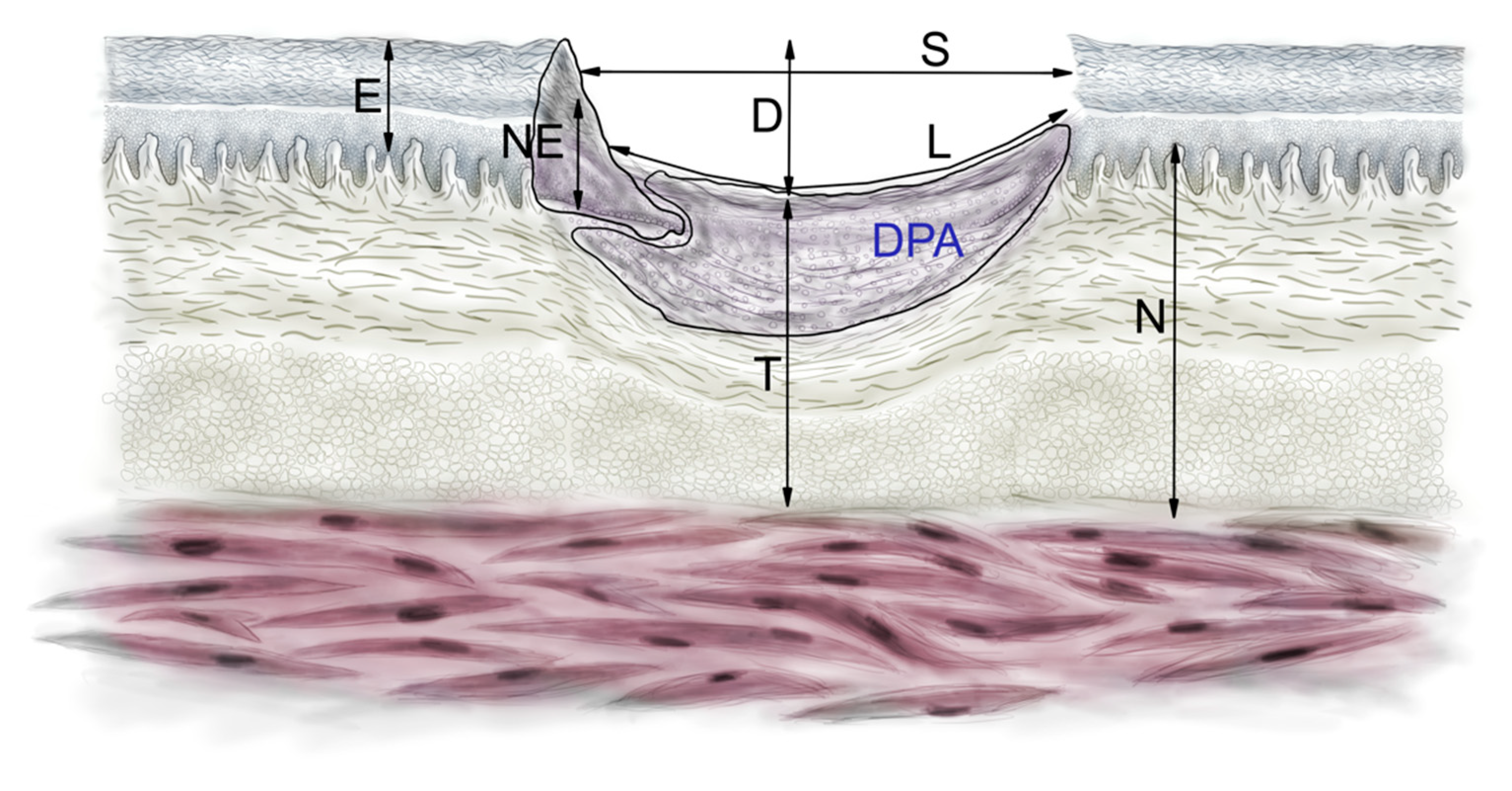
4.6. Histomorphometry
4.7. Collagen Fiber Arrangement Analysis
4.8. Statistical Analysis
5. Conclusions
- Including 0.03% NAC injected intradermally prior to incision significantly decreases the area and width of a wound at an early stage of healing (3rd and 4th day) and non-significantly at later time points.
- This treatment increases the contraction and proliferation of the wound bed at an early stage. It does not affect other histologic features.
