The Epithelial Cell Leak Pathway
Abstract
:1. The Tight Junction Is the Paracellular Permeability Barrier
2. Strands, Pores, and Paracellular Permeability
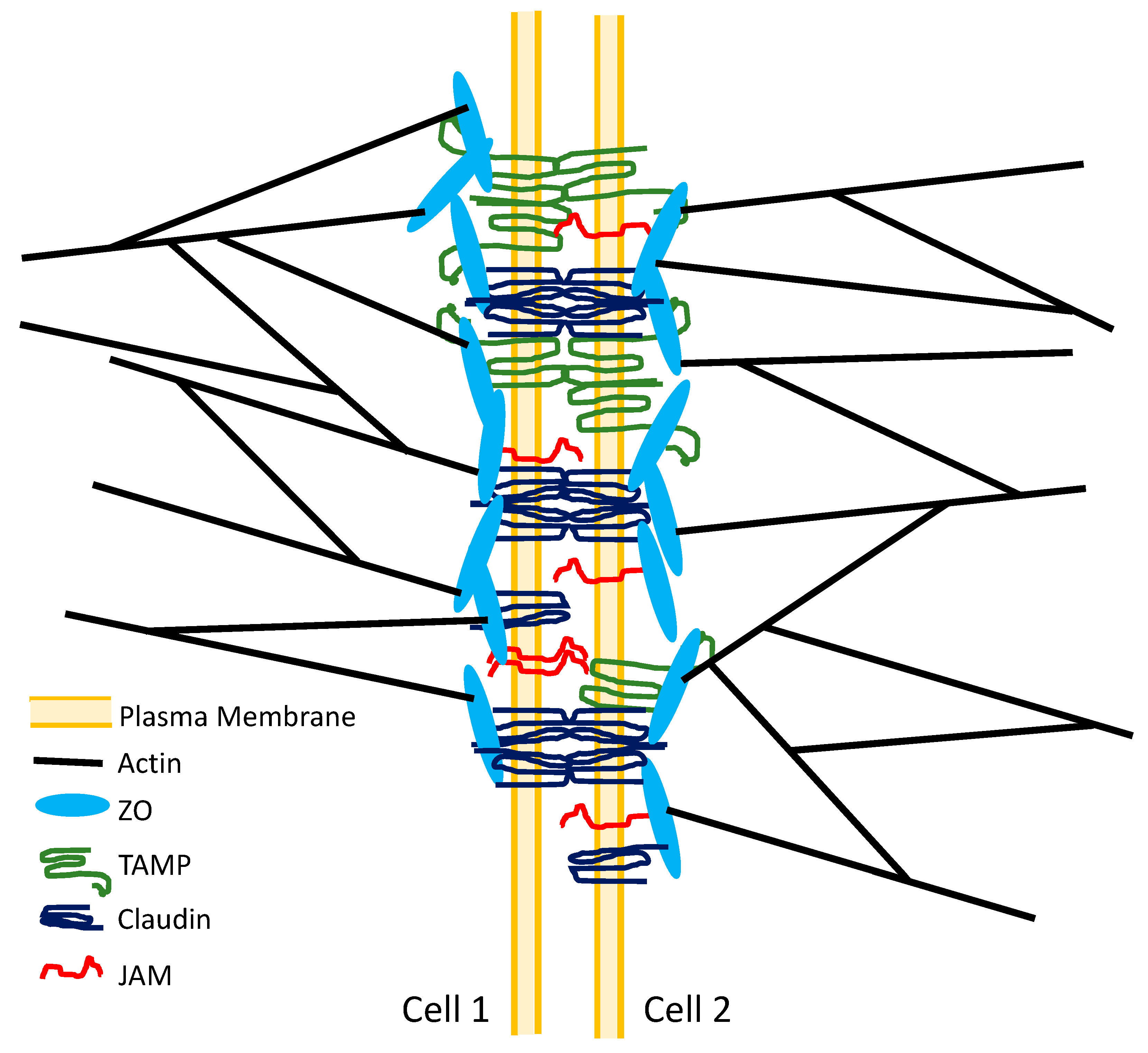
3. Tight Junction Structure
4. What Constitutes the Paracellular Permeability Barrier?
5. Two Paracellular Permeability Pathways: The Pore Pathway and the Leak Pathway
6. Pore Pathway Versus Leak Pathway: The Same or Different?
7. What Is the Leak Pathway?
= π(50 Å)2 × 35
= 274,889 Å2
= (27.4889 × 104 Å2)/(36 × 1010 Å2)
= 7.636 × 10−7
8. What Cell Components Are Part of the Leak Pathway?
9. Does Tight Junction Stress/Tension Affect Leak Pathway Permeability?
10. How Can Leak Pathway Permeability Be Regulated Independently from Pore Pathway Permeability?
11. TAMPs, Tight Junction Structure, and Leak Pathway Permeability
12. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Bizzozero, G. Sulla struttura degli epiteli pavimentosi stratificati. Arch. Sci. Med. 1885, 9, 378. [Google Scholar]
- Farquhar, M.G.; Palade, G.E. Junctional complexes in various epithelia. J. Cell Biol. 1963, 17, 375–412. [Google Scholar] [CrossRef] [Green Version]
- Ussing, H.H.; Windhager, E.E. Nature of shunt path and active sodium transport path through frog skin epithelium. Acta Physiol. Scand. 1964, 61, 484–504. [Google Scholar]
- Machen, T.E.; Erlij, D.; Wooding, F.B. Permeable junctional complexes. The movement of lanthanum across rabbit gallbladder and intestine. J. Cell Biol. 1972, 54, 302–312. [Google Scholar] [CrossRef] [Green Version]
- Whittembury, G.; Rawlins, F.A. Evidence of a paracellular pathway for ion flow in the kidney proximal tubule: Electromicroscopic demonstration of lanthanum precipitate in the tight junction. Pflügers Arch. 1971, 330, 302–309. [Google Scholar] [CrossRef]
- Martinez-Palomo, A.; Erlij, D.; Bracho, H. Localization of permeability barriers in the frog skin epithelium. J. Cell Biol. 1971, 50, 277–287. [Google Scholar] [CrossRef] [Green Version]
- Frömter, E.; Diamond, J. Route of passive ion permeation in epithelia. Nat. New Biol. 1972, 235, 9–13. [Google Scholar] [CrossRef]
- Goodenough, D.A.; Revel, J.P. A fine structural analysis of intercellular junctions in the mouse liver. J. Cell Biol. 1970, 45, 272–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staehelin, A.; Mukherjee, T.M.; Williams, A.W. Freeze-etch appearance of the tight junctions in the epithelium of small and large intestine of mice. Protoplasma 1969, 67, 165–184. [Google Scholar] [CrossRef] [PubMed]
- Humbert, F.; Grandchamp, A.; Pricam, C.; Perrelet, A.; Orci, L. Morphological changes in tight junctions of Necturus maculosus proximal tubules undergoing saline diuresis. J. Cell Biol. 1976, 69, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Wade, J.B.; Karnovsky, M.J. Fracture faces of osmotically disrupted zonulae occludentes. J. Cell Biol. 1974, 62, 344–350. [Google Scholar] [CrossRef]
- Claude, P.; Goodenough, D.A. Fracture faces of zonulae occludentes from “tight” and “leaky” epithelia. J. Cell Biol. 1973, 58, 390–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claude, P. Morphological factors influencing transepithelial permeability: A model for the resistance of the zonula occludens. J. Membr. Biol. 1978, 39, 219–232. [Google Scholar] [CrossRef]
- Stevenson, B.R.; Anderson, J.M.; Goodenough, D.A.; Mooseker, M.S. Tight junction structure and ZO-1 content are identical in two strains of Madin-Darby canine kidney cells which differ in transepithelial resistance. J. Cell Biol. 1988, 107, 2401–2408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevenson, B.R.; Siliciano, J.D.; Mooseker, M.S.; Goodenough, D.A. Identification of ZO-1: A high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J. Cell Biol. 1986, 103, 755–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanning, A.S.; Jameson, B.J.; Jesaitis, L.A.; Anderson, J.M. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 1998, 273, 29745–29753. [Google Scholar] [CrossRef] [Green Version]
- Itoh, M.; Nagafuchi, A.; Moroi, S.; Tsukita, S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J. Cell Biol. 1997, 138, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Gumbiner, B.; Lowenkopf, T.; Apatira, D. Identification of a 160-kDa polypeptide that binds to the tight junction protein ZO-1. Proc. Natl. Acad. Sci. USA 1991, 88, 3460–3464. [Google Scholar] [CrossRef] [Green Version]
- Haskins, J.; Gu, L.; Wittchen, E.S.; Hibbard, J.; Stevenson, B.R. ZZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J. Cell Biol. 1998, 141, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Jesaitis, L.A.; Goodenough, D.A. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila discs-large tumor suppressor protein. J. Cell Biol. 1994, 124, 949–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuse, M.; Hirase, T.; Itoh, M.; Nagafuchi, A.; Yonemura, S.; Tsukita, S. Occludin: A novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993, 123, 1777–1788. [Google Scholar] [CrossRef]
- Raleigh, D.R.; Marchiando, A.M.; Zhang, Y.E.; Shen, L.; Sasaki, H.; Wang, Y.; Long, M.; Turner, J.R. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol. Biol. Cell 2010, 21, 1200–1213. [Google Scholar] [CrossRef] [Green Version]
- Ikenouchi, J.; Furuse, M.; Furuse, K.; Sasaki, H.; Tsukita, S.; Tsukita, S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J. Cell Biol. 2005, 171, 939–945. [Google Scholar] [CrossRef]
- Higashi, T.; Tokuda, S.; Kitajiri, S.-I.; Masuda, S.; Nakamura, H.; Oda, Y.; Furuse, M. Analysis of the angulin family consisting of LSR, ILDR1 and ILDR2: Tricellulin recruitment, epithelial barrier function and implication in deafness pathogenesis. J. Cell Sci. 2013, 126, 966–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, S.; Oda, Y.; Sasaki, H.; Ikenouchi, J.; Higashi, T.; Akashi, M.; Nishi, E.; Furuse, M. LSR defines cell corners for tricellular tight junction formation in epithelial cells. J. Cell Sci. 2011, 124, 548–555. [Google Scholar] [CrossRef] [Green Version]
