Early Microvascular Dysfunction: Is the Vasa Vasorum a “Missing Link” in Insulin Resistance and Atherosclerosis
Abstract
:1. Introduction
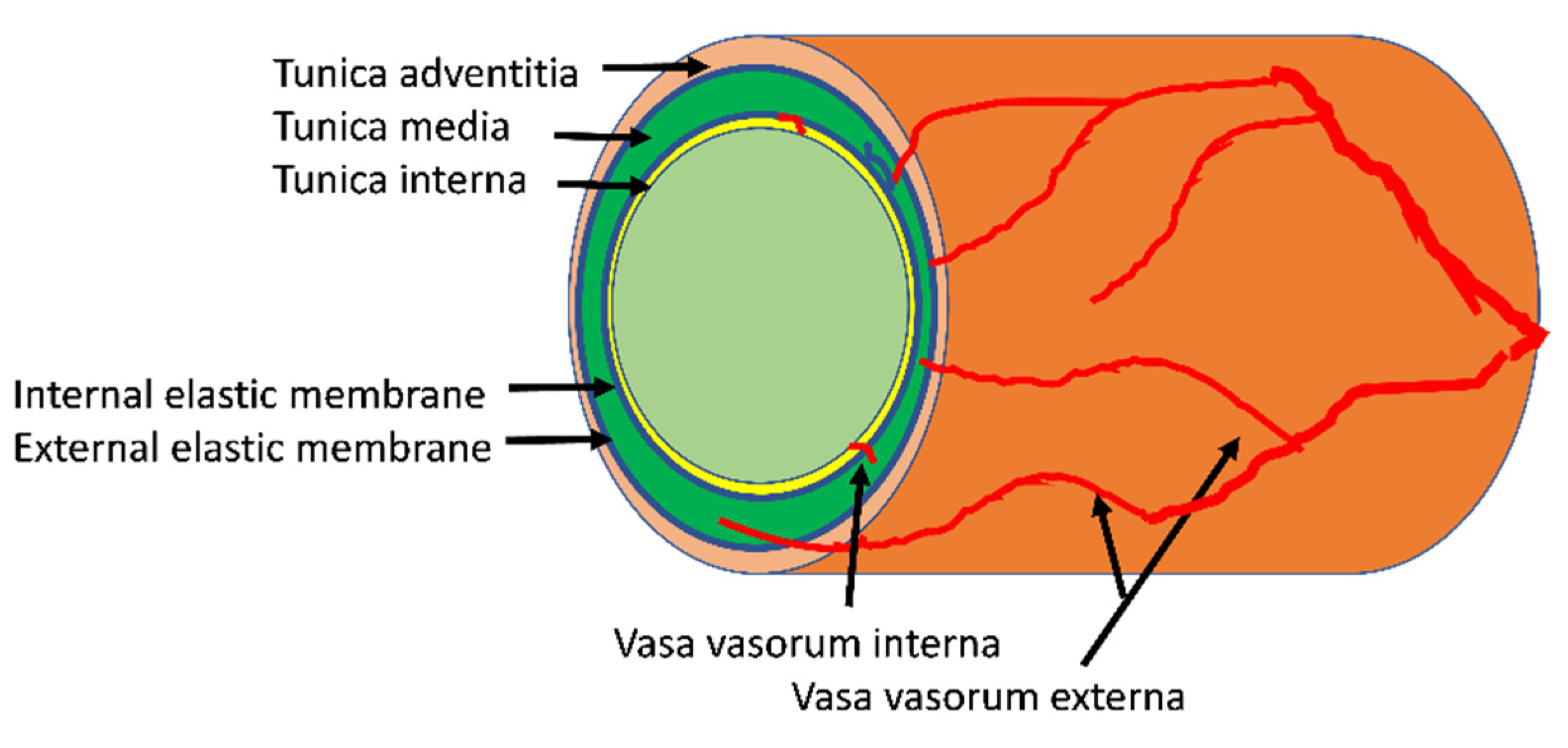
2. Structure and Function of the Arterial Vasa Vasorum
3. Normal Growth of the Vasa Vasorum
4. Insulin, Insulin Resistance, and the Microvasculature
5. Experimental Approaches to Studying Vasa Vasorum Structure and Function
6. Aberrant Vaso Vasorum Growth in Atherosclerosis
7. Microvascular Dysfunction, Insulin Resistance and Accelerated Atherosclerosis: Is Vasa Vasorum a Missing Link?
8. Research Challenges: Knowable Unknowns
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ritman, E.L.; Lerman, A. The dynamic vasa vasorum. Cardiovasc. Res. 2007, 75, 649–658. [Google Scholar] [CrossRef]
- Wolinsky, H.; Glagov, S. Nature of species differences in the medial distribution of aortic vasa vasorum in mammals. Circ. Res. 1967, 20, 409–421. [Google Scholar] [CrossRef] [Green Version]
- Mulligan-Kehoe, M.J.; Simons, M. Vasa vasorum in normal and diseased arteries. Circulation 2014, 129, 2557–2566. [Google Scholar] [CrossRef] [PubMed]
- Scotland, R.; Vallance, P.; Ahluwalia, A. On the regulation of tone in vasa vasorum. Cardiovasc. Res. 1999, 41, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Wilens, S.; Malcolm, J.; Vazquez, J. Experimental infarction (medial necrosis) of the dog’s aorta. Am. J. Pathol. 1965, 47, 695. [Google Scholar] [PubMed]
- Heistad, D.D.; Marcus, M.L.; Larsen, G.E.; Armstrong, M.L. Role of vasa vasorum in nourishment of the aortic wall. Am. J. Physiol. Heart Circ. Physiol. 1981, 240, H781–H787. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.K.; Armstrong, M.L.; Heistad, D.D. Vasa vasorum in atherosclerotic coronary arteries: Responses to vasoactive stimuli and regression of atherosclerosis. Circ. Res. 1988, 62, 515–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heistad, D.D.; Marcus, M.L.; Martins, J.B. Effects of neural stimuli on blood flow through vasa vasorum in dogs. Circ. Res. 1979, 45, 615–620. [Google Scholar] [CrossRef] [Green Version]
- Gössl, M.; Zamir, M.; Ritman, E.L. Vasa vasorum growth in the coronary arteries of newborn pigs. Anat. Embryol. 2004, 208, 351–357. [Google Scholar] [CrossRef]
- Gössl, M.; Versari, D.; Mannheim, D.; Ritman, E.L.; Lerman, L.O.; Lerman, A. Increased spatial vasa vasorum density in the proximal LAD in hypercholesterolemia—Implications for vulnerable plaque-development. Atherosclerosis 2007, 192, 246–252. [Google Scholar] [CrossRef]
- Barrett, E.J.; Eggleston, E.M.; Inyard, A.C.; Wang, H.; Li, G.; Chai, W.; Liu, Z. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia 2009, 52, 752–764. [Google Scholar] [CrossRef] [Green Version]
- Baron, A. Hemodynamic actions of insulin. Am. J. Physiol. Endocrinol. Metab. 1994, 267, E187–E202. [Google Scholar] [CrossRef] [Green Version]
- Coggins, M.P.; Lindner, J.; Rattigan, S.; Fasy, E.; Jahn, L.; Kaul, S.; Barrett, E.J. Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes 2001, 50, 2682–2690. [Google Scholar] [CrossRef] [Green Version]
