In Vitro and In Vivo Characterization of PLLA-316L Stainless Steel Electromechanical Devices for Bone Tissue Engineering—A Preliminary Study
Abstract
:1. Introduction
2. Results
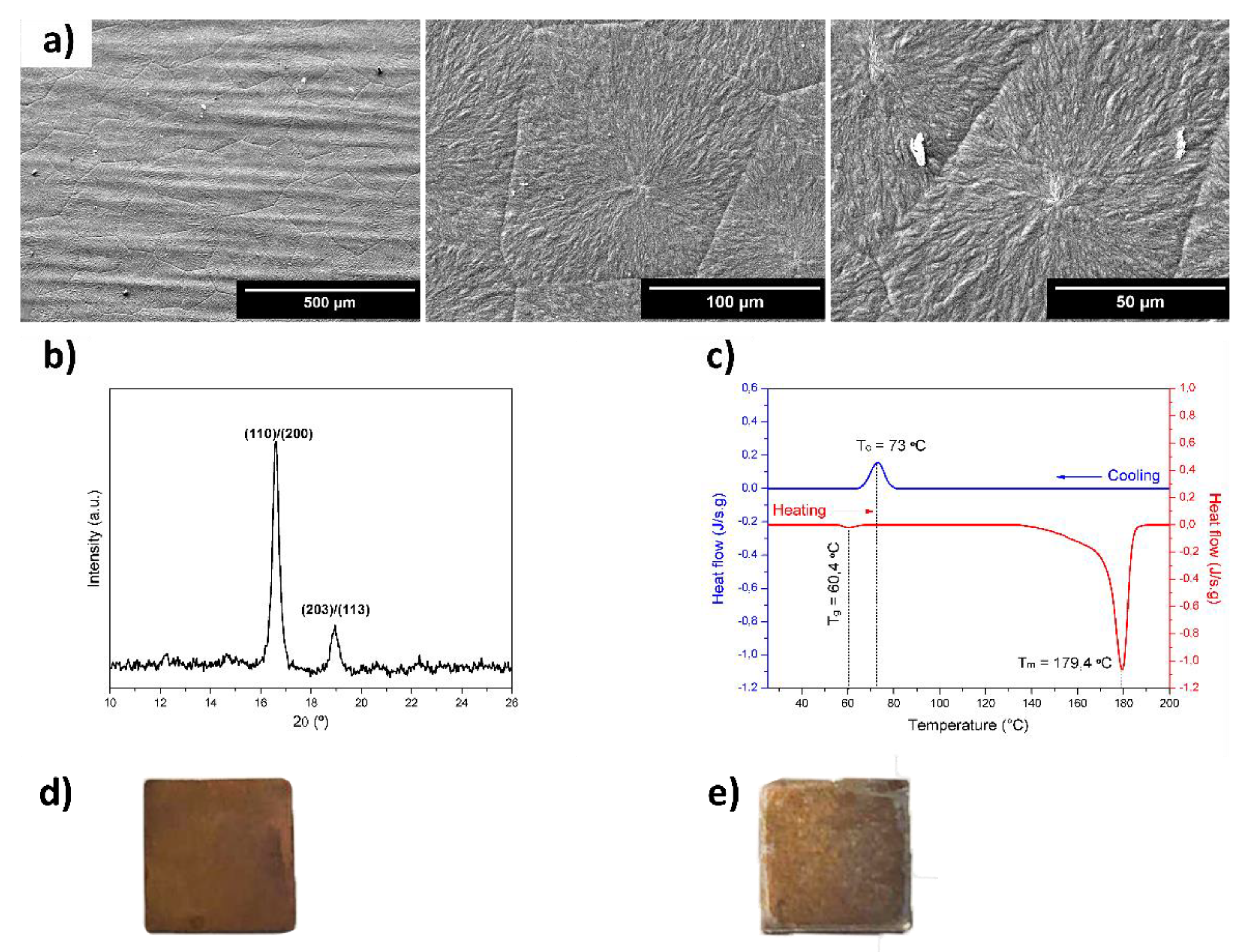
2.1. Characterization of the Devices
2.2. Cytocompatibility Assessment
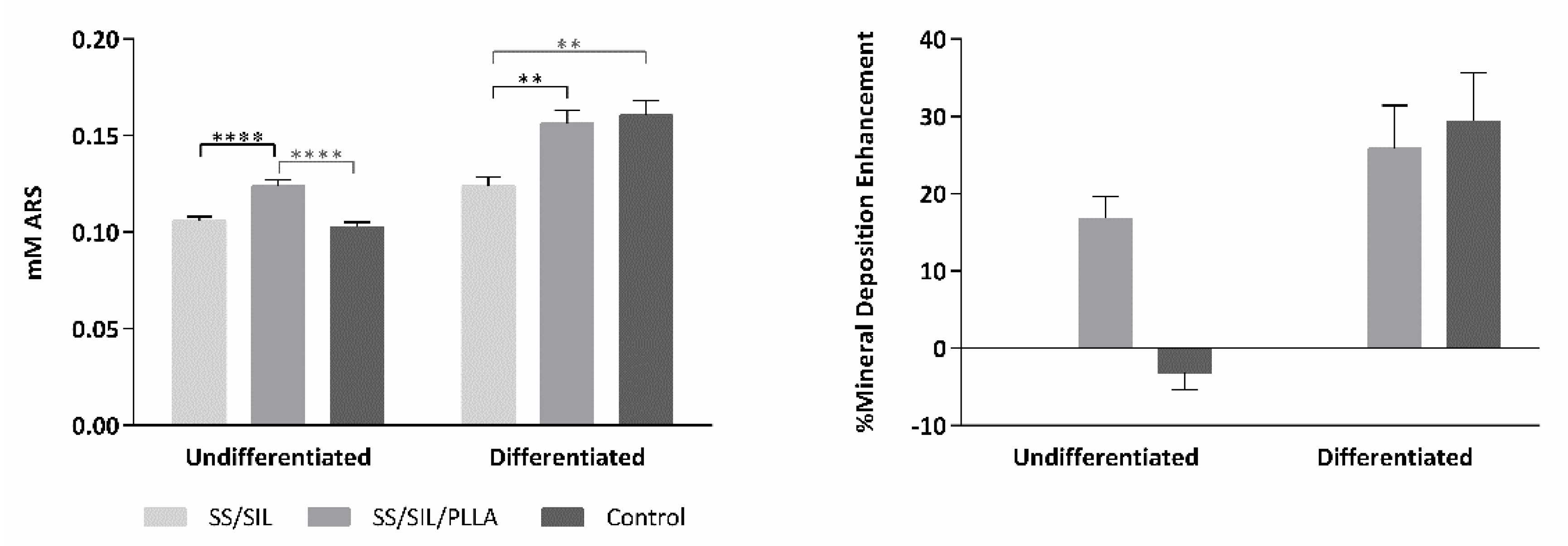
2.3. Osteogenic Differentiation Assay
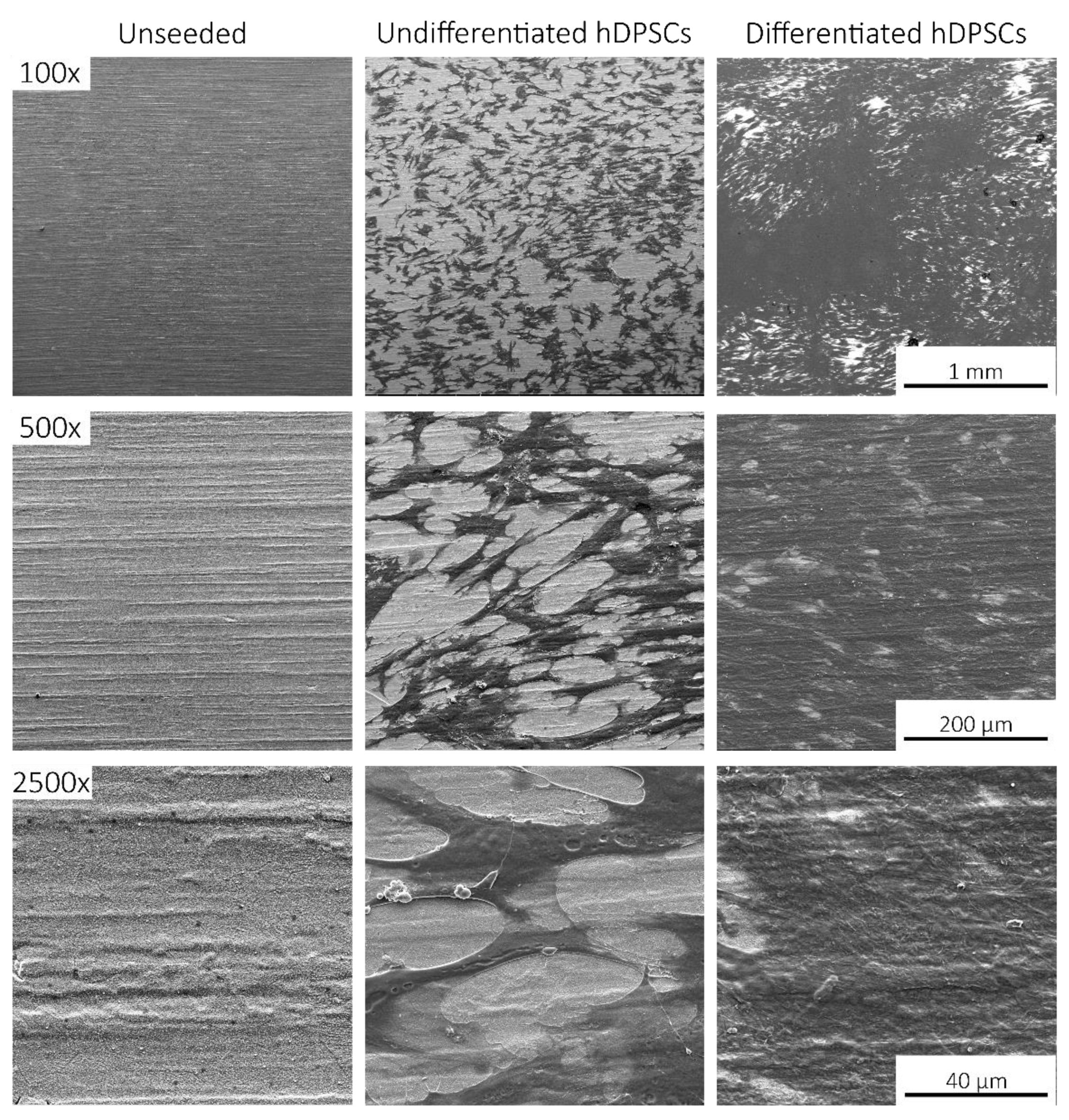
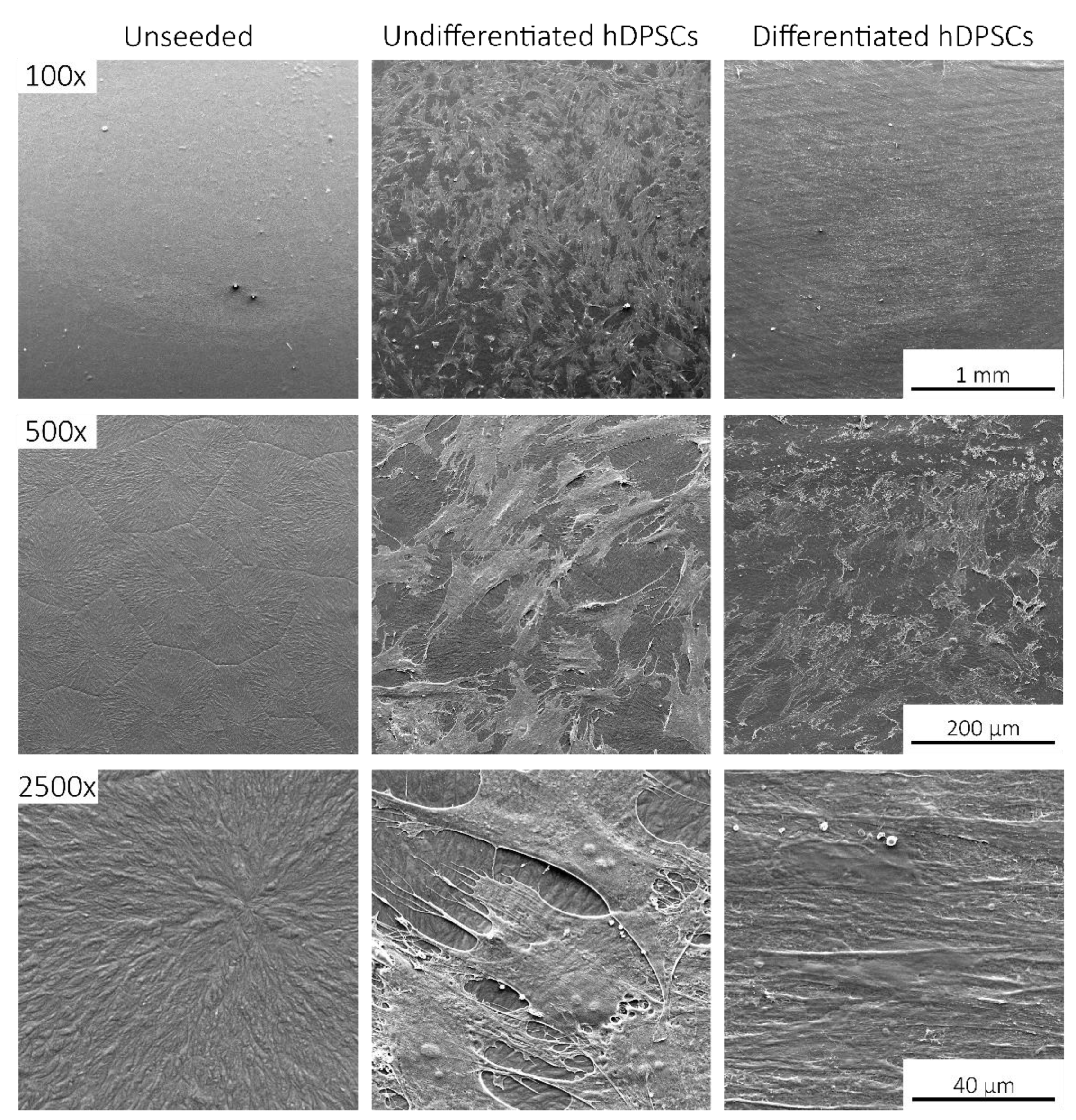
2.4. Scanning Electronic Microscopy (SEM)
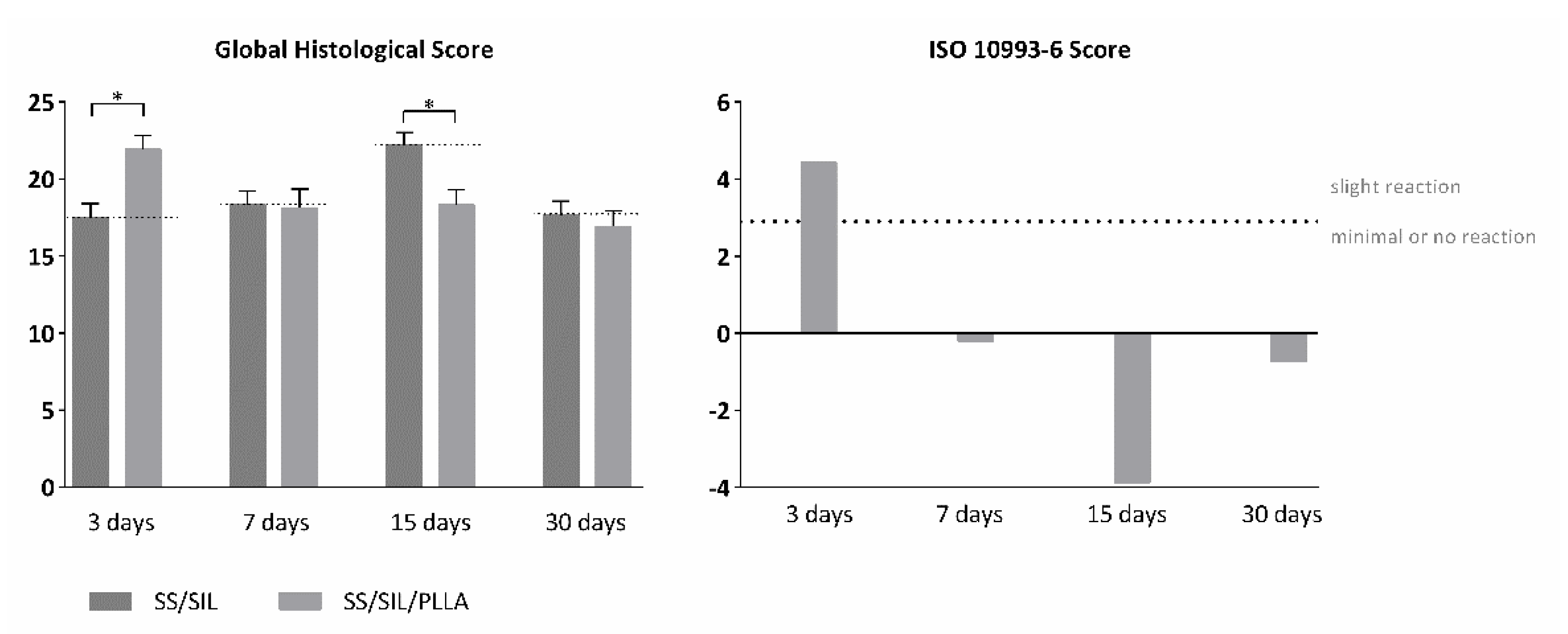
2.5. In Vivo Biocompatibility Assessment of Implantable Devices in Subcutaneous Tissue
3. Discussion
4. Materials and Methods
4.1. Devices’ Preparation
4.2. Characterization of the Devices
4.3. In Vitro Assays
4.3.1. Cell Culture and Maintenance
4.3.2. Cytocompatibility Assessment
4.3.3. Osteogenic Differentiation Assay
4.3.4. Scanning Electronic Microscopy (SEM)
4.4. In Vivo Biocompatibility Assessment of Implantable Devices in Subcutaneous Tissue
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARS | Alizarin Red S |
| DMF | Dimethylformamide |
| DPBS | Dulbecco’s phosphate-buffered saline solution |
| DSC | Differential scanning calorimetry |
| ECM | Extracellular matrix |
| EDS | Energy dispersive X-ray spectroscopy |
| FBS | Fetal bovine serum |
| H&E | Haematoxylin-Eosin |
| hDPSCs | Human dental pulp stem/stromal cells |
| HMDS | hexamethyldisilazane |
| (J/g) | Enthalpy of fusion |
| (MSCs) | Mesenchymal stem cells |
| PLLA | Poly (L-lactic acid) |
| SEM | Scanning electron microscopy |
| SIL | Silanization |
| SS | Stainless steel |
| Tc | Crystallization temperature |
| Tg | Glass transition temperature |
| Tm | Melting temperature |
| Xc | Degree of crystallinity |
| XRD | X-ray diffraction |
| ΔHf | Specific enthalpy |
References
- Williamson, S.; Landeiro, F.; McConnell, T.; Fulford-Smith, L.; Javaid, M.K.; Judge, A.; Leal, J. Costs of fragility hip fractures globally: A systematic review and meta-regression analysis. Osteoporos. Int. 2017, 28, 2791–2800. [Google Scholar] [CrossRef] [PubMed]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Pinto, P.; Branquinho, M.; Caseiro, A.; Sousa, A.; Brandão, A.; Pedrosa, S.; Alvites, R.; Campos, J.; Santos, F.; Santos, J. The application of bonelike® Poro as a synthetic bone substitute for the management of critical-sized bone defects-a comparative approach to the autograft technique-a preliminary study. Bone Rep. 2021, 14, 101064. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Merchán, E.C. A Review of Recent Developments in the Molecular Mechanisms of Bone Healing. Int. J. Mol. Sci. 2021, 22, 767. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, K.; Nakajima, H. Metallic scaffolds for bone regeneration. Materials 2009, 2, 790–832. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, A.; Qiu, H.; Yang, Y. Mesenchymal stem cell and endothelial cell interaction restores endothelial permeability via paracrine hepatocyte growth factor in vitro. Crit. Care 2015, 19, P236. [Google Scholar] [CrossRef] [Green Version]
- Salahinejad, E.; Hadianfard, M.; Macdonald, D.; Mozafari, M.; Walker, K.; Rad, A.T.; Madihally, S.; Vashaee, D.; Tayebi, L. Surface modification of stainless steel orthopedic implants by sol–gel ZrTiO4 and ZrTiO4–PMMA coatings. J. Biomed. Nanotechnol. 2013, 9, 1327–1335. [Google Scholar] [CrossRef]
- Saleh, M.M.; Touny, A.; Al-Omair, M.A.; Saleh, M. Biodegradable/biocompatible coated metal implants for orthopedic applications. Bio-Med. Mater. Eng. 2016, 27, 87–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barroca, N.; Vilarinho, P.M.; Daniel-da-Silva, A.L.; Wu, A.; Fernandes, M.H.; Gruverman, A. Protein adsorption on piezoelectric poly (L-lactic) acid thin films by scanning probe microscopy. Appl. Phys. Lett. 2011, 98, 133705. [Google Scholar] [CrossRef]
- Jianqing, F.; Huipin, Y.; Xingdong, Z. Promotion of osteogenesis by a piezoelectric biological ceramic. Biomaterials 1997, 18, 1531–1534. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, W.; He, C.; Peng, S.; Gao, C.; Yang, Y.; Qi, F.; Feng, P. A magnetic micro-environment in scaffolds for stimulating bone regeneration. Mater. Des. 2020, 185, 108275. [Google Scholar] [CrossRef]
- Fukada, E.; Yasuda, I. On the piezoelectric effect of bone. J. Phys. Soc. Jpn. 1957, 12, 1158–1162. [Google Scholar] [CrossRef]
- Pawlikowski, M. Biomineralogical investigationof apatite piezoelectricity. Tpaвмaтoлoгия и opтoпeдия Poccии 2016, 22. [Google Scholar] [CrossRef] [Green Version]
- Pawlikowski, M. Electric phenomenon in bones as a result of piezoelectricity of hydroxyapatite. Arch. Clin. Biomed. Res 2017, 1, 132–139. [Google Scholar] [CrossRef]