- Martìn-Padura, I.; Lostaglio, S.; Schneemann, M.; Williams, L.; Romano, M.; Fruscella, P.; Panzeri, M.C.; Stoppacciaro, A.; Ruco, L.; Villa, A.; et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J. Cell Biol. 1998, 142, 117–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuse, M.; Fujita, K.; Hiiragi, T.; Fujimoto, K.; Tsukita, S. Claudin-1 and -2: Novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 1998, 141, 1539–1550. [Google Scholar] [CrossRef]
- Tsukita, S.; Tanaka, H.; Tamura, A. The claudins: From tight junctions to biological systems. Trends Biochem. Sci. 2019, 44, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Citi, S.; Sabanay, H.; Jakes, R.; Geiger, B.; Kendrick-Jones, J. Cingulin, a new peripheral component of tight junctions. Nat. Cell Biol. 1988, 333, 272–276. [Google Scholar] [CrossRef]
- Zihni, C.; Terry, S.J. RhoGTPase signalling at epithelial tight junctions: Bridging the GAP between polarity and cancer. Int. J. Biochem. Cell Biol. 2015, 64, 120–125. [Google Scholar] [CrossRef] [Green Version]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef]
- Fuladi, S.; Jannat, R.-W.; Shen, L.; Weber, C.R.; Khalili-Araghi, F. Computational modeling of claudin structure and function. Int. J. Mol. Sci. 2020, 21, 742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinemann, U.; Schuetz, A. Structural features of tight-junction proteins. Int. J. Mol. Sci. 2019, 20, 6020. [Google Scholar] [CrossRef] [Green Version]
- Cording, J.; Berg, J.; Käding, N.; Bellmann, C.; Tscheik, C.; Westphal, J.; Milatz, S.; Günzel, D.; Wolburg, H.; Piontek, J.; et al. Tight junctions: Claudins regulate the interactions between occludin, tricellulin and marvelD3, which, inversely, modulate claudin oligomerization. J. Cell Sci. 2013, 126, 554–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehder, D.; Iden, S.; Nasdala, I.; Wegener, J.; Zu Brickwedde, M.-K.M.; Vestweber, D.; Ebnet, K. Junctional adhesion molecule-A participates in the formation of apico-basal polarity through different domains. Exp. Cell Res. 2006, 312, 3389–3403. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.C.C.; Luissint, A.-C.; Sumagin, R.; Lai, C.; Vielmuth, F.; Wolf, M.F.; Laur, O.; Reiss, K.; Spindler, V.; Stehle, T.; et al. Trans-dimerization of JAM-A regulates Rap2 and is mediated by a domain that is distinct from the cis-dimerization interface. Mol. Biol. Cell 2014, 25, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, G.; Weber, K.S.C.; Zernecke, A.; Schröder, A.; Weber, C. JAM-1 is a ligand of the β2 integrin LFA-1 involved in transendothelial migration of leukocytes. Nat. Immunol. 2002, 3, 151–158. [Google Scholar] [CrossRef]
- Itoh, M.; Furuse, M.; Morita, K.; Kubota, K.; Saitou, M.; Tsukita, S. Direct binding of three tight junction-associated maguks, Zo-1, Zo-2, and Zo-3, with the cooh termini of claudins. J. Cell Biol. 1999, 147, 1351–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuse, M.; Itoh, M.; Hirase, T.; Nagafuchi, A.; Yonemura, S.; Tsukita, S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J. Cell Biol. 1994, 127, 1617–1626. [Google Scholar] [CrossRef] [Green Version]
- Ebnet, K.; Schulz, C.U.; zu Brickwedde, M.-K.M.; Pendl, G.G.; Vestweber, D. Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J. Biol. Chem. 2000, 275, 27979–27988. [Google Scholar] [CrossRef] [Green Version]
- Nomme, J.; Fanning, A.S.; Caffrey, M.; Lye, M.F.; Anderson, J.M.; Lavie, A. The Src homology 3 domain is required for junctional adhesion molecule binding to the third PDZ domain of the scaffolding protein ZO-1. J. Biol. Chem. 2011, 286, 43352–43360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Itallie, C.M.; Tietgens, A.J.; Krystofiak, E.; Kachar, B.; Anderson, J.M. A complex of ZO-1 and the BAR-domain protein TOCA-1 regulates actin assembly at the tight junction. Mol. Biol. Cell 2015, 26, 2769–2787. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.C.; Li, X.; Perreault, H.; Nagy, J.I. Interaction of zonula occludens-1 (ZO-1) with α-actinin-4: Application of functional proteomics for identification of PDZ domain-associated proteins. J. Proteom. Res. 2006, 5, 2123–2134. [Google Scholar] [CrossRef]
- Katsube, T.; Takahisa, M.; Ueda, R.; Hashimoto, N.; Kobayashi, M.; Togashi, S. Cortactin associates with the cell-cell junction protein ZO-1 in both drosophila and mouse. J. Biol. Chem. 1998, 273, 29672–29677. [Google Scholar] [CrossRef] [Green Version]
- Van Itallie, C.M.; Fanning, A.S.; Bridges, A.; Anderson, J.M. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol. Biol. Cell 2009, 20, 3930–3940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Itallie, C.M.; Anderson, J.M. Architecture of tight junctions and principles of molecular composition. Semin. Cell Dev. Biol. 2014, 36, 157–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glotfelty, L.G.; Zahs, A.; Iancu, C.; Shen, L.; Hecht, G.A. Microtubules are required for efficient epithelial tight junction homeostasis and restoration. Am. J. Physiol. Physiol. 2014, 307, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Vasileva, E.; Citi, S. The role of microtubules in the regulation of epithelial junctions. Tissue Barriers 2018, 6, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Dörfel, M.J.; Huber, O. Modulation of tight junction structure and function by kinases and phosphatases targeting occludin. J. Biomed. Biotechnol. 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Fan, S.; Weight, C.M.; Luissint, A.-C.; Hilgarth, R.S.; Brazil, J.C.; Ettel, M.; Nusrat, A.; Parkos, C.A. Role of JAM-A tyrosine phosphorylation in epithelial barrier dysfunction during intestinal inflammation. Mol. Biol. Cell 2019, 30, 566–578. [Google Scholar] [CrossRef]