- Scognamiglio, R.; Negut, C.; de Kreutzenberg, S.V.; Tiengo, A.; Avogaro, A. Effects of different insulin regimes on postprandial myocardial perfusion defects in type 2 diabetic patients. Diabetes Care 2006, 29, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Sjøberg, K.A.; Rattigan, S.; Hiscock, N.; Richter, E.A.; Kiens, B. A new method to study changes in microvascular blood volume in muscle and adipose tissue: Real-time imaging in humans and rat. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H450–H458. [Google Scholar] [CrossRef]
- Meijer, R.I.; De Boer, M.P.; Groen, M.R.; Eringa, E.C.; Rattigan, S.; Barrett, E.J.; Smulders, Y.M.; Serne, E.H. Insulin-induced microvascular recruitment in skin and muscle are related and both are associated with whole-body glucose uptake. Microcirculation 2012, 19, 494–500. [Google Scholar] [CrossRef]
- Hoscheidt, S.M.; Kellawan, J.M.; Berman, S.E.; Rivera-Rivera, L.A.; Krause, R.A.; Oh, J.M.; Beeri, M.S.; Rowley, H.A.; Wieben, O.; Carlsson, C.M.; et al. Insulin resistance is associated with lower arterial blood flow and reduced cortical perfusion in cognitively asymptomatic middle-aged adults. J. Cereb. Blood Flow Metab. 2017, 37, 2249–2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, V.; Trombetta, B.; Jafri, R.; Koenig, A.M.; Wennick, C.; Carlyle, B.; Ekhlaspur, L.; Ahima, R.; Russell, S.; Salat, D.H.; et al. Task-related fMRI Bold response to Hyperinsulinemia in Healthy Older Adults. JCI Insight 2019, 4, e129700. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Nystrom, F.H.; Ravichandran, L.V.; Cong, L.N.; Kirby, M.; Mostowski, H.; Quon, M.J. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation 2000, 101, 1539–1545. [Google Scholar] [CrossRef] [Green Version]
- Zeng, G.; Quon, M.J. Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J. Clin. Investig. 1996, 98, 894–898. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Sellers, S.; Stefanovic, N.; Leung, C.; Tan, S.M.; Huet, O.; Granville, D.J.; Cooper, M.E.; de Haan, J.B.; Bernatchez, P. Direct endothelial nitric oxide synthase activation provides atheroprotection in diabetes-accelerated atherosclerosis. Diabetes 2015, 64, 3937–3950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, W.; Dong, Z.; Wang, N.; Wang, W.; Tao, L.; Cao, W.; Liu, Z. Glucagon-Like Peptide 1 Recruits Microvasculature and Increases Glucose Use in Muscle via a Nitric Oxide-Dependent Mechanism. Diabetes 2012, 61, 888–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Montagnani, M.; Funahashi, T.; Shimomura, I.; Quon, M.J. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J. Biol. Chem. 2003, 278, 45021–45026. [Google Scholar] [CrossRef] [Green Version]