- Jacob, J.; More, N.; Kalia, K.; Kapusetti, G. Piezoelectric smart biomaterials for bone and cartilage tissue engineering. Inflamm. Regen. 2018, 38, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, C.; Correia, D.; Ribeiro, S.; Fernandes, M.; Lanceros-Mendez, S. Piezo-and magnetoelectric polymers as biomaterials for novel tissue engineering strategies. MRS Adv. 2018, 3, 1671–1676. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, C.; Sencadas, V.; Correia, D.M.; Lanceros-Méndez, S. Piezoelectric polymers as biomaterials for tissue engineering applications. Colloids Surf. B Biointerfaces 2015, 136, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.; Chalklen, T.; Lindackers, C.; Calahorra, Y.; Howe, C.; Tamboli, A.; Bax, D.V.; Barrett, D.J.; Cameron, R.E.; Best, S.M. Poly-L-lactic acid nanotubes as soft piezoelectric interfaces for biology: Controlling cell attachment via polymer crystallinity. ACS Appl. Bio Mater. 2020, 3, 2140–2149. [Google Scholar] [CrossRef] [Green Version]
- Barroca, N.; Daniel-da-Silva, A.; Gomes, P.; Fernandes, M.; Lanceros-Méndez, S.; Sharma, P.; Gruverman, A.; Fernandes, M.; Vilarinho, P.M. Suitability of PLLA as piezoelectric substrates for tissue engineering evidenced by microscopy techniques. Microsc. Microanal. 2012, 18, 63–64. [Google Scholar] [CrossRef] [Green Version]
- Magueta, A.F.; Fernandes, M.H.; Hortigüela, M.J.; Otero-Irurueta, G.; Vilarinho, P.M. Poly (L-lactic acid) coatings on 316 SS substrates for biomedical devices: The impact of surface silanization. Prog. Org. Coat. 2021, 157, 106289. [Google Scholar] [CrossRef]
- Bernardo, V.; Luz, G.M.; Alves, N.M.; Mano, J.F. Cell behaviour in new poly (l-lactic acid) films with crystallinity gradients. Mater. Lett. 2012, 87, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Kong, Y.; Hay, J. The measurement of the crystallinity of polymers by DSC. Polymer 2002, 43, 3873–3878. [Google Scholar] [CrossRef]
- Righetti, M.C.; Gazzano, M.; Di Lorenzo, M.L.; Androsch, R. Enthalpy of melting of α′-and α-crystals of poly (l-lactic acid). Eur. Polym. J. 2015, 70, 215–220. [Google Scholar] [CrossRef]
- Emter, R.; Natsch, A. A fast Resazurin-based live viability assay is equivalent to the MTT-test in the KeratinoSens assay. Toxicol. In Vitro 2015, 29, 688–693. [Google Scholar] [CrossRef]
