Small Rab GTPases in Intracellular Vesicle Trafficking: The Case of Rab3A/Raphillin-3A Complex in the Kidney
Abstract
1. Introduction
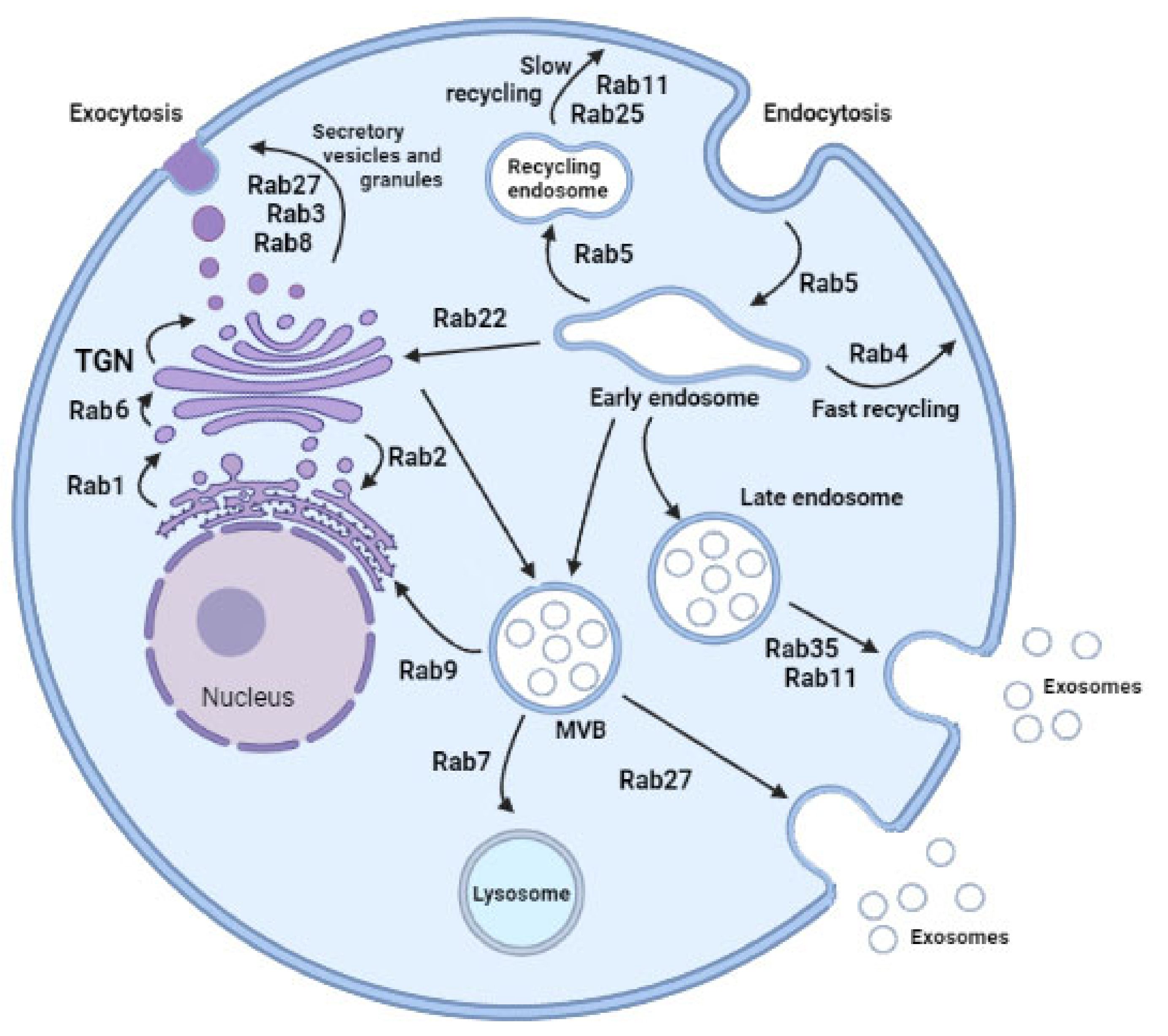
2. Rab GTPases and Vesicle Trafficking
3. Rab-Mediated Vesicular Traffic and Diseases
| Rab Protein | Primordial Function | Diseases Related |
|---|---|---|
| ENDOSOME RECYCLING | ||
| Rab11 | Slow transport endolysosomal vesicles from perinuclear recycling endosome compartment toward plasma membrane Three members: Rab11a, widely distributed; Rab11b, Rab 11 (Rab 25) restricted tissue expression pattern | Facilitate spread of colon cancer cells [33] |
| Rab35 | Slow transport endolysosomal vesicles from perinuclear recycling endosome compartment toward plasma membrane Role not well established | |
| Rab4 | Fast recycling after endocytosis | |
| Rab9 | Transport to the Golgi network Autophagosome recognition and engulfing of damaged mitochondria. | Mitochondrial fission in cardiac myocytes. Eventually decreased cardiomyocyte viability [37] |
| Rab5A | Involved in exosome secretion regulatory pathways Fast delivery of cargo to the plasma membrane Overexpression inhibits progression of endocytosed material from early endosomes | |
| ENDOSOME MATURATION | ||
| Rab5ARab7 | Interacts with 37 genes involved in exosome secretion regulatory pathways Maturation of late endosomes and their fusion with lysosomes Sorting and degradation Release Rab5 | |
| VESICLE SECRETION | ||
| Rab27 | Secretory protein Transport of late endosomal/lysosome-like compartments to the plasma membrane. Regulate exocytic events in a sequential manner together with Rab3 and Rab11 | The first found to be involved in human disease. Important role in cancer progression and metastasis. High expression was associated with poor survival, lymph node, and distant metastasis [43] Increased in serum of diabetic patients [44] |
| Rab3 | Secretory protein Regulate exocytic events | Renal dysfunction * Cardiac dysfunction * |
| Rab22A | Endosomal associated protein in different cell linesEctosome formation in other models |
| Rab Protein | Primordial Function | Experimental Model | Diseases Related |
|---|---|---|---|
| Multiple | Transport endolysosomal vesicles | Multiple | |
| Rab5 | Endocytosis Endocytic traffic of nephrin | Cultured podocyte Drosophila nephrocyte | SRNS with FGS by mutation in GAPVD1 and ANKFY1 genes [45] |
| Rab7 | Protein degradation | Drosophila nephrocyte | |