- De Boer, M.P.; Meijer, R.I.; Richter, E.A.; van Nieuw Amerongen, G.P.; Sipkema, P.; van Poelgeest, E.M.; Aman, J.; Kokhuis, T.J.; Koolwijk, P.; van Hinsbergh, V.W.; et al. Globular adiponectin controls insulin-mediated vasoreactivity in muscle through AMPKalpha2. Vasc. Pharmacol. 2016, 78, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Subaran, S.C.; Sauder, M.A.; Chai, W.; Jahn, L.A.; Fowler, D.E.; Aylor, K.W.; Basu, A.; Liu, Z. GLP-1 at physiological concentrations recruits skeletal and cardiac muscle microvasculature in healthy humans. Clin. Sci. 2014, 127, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Jahn, L.A.; Hartline, L.; Rao, N.; Logan, B.; Kim, J.J.; Aylor, K.; Gan, L.-M.; Westergren, H.U.; Barrett, E.J. Insulin Enhances Endothelial Function Throughout the Arterial Tree in Healthy But Not Metabolic Syndrome Subjects. J. Clin. Endocrinol. Metab. 2016, 101, 1198–1206. [Google Scholar] [CrossRef] [Green Version]
- Cardillo, C.; Nambi, S.S.; Kilcoyne, C.M.; Choucair, W.K.; Katz, A.; Quon, M.J.; Panza, J.A. Insulin stimulates both endothelin and nitric oxide activity in the human forearm. Circulation 1999, 100, 820–825. [Google Scholar] [CrossRef] [Green Version]
- Muniyappa, R.; Montagnani, M.; Koh, K.K.; Quon, M.J. Cardiovascular Actions of Insulin. Endocr. Rev. 2007, 28, 463–491. [Google Scholar] [CrossRef]
- Eggleston, E.M.; Jahn, L.A.; Barrett, E.J. Hyperinsulinemia Rapidly Increases Human Muscle Microvascular Perfusion but Fails to Increase Muscle Insulin Clearance: Evidence That a Saturable Process Mediates Muscle Insulin Uptake. Diabetes 2007, 56, 2958–2963. [Google Scholar] [CrossRef] [Green Version]
- Vincent, M.A.; Barrett, E.J.; Lindner, J.R.; Clark, M.G.; Rattigan, S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E123–E129. [Google Scholar] [CrossRef]
- Zhao, L.; Fu, Z.; Wu, J.; Aylor, K.W.; Barrett, E.J.; Cao, W.; Liu, Z. Inflammation-induced microvascular insulin resistance is an early event in diet-induced obesity. Clin. Sci. 2015, 129, 1025–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggleston, E.M.; Jahn, L.A.; Barrett, E.J. Early microvascular recruitment modulates subsequent insulin-mediated skeletal muscle glucose metabolism during lipid infusion. Diabetes Care 2013, 36, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Vogel, R.A.; Corretti, M.C.; Plotnick, G.D. Effect of a single high-fat meal on endothelial function in healthy subjects. Am. J. Cardiol. 1997, 79, 350–354. [Google Scholar] [CrossRef]
- Liu, J.; Jahn, L.A.; Fowler, D.E.; Barrett, E.J.; Cao, W.; Liu, Z. Free Fatty Acids Induce Insulin Resistance in Both Cardiac and Skeletal Muscle Microvasculature in Humans. J. Clin. Endocrinol. Metab. 2011, 96, 438–446. [Google Scholar] [CrossRef] [Green Version]
- Virtanen, K.A.; Lonnroth, P.; Parkkola, R.; Peltoniemi, P.; Asola, M.; Viljanen, T.; Tolvanen, T.; Knuuti, J.; Ronnemaa, T.; Huupponen, R.; et al. Glucose Uptake and Perfusion in Subcutaneous and Visceral Adipose Tissue during Insulin Stimulation in Nonobese and Obese Humans. J. Clin. Endocrinol. Metab. 2002, 87, 3902–3910. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, A.L.; Meijer, R.I.; Muskiet, M.H.; Van Raalte, D.H.; Eringa, E.C.; Serné, E.H. Role of insulin-stimulated adipose tissue perfusion in the development of whole-body insulin resistance. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 411–418. [Google Scholar] [CrossRef] [Green Version]
