Adenosine Receptor Reserve and Long-Term Potentiation: Unconventional Adaptive Mechanisms in Cardiovascular Diseases?
Abstract
:1. Introduction
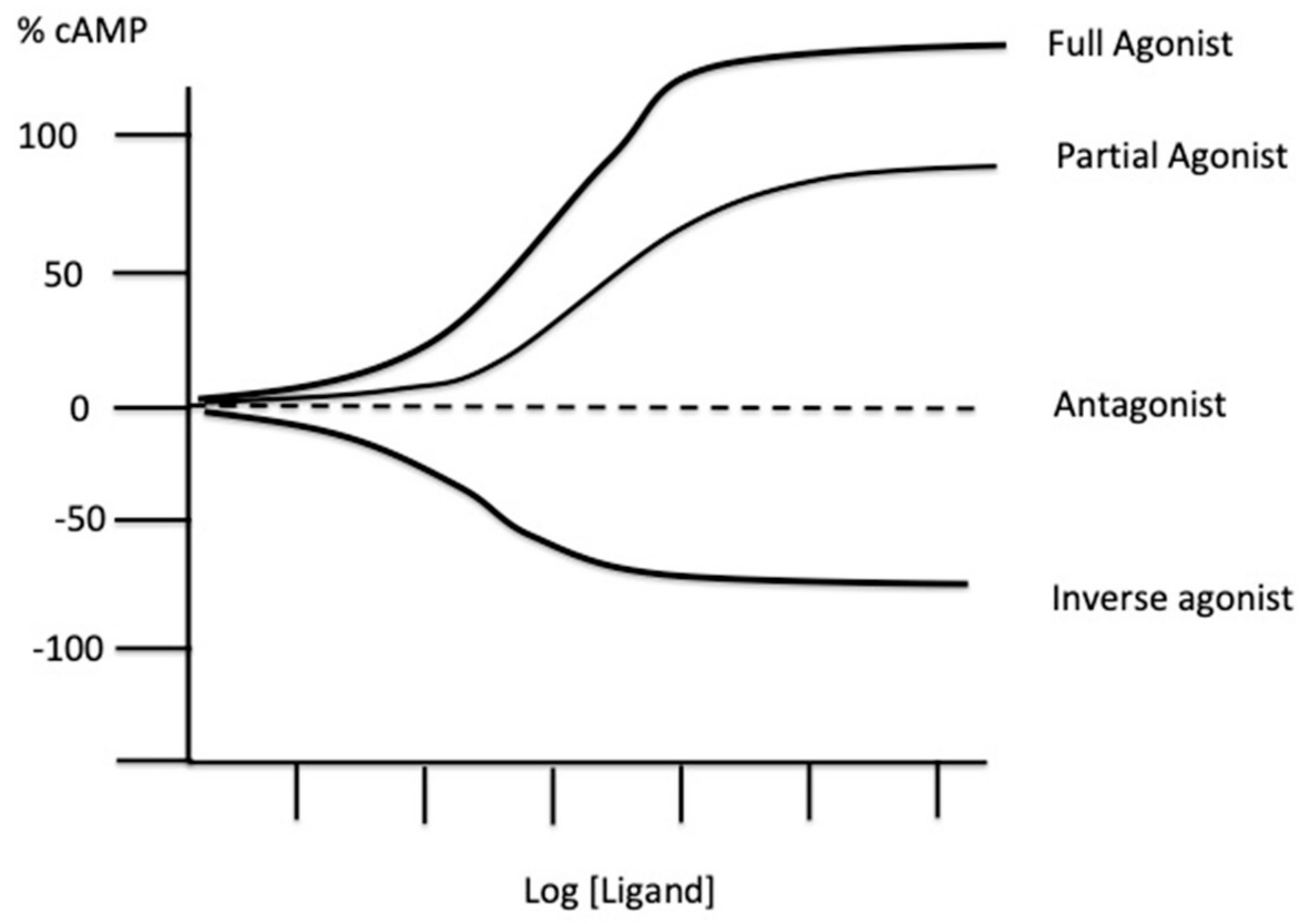
2. Experimental Tools to Evaluate the Presence of Spare Receptors
3. Spare Receptors in the Adenosinergic System
4. Biological Consequences of the Presence of Spare Receptors
5. Possible Clinical Consequences of the Presence of Spare Receptors
5.1. Spare Receptors and Coronary Artery Disease (CAD)
5.2. Spare Receptors and Arrhythmia
5.3. Spare Receptors and Atrial Fibrillation
5.4. Role of Adenosine Receptors in the Induction of Long-Term Potentiation
6. Possible Clinical Consequences of LTP
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clark, A.J. Mode of Action of Drugs on Cells; Edward Arnold and Co.: London, UK, 1933. [Google Scholar]
- Stephenson, R.P. A modification of receptor theory. Br. J. Pharmacol. Chemother. 1956, 11, 379–393. [Google Scholar] [CrossRef]
- Ariens, E.J. Affinity and intrinsic activity in the theory of competitive inhibition. I. Problems and theory. Arch. Int. Pharmacodyn. Ther. 1954, 99, 32–49. [Google Scholar]
- Kenakin, T. Principles: Receptor theory in pharmacology. Trends Pharmacol. Sci. 2004, 25, 186–192. [Google Scholar] [CrossRef]
- Buchwald, P. A receptor model with binding affinity, activation efficacy, and signal amplification parameters for complex fractional response versus occupancy data. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Limbird, L.E. The receptor concept: A continuing evolution. Mol. Interv. 2004, 4, 326–336. [Google Scholar] [CrossRef]
- Nickerson, M. Receptor occupancy and tissue response. Nat. Cell Biol. 1956, 178, 697–698. [Google Scholar] [CrossRef]
- Baker, S.P.; Scammells, P.J.; Belardinelli, L. Differential A1-adenosine receptor reserve for inhibition of cyclic AMP accumulation and G-protein activation in DDT1 MF-2 cells. Br. J. Pharmacol. 2000, 130, 1156–1164. [Google Scholar] [CrossRef] [Green Version]
- Fenouillet, E.; Mottola, G.; Kipson, N.; Paganelli, F.; Guieu, R.; Ruf, J. Adenosine receptor profiling reveals an association between the presence of spare receptors and cardiovascular disorders. Int. J. Mol. Sci. 2019, 20, 5964. [Google Scholar] [CrossRef] [Green Version]
- Zsuga, J.; Erdei, T.; Szabó, K.; Lampe, N.; Papp, C.; Pinter, A.; Szentmiklosi, A.J.; Juhasz, B.; Szilvássy, Z.; Gesztelyi, R. Methodical challenges and a possible resolution in the assessment of receptor reserve for adenosine, an agonist with short half-life. Molecules 2017, 22, 839. [Google Scholar] [CrossRef] [Green Version]
- Timmerman, H.; van der Goot, H. Histamine receptors and their ligands: Mechanisms and applications. In Encyclopedia of Neurosciences; Academic Press: Cambridge, MA, USA, 2009; p. 1149. [Google Scholar]
- Paganelli, F.; Resseguier, N.; Marlinge, M.; Laine, M.; Malergue, F.; Kipson, N.; Armangau, P.; Pezzoli, N.; Kerbaul, F.; Bonello, L.; et al. Specific pharmacological profile of A 2A adenosine receptor predicts reduced fractional flow reserve in patients with suspected coronary artery disease. J. Am. Heart Assoc. 2018, 7, e008290. [Google Scholar] [CrossRef]