| Rab11 | Endocytic recycling | Drosophila nephrocyte | |
| Rab11b | Architectural structure Endocytic recycling Migration | Cultured podocyte Cultured fibroblast Drosophila nephrocyte | SRNS with FGS by mutation in TBC1D8B gene [46] |
| Rab3A | Architectural structure | Cultured podocyte Drosophila nephrocyte | Proteinuria [47,48] |
| Rab38 | Endocytosis of albumin | Transgenic rats Cultured cells proximal tubule LLC-PK1) | Fawn-hooded hypertensive rat [49] Proteinuria [49] |
| Rab7 | Maturation of late endosomes and their fusion with lysosomes Reduce activation of MMP-2 Aquaporine2 sorting in collecting duct cells | Cultured resident fibroblasts and tubular cells Collecting duct mpkCCD cells | Endothelial mesenchymal transition in diabetic nephropathy [50] |
| Rab27a | Cell polarization | Madin-Darby canine kidney II cells | Reduction of tight junction protein in tubular cells [51] |
| Rab27a | Reduce exosome release | Cultured podocyte Cultured renal tubular epithelial cells | Reduce inflammation of diabetic renal disease through the miR-26a-5p/CHAC1/NF-kB pathway [52] |
3.1. Rab-Mediated Actions in Health and Disease in the Kidney
3.1.1. Podocytes
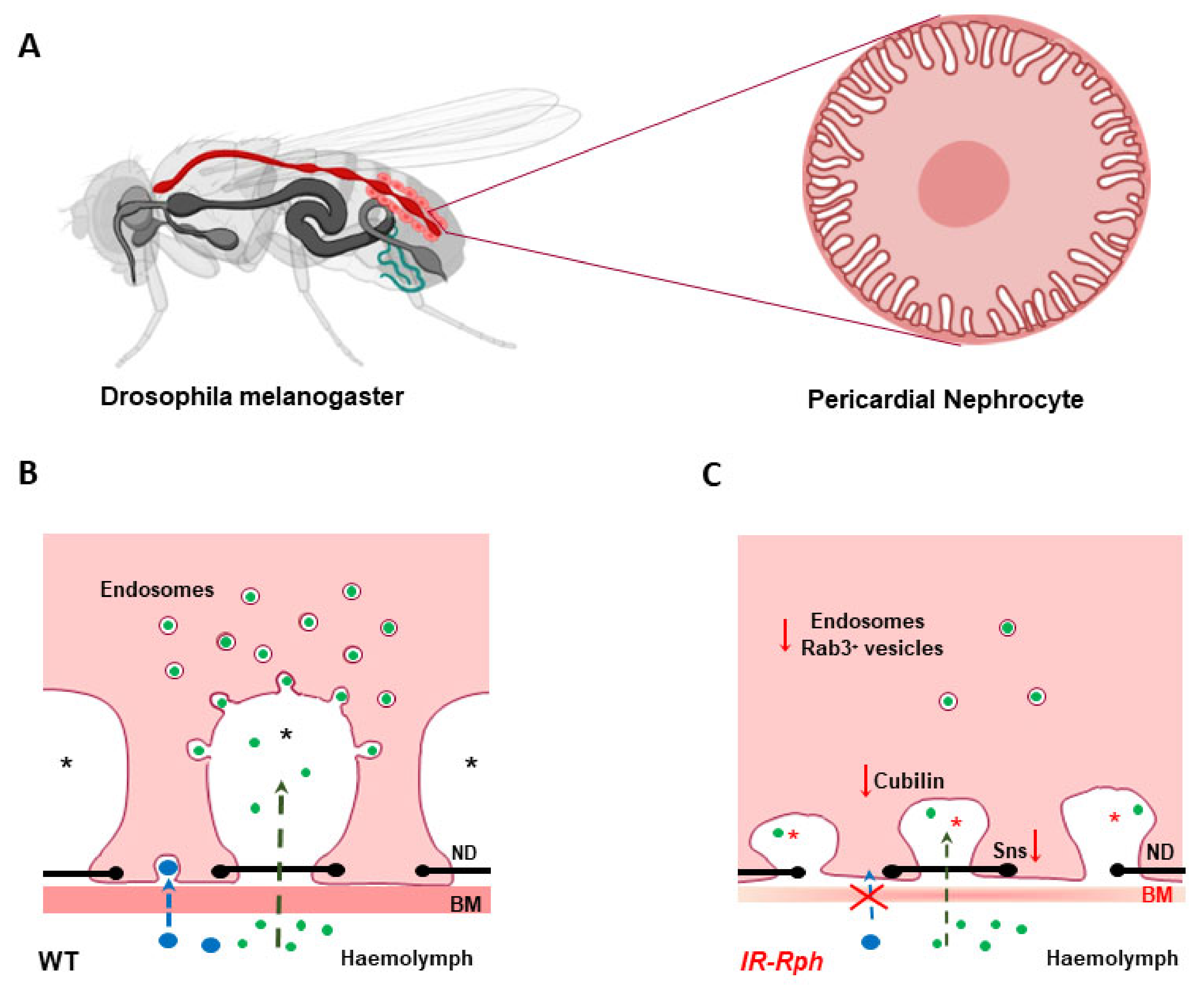
3.1.2. Renal Tubules
3.1.3. Exosome Release
3.2. Rab-3A/Rabphilin-3A Complex in Kidney Disease
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Homma, Y.; Hiragi, S.; Fukuda, M. Rab family of small GTPases: An updated view on their regulation and functions. FEBS J. 2021, 288, 36–55. [Google Scholar] [CrossRef]
- Aheget, H.; Mazini, L.; Martin, F.; Belqat, B.; Marchal, J.A.; Benabdellah, K. Exosomes: Their Role in Pathogenesis, Diagnosis and Treatment of Diseases. Cancers 2020, 13, 84. [Google Scholar] [CrossRef]
- Dreyer, F.; Baur, A. Biogenesis and Functions of Exosomes and Extracellular Vesicles. Methods Mol. Biol. 2016, 1448, 201–216. [Google Scholar] [CrossRef]
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: Composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 2019, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Tan, J.; Miao, Y.Y.; Zhang, Q. Extracellular vesicles degradation pathway based autophagy lysosome pathway. Am. J. Transl. Res. 2019, 11, 1170–1183. [Google Scholar] [PubMed]
- Banworth, M.J.; Li, G. Consequences of Rab GTPase dysfunction in genetic or acquired human diseases. Small GTPases 2018, 9, 158–181. [Google Scholar] [CrossRef] [PubMed]