- de Jongh, R.T.; Serne, E.H.; Ijzerman, R.G.; Jorstad, H.T.; Stehouwer, C.D. Impaired local microvascular vasodilatory effects of insulin and reduced skin microvascular vasomotion in obese women. Microvasc. Res. 2008, 75, 256–262. [Google Scholar] [CrossRef]
- Pereira, T.; Betriu, A.; Alves, R. Non-invasive imaging techniques and assessment of carotid vasa vasorum neovascularization: Promises and pitfalls. Trends Cardiovasc. Med. 2019, 29, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Feinstein, S.B. Contrast ultrasound imaging of the carotid artery vasa vasorum and atherosclerotic plaque neovascularization. J. Am. Coll. Cardiol. 2006, 48, 236–243. [Google Scholar] [CrossRef] [Green Version]
- Staub, D.; Schinkel, A.F.; Coll, B.; Coli, S.; van der Steen, A.F.; Reed, J.D.; Krueger, C.; Thomenius, K.E.; Adam, D.; Sijbrands, E.J.; et al. Contrast-enhanced ultrasound imaging of the vasa vasorum: From early atherosclerosis to the identification of unstable plaques. JACC Cardiovasc. Imaging 2010, 3, 761–771. [Google Scholar] [CrossRef]
- Sampson, U.K.; Harrell Jr, F.E.; Fazio, S.; Nwosu, S.; Mercaldo, N.; Mensah, G.A.; Davidson, M.H.; Coll, B.; Feinstein, S.B. Carotid Adventitial Vasa Vasorum and Intima—Media Thickness in a Primary Prevention Population. Echocardiography 2015, 32, 264–270. [Google Scholar] [CrossRef]
- Thapar, A.; Shalhoub, J.; Averkiou, M.; Mannaris, C.; Davies, A.H.; Leen, E.L. Dose-dependent artifact in the far wall of the carotid artery at dynamic contrast-enhanced US. Radiology 2012, 262, 672–679. [Google Scholar] [CrossRef]
- Rubinat, E.; Ortega, E.; Traveset, A.; Arcidiacono, M.V.; Alonso, N.; Betriu, A.; Granado-Casas, M.; Hernández, M.; Soldevila, J.; Puig-Domingo, M. Microangiopathy of common carotid vasa vasorum in type 1 diabetes mellitus. Atherosclerosis 2015, 241, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Nair, V.; Chaube, R.; Stoute, H.; Werstuck, G. Dysglycemia and the density of the coronary vasa vasorum. Diabetes Care 2019, 42, 980–982. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-J.; Matsuo, Y.; Aoki, T.; Kwon, T.-G.; Prasad, A.; Gulati, R.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Coronary endothelial dysfunction is associated with inflammation and vasa vasorum proliferation in patients with early atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2473–2477. [Google Scholar] [CrossRef] [Green Version]
- Boyle, E.C.; Sedding, D.G.; Haverich, A. Targeting vasa vasorum dysfunction to prevent atherosclerosis. Vasc. Pharmacol. 2017, 96, 5–10. [Google Scholar] [CrossRef]