- Gaudry, M.; Vairo, D.; Marlinge, M.; Gaubert, M.; Guiol, C.; Mottola, G.; Gariboldi, V.; Deharo, P.; Sadrin, S.; Maixent, J.M.; et al. Adenosine and its receptors: An expected tool for the diagnosis and treatment of coronary artery and ischemic heart diseases. Int. J. Mol. Sci. 2020, 21, 5321. [Google Scholar] [CrossRef]
- Kenakin, T.P. A Pharmacology Primer: Techniques for More Effective and Strategic Drug Discovery, 5th ed.; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Shryock, J.C.; Snowdy, S.; Baraldi, P.G.; Cacciari, B.; Spalluto, G.; Monopoli, A.; Ongini, E.; Baker, S.P.; Belardinelli, L. A 2A-adenosine receptor reserve for coronary vasodilation. Circulation 1998, 98, 711–718. [Google Scholar] [CrossRef] [Green Version]
- Dripps, I.J.; Chen, R.; Shafer, A.M.; Livingston, K.E.; Disney, A.; Husbands, S.; Traynor, J.R.; Rice, K.C.; Jutkiewicz, E.M. Pharmacological properties of δ-opioid receptor–mediated behaviors: Agonist efficacy and receptor reserve. J. Pharmacol. Exp. Ther. 2020, 374, 319–330. [Google Scholar] [CrossRef]
- Brown, L.; Deighton, N.M.; Bals, S.; Söhlmann, W.; Zerkowski, H.-R.; Michel, M.C.; Brodde, O.-E. Spare receptors for β-adrenoceptor-mediated positive inotropic effects of catecholamines in the human heart. J. Cardiovasc. Pharmacol. 1992, 19, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Lodish, H.; Berk, A.; Zipursky, S.L. Molecular Cell Biology: Section 20.3 G Protein-Coupled Receptors and Their Effectors; WH Freeman editor: New York, NY, USA, 2000. [Google Scholar]
- Ruffolo, R.R., Jr.; Yaden, E.L. Existence of spare alpha 1-adrenoreceptors, but not alpha 2-adrenoreceptors, for respective vasopressor effects of cirazoline and B-HT 933 in the pithed rat. J. Cardiovasc. Pharmacol. 1984, 6, 1011–1019. [Google Scholar] [CrossRef]
- Brown, J.H.; Goldstein, D. Differences in muscarinic receptor reserve for inhibition of adenylate cyclase and stimulation of phosphoinositide hydrolysis in chick heart cells. Mol. Pharmacol. 1986, 30, 566–570. [Google Scholar]
- Meller, E.; Bohmaker, K.; Namba, Y.; Friedhoff, A.J.; Goldstein, M. Relationship between receptor occupancy and response at striatal dopamine autoreceptors. Mol. Pharmacol. 1987, 31, 592–598. [Google Scholar] [PubMed]
- Zhang, J.; Belardinelli, L.; Jacobson, K.A.; Otero, D.H.; Baker, S.P. Persistent activation by and receptor reserve for an irreversible a1-adenosine receptor agonist in DDT1 MF-2 cells and in guinea pig heart. Mol. Pharmacol. 1997, 52, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Adham, N.; Ellerbrock, B.; Hartig, P.; Weinshank, R.L.; Branchek, T. Receptor reserve masks partial agonist activity of drugs in a cloned rat 5-hydroxytryptamine1B receptor expression system. Mol. Pharmacol. 1993, 43, 427–433. [Google Scholar] [PubMed]
- McNeil, L.K.; Evavold, B.D. TCR reserve: A novel principle of CD4 T cell activation by weak ligands. J. Immunol. 2003, 170, 1224–1230. [Google Scholar] [CrossRef] [Green Version]