- Raleigh, D.R.; Boe, D.M.; Yu, D.; Weber, C.; Marchiando, A.M.; Bradford, E.; Wang, Y.; Wu, L.; Schneeberger, E.E.; Shen, L.; et al. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J. Cell Biol. 2011, 193, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.K.; Basuroy, S.; Rao, V.U.; Karnaky, J.K.J.; Gupta, A. Tyrosine phosphorylation and dissociation of occludin–ZO-1 and E-cadherin–β-catenin complexes from the cytoskeleton by oxidative stress. Biochem. J. 2002, 368, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.; Sheth, P.; Elias, B.C.; Rao, R. Protein Phosphatases 2A and 1 Interact with occludin and negatively regulate the assembly of tight junctions in the CACO-2 cell monolayer. J. Biol. Chem. 2007, 282, 11487–11498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Itallie, C.M.; Anderson, J.M. Phosphorylation of tight junction transmembrane proteins: Many sites, much to do. Tissue Barriers 2017, 6, e1382671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kachar, B.; Reese, T.S. Evidence for the lipidic nature of tight junction strands. Nat. Cell Biol. 1982, 296, 464–466. [Google Scholar] [CrossRef]
- Nusrat, A.; Parkos, C.; Verkade, P.; Foley, C.; Liang, T.; Innis-Whitehouse, W.; Eastburn, K.; Madara, J. Tight junctions are membrane microdomains. J. Cell Sci. 2000, 113, 1771–1781. [Google Scholar] [CrossRef]
- Lambert, D.; O’Neill, C.; Padfield, P.J. Depletion of Caco-2 cell cholesterol disrupts barrier function by altering the detergent solubility and distribution of specific tight-junction proteins. Biochem. J. 2005, 387, 553–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowie, R.V.; Donatello, S.; Lyes, C.; Owens, M.B.; Babina, I.S.; Hudson, L.; Walsh, S.V.; O’Donoghue, D.P.; Amu, S.; Barry, S.P.; et al. Lipid rafts are disrupted in mildly inflamed intestinal microenvironments without overt disruption of the epithelial barrier. Am. J. Physiol. Liver Physiol. 2012, 302, 781–793. [Google Scholar] [CrossRef]
- Suzuki, H.; Tani, K.; Fujiyoshi, Y. Crystal structures of claudins: Insights into their intermolecular interactions. Ann. N. Y. Acad. Sci. 2017, 1397, 25–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hempel, C.; Protze, J.; Altun, E.; Riebe, B.; Piontek, A.; Fromm, A.; Lee, I.; Saleh, T.; Günzel, D.; Krause, G. Assembly of tight junction strands: Claudin-10b and claudin-3 form homo-tetrameric building blocks that polymerise in a channel-independent manner. J. Mol. Biol. 2020, 432, 2405–2427. [Google Scholar] [CrossRef]
- Suzuki, H.; Tani, K.; Tamura, A.; Tsukita, S.; Fujiyoshi, Y. Model for the Architecture of claudin-based paracellular ion channels through tight junctions. J. Mol. Biol. 2015, 427, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Van Itallie, C.M.; Tietgens, A.J.; Anderson, J.M. Visualizing the dynamic coupling of claudin strands to the actin cytoskeleton through ZO-1. Mol. Biol. Cell 2017, 28, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Weber, C.; Turner, J.R. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J. Cell Biol. 2008, 181, 683–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazzoni, G.; Estrada, O.M.M.; Orsenigo, F.; Cordenonsi, M.; Citi, S.; Dejana, E. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J. Biol. Chem. 2000, 275, 20520–20526. [Google Scholar] [CrossRef] [Green Version]
- Yano, T.; Matsui, T.; Tamura, A.; Uji, M.; Tsukita, S. The association of microtubules with tight junctions is promoted by cingulin phosphorylation by AMPK. J. Cell Biol. 2013, 203, 605–614. [Google Scholar] [CrossRef] [Green Version]
- Yano, T.; Torisawa, T.; Oiwa, K.; Tsukita, S. AMPK-dependent phosphorylation of cingulin reversibly regulates its binding to actin filaments and microtubules. Sci. Rep. 2018, 8, 15550. [Google Scholar] [CrossRef]
- Otani, T.; Furuse, M. Tight junction structure and function revisited. Trends Cell Biol. 2020, 30, 805–817. [Google Scholar] [CrossRef]
- Furuse, M.; Sasaki, H.; Fujimoto, K.; Tsukita, S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J. Cell Biol. 1998, 143, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Krystofiak, E.S.; Ballesteros, A.; Cui, R.; Van Itallie, C.M.; Anderson, J.M.; Fenollar-Ferrer, C.; Kachar, B. Multiple claudin–claudin cis interfaces are required for tight junction strand formation and inherent flexibility. Commun. Biol. 2018, 1, 50. [Google Scholar] [CrossRef] [PubMed]
- Piontek, J.; Krug, S.M.; Protze, J.; Krause, G.; Fromm, M. Molecular architecture and assembly of the tight junction backbone. BBA Biomembr. 2020, 1862, 183279. [Google Scholar] [CrossRef]
- Inai, T.; Kobayashi, J.; Shibata, Y. Claudin-1 contributes to the epithelial barrier function in MDCK cells. Eur. J. Cell Biol. 1999, 78, 849–855. [Google Scholar] [CrossRef]
- Milatz, S.; Krug, S.; Rosenthal, R.; Günzel, D.; Müller, D.; Schulzke, J.-D.; Amasheh, S.; Fromm, M. Claudin-3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. BBA Biomembr. 2010, 1798, 2048–2057. [Google Scholar] [CrossRef] [Green Version]
- Zwanziger, D.; Hackel, D.; Staat, C.; Böcker, A.; Brack, A.; Beyermann, M.; Rittner, H.; Blasig, I.E. A peptidomimetic tight junction modulator to improve regional analgesia. Mol. Pharm. 2012, 9, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Staat, C.; Coisne, C.M.; Dabrowski, S.; Stamatovic, S.M.; Andjelkovic, A.V.; Wolburg, H.; Engelhardt, B.; Blasig, I.E. Mode of action of claudin peptidomimetics in the transient opening of cellular tight junction barriers. Biomaterials 2015, 54, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, N.; Furuse, M.; Sasaki, H.; Yonemura, S.; Katahira, J.; Horiguchi, Y.; Tsukita, S. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands. J. Cell Biol. 1999, 147, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Piehl, C.; Piontek, J.; Cording, J.; Wolburg, H.; Blasig, I.E. Participation of the second extracellular loop of claudin-5 in paracellular tightening against ions, small and large molecules. Cell. Mol. Life Sci. 2010, 67, 2131–2140. [Google Scholar] [CrossRef]
- Gan, H.; Wang, G.; Hao, Q.; Wang, Q.J.; Tang, H. Protein kinase D promotes airway epithelial barrier dysfunction and permeability through down-regulation of claudin-1. J. Biol. Chem. 2013, 288, 37343–37354. [Google Scholar] [CrossRef] [Green Version]