- Campos, J.; Sousa, A.; Caseiro, A.; Pedrosa, S.; Pinto, P.; Branquinho, M.; Amorim, I.; Santos, J.; Pereira, T.; Mendonça, C. Dental pulp stem cells and Bonelike® for bone regeneration in ovine model. Regen. Biomater. 2019, 6, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Caseiro, A.; Alvites, R.; Pedrosa, S.; Miguel, J.; Reis, I.; Santos, J.; Mendonça, C.; Atayde, M.; Maurício, A. The potential clinical application of mesenchymal stem cells from the dental pulp (DPSCs) for bone regeneration. Front. Stem Cell Regen. Med. Res. 2017, 6, 3–52. [Google Scholar]
- Caseiro, A.R.; Santos Pedrosa, S.; Ivanova, G.; Vieira Branquinho, M.; Almeida, A.; Faria, F.; Amorim, I.; Pereira, T.; Maurício, A.C. Mesenchymal Stem/Stromal Cells metabolomic and bioactive factors profiles: A comparative analysis on the umbilical cord and dental pulp derived Stem/Stromal Cells secretome. PLoS ONE 2019, 14, e0221378. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, A.H.; Jaffe, M.; Arinzeh, T.L. Piezoelectric materials for tissue regeneration: A review. Acta Biomater. 2015, 24, 12–23. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Yamamoto, A. Characteristics and cytocompatibility of biodegradable polymer film on magnesium by spin coating. Colloids Surf. B Biointerfaces 2012, 93, 67–74. [Google Scholar] [CrossRef]
- Alvites, R.D.; Branquinho, M.V.; Caseiro, A.R.; Amorim, I.; Santos Pedrosa, S.; Rêma, A.; Faria, F.; Porto, B.; Oliveira, C.; Teixeira, P. Rat olfactory mucosa mesenchymal stem/stromal cells (OM-MSCs): A characterization study. Int. J. Cell Biol. 2020, 2020. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Xiao, E.; Lv, W.; Dong, X.; He, L.; Wang, Y.; Zhang, Y. A chemically defined serum-free culture system for spontaneous human mesenchymal stem cell spheroid formation. Stem Cells Int. 2020, 2020. [Google Scholar] [CrossRef]
- Egger, D.; Tripisciano, C.; Weber, V.; Dominici, M.; Kasper, C. Dynamic cultivation of mesenchymal stem cell aggregates. Bioengineering 2018, 5, 48. [Google Scholar] [CrossRef] [Green Version]