- McNeil, L.K.; Evavold, B.D. Dissociation of peripheral T cell responses from thymocyte negative selection by weak agonists supports a spare receptor model of T cell activation. Proc. Natl. Acad. Sci. USA 2002, 99, 4520–4525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furchgott, R.F.; Bursztyn, P. Comparison of dissociation constants and of relative efficacies of selected agonists acting on parasympathetic receptors. Ann. N. Y. Acad. Sci. 1967, 144, 882–899. [Google Scholar] [CrossRef]
- Jasper, J.R.; Motulsky, H.J.; Insel, P.A. Characterization of a bromoacetylated derivative of pindolol as a high affinity, irreversible beta adrenergic antagonist in cultured cells. J. Pharmacol. Exp. Ther. 1988, 244, 820–824. [Google Scholar] [PubMed]
- Dennis, D.; Jacobson, K.; Belardinelli, L. Evidence of spare A1-adenosine receptors in guinea pig atrioventricular node. Am. J. Physiol. Circ. Physiol. 1992, 262, H661–H671. [Google Scholar] [CrossRef] [PubMed]
- By, Y.; Durand-Gorde, J.-M.; Condo, J.; Lejeune, P.-J.; Mallet, B.; Carayon, P.; Guieu, R.; Ruf, J. Production of an agonist-like monoclonal antibody to the human A2A receptor of adenosine for clinical use. Mol. Immunol. 2009, 46, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Ruf, J.; Paganelli, F.; Bonello, L.; Kipson, N.; Mottola, G.; Fromonot, J.; Condo, J.; Boussuges, A.; Bruzzese, L.; Kerbaul, F.; et al. Spare adenosine A2a receptors are associated with positive exercise stress test in coronary artery disease. Mol. Med. 2016, 22, 530–536. [Google Scholar] [CrossRef]
- Gaudry, M.; Marlinge, M.; Deharo, P.; Vairo, D.; Bottone, S.; Mottola, G.; Kipson, N.; Criado, C.; Mace, P.; Chefrour, M.; et al. Pharmacological profile of adenosine A2A receptors in patients with lower extremity peripheral artery disease and associated coronary artery disease: A pilot study. Int. J. Cardiol. 2019, 285, 121–127. [Google Scholar] [CrossRef]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of adenosine receptors: The state of the art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef]
- Iwamoto, T.; Umemura, S.; Toya, Y.; Uchibori, T.; Kogi, K.; Takagi, N.; Ishii, M. Identification of adenosine A2 receptor-cAMP System in human aortic endothelial cells. Biochem. Biophys. Res. Commun. 1994, 199, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Ponnoth, D.S.; Sanjani, M.S.; Ledent, C.; Roush, K.; Krahn, T.; Mustafa, S.J. Absence of adenosine-mediated aortic relaxation in A2A adenosine receptor knock out m ice. Am. J. Physiol. Circ. Physiol. 2009, 297, H1655–H1660. [Google Scholar] [CrossRef] [Green Version]
- Kusano, Y.; Echeverry, G.; Miekisiak, G.; Kulik, T.B.; Aronhime, S.N.; Chen, J.F.; Winn, H.R. Role of adenosine A2 receptors in regulmation of cerebral blood flow during induced hypotension. Br. J. Pharmacol. 2009, 30, 808–815. [Google Scholar]
- Kleppish, T.; Nelson, M.T. Adenosine activates ATP-sensitive potassium channels in arterial myocytes via A2 receptors and cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 1995, 92, 12441–12445. [Google Scholar] [CrossRef] [Green Version]