- Carthy, K.M.; Francis, S.A.; Cormack, J.M.; Lai, J.; Rogers, R.A.; Skare, I.B.; Lynch, R.D.; Schneeberger, E.E. Inducible expression of claudin-1-myc but not occludin-VSV-G results in aberrant tight junction strand formation in MDCK cells. J. Cell Sci. 2000, 19, 3387–3398. [Google Scholar]
- Saito, A.C.; Higashi, T.; Fukazawa, Y.; Otani, T.; Tauchi, M.; Higashi, A.Y.; Furuse, M.; Chiba, H. Occludin and tricellulin facilitate formation of anastomosing tight-junction strand network to improve barrier function. Mol. Biol. Cell 2021, 32, 722–738. [Google Scholar] [CrossRef] [PubMed]
- Otani, T.; Nguyen, T.P.; Tokuda, S.; Sugihara, K.; Sugawara, T.; Furuse, K.; Miura, T.; Ebnet, K.; Furuse, M. Claudins and JAM-A coordinately regulate tight junction formation and epithelial polarity. J. Cell Biol. 2019, 218, 3372–3396. [Google Scholar] [CrossRef] [Green Version]
- Meyer, H.W. Tight junction strands are lipidic cylinders. Naturwissenschaften 1983, 70, 251–252. [Google Scholar] [CrossRef]
- Verkleij, A.J.; Mombers, C.; Leunissen-Bijvelt, J.; Ververgaert, P.H. Lipidic intramembranous particles. Nat. Cell Biol. 1979, 279, 162–163. [Google Scholar] [CrossRef]
- Miller, R.G. Do ‘lipidic particles’ represent intermembrane attachment sites? Nat. Cell Biol. 1980, 287, 166–167. [Google Scholar] [CrossRef] [PubMed]
- van Meer, G.; Gumbiner, B.; Simons, K. The tight junction does not allow lipid molecules to diffuse from one epithelial cell to the next. Nat. Cell Biol. 1986, 322, 639–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grebenkämper, K.; Galla, H.-J. Translational diffusion measurements of a fluorescent phospholipid between MDCK-I cells support the lipid model of the tight junctions. Chem. Phys. Lipids 1994, 71, 133–143. [Google Scholar] [CrossRef]
- Lee, D.B.N.; Jamgotchian, N.; Allen, S.G.; Abeles, M.B.; Ward, H.J. A lipid-protein hybrid model for tight junction. Am. J. Physiol. Physiol. 2008, 295, 1601–1612. [Google Scholar] [CrossRef]
- Lürick, A.; Kümmel, D.; Ungermann, C. Multisubunit tethers in membrane fusion. Curr. Biol. 2018, 28, 417–420. [Google Scholar] [CrossRef] [Green Version]
- Brukman, N.G.; Uygur, B.; Podbilewicz, B.; Chernomordik, L.V. How cells fuse. J. Cell Biol. 2019, 218, 1436–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lingaraju, A.; Long, T.M.; Wang, Y.; Austin, J.R.; Turner, J.R. Conceptual barriers to understanding physical barriers. Semin. Cell Dev. Biol. 2015, 42, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Durbin, R.P.; Frank, H.; Solomon, A.K. Water flow through frog gastric mucosa. J. Gen. Physiol. 1956, 39, 535–551. [Google Scholar] [CrossRef] [Green Version]
- Van Os, C.H.; De Jong, M.D.; Slegers, J.F.G. Dimensions of polar pathways through rabbit gallbladder epithelium. J. Membr. Biol. 1974, 15, 363–382. [Google Scholar] [CrossRef]
- Watson, C.J.; Rowland, M.; Warhurst, G. Functional modeling of tight junctions in intestinal cell monolayers using polyethylene glycol oligomers. Am. J. Physiol. Physiol. 2001, 281, 388–397. [Google Scholar] [CrossRef]
- France, M.M.; Turner, J.R. The mucosal barrier at a glance. J. Cell Sci. 2017, 130, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Cooke, K.R.; Hill, G.; Crawford, J.M.; Bungard, D.; Brinson, Y.S.; Delmonte, J.; Ferrara, J.L. Tumor necrosis factor-alpha production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. J. Clin. Investig. 1998, 102, 1882–1891. [Google Scholar] [CrossRef]
- Nalle, S.C.; Kwak, H.A.; Edelblum, K.L.; Joseph, N.E.; Singh, G.; Khramtsova, G.; Mortenson, E.D.; Savage, P.A.; Turner, J.R. Recipient NK cell inactivation and intestinal barrier loss are required for MHC-matched graft-versus-host disease. Sci. Transl. Med. 2014, 6, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amasheh, S.; Meiri, N.; Gitter, A.H.; Schöneberg, T.; Mankertz, J.; Schulzke, J.D.; Fromm, M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 2002, 115, 4969–4976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokuda, S.; Furuse, M. Claudin-2 knockout by talen-mediated Gene targeting in MDCK cells: Claudin-2 independently determines the leaky property of tight junctions in MDCK cells. PLoS ONE 2015, 10, e0119869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oshima, T.; Miwa, H.; Joh, T. Aspirin induces gastric epithelial barrier dysfunction by activating p38 MAPK via claudin-7. Am. J. Physiol. Physiol. 2008, 295, 800–806. [Google Scholar] [CrossRef] [Green Version]
- Weber, C.; Raleigh, D.R.; Su, L.; Shen, L.; Sullivan, E.A.; Wang, Y.; Turner, J.R. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J. Biol. Chem. 2010, 285, 12037–12046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buschmann, M.M.; Shen, L.; Rajapakse, H.; Raleigh, D.R.; Wang, Y.; Wang, Y.; Lingaraju, A.; Zha, J.; Abbott, E.; McAuley, E.; et al. Occludin OCEL-domain interactions are required for maintenance and regulation of the tight junction barrier to macromolecular flux. Mol. Biol. Cell 2013, 24, 3056–3068. [Google Scholar] [CrossRef]
- Cao, M.; Wang, P.; Sun, C.; He, W.; Wang, F. Amelioration of IFN-γ and TNF-α-induced intestinal epithelial barrier dysfunction by berberine via suppression of MLCK-MLC phosphorylation signaling pathway. PLoS ONE 2013, 8, e61944. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Blikslager, A.T. Myosin light chain kinase mediates intestinal barrier dysfunction via occludin endocytosis during anoxia/reoxygenation injury. Am. J. Physiol. Physiol. 2016, 311, 996–1004. [Google Scholar] [CrossRef]