- Pre-incisional NAC mildly moderates collagen fiber orientation on the 7th and 14th day of healing and decreases variance in the scar surrounding and increases in the scar itself.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Landen, N.X.; Li, D.; Stahle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [Green Version]
- Becker, D.E.; Reed, K.L. Local anesthetics: Review of pharmacological considerations. Anesth. Prog. 2012, 59, 90–101, quiz 102–103. [Google Scholar] [CrossRef] [Green Version]
- Geers, N.C.; Zegel, M.; Huybregts, J.G.J.; Niessen, F.B. The Influence of Preoperative Interventions on Postoperative Surgical Wound Healing in Patients Without Risk Factors: A Systematic Review. Aesthetic Surg. J. 2018, 38, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, N.; Zuin, V.; Burro, R.; Ottolenghi, A.; Camoglio, F.S. A prospective study in children: Pre- and post-surgery use of vitamin E in surgical incisions. J. Plast. Reconstr. Aesthetic Surg. 2010, 63, 1474–1478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lau, S.S.; Monks, T.J. The cytoprotective effect of N-acetyl-L-cysteine against ROS-induced cytotoxicity is independent of its ability to enhance glutathione synthesis. Toxicol. Sci. 2011, 120, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Rushworth, G.F.; Megson, I.L. Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014, 141, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Aktunc, E.; Ozacmak, V.H.; Ozacmak, H.S.; Barut, F.; Buyukates, M.; Kandemir, O.; Demircan, N. N-acetyl cysteine promotes angiogenesis and clearance of free oxygen radicals, thus improving wound healing in an alloxan-induced diabetic mouse model of incisional wound. Clin. Exp. Dermatol. 2010, 35, 902–909. [Google Scholar] [CrossRef]
- Tsai, M.-L.; Huang, H.-P.; Hsu, J.-D.; Lai, Y.-R.; Hsiao, Y.-P.; Lu, F.-J.; Chang, H.-R. Topical N-Acetylcysteine Accelerates Wound Healing in Vitro and in Vivo via the PKC/Stat3 Pathway. Int. J. Mol. Sci. 2014, 15, 7563–7578. [Google Scholar] [CrossRef] [Green Version]
- Ozkaya, H.; Omma, T.; Bag, Y.M.; Uzunoglu, K.; Isildak, M.; Duymus, M.E.; Kismet, K.; Senes, M.; Fidanci, V.; Celepli, P.; et al. Topical and Systemic Effects of N-acetyl Cysteine on Wound Healing in a Diabetic Rat Model. Wounds Compend. Clin. Res. Pract. 2019, 31, 91–96. [Google Scholar]
- Gomez-Aparicio, L.S.; Bernaldez-Sarabia, J.; Camacho-Villegas, T.A.; Lugo-Fabres, P.H.; Diaz-Martinez, N.E.; Padilla-Camberos, E.; Licea-Navarro, A.; Castro-Cesena, A.B. Improvement of the wound healing properties of hydrogels with N-acetylcysteine through their modification with methacrylate-containing polymers. Biomater. Sci. 2021, 9, 726–744. [Google Scholar] [CrossRef]