- Arsyad, A.; Dobson, G.P. Adenosine relaxation in isolated rat aortic rings and possible roles of smooth muscle Kv channels, KATP channels and A2a receptors. BMC Pharmacol. Toxicol. 2016, 17, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berwick, Z.C.; Payne, G.A.; Lynch, B.; Dick, G.M.; Sturek, M.; Tune, J.D. Contribution of Adenosine A(2A) and A(2B) Receptors to Ischemic Coronary Dilation: Role of KV and KATP Channels. Microcirculation 2010, 17, 600–607. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.N.; Arner, A.; Boels, P.J.M.; Fredholm, B.B. Adenosine A1 receptors and vascular reactivity. Acta Physiol. 2010, 199, 211–220. [Google Scholar] [CrossRef]
- Yadav, V.R.; Teng, B.; Mustafa, S.J. Enhanced A1 adenosine receptor-induced vascular contractions in mesenteric artery and aortaz on in L-NAME mouse model of hypertension. Eur. J. Pharmacol. 2019, 842, 111–117. [Google Scholar] [CrossRef]
- Jackson, E.K.; Gillespie, D.G.; Mi, Z.; Cheng, D. Adenosine receptors influence hypertension in dahl salt-sensitive rats. Hypertension 2018, 72, 511–521. [Google Scholar] [CrossRef]
- Adenosine induces vasoconstriction through Gi-dependent activation of phospholipase C in isolated perfused afferent arterioles of mice. J. Am. Soc. Nephrol. 2003, 14, 2457–2465. [CrossRef] [PubMed] [Green Version]
- Lai, E.; Patzak, A.; Steege, A.; Mrowka, R.; Brown, R.; Spielmann, N.; Persson, P.; Fredholm, B.; Persson, A. Contribution of adenosine receptors in the control of arteriolar tone and adenosine–angiotensin II interaction. Kidney Int. 2006, 70, 690–698. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Lai, E.Y.; Huang, Y.; Eisner, C.; Mizel, D.; Wilcox, C.S.; Schnermann, J. Renal afferent arteriolar and tubuloglomerular feedback reactivity in mice with conditional deletions of adenosine 1 receptors. Am. J. Physiol. Physiol. 2012, 303, F1166–F1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guieu, R.; Deharo, J.-C.; Maille, B.; Crotti, L.; Torresani, E.; Brignole, M.; Parati, G. Adenosine and the cardiovascular system: The good and the bad. J. Clin. Med. 2020, 9, 1366. [Google Scholar] [CrossRef] [PubMed]
- Sebastião, A.M.; Ribeiro, J.A. Adenosine Receptors and the Central Nervous System. Organotypic Models Drug Dev. 2009, 193, 471–534. [Google Scholar]
- Ribeiro, J.; Sebastião, A.; de Mendonça, A. Adenosine receptors in the nervous system: Pathophysiological implications. Prog. Neurobiol. 2002, 68, 377–392. [Google Scholar] [CrossRef]
- Chin, J.H. Adenosine receptors in brain: Neuromodulation and role in epilepsy. Ann. Neurol. 1989, 26, 695–698. [Google Scholar] [CrossRef]
- Masino, S.A.; Kawamura, M., Jr.; Ruskin, D.N. Adenosine receptors and epilepsy. Int. Rev. Neurobiol. 2014, 119, 233–255. [Google Scholar] [CrossRef]
- Guieu, R.; Peragut, J.C.; Hassani, H.; Sampieri, F.; Bechis, G.; Gola, R.; Rochat, H. Adenosine and neuropathic pain. Pain 1996, 68, 271–274. [Google Scholar] [CrossRef]
- Antonioli, L.; Fornai, M.; Blandizzi, C.; Pacher, P.; Haskó, G. Adenosine signaling and the immune system: When a lot could be too much. Immunol. Lett. 2019, 205, 9–15. [Google Scholar] [CrossRef]