- Shekaran, A.; Sim, E.; Tan, K.Y.; Chan, J.K.Y.; Choolani, M.; Reuveny, S.; Oh, S. Enhanced in vitro osteogenic differentiation of human fetal MSCs attached to 3D microcarriers versus harvested from 2D monolayers. BMC Biotechnol. 2015, 15, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, H.F.; Cengiz, I.F.; Silva, F.S.; Reis, R.L.; Oliveira, J.M. Scaffolds and coatings for bone regeneration. J. Mater. Sci. Mater. Med. 2020, 31, 27. [Google Scholar] [CrossRef] [Green Version]
- Barroca, N.; Collins, L.; Rodriguez, B.J.; Fernandes, M.H.V.; Vilarinho, P.M. Mechanical writing of electrical polarization in poly (L-lactic) acid. Acta Biomater. 2021. [Google Scholar] [CrossRef]
- Barroca, N.; Vilarinho, P.M.; Fernandes, M.H.V.; Sharma, P.; Gruverman, A. Stability of electrically induced-polarization in poly (L-lactic) acid for bone regeneration. Appl. Phys. Lett. 2012, 101, 023701. [Google Scholar] [CrossRef]
- Cui, H.; Sinko, P.J. The role of crystallinity on differential attachment/proliferation of osteoblasts and fibroblasts on poly (caprolactone-co-glycolide) polymeric surfaces. Front. Mater. Sci. 2012, 6, 47–59. [Google Scholar] [CrossRef]
- Maqsood, M.; Kang, M.; Wu, X.; Chen, J.; Teng, L.; Qiu, L. Adult mesenchymal stem cells and their exosomes: Sources, characteristics, and application in regenerative medicine. Life Sci. 2020, 256, 118002. [Google Scholar] [CrossRef]
- Iijima, K.; Otsuka, H. Cell Scaffolds for Bone Tissue Engineering. Bioengineering 2020, 7, 119. [Google Scholar] [CrossRef] [PubMed]
- Shang, F.; Yu, Y.; Liu, S.; Ming, L.; Zhang, Y.; Zhou, Z.; Zhao, J.; Jin, Y. Advancing application of mesenchymal stem cell-based bone tissue regeneration. Bioact. Mater. 2021, 6, 666–683. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, T.W. Dental pulp stem cells: Advances to applications. Stem Cells Cloning Adv. Appl. 2020, 13, 33. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, S.; Tomokiyo, A.; Hasegawa, D.; Hamano, S.; Sugii, H.; Maeda, H. Insight into the role of dental pulp stem cells in regenerative therapy. Biology 2020, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Alge, D.L.; Zhou, D.; Adams, L.L.; Wyss, B.K.; Shadday, M.D.; Woods, E.J.; Gabriel Chu, T.; Goebel, W.S. Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J. Tissue Eng. Regen. Med. 2010, 4, 73–81. [Google Scholar] [CrossRef]