- Oguz, A.; Uslukaya, O.; Alabalik, U.; Turkoglu, A.; Kapan, M.; Bozdag, Z. Topical N-acetylcysteine improves wound healing comparable to dexpanthenol: An experimental study. Int. Surg. 2015, 100, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Parasassi, T.; Brunelli, R.; Bracci-Laudiero, L.; Greco, G.; Gustafsson, A.C.; Krasnowska, E.K.; Lundeberg, J.; Lundeberg, T.; Pittaluga, E.; Romano, M.C.; et al. Differentiation of normal and cancer cells induced by sulfhydryl reduction: Biochemical and molecular mechanisms. Cell Death Differ. 2005, 12, 1285–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abramov, Y.; Golden, B.; Sullivan, M.; Botros, S.M.; Miller, J.J.; Alshahrour, A.; Goldberg, R.P.; Sand, P.K. Histologic characterization of vaginal vs. abdominal surgical wound healing in a rabbit model. Wound Repair Regen. 2007, 15, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Lemo, N.; Marignac, G.; Reyes-Gomez, E.; Lilin, T.; Crosaz, O.; Dohan Ehrenfest, D.M. Cutaneous reepithelialization and wound contraction after skin biopsies in rabbits: A mathematical model for healing and remodelling index. Vet. Arh. 2010, 80, 637–652. [Google Scholar]
- Van Winkle, W., Jr. The epithelium in wound healing. Surg. Gynecol. Obstet. 1968, 127, 1089–1115. [Google Scholar]
- Tascilar, O.; Cakmak, G.; Emre, A.; Bakkal, H.; Kandemir, N.; Turkcu, U.; Demir, E. N-acetylcycsteine attenuates the deleterious effects of radiation therapy on inci-sional wound healing in rats. Hippokratia 2014, 18, 17–23. [Google Scholar]
- Quinn, K.P.; Golberg, A.; Broelsch, G.F.; Khan, S.; Villiger, M.; Bouma, B.; Austen, W.G.; Sheridan, R.L.; Mihm, M.C.; Yarmush, M.L.; et al. An automated image processing method to quantify collagen fiber organization within cutaneous scar tissue. Exp. Dermatol. 2015, 24, 78–80. [Google Scholar] [CrossRef] [Green Version]
- Zafarullah, M.; Li, W.Q.; Sylvester, J.; Ahmad, M. Molecular mechanisms of N-acetylcysteine actions. Cell. Mol. Life Sci. 2003, 60, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Parnell, L.K.S.; Volk, S.W. The Evolution of Animal Models in Wound Healing Research: 1993–2017. Adv. Wound Care 2019, 8, 692–702. [Google Scholar] [CrossRef]
- Mustoe, T.A.; Pierce, G.F.; Thomason, A.; Gramates, P.; Sporn, M.B.; Deuel, T.F. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science 1987, 237, 1333–1336. [Google Scholar] [CrossRef]
- Krischak, G.D.; Augat, P.; Claes, L.; Kinzl, L.; Beck, A. The effects of non-steroidal anti-inflammatory drug application on incisional wound healing in rats. J. Wound Care 2007, 16, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Yaman, I.; Derici, H.; Kara, C.; Kamer, E.; Diniz, G.; Ortac, R.; Sayin, O. Effects of resveratrol on incisional wound healing in rats. Surg. Today 2013, 43, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Yagiela, J.A. Vasoconstrictor agents for local anesthesia. Anesth. Prog. 1995, 42, 116–120. [Google Scholar] [PubMed]