- Antonioli, L.; Blandizzi, C.; Pacher, P.; Haskó, G. The Purinergic system as a pharmacological target for the treatment of immune-mediated inflammatory diseases. Pharmacol. Rev. 2019, 71, 345–382. [Google Scholar] [CrossRef] [PubMed]
- Varani, K.; Pasini, F.L.; Camurri, A.; Capecchi, P.L.; Maccherini, M.; Diciolla, F.; Ceccatelli, L.; Lazzerini, P.E.; Ulouglu, C.; Cattabeni, F.; et al. Changes of peripheral A 2A adenosine receptors in chronic heart failure and cardiac transplantation. FASEB J. 2002, 17, 280–282. [Google Scholar] [CrossRef] [Green Version]
- Gariboldi, V.; Vairo, D.; Guieu, R.; Marlinge, M.; Ravis, E.; Lagier, D.; Mari, A.; Thery, E.; Collart, F.; Gaudry, M.; et al. Expressions of adenosine A2A receptors in coronary arteries and peripheral blood mononuclear cells are correlated in coronary artery disease patients. Int. J. Cardiol. 2017, 230, 427–431. [Google Scholar] [CrossRef]
- Srinivas, M.; Shryock, J.C.; Dennis, D.M.; Baker, S.P.; Belardinelli, L. Differential A1 nadenosine receptor reserve for two actions of adenosine on guinea pig atrial myocytes. Mol. Pharmacol. 1997, 52, 683–691. [Google Scholar] [CrossRef] [Green Version]
- Belardinelli, L.; Shryock, J. Does adenosine function as a retaliatory metabolite in the heart? Physiology 1992, 7, 52–56. [Google Scholar] [CrossRef]
- Sollevi, A. Cardiovascular effects of adenosine in man; possible clinical implications. Prog. Neurobiol. 1986, 27, 319–349. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Sollevi, A. Cardiovascular effects of adenosine. Clin. Physiol. 1986, 6, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. American heart association council on epidemiology and prevention statistics committee and stroke statistics subcommittee. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Duncker, D.J.; Stubenitsky, R.; Verdouw, P.D. Role of adenosine in the regulation of coronary blood flow in swine at rest and during treadmill exercise. Am. J. Physiol. Content 1998, 275, H1663–H1672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laxson, D.D.; Homans, D.C.; Bache, R.J. Inhibition of adenosine-mediated coronary vasodilation exacerbates myocardial ischemia during exercise. Am. J. Physiol. 1993, 265, H1471–H1477. [Google Scholar] [CrossRef]
- Zhou, Z.; Rajamani, U.; Labazi, H.; Tilley, S.L.; Ledent, C.; Teng, B.; Mustafa, S.J. Involvement of NADPH oxidase in A2A adenosine receptor-mediated increase in coronary flow in isolated mouse hearts. Purinergic Signal. 2015, 11, 263–273. [Google Scholar] [CrossRef]
- Marunaka, Y.; Niisato, N.; Miyazaki, H. New Concept of Spare Receptors and Effectors. J. Membr. Biol. 2005, 203, 31–39. [Google Scholar] [CrossRef]
- Ruf, J.; Vairo, D.; Paganelli, F.; Guieu, R. Extracellular vesicles with ubiquitinated adenosine A2A receptor in plasma of patients with coronary artery disease. J. Cell. Mol. Med. 2019, 23, 6805–6811. [Google Scholar] [CrossRef] [Green Version]