- Sedding, D.G.; Boyle, E.C.; Demandt, J.A.; Sluimer, J.C.; Dutzmann, J.; Haverich, A.; Bauersachs, J. Vasa vasorum angiogenesis: Key player in the initiation and progression of atherosclerosis and potential target for the treatment of cardiovascular disease. Front. Immunol. 2018, 9, 706. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.M.; Sangiorgi, G.; Ritman, E.L.; Lerman, A.; McKenna, C.; Virmani, R.; Edwards, W.D.; Holmes, D.R.; Schwartz, R.S. Adventitial vasa vasorum in balloon-injured coronary arteries: Visualization and quantitation by a microscopic three-dimensional computed tomography technique. J. Am. Coll. Cardiol. 1998, 32, 2072–2079. [Google Scholar] [CrossRef] [Green Version]
- Hayden, M.; Tyagi, S. Is type 2 diabetes mellitus a vascular disease (atheroscleropathy) with hyperglycemia a late manifestation? The role of NOS, NO, and redox stress. Cardiovasc. Diabetol. 2003, 2, 2. [Google Scholar] [CrossRef]
- Herrmann, J.; Lerman, L.O.; Rodriguez-Porcel, M.; Holmes Jr, D.R.; Richardson, D.M.; Ritman, E.L.; Lerman, A. Coronary vasa vasorum neovascularization precedes epicardial endothelial dysfunction in experimental hypercholesterolemia. Cardiovasc. Res. 2001, 51, 762–766. [Google Scholar] [CrossRef]
- Baron, A.D.; Steinberg, H.; Brechtel, G.; Johnson, A. Skeletal muscle blood flow independently modulates insulin-mediated glucose uptake. Am. J. Physiol. Endocrinol. Metab. 1994, 266, E248–E253. [Google Scholar] [CrossRef] [PubMed]
- Duncan, E.R.; Walker, S.J.; Ezzat, V.A.; Wheatcroft, S.B.; Li, J.-M.; Shah, A.M.; Kearney, M.T. Accelerated endothelial dysfunction in mild prediabetic insulin resistance: The early role of reactive oxygen species. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1311–E1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talukdar, S.; Oh, D.Y.; Bandyopadhyay, G.; Li, D.; Xu, J.; McNelis, J.; Lu, M.; Li, P.; Yan, Q.; Zhu, Y.; et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 2012, 18, 1407–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, K.D.; McDonald, T.O.; Chait, A.; Allen, M.D.; Alpers, C.E. Neovascular expression of E-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in human atherosclerosis and their relation to intimal leukocyte content. Circulation 1996, 93, 672–682. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owusu, J.; Barrett, E. Early Microvascular Dysfunction: Is the Vasa Vasorum a “Missing Link” in Insulin Resistance and Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 7574. https://doi.org/10.3390/ijms22147574
Owusu J, Barrett E. Early Microvascular Dysfunction: Is the Vasa Vasorum a “Missing Link” in Insulin Resistance and Atherosclerosis. International Journal of Molecular Sciences. 2021; 22(14):7574. https://doi.org/10.3390/ijms22147574
Chicago/Turabian StyleOwusu, Jeanette, and Eugene Barrett. 2021. "Early Microvascular Dysfunction: Is the Vasa Vasorum a “Missing Link” in Insulin Resistance and Atherosclerosis" International Journal of Molecular Sciences 22, no. 14: 7574. https://doi.org/10.3390/ijms22147574
APA StyleOwusu, J., & Barrett, E. (2021). Early Microvascular Dysfunction: Is the Vasa Vasorum a “Missing Link” in Insulin Resistance and Atherosclerosis. International Journal of Molecular Sciences, 22(14), 7574. https://doi.org/10.3390/ijms22147574