- Margiotta, A.; Bucci, C. Coordination between Rac1 and Rab Proteins: Functional Implications in Health and Disease. Cells 2019, 8, 396. [Google Scholar] [CrossRef]
- Stenmark, H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009, 10, 513–525. [Google Scholar] [CrossRef]
- Pfeffer, S.R. Rab GTPase regulation of membrane identity. Curr. Opin. Cell Biol. 2013, 25, 414–419. [Google Scholar] [CrossRef]
- Zerial, M.; McBride, H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2001, 2, 107–117. [Google Scholar] [CrossRef]
- Fukuda, M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell. Mol. Life Sci. 2008, 65, 2801–2813. [Google Scholar] [CrossRef]
- Saraste, J.; Lahtinen, U.; Goud, B. Localization of the small GTP-binding protein rab1p to early compartments of the secretory pathway. J. Cell Sci. 1995, 108, 1541–1552. [Google Scholar] [CrossRef]
- Quevedo, M.F.; Bustos, M.A.; Masone, D.; Roggero, C.M.; Bustos, D.M.; Tomes, C.N. Grab recruitment by Rab27A-Rabphilin3a triggers Rab3A activation in human sperm exocytosis. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Savina, A.; Vidal, M.; Colombo, M.I. The exosome pathway in K562 cells is regulated by Rab11. J. Cell Sci. 2002, 115, 2505–2515. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Morohashi, Y.; Yoshimura, S.; Manrique-Hoyos, N.; Jung, S.; Lauterbach, M.A.; Bakhti, M.; Grønborg, M.; Möbius, W.; Rhee, J.; et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J. Cell Biol. 2010, 189, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Wandinger-Ness, A.; Zerial, M. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb. Perspect. Biol. 2014, 6, a022616. [Google Scholar] [CrossRef]
- Duhamel, S.; Zaoui, K. Real-time Three-dimensional Tracking of Endocytic Vesicles. Bio-Protocol 2020, 10, e3794. [Google Scholar] [CrossRef] [PubMed]
- Olkkonen, V.M.; Ikonen, E. Genetic defects of intracellular-membrane transport. N. Engl. J. Med. 2000, 343, 1095–1104. [Google Scholar] [CrossRef]
- Hutagalung, A.H.; Novick, P.J. Role of Rab GTPases in membrane traffic and cell physiology. Physiol. Rev. 2011, 91, 119–149. [Google Scholar] [CrossRef]
- Somasundaram, A.; Taraska, J.W. Local protein dynamics during microvesicle exocytosis in neuroendocrine cells. Mol. Biol. Cell 2018, 29, 1891–1903. [Google Scholar] [CrossRef]
- Kiral, F.R.; Kohrs, F.E.; Jin, E.J.; Hiesinger, P.R. Rab GTPases and Membrane Trafficking in Neurodegeneration. Curr. Biol. 2018, 28, R471–R486. [Google Scholar] [CrossRef]