- Jacquin, L.; Franceschi, F.; By, Y.; Durand-Gorde, J.-M.; Condo, J.; Deharo, J.-C.; Michelet, P.; Fenouillet, E.; Guieu, R.; Ruf, J. Search for adenosine A2A spare receptors on peripheral human lymphocytes. FEBS Open Bio. 2012, 3, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Franceschi, F.; By, Y.; Peyrouse, E.; Fromonot, J.; Gerolami, V.; Kipson, N.; Boussuges, A.; Brignole, M.; Fenouillet, E.; Deharo, J.C.; et al. A2A adenosine receptor function in patients with vasovagal syncope. Europace 2013, 15, 1328–1332. [Google Scholar] [CrossRef]
- Brignole, M.; Guieu, R.; Tomaino, M.; Iori, M.; Ungar, A.; Bertolone, C.; Unterhuber, M.; Bottoni, N.; Tesi, F.; Deharo, J.C. Mechanism of syncope without prodromes with normal heart and normal electrocardiogram. Heart Rhythm. 2017, 14, 234–239. [Google Scholar] [CrossRef]
- Guieu, R.; Deharo, J.-C.; Ruf, J.; Mottola, G.; Kipson, N.; Bruzzese, L.; Gerolami, V.; Franceschi, F.; Ungar, A.; Tomaino, M.; et al. Adenosine and Clinical Forms of Neurally-Mediated Syncope. J. Am. Coll. Cardiol. 2015, 66, 204–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deharo, J.-C.; Mechulan, A.; Giorgi, R.; Franceschi, F.; Prevot, S.; Peyrouse, E.; Condo, J.; By, Y.; Ruf, J.; Brignole, M.; et al. Adenosine plasma level and A2Aadenosine receptor expression: Correlation with laboratory tests in patients with neurally mediated syncope. Heart 2012, 98, 855–859. [Google Scholar] [CrossRef]
- Deharo, J.-C.; Brignole, M.; Guieu, R. Adenosine hypersensitivity and atrioventricular block. Herzschrittmachertherapie und Elektrophysiologie 2018, 29, 166–170. [Google Scholar] [CrossRef]
- Deharo, J.-C.; Guieu, R.; Mechulan, A.; Peyrouse, E.; Kipson, N.; Ruf, J.; Gérolami, V.; Devoto, G.; Marrè, V.; Brignole, M. Syncope without prodromes in patients with normal heart and normal electrocardiogram: A distinct entity. J. Am. Coll. Cardiol. 2013, 62, 1075–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brignole, M.; Iori, M.; Solari, D.; Bottoni, N.; Rivasi, G.; Ungar, A.; Deharo, J.C.; Guieu, R. Efficacy of theophylline in patients with syncope without prodromes with normal heart and normal ECG. Int. J. Cardiol. 2019, 289, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Brignole, M.; Solari, D.; Iori, M.; Bottoni, N.; Guieu, R.; Deharo, J.C. Efficacy of theophylline in patients affected by low adenosine syncope. Heart Rhythm. 2016, 13, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Strickberger, S.A.; Man, K.C.; Daoud, E.G.; Goyal, R.; Brinkman, K.; Knight, B.P.; Weiss, R.; Bahu, M.; Morady, F. Adenosine-induced atrial arrhythmia: A prospective analysis. Ann. Intern. Med. 1997, 127, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Maille, B.; Marlinge, M.; Vairo, D.; Mottola, G.; Koutbi, L.; Deharo, P.; Gastaldi, M.; Gaudry, M.; Guiol, C.; Bottone, S.; et al. Adenosine plasma level in patients with paroxysmal or persistent atrial fibrillation and normal heart during ablation procedure and/or cardioversion. Purinergic Signal. 2019, 15, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Llach, A.; Molina, C.E.; Prat-Vidal, C.; Fernandes, J.; Casadó, V.; Ciruela, F.; Lluís, C.; Franco, R.; Cinca, J.; Hove-Madsen, L. Abnormal calcium handling in atrial fibrillation is linked to up-regulation of adenosine A2A receptors. Eur. Heart J. 2010, 32, 721–729. [Google Scholar] [CrossRef] [Green Version]