- Watson, C.J.; Hoare, C.J.; Garrod, D.; Carlson, G.L.; Warhurst, G. Interferon-γ selectively increases epithelial permeability to large molecules by activating different populations of paracellular pores. J. Cell Sci. 2005, 118, 5221–5230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Itallie, C.M.; Fanning, A.S.; Holmes, J.; Anderson, J.M. Occludin is required for cytokine-induced regulation of tight junction barriers. J. Cell Sci. 2010, 123, 2844–2852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercado, J.; Valenzano, M.C.; Jeffers, C.; Sedlak, J.; Cugliari, M.K.; Papanikolaou, E.; Clouse, J.; Miao, J.; Wertan, N.E.; Mullin, J.M. Enhancement of tight junctional barrier function by micronutrients: Compound-specific effects on permeability and claudin composition. PLoS ONE 2013, 8, e78775. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, H.; Fujita, H.; Katoh, H.; Aoki, J.; Nakamura, K.; Ichikawa, A.; Negishi, M. Opposite regulation of transepithelial electrical resistance and paracellular permeability by Rho in madin-darby canine kidney cells. J. Biol. Chem. 1999, 274, 20982–20988. [Google Scholar] [CrossRef] [Green Version]
- Jou, T.-S.; Schneeberger, E.E.; Nelson, W.J. Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J. Cell Biol. 1998, 142, 101–115. [Google Scholar] [CrossRef] [Green Version]
- Janosevic, D.; Axis, J.; Bacallao, R.L.; Amsler, K. Occludin content modulates hydrogen peroxide-induced increase in renal epithelial paracellular permeability. J. Cell. Biochem. 2015, 117, 769–779. [Google Scholar] [CrossRef] [Green Version]
- Patrick, D.; Leone, A.K.; Shellenberger, J.J.; A Dudowicz, K.; King, J.M. Proinflammatory cytokines tumor necrosis factor-α and interferon-γ modulate epithelial barrier function in madin-darby canine kidney cells through mitogen activated protein kinase signaling. BMC Physiol. 2006, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Krug, S.; Amasheh, S.; Richter, J.F.; Milatz, S.; Günzel, D.; Westphal, J.; Huber, O.; Schulzke, J.D.; Fromm, M. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol. Biol. Cell 2009, 20, 3713–3724. [Google Scholar] [CrossRef] [Green Version]
- Krug, S.M. Contribution of the tricellular tight junction to paracellular permeability in leaky and tight epithelia. Ann. N. Y. Acad. Sci. 2017, 1397, 219–230. [Google Scholar] [CrossRef]
- Staehelin, L.A. Further observations on the fine structure of freeze-cleaved tight junctions. J. Cell Sci. 1973, 13, 763–786. [Google Scholar] [CrossRef]
- Raya-Sandino, A.; Castillo-Kauil, A.; Domínguez-Calderón, A.; Alarcón, L.; Flores-Benitez, D.; Cuellar-Perez, F.; López-Bayghen, B.; Chávez-Munguía, B.; Vazquez-Prado, J.; González-Mariscal, L. Zonula occludens-2 regulates Rho proteins activity and the development of epithelial cytoarchitecture and barrier function. BBA Bioenerg. 2017, 1864, 1714–1733. [Google Scholar] [CrossRef] [PubMed]
- Tervonen, A.; Ihalainen, T.O.; Nymark, S.; Hyttinen, J. Structural dynamics of tight junctions modulate the properties of the epithelial barrier. PLoS ONE 2019, 14, e0214876. [Google Scholar] [CrossRef]
- Sasaki, H.; Matsui, C.; Furuse, K.; Mimori-Kiyosue, Y.; Furuse, M.; Tsukita, S. Dynamic behavior of paired claudin strands within apposing plasma membranes. Proc. Natl. Acad. Sci. USA 2003, 100, 3971–3976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Itallie, C.M.; Lidman, K.F.; Tietgens, A.J.; Anderson, J.M. Newly synthesized claudins but not occludin are added to the basal side of the tight junction. Mol. Biol. Cell 2019, 30, 1406–1424. [Google Scholar] [CrossRef] [PubMed]
- Zeissig, S.; Bürgel, N.; Günzel, D.; Richter, J.; Mankertz, J.; Wahnschaffe, U.; Kroesen, A.J.; Zeitz, M.; Fromm, M.; Schulzke, J.D. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 2007, 56, 61–72. [Google Scholar] [CrossRef]
- Schulzke, J.D.; Ploeger, S.; Amasheh, M.; Fromm, A.; Zeissig, S.; Troeger, H.; Richter, J.; Bojarski, C.; Schumann, M.; Fromm, M. Epithelial tight junctions in intestinal inflammation. Ann. N. Y. Acad. Sci. 2009, 1165, 294–300. [Google Scholar] [CrossRef]
- Anderson, J.M.; Van Itallie, C.M.; Fanning, A.S. Setting up a selective barrier at the apical junction complex. Curr. Opin. Cell Biol. 2004, 16, 140–145. [Google Scholar] [CrossRef]
- Belardi, B.; Hamkins-Indik, T.; Harris, A.R.; Kim, J.; Xu, K.; Fletcher, D.A. A weak link with actin organizes tight junctions to control epithelial permeability. Dev. Cell 2020, 54, 792–804.e7. [Google Scholar] [CrossRef]
- Schlingmann, B.; Molina, S.A.; Koval, M. Claudins: Gatekeepers of lung epithelial function. Semin. Cell Dev. Biol. 2015, 42, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Oshima, T.; Miwa, H. Gastrointestinal mucosal barrier function and diseases. J. Gastroenterol. 2016, 51, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Meoli, L.; Günzel, D. Channel functions of claudins in the organization of biological systems. BBA Biomembr. 2020, 1862, 183344. [Google Scholar] [CrossRef]
- Günzel, D.; Yu, A. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Souza, T.; Agarwal, R.; Morin, P.J. Phosphorylation of claudin-3 at threonine 192 by cAMP-dependent protein kinase regulates tight junction barrier function in ovarian cancer cells. J. Biol. Chem. 2005, 280, 26233–26240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomme, J.; Antanasijevic, A.; Caffrey, M.; Van Itallie, C.M.; Anderson, J.M.; Fanning, A.S.; Lavie, A. Structural basis of a key factor regulating the affinity between the zonula occludens first PDZ domain and claudins. J. Biol. Chem. 2015, 290, 16595–16606. [Google Scholar] [CrossRef] [Green Version]
- Twiss, F.; Oldenkamp, M.; Hiemstra, A.; Zhou, H.; Matheron, L.; Mohammed, S.; De Rooij, J. HGF signaling regulates claudin-3 dynamics through its C-terminal tyrosine residues. Tissue Barriers 2013, 1, e27425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulzke, J.; Gitter, A.; Mankertz, J.; Spiegel, S.; Seidler, U.; Amasheh, S.; Saitou, M.; Tsukita, S.; Fromm, M. Epithelial transport and barrier function in occludin-deficient mice. BBA Biomembr. 2005, 1669, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Saitou, M.; Fujimoto, K.; Doi, Y.; Itoh, M.; Fujimoto, T.; Furuse, M.; Takano, H.; Noda, T.; Tsukita, S. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J. Cell Biol. 1998, 141, 397–408. [Google Scholar] [CrossRef]