- Yamada, Y.; Ito, K.; Nakamura, S.; Ueda, M.; Nagasaka, T. Promising cell-based therapy for bone regeneration using stem cells from deciduous teeth, dental pulp, and bone marrow. Cell Transplant. 2011, 20, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, F.; Nouri Khorasani, S.; Khalili, S.; Esmaeely Neisiany, R.; Rezvani Ghomi, E.; Ejeian, F.; Das, O.; Nasr-Esfahani, M.H. Development of a highly proliferated bilayer coating on 316L stainless steel implants. Polymers 2020, 12, 1022. [Google Scholar] [CrossRef]
- Majee, P.; Dhar, S.; Mitra, P.; Lalzawmliana, V.; Nandi, S.K.; Basak, P.; Kundu, B. In vivo bone regeneration analysis of trilayer coated 316L stainless steel implant in rabbit model. J. Mater. Res. 2018, 33, 2106–2117. [Google Scholar] [CrossRef]
- Ciapetti, G.; Granchi, D.; Devescovi, V.; Baglio, S.R.; Leonardi, E.; Martini, D.; Jurado, M.J.; Olalde, B.; Armentano, I.; Kenny, J.M. Enhancing osteoconduction of PLLA-based nanocomposite scaffolds for bone regeneration using different biomimetic signals to MSCs. Int. J. Mol. Sci. 2012, 13, 2439–2458. [Google Scholar] [CrossRef] [Green Version]
- Santos, D.; Silva, D.M.; Gomes, P.S.; Fernandes, M.H.; Santos, J.D.; Sencadas, V. Multifunctional PLLA-ceramic fiber membranes for bone regeneration applications. J. Colloid Interface Sci. 2017, 504, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Liu, Y.; Ouyang, Z.; Feng, Q. Comparing the regeneration potential between PLLA/Aragonite and PLLA/Vaterite pearl composite scaffolds in rabbit radius segmental bone defects. Bioact. Mater. 2020, 5, 980–989. [Google Scholar] [CrossRef]
- Sathishkumar, S.; Louis, K.; Shinyjoy, E.; Gopi, D. Tailoring the Sm/Gd-substituted hydroxyapatite coating on biomedical AISI 316L SS: Exploration of corrosion resistance, protein profiling, osteocompatibility, and osteogenic differentiation for orthopedic implant applications. Ind. Eng. Chem. Res. 2016, 55, 6331–6344. [Google Scholar] [CrossRef]
- Lodhi, M.; Deen, K.; Greenlee-Wacker, M.; Haider, W. Additively manufactured 316L stainless steel with improved corrosion resistance and biological response for biomedical applications. Addit. Manuf. 2019, 27, 8–19. [Google Scholar] [CrossRef]
- Bordjih, K.; Jouzeau, J.-Y.; Mainard, D.; Payan, E.; Delagoutte, J.-P.; Netter, P. Evaluation of the effect of three surface treatments on the biocompatibility of 316L stainless steel using human differentiated cells. Biomaterials 1996, 17, 491–500. [Google Scholar] [CrossRef]