- Godoy-Marín, H.; Duroux, R.; Jacobson, K.; Soler, C.; Colino-Lage, H.; Jiménez-Sábado, V.; Montiel, J.; Hove-Madsen, L.; Ciruela, F. Adenosine A2A Receptors Are Upregulated in Peripheral Blood Mononuclear Cells from Atrial Fibrillation Patients. Int. J. Mol. Sci. 2021, 22, 3467. [Google Scholar] [CrossRef]
- Kabell, G.; Buchanan, L.V.; Gibson, J.K.; Belardinelli, L. Effects of adenosine on atrial refractoriness and arrhythmias. Cardiovasc. Res. 1994, 28, 1385–1389. [Google Scholar] [CrossRef]
- Gillinov, A.M.; Bagiella, E.; Moskowitz, A.; Raiten, J.M.; Groh, M.A.; Bowdish, M.E.; Ailawadi, G.; Kirkwood, K.A.; Perrault, L.P.; Parides, M.K.; et al. Rate Control versus Rhythm Control for Atrial Fibrillation after Cardiac Surgery. N. Engl. J. Med. 2016, 374, 1911–1921. [Google Scholar] [CrossRef] [PubMed]
- McElderry, H.T.; McGiffin, D.C.; Plumb, V.J.; Nanthakumar, K.; Epstein, A.E.; Yamada, T.; Kay, G.N. Proarrhythmic aspects of atrial fibrillation surgery: Mechanisms of postoperative macroreentrant tachycardias. Circulation 2008, 117, 155–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nee, L.; Franceschi, F.; Resseguier, N.; Gravier, G.; Giorgi, R.; Gariboldi, V.; Collart, F.; Michelet, P.; Deharo, J.C.; Guieu, R.; et al. High endogenous adenosine plasma concentration is associated with atrial fibrillation during cardiac surgery with cardiopulmonary bypass. Int. J. Cardiol. 2013, 165, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Lagier, D.; Nee, L.; Guieu, R.; Kerbaul, F.; Fenouillet, E.; Roux, N.; Giorgi, R.; Theron, A.; Grisoli, D.; Gariboldi, V.; et al. Peri-operative oral caffeine does not prevent postoperative atrial fibrillation after heart valve surgery with cardiopulmonary bypass. Eur. J. Anaesthesiol. 2018, 35, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Mattiolli, A.V. Beverages of daily life: Impact Of caffeine on atrial fibrillation. J. Atr. Fibrillation 2014, 7, 1133. [Google Scholar] [CrossRef]
- Conlay, L.A.; Conant, J.A.; Debros, F.; Wurtman, R. Caffeine alters plasma adenosine levels. Nat. Cell Biol. 1997, 389, 136. [Google Scholar] [CrossRef]
- Varani, K.; Portaluppi, F.; Gessi, S.; Merighi, S.; Ongini, E.; Belardinelli, L.; Borea, P.A. Dose and time effects of caffeine intake on human platelet adenosine A(2A) receptors: Functional and biochemical aspects. Circulation 2000, 102, 285–289. [Google Scholar] [CrossRef] [Green Version]
- Daly, J.W.; Shi, D.; Nikodijevic, O.; Jacobson, K.A. The role of adenosine receptors in the central action of caffeine. Pharmacopsychoecologia 2015, 7, 201–213. [Google Scholar]
- Vauquelin, G.; Vanliefde, I. Slow antagonist dissociation and long-lasting in vivo receptor protection. Trends Pharmacol. Sci. 2006, 27, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Bliss, T.V.P.; Lømo, T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 1973, 232, 331–356. [Google Scholar] [CrossRef]