- Olsson, B.; Johansson, M.; Gabrielsson, J.; Bolme, P. Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. Eur. J. Clin. Pharmacol. 1988, 34, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Cotter, M.A.; Thomas, J.; Cassidy, P.; Robinette, K.; Jenkins, N.; Florell, S.R.; Leachman, S.; Samlowski, W.E.; Grossman, D. N-acetylcysteine protects melanocytes against oxidative stress/damage and delays onset of ultraviolet-induced melanoma in mice. Clin. Cancer Res. 2007, 13, 5952–5958. [Google Scholar] [CrossRef] [Green Version]
- Gillis, J.; Gebremeskel, S.; Phipps, K.D.; MacNeil, L.A.; Sinal, C.J.; Johnston, B.; Hong, P.; Bezuhly, M. Effect of N-Acetylcysteine on Adipose-Derived Stem Cell and Autologous Fat Graft Survival in a Mouse Model. Plast. Reconstr. Surg. 2015, 136, 179e–188e. [Google Scholar] [CrossRef]
- Borgstrom, L.; Kagedal, B.; Paulsen, O. Pharmacokinetics of N-acetylcysteine in man. Eur. J. Clin. Pharmacol. 1986, 31, 217–222. [Google Scholar] [CrossRef]
- Buur, J.L.; Diniz, P.P.; Roderick, K.V.; KuKanich, B.; Tegzes, J.H. Pharmacokinetics of N-acetylcysteine after oral and intravenous administration to healthy cats. Am. J. Vet. Res. 2013, 74, 290–293. [Google Scholar] [CrossRef]
- Gouma, E.; Simos, Y.; Verginadis, I.; Lykoudis, E.; Evangelou, A.; Karkabounas, S. A simple procedure for estimation of total body surface area and determination of a new value of Meeh’s constant in rats. Lab. Anim. 2012, 46, 40–45. [Google Scholar] [CrossRef]
- Schencke, C.; Vasconcellos, A.; Sandoval, C.; Torres, P.; Acevedo, F.; del Sol, M. Morphometric evaluation of wound healing in burns treated with Ulmo (Eucryphia cordifolia) honey alone and supplemented with ascorbic acid in guinea pig (Cavia porcellus). Burns Trauma 2016, 4, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bankhead, P.; Loughrey, M.B.; Fernandez, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinn, K.P.; Georgakoudi, I. Rapid quantification of pixel-wise fiber orientation data in micrographs. J. Biomed. Opt. 2013, 18, 046003. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Day 7 | Day 14 | Day 60 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CONT | gNAC | p 1 | NAC30 | p 2 | CONT | gNAC | p 1 | CONT | gNAC | p 1 | |
| D (µm) | 527.2 ± 279.3 | 627.5 ± 193.2 | 0.34 | 558.3 ± 251.7 | 0.86 | 313.0 ± 96.8 | 375.8 ± 191.8 | 0.76 | 157.2 ± 98.2 | 143.4 ± 88.0 | 0.67 |
| DPA (µm2) | 134,280.2 ± 60364.62 | 180,923.8 ± 190501.1 | 0.46 | 353,591.2 ± 314148.8 | 0.04 | 405,838.2 ± 226281.4 | 550,007.5 ± 508,475.6 | 0.27 | 906,748.8 ± 572,360.8 | 743,650.1 ± 336,697.1 | 0.30 |
| EPI (µm) | 50.9 ± 13.1 | 43.3 ± 11.5 | 0.17 | 42.4 ± 12.2 | 0.34 | 44.4 ± 10.2 | 46.0 ± 14.3 | 0.71 | 28.1 ± 6.04 | 28.4 ± 5.21 | 0.85 |
| L(µm) | 1909.8 ± 901.6 | 1932.1 ± 649.9 | 0.94 | 1471.0 ± 810.9 | 0.46 | 1272.9 ± 484.5 | 1382.7 ± 570.5 | 0.58 | 793.99 ± 456.6 | 919.22 ± 815.1 | 0.59 |
| N (µm) | 1434.4 ± 196.7 | 1427.2 ± 169.5 | 0.92 | 1459.5 ± 65.5 | 0.83 | 1404.1 ± 187.8 | 1496.0 ± 393.1 | 0.96 | 1680.7 ± 369.3 | 1625.0 ± 246.4 | 0.60 |