- Harvey, J.L. Title 21—Food and drugs chapter I—Food and drug administration, department of health, education, and welfare subchapter B—Food and food products part 121—Food additives definitions and procedural and interpretative regulations. Food Drug Cosmet. Law J. 1959, 14, 269–290. [Google Scholar]
- Canevarolo, J.; dos Polímeros, S.C. Um Texto Básico para Tecnólogos e Engenheiros, 3rd ed.; Artliber: São Paulo, Brazil, 2010. [Google Scholar]
- Alvites, R.D.; Branquinho, M.V.; Sousa, A.C.; Amorim, I.; Magalhães, R.; João, F.; Almeida, D.; Amado, S.; Prada, J.; Pires, I. Combined Use of Chitosan and Olfactory Mucosa Mesenchymal Stem/Stromal Cells to Promote Peripheral Nerve Regeneration In Vivo. Stem Cells Int. 2021, 2021, 6613029. [Google Scholar] [CrossRef]
- Caseiro, A.; Ivanova, G.; Pedrosa, S.; Branquinho, M.; Georgieva, P.; Barbosa, P.; Santos, J.; Magalhães, R.; Teixeira, P.; Pereira, T. Human umbilical cord blood plasma as an alternative to animal sera for mesenchymal stromal cells in vitro expansion–A multicomponent metabolomic analysis. PLoS ONE 2018, 13, e0203936. [Google Scholar] [CrossRef]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef]
- Pinho, A.C.; Branquinho, M.V.; Alvites, R.D.; Fonseca, A.C.; Caseiro, A.R.; Pedrosa, S.S.; Luís, A.L.; Pires, I.; Prada, J.; Muratori, L. Dextran-based tube-guides for the regeneration of the rat sciatic nerve after neurotmesis injury. Biomater. Sci. 2020, 8, 798–811. [Google Scholar] [CrossRef] [PubMed]
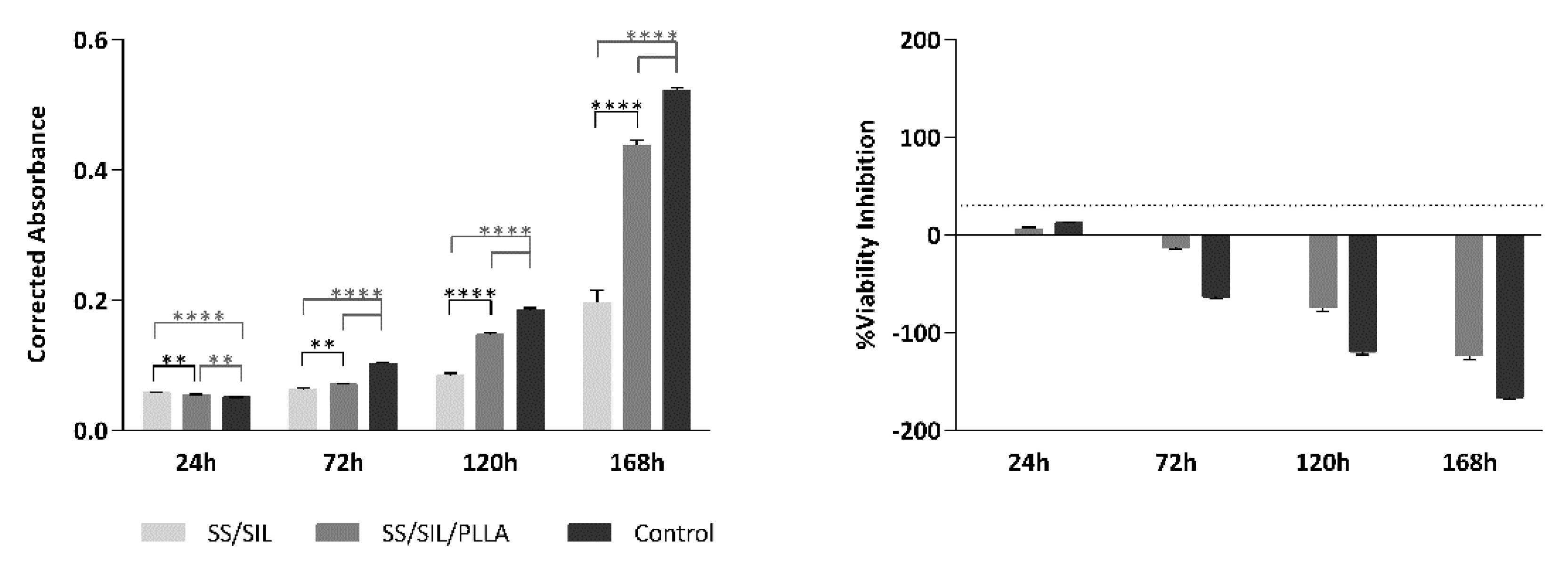


| SS/SIL | SS/SIL/PLLA | Control | ||||
|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | |
| 24 h | 0.058 | 0.001 | 0.054 | 0.001 | 0.051 | 0.001 |
| 72 h | 0.063 | 0.003 | 0.071 | 0.001 | 0.102 | 0.001 |
| 120 h | 0.084 | 0.004 | 0.147 | 0.003 | 0.185 | 0.003 |
| 168 h | 0.196 | 0.019 | 0.438 | 0.008 | 0.522 | 0.004 |
| SS/SIL/PLLA | Control | |||
|---|---|---|---|---|
| Mean | SE | Mean | SE | |
| 24 h | 6.317 | 1.532 | 12.635 | 1.024 |
| 72 h | −12.948 | 2.031 | −63.214 | 2.147 |
| 120 h | −74.198 | 3.935 | −119.568 | 3.371 |
| 168 h | −123.500 | 4.059 | −166.622 | 1.934 |
| SS/SIL | SS/SIL/PLLA | Control | ||||
|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | |
| Undifferentiated | 0.106 | 0.002 | 0.124 | 0.003 | 0.103 | 0.002 |