- Caswell, D.; Jaggi, S.; Axis, J.; Amsler, K. Src family kinases regulate renal epithelial paracellular permeability barrier through an occludin-independent mechanism. J. Cell. Physiol. 2013, 228, 1210–1220. [Google Scholar] [CrossRef]
- Yu, A.; McCarthy, K.M.; Francis, S.A.; McCormack, J.M.; Lai, J.; Rogers, R.A.; Lynch, R.D.; Schneeberger, E.E. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am. J. Physiol. Physiol. 2005, 288, 1231–1241. [Google Scholar] [CrossRef] [Green Version]
- Bilal, S.; Jaggi, S.; Janosevic, D.; Shah, N.; Teymour, S.; Voronina, A.; Watari, J.; Axis, J.; Amsler, K. ZO-1 protein is required for hydrogen peroxide to increase MDCK cell paracellular permeability in an ERK 1/2-dependent manner. Am. J. Physiol. Physiol. 2018, 315, 422–431. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Khatib, K.; Guo, S.; Ye, D.; Youssef, M.; Ma, T. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am. J. Physiol. Liver Physiol. 2011, 300, 1054–1064. [Google Scholar] [CrossRef] [Green Version]
- Richter, J.F.; Hildner, M.; Schmauder, R.; Turner, J.R.; Schumann, M.; Reiche, J. Occludin knockdown is not sufficient to induce transepithelial macromolecule passage. Tissue Barriers 2019, 7, 1612661. [Google Scholar] [CrossRef] [Green Version]
- Balda, M.; A Whitney, J.; Flores, C.; González, S.; Cereijido, M.; Matter, K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J. Cell Biol. 1996, 134, 1031–1049. [Google Scholar] [CrossRef]
- McCarthy, K.; Skare, I.; Stankewich, M.; Furuse, M.; Tsukita, S.; Rogers, R.; Lynch, R.; Schneeberger, E. Occludin is a functional component of the tight junction. J. Cell Sci. 1996, 109, 2287–2298. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Qiu, W.-Y.; Huo, Y.-N.; Yao, Y.-F.; Lou, M.F. Low levels of hydrogen peroxide stimulate corneal epithelial cell Adhesion, migration, and wound healing. Investig. Opthalmol. Vis. Sci. 2011, 52, 1723–1734. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.-R.; Wang, Q.; Xu, X.-F.; Zhang, Z.; Lu, Y.-B.; Shen, G.; Wu, M. Phospholipase D participates in H2O2-induced A549 alveolar epithelial cell migration. Exp. Lung Res. 2012, 38, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Shibata, A.; Tanabe, E.; Inoue, S.; Kitayoshi, M.; Okimoto, S.; Hirane, M.; Araki, M.; Fukushima, N.; Tsujiuchi, T. Hydrogen peroxide stimulates cell motile activity through LPA receptor-3 in liver epithelial WB-F344 cells. Biochem. Biophys. Res. Commun. 2013, 433, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Basuroy, S.; Dunagan, M.; Sheth, P.; Seth, A.; Rao, R.K. Hydrogen peroxide activates focal adhesion kinase and c-Src by a phosphatidylinositol 3 kinase-dependent mechanism and promotes cell migration in Caco-2 cell monolayers. Am. J. Physiol. Liver Physiol. 2010, 299, 186–195. [Google Scholar] [CrossRef] [Green Version]
- Marchiando, A.M.; Shen, L.; Graham, W.; Weber, C.; Schwarz, B.T.; Austin, J.R.; Raleigh, D.R.; Guan, Y.; Watson, A.J.; Montrose, M.H.; et al. Caveolin-1–dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J. Cell Biol. 2010, 189, 111–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manda, B.; Mir, H.; Gangwar, R.; Meena, A.S.; Amin, S.; Shukla, P.K.; Dalal, K.; Suzuki, T.; Rao, R.K. Phosphorylation hotspot in the C-terminal domain of occludin regulates the dynamics of epithelial junctional complexes. J. Cell Sci. 2018, 131, 206789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Song, D.; Song, Y.; Wang, X. Interferon gamma induces inflammatory responses through the interaction of CEACAM1 and PI3K in airway epithelial cells. J. Transl. Med. 2019, 17, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, Q.; Vassilieva, E.V.; Ivanov, A.I.; Wang, Z.; Brown, G.T.; Parkos, C.A.; Nusrat, A. Interferon-γ inhibits T84 epithelial cell migration by redirecting transcytosis of β1 integrin from the migrating leading edge. J. Immunol. 2005, 175, 4030–4038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balda, M.S.; Flores-Maldonado, C.; Cereijido, M.; Matter, K. Multiple domains of occludin are involved in regulation of paracellular permeability. J. Cell. Biochem. 2000, 78, 85–96. [Google Scholar] [CrossRef]
- Wong, V.; Gumbiner, B.M. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight Junction permeability barrier. J. Cell Biol. 1997, 136, 399–409. [Google Scholar] [CrossRef]
- Rao, R. Occludin phosphorylation in regulation of epithelial tight junctions. Ann. N. Y. Acad. Sci. 2009, 1165, 62–68. [Google Scholar] [CrossRef]
- Cummins, P.M. Occludin: One protein, many forms. Mol. Cell. Biol. 2012, 32, 242–250. [Google Scholar] [CrossRef] [Green Version]
- Reiche, J.; Huber, O. Post-translational modifications of tight junction transmembrane proteins and their direct effect on barrier function. BBA Biomembr. 2020, 1862, 183330. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Lu, Q.; Goodenough, D.A.; Jeansonne, B. Nonreceptor tyrosine kinase c-yes interacts with occludin during tight junction formation in canine kidney epithelial cells. Mol. Biol. Cell 2002, 13, 1227–1237. [Google Scholar] [CrossRef] [Green Version]
- Basuroy, S.; Sheth, P.; Kuppuswamy, D.; Balasubramanian, S.; Ray, R.M.; Rao, R.K. Expression of kinase-inactive c-Src delays oxidative stress-induced disassembly and accelerates calcium-mediated reassembly of tight junctions in the Caco-2 cell monolayer. J. Biol. Chem. 2003, 278, 11916–11924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elias, B.C.; Suzuki, T.; Seth, A.; Giorgianni, F.; Kale, G.; Shen, L.; Turner, J.R.; Naren, A.; Desiderio, D.M.; Rao, R. Phosphorylation of Tyr-398 and Tyr-402 in occludin prevents its interaction with ZO-1 and destabilizes its assembly at the tight junctions. J. Biol. Chem. 2009, 284, 1559–1569. [Google Scholar] [CrossRef] [Green Version]
- Olivera, D.; Knall, C.; Boggs, S.; Seagrave, J. Cytoskeletal modulation and tyrosine phosphorylation of tight junction proteins are associated with mainstream cigarette smoke-induced permeability of airway epithelium. Exp. Toxicol. Pathol. 2010, 62, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Samak, G.; Chaudhry, K.; Gangwar, R.; Narayanan, D.; Jaggar, J.H.; Rao, R. Calcium/Ask1/MKK7/JNK2/c-Src signalling cascade mediates disruption of intestinal epithelial tight junctions by dextran sulfate sodium. Biochem. J. 2015, 465, 503–515. [Google Scholar] [CrossRef]