- Ben-Ari, Y.; Represa, A. Brief seizure episodes induce long-term potentiation and mossy fibre sprouting in the hippocampus. Trends Neurosci. 1990, 13, 312–318. [Google Scholar] [CrossRef]
- Dunwiddie, T.; Lynch, G. Long-term potentiation and depression of synaptic responses in the rat hippocampus: Localization and frequency dependency. J. Physiol. 1978, 276, 353–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Düster, R.; Prickaerts, J.; Blokland, A. Purinergic signaling and hippocampal long-term potentiation. Curr. Neuropharmacol. 2014, 12, 37–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Mendonça, A.; Ribeiro, J. Endogenous adenosine modulates long-term potentiation in the hippocampus. Neuroscience 1994, 62, 385–390. [Google Scholar] [CrossRef]
- Arai, A.; Kessler, M.; Lynch, G. The effects of adenosine on the development of long-term potentiation. Neurosci. Lett. 1990, 119, 41–44. [Google Scholar] [CrossRef]
- D’Alcantara, P.; Ledent, C.; Swillens, S.; Schiffmann, S. Inactivation of adenosine A2A receptor impairs long term potentiation in the accumbens nucleus without altering basal synaptic transmission. Neuroscience 2001, 107, 455–464. [Google Scholar] [CrossRef]
- Rebola, N.; Lujan, R.; Cunha, R.; Mulle, C. Adenosine A2A receptors are essential for long-term potentiation of nmda-epscs at hippocampal mossy fiber synapses. Neuron 2008, 57, 121–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkadhi, K.; Alzoubi, K.; Aleisa, A. Plasticity of synaptic transmission in autonomic ganglia. Prog. Neurobiol. 2005, 75, 83–108. [Google Scholar] [CrossRef] [PubMed]
- Alkadhi, K.A. Long-term potentiation in autonomic ganglia: Potential role in cardiovascular disorders. World J. Pharmacol. 2016, 5, 51. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guieu, R.; Brignole, M.; Deharo, J.C.; Deharo, P.; Mottola, G.; Groppelli, A.; Paganelli, F.; Ruf, J. Adenosine Receptor Reserve and Long-Term Potentiation: Unconventional Adaptive Mechanisms in Cardiovascular Diseases? Int. J. Mol. Sci. 2021, 22, 7584. https://doi.org/10.3390/ijms22147584
Guieu R, Brignole M, Deharo JC, Deharo P, Mottola G, Groppelli A, Paganelli F, Ruf J. Adenosine Receptor Reserve and Long-Term Potentiation: Unconventional Adaptive Mechanisms in Cardiovascular Diseases? International Journal of Molecular Sciences. 2021; 22(14):7584. https://doi.org/10.3390/ijms22147584
Chicago/Turabian StyleGuieu, Régis, Michele Brignole, Jean Claude Deharo, Pierre Deharo, Giovanna Mottola, Antonella Groppelli, Franck Paganelli, and Jean Ruf. 2021. "Adenosine Receptor Reserve and Long-Term Potentiation: Unconventional Adaptive Mechanisms in Cardiovascular Diseases?" International Journal of Molecular Sciences 22, no. 14: 7584. https://doi.org/10.3390/ijms22147584
APA StyleGuieu, R., Brignole, M., Deharo, J. C., Deharo, P., Mottola, G., Groppelli, A., Paganelli, F., & Ruf, J. (2021). Adenosine Receptor Reserve and Long-Term Potentiation: Unconventional Adaptive Mechanisms in Cardiovascular Diseases? International Journal of Molecular Sciences, 22(14), 7584. https://doi.org/10.3390/ijms22147584