- Cherry, S.; Jin, E.J.; Ozel, M.N.; Lu, Z.; Agi, E.; Wang, D.; Jung, W.H.; Epstein, D.; Meinertzhagen, I.A.; Chan, C.C.; et al. Charcot-Marie-Tooth 2B mutations in rab7 cause dosage-dependent neurodegeneration due to partial loss of function. Elife 2013, 2, e01064. [Google Scholar] [CrossRef]
- Veleri, S.; Punnakkal, P.; Dunbar, G.L.; Maiti, P. Molecular Insights into the Roles of Rab Proteins in Intracellular Dynamics and Neurodegenerative Diseases. Neuromol. Med. 2018, 20, 18–36. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, S.; Gerondopoulos, A.; Linford, A.; Rigden, D.J.; Barr, F.A. Family-wide characterization of the DENN domain Rab GDP-GTP exchange factors. J. Cell Biol. 2010, 191, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.C.; Peden, A.A.; Buss, F.; Bright, N.A.; Latouche, M.; Reilly, M.M.; Kendrick-Jones, J.; Luzio, J.P. Mistargeting of SH3TC2 away from the recycling endosome causes Charcot-Marie-Tooth disease type 4C. Hum. Mol. Genet. 2010, 19, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, C.; Liu, W.; Wang, P.; Zhang, X. A novel RAB7 mutation in a Chinese family with Charcot-Marie-Tooth type 2B disease. Gene 2014, 534, 431–434. [Google Scholar] [CrossRef]
- Wilson, G.R.; Sim, J.C.; McLean, C.; Giannandrea, M.; Galea, C.A.; Riseley, J.R.; Stephenson, S.E.; Fitzpatrick, E.; Haas, S.A.; Pope, K.; et al. Mutations in RAB39B cause X-linked intellectual disability and early-onset Parkinson disease with α-synuclein pathology. Am. J. Hum. Genet. 2014, 95, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Muqit, M.M.K. Parkinson’s: A Disease of Aberrant Vesicle Trafficking. Annu. Rev. Cell Dev. Biol. 2020, 36, 237–264. [Google Scholar] [CrossRef] [PubMed]
- Breda, C.; Nugent, M.L.; Estranero, J.G.; Kyriacou, C.P.; Outeiro, T.F.; Steinert, J.R.; Giorgini, F. Rab11 modulates α-synuclein-mediated defects in synaptic transmission and behaviour. Hum. Mol. Genet. 2015, 24, 1077–1091. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Yussman, M.G.; Barrett, T.J.; Hahn, H.S.; Osinska, H.; Hilliard, G.M.; Wang, X.; Toyokawa, T.; Yatani, A.; Lynch, R.A.; et al. Increased myocardial Rab GTPase expression: A consequence and cause of cardiomyopathy. Circ. Res. 2001, 89, 1130–1137. [Google Scholar] [CrossRef]
- Dhanabalan, K.; Huisamen, B.; Lochner, A. Mitochondrial oxidative phosphorylation and mitophagy in myocardial ischaemia/reperfusion: Effects of chloroquine. Cardiovasc. J. Afr. 2020, 31, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Nah, J.; Oka, S.I.; Mukai, R.; Monden, Y.; Maejima, Y.; Ikeda, Y.; Sciarretta, S.; Liu, T.; Li, H.; et al. An alternative mitophagy pathway mediated by Rab9 protects the heart against ischemia. J. Clin. Investig. 2019, 129, 802–819. [Google Scholar] [CrossRef]