| NEO (µm) | 83.6 ± 23.4 | 98.8 ± 24.4 | 0.16 | 102.3 ± 43.85 | 0.33 | 110.1 ± 28.9 | 114.2 ± 33.2 | 0.72 | 38.1 ± 7.82 | 37.3 ± 12.9 | 0.82 |
| S (µm) | 1533.2 ± 791.5 | 1312.6 ± 630.3 | 0.48 | 820.3 ± 670.0 | 0.18 | 1150.8 ± 541.2 | 1268.9 ± 676.7 | 0.61 | 696.69 ± 403.1 | 836.99 ± 776.4 | 0.52 |
| T (µm) | 915.5 ± 298.8 | 802.9 ± 270.5 | 0.37 | 794.5 ± 145.9 | 0.52 | 1289 ± 192.6 | 1484.3 ± 381.8 | 0.21 | 1650.1 ± 435.3 | 1542.3 ± 275.6 | 0.40 |
| H (µm) | 1604.5 ± 980.1 | 1448.4 ± 1152.6 | 0.66 | 1731.1 ± 762.5 | 0.79 | 1890.3 ± 1082.3 | 1679.2 ± 1134.4 | 0.57 | 542.48 ± 248.6 | 736.08 ± 705.2 | 0.27 |
| B (µm2) | 189,079.1 ± 127,604.5 | 155,889.7 ± 140,034.4 | 0.46 | 222,271.6 ± 196,386.6 | 0.65 | 652,444.9 ± 293,520.0 | 622,769.1 ± 387,104.4 | 0.79 | 619,803.1 ± 407,497.9 | 645,390.3 ± 409,208.7 | 0.85 |
| SCI | 0.21 ± 0.15 | 0.34 ± 0.15 | 0.07 | 0.46 ± 0.18 | 0.03 | 0.11 ± 0.21 | 0.11 ± 0.25 | 0.94 | 0.12 ± 0.08 | 0.10 ± 0.07 | 0.44 |
| DCI | 0.63 ± 0.15 | 0.55 ± 0.13 | 0.22 | 0.62 ± 0.16 | 0.86 | 0.77 ± 0.06 | 0.77 ± 0.07 | 0.84 | 0.90 ± 0.06 | 0.91 ± 0.05 | 0.58 |
| WSI | 0.35 ± 0.22 | 0.44 ± 0.14 | 0.27 | 0.45 ± 0.12 | 0.48 | 0.09 ± 0.14 | 0.04 ± 0.17 | 0.40 | 0.01 ± 0.11 | 0.05 ± 0.10 | 0.34 |
| GHI | 0.49 ± 0.32 | 0.45 ± 0.31 | 0.76 | 0.63 ± 0.39 | 0.55 | 0.80 ± 0.22 | 0.84 ± 0.30 | 0.69 | 1.01 ± 0.18 | 0.96 ± 0.15 | 0.42 |
| GCI | 0.85 ± 0.21 | 0.90 ± 0.21 | 0.59 | 1.08 ± 0.27 | 0.13 | 0.89 ± 0.22 | 0.88 ± 0.28 | 0.90 | 1.02 ± 0.10 | 1.01 ± 0.09 | 0.75 |
| RHI | −2.76 ± 0.61 | 0.09 ± 0.71 | 0.66 | −0.07 ± 0.47 | 0.79 | −0.17 ± 0.67 | −0.04 ± 0.70 | 0.57 | 0.66 ± 0.15 | 0.54 ± 0.43 | 0.27 |
| MRI | 2.35 ± 0.67 | 0.17 ± 0.74 | 0.46 | −0.17 ± 1.03 | 0.65 | −2.45 ± 1.55 | −2.29 ± 2.04 | 0.79 | −2.27 ± 2.15 | −2.41 ± 2.16 | 0.85 |
| GRI | −2.09 ± 0.44 | 0.13 ± 0.61 | 0.45 | −0.12 ± 0.51 | 0.58 | −1.31 ± 1.06 | −1.17 ± 1.02 | 0.68 | −0.80 ± 1.11 | −0.93 ± 1.22 | 0.74 |
| Day 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Distal Areas (RD&LD) (n = 36) | Proximal Areas (RP&LP) (n = 36) | Scar Area (S) (n = 18) | |||||||
| CONT | gNAC | p 1 | CONT | gNAC | p 1 | CONT | gNAC | p 1 | |
| Directional Variance | 0.86 ± 0.05 | 0.85 ± 0.05 | >0.05 | 0.84 ± 0.06 | 0.82 ± 0.07 | >0.05 | 0.88 ± 0.05 | 0.89 ± 0.05 | >0.05 |
| Local Directional Variance | 0.78 ± 0.03 | 0.79 ± 0.04 | >0.05 | 0.78 ± 0.03 | 0.76 ± 0.05 | >0.05 | 0.76 ± 0.04 | 0.76 ± 0.02 | >0.05 |
| Fiber Density | 0.74 ± 0.07 | 0.74 ± 0.07 | >0.05 | 0.74 ± 0.06 | 0.75 ± 0.07 | >0.05 | 0.62 ± 0.09 | 0.60 ± 0.08 | >0.05 |
| Blue Intensity | 0.71 ± 0.03 | 0.71 ± 0.04 | >0.05 | 0.71 ± 0.02 | 0.71 ± 0.03 | >0.05 | 0.72 ± 0.02 | 0.71 ± 0.02 | >0.05 |
| Day 7 | |||||||||
| Directional Variance | 0.88 ± 0.06 | 0.87 ± 0.07 | >0.05 | 0.88 ± 0.05 | 0.85 ± 0.05 | 0.02 | 0.92 ± 0.03 | 0.90 ± 0.04 | >0.05 |
| Local Directional Variance | 0.78 ± 0.03 | 0.78 ± 0.04 | >0.05 | 0.79 ± 0.03 | 0.77 ± 0.03 | >0.05 | 0.76 ± 0.02 | 0.76 ± 0.02 | >0.05 |