- Suzuki, T.; Elias, B.C.; Seth, A.; Shen, L.; Turner, J.R.; Giorgianni, F.; Desiderio, D.; Guntaka, R.; Rao, R. PKC regulates occludin phosphorylation and epithelial tight junction integrity. Proc. Natl. Acad. Sci. USA 2009, 106, 61–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, S.; Suzuki, T.; Seth, A.; Samak, G.; Rao, R. Protein kinase Cζ phosphorylates occludin and promotes assembly of epithelial tight junctions. Biochem. J. 2011, 437, 289–299. [Google Scholar] [CrossRef] [Green Version]
- Kalsi, K.K.; Garnett, J.P.; Patkee, W.; Weekes, A.; Dockrell, M.E.; Baker, E.H.; Baines, D.L. Metformin attenuates the effect of Staphylococcus aureus on airway tight junctions by increasing PKCζ-mediated phosphorylation of occludin. J. Cell. Mol. Med. 2019, 23, 317–327. [Google Scholar] [CrossRef] [Green Version]
- Mandell, K.J.; Babbin, B.A.; Nusrat, A.; Parkos, C.A. Junctional adhesion molecule 1 regulates epithelial cell morphology through effects on β1 integrins and Rap1 activity. J. Biol. Chem. 2005, 280, 11665–11674. [Google Scholar] [CrossRef] [Green Version]
- Laukoetter, M.G.; Nava, P.; Lee, W.Y.; Severson, E.A.; Capaldo, C.T.; Babbin, B.A.; Williams, I.R.; Koval, M.; Peatman, E.; Campbell, J.A.; et al. JAM-A regulates permeability and inflammation in the intestine in vivo. J. Exp. Med. 2007, 204, 3067–3076. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.C.C.; Sumagin, R.; Rankin, C.R.; Leoni, G.; Mina, M.; Reiter, D.M.; Stehle, T.; Dermody, T.S.; Schaefer, S.A.; Hall, R.; et al. JAM-A associates with ZO-2, afadin, and PDZ-GEF1 to activate Rap2c and regulate epithelial barrier function. Mol. Biol. Cell 2013, 24, 2849–2860. [Google Scholar] [CrossRef]
- Fanning, A.S.; Van Itallie, C.M.; Anderson, J.M. Zonula occludens-1 and -2 regulate apical cell structure and the zonula adherens cytoskeleton in polarized epithelia. Mol. Biol. Cell 2012, 23, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fanning, A.S.; Anderson, J.M.; Lavie, A. Structure of the conserved cytoplasmic C-terminal domain of occludin: Identification of the ZO-1 binding surface. J. Mol. Biol. 2005, 352, 151–164. [Google Scholar] [CrossRef]
- Tokuda, S.; Higashi, T.; Furuse, M. ZO-1 knockout by talen-mediated gene targeting in MDCK cells: Involvement of ZO-1 in the regulation of cytoskeleton and cell shape. PLoS ONE 2014, 9, e104994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, S.; Munguia, B.C.; Gonzalez-Mariscal, L. ZO-2 silencing in epithelial cells perturbs the gate and fence function of tight junctions and leads to an atypical monolayer architecture. Exp. Cell Res. 2007, 313, 1533–1547. [Google Scholar] [CrossRef]
- Rodgers, L.S.; Beam, M.T.; Anderson, J.M.; Fanning, A.S. Epithelial barrier assembly requires coordinated activity of multiple domains of the tight junction protein ZO-1. J. Cell Sci. 2013, 126, 1565–1575. [Google Scholar] [CrossRef] [Green Version]
- Spadaro, D.; LE, S.; Laroche, T.; Mean, I.; Jond, L.; Yan, J.; Citi, S. Tension-dependent stretching activates ZO-1 to control the junctional localization of its interactors. Curr. Biol. 2017, 27, 3783–3795.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odenwald, M.A.; Choi, W.; Kuo, W.-T.; Singh, G.; Sailer, A.; Wang, Y.; Shen, L.; Fanning, A.S.; Turner, J.R. The scaffolding protein ZO-1 coordinates actomyosin and epithelial apical specializations in vitro and in vivo. J. Biol. Chem. 2018, 293, 17317–17335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodgers, L.S.; Fanning, A.S. Regulation of epithelial permeability by the actin cytoskeleton. Cytoskeleton 2011, 68, 653–660. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, K.E.; Turner, J.R. Myosin light chain kinase: Pulling the strings of epithelial tight junction function. Ann. N. Y. Acad. Sci. 2012, 1258, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Quiros, M.; Nusrat, A. RhoGTPases, actomyosin signaling and regulation of the epithelial apical junctional complex. Semin. Cell Dev. Biol. 2014, 36, 194–203. [Google Scholar] [CrossRef] [Green Version]
- He, W.-Q.; Wang, J.; Sheng, J.-Y.; Zha, J.-M.; Graham, W.V.; Turner, J.R. Contributions of myosin light chain kinase to regulation of epithelial paracellular permeability and mucosal homeostasis. Int. J. Mol. Sci. 2020, 21, 993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, J.R.; Rill, B.K.; Carlson, S.L.; Carnes, D.; Kerner, R.; Mrsny, R.J.; Madara, J.L. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am. J. Physiol. Content 1997, 273, 1378–1385. [Google Scholar] [CrossRef]
- Graham, W.V.; He, W.; Marchiando, A.M.; Zha, J.; Singh, G.; Li, H.-S.; Biswas, A.; Ong, M.L.D.M.; Jiang, Z.-H.; Choi, W.; et al. Intracellular MLCK1 diversion reverses barrier loss to restore mucosal homeostasis. Nat. Med. 2019, 25, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xiong, X.; Zhao, X.; Yang, X.; Wang, H. F-BAR family proteins, emerging regulators for cell membrane dynamic changes—from structure to human diseases. J. Hematol. Oncol. 2015, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Arnold, T.; Stephenson, R.E.; Miller, A.L. Rho GTPases and actomyosin: Partners in regulating epithelial cell-cell junction structure and function. Exp. Cell Res. 2017, 358, 20–30. [Google Scholar] [CrossRef]
- Kotelevets, L.; Chastre, E. Rac1 signaling: From intestinal homeostasis to colorectal cancer metastasis. Cancers 2020, 12, 665. [Google Scholar] [CrossRef] [Green Version]
- Salloum, G.; Jaafar, L.; El-Sibai, M. Rho A and Rac1: Antagonists moving forward. Tissue Cell 2020, 65, 101364. [Google Scholar] [CrossRef]
- Rusu, A.D.; Georgiou, M. The multifarious regulation of the apical junctional complex. Open Biol. 2020, 10, 190278. [Google Scholar] [CrossRef]