- Huang, C.Y.; Kuo, W.W.; Ho, T.J.; Chiang, S.F.; Pai, P.Y.; Lin, J.Y.; Lin, D.Y.; Kuo, C.H.; Huang, C.Y. Rab9-dependent autophagy is required for the IGF-IIR triggering mitophagy to eliminate damaged mitochondria. J. Cell. Physiol. 2018, 233, 7080–7091. [Google Scholar] [CrossRef]
- Teumer, A.; Tin, A.; Sorice, R.; Gorski, M.; Yeo, N.C.; Chu, A.Y.; Li, M.; Li, Y.; Mijatovic, V.; Ko, Y.A.; et al. Genome-wide Association Studies Identify Genetic Loci Associated With Albuminuria in Diabetes. Diabetes 2016, 65, 803–817. [Google Scholar] [CrossRef]
- Walsh, T.G.; Li, Y.; Wersäll, A.; Poole, A.W. Small GTPases in platelet membrane trafficking. Platelets 2019, 30, 31–40. [Google Scholar] [CrossRef]
- Taefehshokr, N.; Yin, C.; Heit, B. Rab GTPases in the differential processing of phagocytosed pathogens versus efferocytosed apoptotic cells. Histol. Histopathol. 2021, 36, 123–135. [Google Scholar] [CrossRef]
- Yuan, Q.; Ren, C.; Xu, W.; Petri, B.; Zhang, J.; Zhang, Y.; Kubes, P.; Wu, D.; Tang, W. PKN1 Directs Polarized RAB21 Vesicle Trafficking via RPH3A and Is Important for Neutrophil Adhesion and Ischemia-Reperfusion Injury. Cell Rep. 2017, 19, 2586–2597. [Google Scholar] [CrossRef]
- Yang, M.Q.; Du, Q.; Goswami, J.; Varley, P.R.; Chen, B.; Wang, R.H.; Morelli, A.E.; Stolz, D.B.; Billiar, T.R.; Li, J.; et al. Interferon regulatory factor 1-Rab27a regulated extracellular vesicles promote liver ischemia/reperfusion injury. Hepatology 2018, 67, 1056–1070. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.M.; Jang, B.G.; Kim, D.C. Prognostic significance of Rab27 expression in solid cancer: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 14136. [Google Scholar] [CrossRef]
- Kowluru, A. Role of G-proteins in islet function in health and diabetes. Diabetes Obes. Metab. 2017, 19 (Suppl. 1), 63–75. [Google Scholar] [CrossRef] [PubMed]
- Hermle, T.; Schneider, R.; Schapiro, D.; Braun, D.A.; van der Ven, A.T.; Warejko, J.K.; Daga, A.; Widmeier, E.; Nakayama, M.; Jobst-Schwan, T.; et al. GAPVD1 and ANKFY1 Mutations Implicate RAB5 Regulation in Nephrotic Syndrome. J. Am. Soc. Nephrol. 2018, 29, 2123–2138. [Google Scholar] [CrossRef] [PubMed]