| Differentiated | 0.124 | 0.004 | 0.156 | 0.007 | 0.161 | 0.008 |
| SS/SIL/PLLA | Control | |||
|---|---|---|---|---|
| Mean | SE | Mean | SE | |
| Undifferentiated | 16.789 | 2.917 | −3.141 | 2.258 |
| Differentiated | 25.851 | 5.553 | 29.384 | 6.203 |
| SS/SIL | SS/SIL/PLLA | |||
|---|---|---|---|---|
| Mean | SE | Mean | SE | |
| 3 days | 17.50 | 0.909 | 21.93 | 0.892 |
| ISO SCORE | 4.43 | |||
| 7 days | 18.33 | 0.882 | 18.13 | 1.230 |
| ISO SCORE | −0.20 | |||
| 15 days | 22.20 | 0.827 | 18.33 | 0964 |
| ISO SCORE | −3.87 | |||
| 30 days | 17.67 | 0.908 | 16.93 | 1.021 |
| ISO SCORE | −0.74 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branquinho, M.V.; Ferreira, S.O.; Alvites, R.D.; Magueta, A.F.; Ivanov, M.; Sousa, A.C.; Amorim, I.; Faria, F.; Fernandes, M.H.V.; Vilarinho, P.M.; et al. In Vitro and In Vivo Characterization of PLLA-316L Stainless Steel Electromechanical Devices for Bone Tissue Engineering—A Preliminary Study. Int. J. Mol. Sci. 2021, 22, 7655. https://doi.org/10.3390/ijms22147655
Branquinho MV, Ferreira SO, Alvites RD, Magueta AF, Ivanov M, Sousa AC, Amorim I, Faria F, Fernandes MHV, Vilarinho PM, et al. In Vitro and In Vivo Characterization of PLLA-316L Stainless Steel Electromechanical Devices for Bone Tissue Engineering—A Preliminary Study. International Journal of Molecular Sciences. 2021; 22(14):7655. https://doi.org/10.3390/ijms22147655
Chicago/Turabian StyleBranquinho, Mariana V., Sheila O. Ferreira, Rui D. Alvites, Adriana F. Magueta, Maxim Ivanov, Ana Catarina Sousa, Irina Amorim, Fátima Faria, M. H. V. Fernandes, Paula M. Vilarinho, and et al. 2021. "In Vitro and In Vivo Characterization of PLLA-316L Stainless Steel Electromechanical Devices for Bone Tissue Engineering—A Preliminary Study" International Journal of Molecular Sciences 22, no. 14: 7655. https://doi.org/10.3390/ijms22147655
APA StyleBranquinho, M. V., Ferreira, S. O., Alvites, R. D., Magueta, A. F., Ivanov, M., Sousa, A. C., Amorim, I., Faria, F., Fernandes, M. H. V., Vilarinho, P. M., & Maurício, A. C. (2021). In Vitro and In Vivo Characterization of PLLA-316L Stainless Steel Electromechanical Devices for Bone Tissue Engineering—A Preliminary Study. International Journal of Molecular Sciences, 22(14), 7655. https://doi.org/10.3390/ijms22147655