- Jin, Y.; Blikslager, A.T. The regulation of intestinal mucosal barrier by myosin light chain kinase/Rho kinases. Int. J. Mol. Sci. 2020, 21, 3550. [Google Scholar] [CrossRef] [PubMed]
- Bruewer, M.; Hopkins, A.M.; Hobert, M.E.; Nusrat, A.; Madara, J.L. RhoA, Rac1, and Cdc42 exert distinct effects on epithelial barrier via selective structural and biochemical modulation of junctional proteins and F-actin. Am. J. Physiol. Physiol. 2004, 287, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, A.; Walsh, S.V.; Verkade, P.; Boquet, P.; Nusrat, A. Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function. J. Cell Sci. 2003, 116, 725–742. [Google Scholar] [CrossRef] [Green Version]
- Terry, S.J.; Zihni, C.; Elbediwy, A.; Vitiello, E.; San, I.V.L.C.; Balda, M.S.; Matter, K. Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis. Nat. Cell Biol. 2011, 13, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Stephenson, R.E.; Higashi, T.; Erofeev, I.S.; Arnold, T.; Leda, M.; Goryachev, A.; Miller, A.L. Rho flares repair local tight junction leaks. Dev. Cell 2019, 48, 445–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acharya, B.; Nestor-Bergmann, A.; Liang, X.; Gupta, S.; Duszyc, K.; Gauquelin, E.; Gomez, G.; Budnar, S.; Marcq, P.; Jensen, O.; et al. A mechanosensitive RhoA pathway that protects epithelia against acute tensile stress. Dev. Cell 2018, 47, 439–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabath, E.; Negoro, H.; Beaudry, S.; Paniagua, M.; Angelow, S.; Shah, J.; Grammatikakis, N.; Yu, A.; Denker, B.M. Gα12 regulates protein interactions within the MDCK cell tight junction and inhibits tight-junction assembly. J. Cell Sci. 2008, 121, 814–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acharya, B.; Wu, S.; Lieu, Z.Z.; Parton, R.; Grill, S.W.; Bershadsky, A.D.; Gomez, G.; Yap, A.S. Mammalian diaphanous 1 mediates a pathway for E-cadherin to stabilize epithelial barriers through junctional contractility. Cell Rep. 2017, 18, 2854–2867. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, S.; Wada, Y.; Watanabe, T.; Nagafuchi, A.; Shibata, M. α-Catenin as a tension transducer that induces adherens junction development. Nat. Cell Biol. 2010, 12, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, K.J.; Cohen, T.S.; Margulies, S.S. Stretch increases alveolar epithelial permeability to uncharged micromolecules. Am. J. Physiol. Physiol. 2006, 290, 1179–1188. [Google Scholar] [CrossRef] [Green Version]
- Cohen, T.S.; Cavanaugh, K.J.; Margulies, S.S. Frequency and peak stretch magnitude affect alveolar epithelial permeability. Eur. Respir. J. 2008, 32, 854–861. [Google Scholar] [CrossRef] [Green Version]
- Cohen, T.S.; Lawrence, G.G.; Khasgiwala, A.; Margulies, S.S. MAPk activation modulates permeability of isolated rat alveolar epithelial cell monolayers following cyclic stretch. PLoS ONE 2010, 5, e10385. [Google Scholar] [CrossRef] [Green Version]
- DiPaolo, B.C.; Margulies, S.S. Rho kinase signaling pathways during stretch in primary alveolar epithelia. Am. J. Physiol. Cell. Mol. Physiol. 2012, 302, 992–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samak, G.; Gangwar, R.; Crosby, L.M.; Desai, L.P.; Wilhelm, K.; Waters, C.M.; Rao, R. Cyclic stretch disrupts apical junctional complexes in Caco-2 cell monolayers by a JNK-2-, c-Src-, and MLCK-dependent mechanism. Am. J. Physiol. Liver Physiol. 2014, 306, 947–958. [Google Scholar] [CrossRef] [Green Version]
- Samak, G.; Suzuki, T.; Bhargava, A.; Rao, R.K. c-Jun NH2-terminal kinase-2 mediates osmotic stress-induced tight junction disruption in the intestinal epithelium. Am. J. Physiol. Liver Physiol. 2010, 299, 572–584. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo, I.; Condorelli, L.; Terrinoni, A.R.; Antiga, L.; Sangalli, F.; Remuzzi, A. Shear stress reverses dome formation in confluent renal tubular cells. Cell. Physiol. Biochem. 2011, 28, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Acharya, B.; Peyret, G.; Fardin, M.-A.; Mège, R.-M.; Ladoux, B.; Yap, A.; Fanning, A.S.; Peifer, M. Remodeling the zonula adherens in response to tension and the role of afadin in this response. J. Cell Biol. 2016, 213, 243–260. [Google Scholar] [CrossRef]
- Charras, G.; Yap, A.S. Tensile forces and mechanotransduction at cell–cell junctions. Curr. Biol. 2018, 28, 445–457. [Google Scholar] [CrossRef] [Green Version]
- Haas, A.; Zihni, C.; Ruppel, A.; Hartmann, C.; Ebnet, K.; Tada, M.; Balda, M.S.; Matter, K. Interplay between extracellular matrix stiffness and JAM-A regulates mechanical load on ZO-1 and tight junction assembly. Cell Rep. 2020, 32, 107924. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Miller, A.L. Tricellular junctions: How to build junctions at the TRICkiest points of epithelial cells. Mol. Biol. Cell 2017, 28, 2023–2034. [Google Scholar] [CrossRef]
- Richter, J.F.; Schmauder, R.; Krug, S.; Gebert, A.; Schumann, M. A novel method for imaging sites of paracellular passage of macromolecules in epithelial sheets. J. Control. Release 2016, 229, 70–79. [Google Scholar] [CrossRef]
- Ikenouchi, J.; Sasaki, H.; Tsukita, S.; Furuse, M.; Tsukita, S. Loss of occludin affects tricellular localization of tricellulin. Mol. Biol. Cell 2008, 19, 4687–4693. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monaco, A.; Ovryn, B.; Axis, J.; Amsler, K. The Epithelial Cell Leak Pathway. Int. J. Mol. Sci. 2021, 22, 7677. https://doi.org/10.3390/ijms22147677
Monaco A, Ovryn B, Axis J, Amsler K. The Epithelial Cell Leak Pathway. International Journal of Molecular Sciences. 2021; 22(14):7677. https://doi.org/10.3390/ijms22147677
Chicago/Turabian StyleMonaco, Ashley, Ben Ovryn, Josephine Axis, and Kurt Amsler. 2021. "The Epithelial Cell Leak Pathway" International Journal of Molecular Sciences 22, no. 14: 7677. https://doi.org/10.3390/ijms22147677
APA StyleMonaco, A., Ovryn, B., Axis, J., & Amsler, K. (2021). The Epithelial Cell Leak Pathway. International Journal of Molecular Sciences, 22(14), 7677. https://doi.org/10.3390/ijms22147677