| Fiber Density | 0.73 ± 0.05 | 0.75 ± 0.05 | >0.05 | 0.75 ± 0.08 | 0.74 ± 0.07 | >0.05 | 0.62 ± 0.07 | 0.63 ± 0.08 | >0.05 |
| Blue Intensity | 0.71 ± 0.03 | 0.71 ± 0.03 | >0.05 | 0.71 ± 0.03 | 0.71 ± 0.03 | >0.05 | 0.72 ± 0.02 | 0.72 ± 0.03 | >0.05 |
| Day 14 | |||||||||
| Directional Variance | 0.86 ± 0.06 | 0.88 ± 0.07 | >0.05 | 0.86 ± 0.09 | 0.87 ± 0.05 | >0.05 | 0.87 ± 0.07 | 0.92 ± 0.03 | 0.05 |
| Local Directional Variance | 0.76 ± 0.04 | 0.77 ± 0.03 | >0.05 | 0.76 ± 0.06 | 0.76 ± 0.03 | >0.05 | 0.74 ± 0.03 | 0.76 ± 0.02 | 0.03 |
| Fiber Density | 0.69 ± 0.15 | 0.72 ± 0.10 | >0.05 | 0.68 ± 0.16 | 0.73 ± 0.09 | >0.05 | 0.61 ± 0.09 | 0.64 ± 0.13 | >0.05 |
| Blue Intensity | 0.76 ± 0.05 | 0.75 ± 0.04 | >0.05 | 0.76 ± 0.05 | 0.75 ± 0.05 | >0.05 | 0.76 ± 0.05 | 0.76 ± 0.04 | >0.05 |
| Day 60 | |||||||||
| Directional Variance | 0.86 ± 0.06 | 0.86 ± 0.06 | >0.05 | 0.84 ± 0.05 | 0.85 ± 0.05 | >0.05 | 0.86 ± 0.03 | 0.89 ± 0.05 | >0.05 |
| Local Directional Variance | 0.77 ± 0.04 | 0.77 ± 0.04 | >0.05 | 0.76 ± 0.03 | 0.76 ± 0.03 | >0.05 | 0.75 ± 0.02 | 0.77 ± 0.03 | >0.05 |
| Fiber Density | 0.73 ± 0.06 | 0.74 ± 0.03 | >0.05 | 0.74 ± 0.06 | 0.72 ± 0.05 | >0.05 | 0.75 ± 0.05 | 0.74 ± 0.06 | >0.05 |
| Blue Intensity | 0.71 ± 0.03 | 0.71 ± 0.04 | >0.05 | 0.71 ± 0.02 | 0.71 ± 0.03 | >0.05 | 0.72 ± 0.02 | 0.71 ± 0.02 | >0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paskal, W.; Paskal, A.M.; Pietruski, P.; Stachura, A.; Pełka, K.; Woessner, A.E.; Quinn, K.P.; Kopka, M.; Galus, R.; Wejman, J.; et al. N-Acetylcysteine Added to Local Anesthesia Reduces Scar Area and Width in Early Wound Healing—An Animal Model Study. Int. J. Mol. Sci. 2021, 22, 7549. https://doi.org/10.3390/ijms22147549
Paskal W, Paskal AM, Pietruski P, Stachura A, Pełka K, Woessner AE, Quinn KP, Kopka M, Galus R, Wejman J, et al. N-Acetylcysteine Added to Local Anesthesia Reduces Scar Area and Width in Early Wound Healing—An Animal Model Study. International Journal of Molecular Sciences. 2021; 22(14):7549. https://doi.org/10.3390/ijms22147549
Chicago/Turabian StylePaskal, Wiktor, Adriana M. Paskal, Piotr Pietruski, Albert Stachura, Kacper Pełka, Alan E. Woessner, Kyle P. Quinn, Michał Kopka, Ryszard Galus, Jarosław Wejman, and et al. 2021. "N-Acetylcysteine Added to Local Anesthesia Reduces Scar Area and Width in Early Wound Healing—An Animal Model Study" International Journal of Molecular Sciences 22, no. 14: 7549. https://doi.org/10.3390/ijms22147549
APA StylePaskal, W., Paskal, A. M., Pietruski, P., Stachura, A., Pełka, K., Woessner, A. E., Quinn, K. P., Kopka, M., Galus, R., Wejman, J., & Włodarski, P. (2021). N-Acetylcysteine Added to Local Anesthesia Reduces Scar Area and Width in Early Wound Healing—An Animal Model Study. International Journal of Molecular Sciences, 22(14), 7549. https://doi.org/10.3390/ijms22147549