- Dorval, G.; Kuzmuk, V.; Gribouval, O.; Welsh, G.I.; Bierzynska, A.; Schmitt, A.; Miserey-Lenkei, S.; Koziell, A.; Haq, S.; Benmerah, A.; et al. TBC1D8B Loss-of-Function Mutations Lead to X-Linked Nephrotic Syndrome via Defective Trafficking Pathways. Am. J. Hum. Genet. 2019, 104, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Rastaldi, M.P.; Armelloni, S.; Berra, S.; Li, M.; Pesaresi, M.; Poczewski, H.; Langer, B.; Kerjaschki, D.; Henger, A.; Blattner, S.M.; et al. Glomerular podocytes possess the synaptic vesicle molecule Rab3A and its specific effector rabphilin-3a. Am. J. Pathol. 2003, 163, 889–899. [Google Scholar] [CrossRef]
- Selma-Soriano, E.; Llamusi, B.; Fernández-Costa, J.M.; Ozimski, L.L.; Artero, R.; Redón, J. Rabphilin involvement in filtration and molecular uptake in Drosophila nephrocytes suggests a similar role in human podocytes. Dis. Model Mech. 2020, 13, dmm041509. [Google Scholar] [CrossRef]
- Rangel-Filho, A.; Lazar, J.; Moreno, C.; Geurts, A.; Jacob, H.J. Rab38 modulates proteinuria in model of hypertension-associated renal disease. J. Am. Soc. Nephrol. 2013, 24, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Y.; Wang, Z.; Ding, F.; Cheng, Z.; Xu, Q.; Cai, Y.; Limbu, M.H.; Yang, Y.; Liu, B.; et al. Rab7 empowers renal tubular epithelial cells with autophagy-mediated protection against albumin-induced injury. Exp. Cell Res. 2018, 370, 198–207. [Google Scholar] [CrossRef]
- Yasuda, T.; Saegusa, C.; Kamakura, S.; Sumimoto, H.; Fukuda, M. Rab27 effector Slp2-a transports the apical signaling molecule podocalyxin to the apical surface of MDCK II cells and regulates claudin-2 expression. Mol. Biol. Cell 2012, 23, 3229–3239. [Google Scholar] [CrossRef]
- Li, S.; Jia, Y.; Xue, M.; Hu, F.; Zheng, Z.; Zhang, S.; Ren, S.; Yang, Y.; Si, Z.; Wang, L.; et al. Inhibiting Rab27a in renal tubular epithelial cells attenuates the inflammation of diabetic kidney disease through the miR-26a-5p/CHAC1/NF-kB pathway. Life Sci. 2020, 261, 118347. [Google Scholar] [CrossRef]
- Grahammer, F.; Schell, C.; Huber, T.B. The podocyte slit diaphragm--from a thin grey line to a complex signalling hub. Nat. Rev. Nephrol. 2013, 9, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.K.; Jou, T.S.; Guo, W.; Lipschutz, J.H. The Rho family of small GTPases is involved in epithelial cystogenesis and tubulogenesis. Kidney Int. 2003, 63, 1632–1644. [Google Scholar] [CrossRef][Green Version]
- Lipschutz, J.H. The role of the exocyst in renal ciliogenesis, cystogenesis, tubulogenesis, and development. Kidney Res. Clin. Pract. 2019, 38, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhu, J.Y.; Zhang, F.; Richman, A.; Zhao, Z.; Han, Z. Comprehensive functional analysis of Rab GTPases in Drosophila nephrocytes. Cell Tissue Res. 2017, 368, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Kampf, L.L.; Schneider, R.; Gerstner, L.; Thünauer, R.; Chen, M.; Helmstädter, M.; Amar, A.; Onuchic-Whitford, A.C.; Loza Munarriz, R.; Berdeli, A.; et al. TBC1D8B Mutations Implicate RAB11-Dependent Vesicular Trafficking in the Pathogenesis of Nephrotic Syndrome. J. Am. Soc. Nephrol. 2019, 30, 2338–2353. [Google Scholar] [CrossRef]
- Wang, W.L.; Su, S.H.; Wong, K.Y.; Yang, C.W.; Liu, C.F.; Yu, M.J. Rab7 involves Vps35 to mediate AQP2 sorting and apical trafficking in collecting duct cells. Am. J. Physiol. Renal. Physiol. 2020, 318, F956–F970. [Google Scholar] [CrossRef]
- Song, L.; Tang, S.; Han, X.; Jiang, Z.; Dong, L.; Liu, C.; Liang, X.; Dong, J.; Qiu, C.; Wang, Y.; et al. KIBRA controls exosome secretion via inhibiting the proteasomal degradation of Rab27a. Nat. Commun. 2019, 10, 1639. [Google Scholar] [CrossRef]
- Marrachelli, V.G.; Monleon, D.; Rentero, P.; Mansego, M.L.; Morales, J.M.; Galan, I.; Segura, R.; Martinez, F.; Martin-Escudero, J.C.; Briongos, L.; et al. Genomic and metabolomic profile associated to microalbuminuria. PLoS ONE 2014, 9, e98227. [Google Scholar] [CrossRef]
- Hwang, S.J.; Yang, Q.; Meigs, J.B.; Pearce, E.N.; Fox, C.S. A genome-wide association for kidney function and endocrine-related traits in the NHLBI’s Framingham Heart Study. BMC Med. Genet. 2007, 8, S10. [Google Scholar] [CrossRef] [PubMed]
- Rastaldi, M.P.; Armelloni, S.; Berra, S.; Calvaresi, N.; Corbelli, A.; Giardino, L.A.; Li, M.; Wang, G.Q.; Fornasieri, A.; Villa, A.; et al. Glomerular podocytes contain neuron-like functional synaptic vesicles. FASEB J. 2006, 20, 976–978. [Google Scholar] [CrossRef]
- Giardino, L.; Armelloni, S.; Corbelli, A.; Mattinzoli, D.; Zennaro, C.; Guerrot, D.; Tourrel, F.; Ikehata, M.; Li, M.; Berra, S.; et al. Podocyte glutamatergic signaling contributes to the function of the glomerular filtration barrier. J. Am. Soc. Nephrol. 2009, 20, 1929–1940. [Google Scholar] [CrossRef]
- Armelloni, S.; Calvaresi, N.; Ikehata, M.; Corbelli, A.; Mattinzoli, D.; Giardino, L.A.; Li, M.; Messa, P.; Rastaldi, M.P. Proteinuria and glomerular damage in Rab3A knockout mice chronically fed a high-glucose diet. Nephron Exp. Nephrol. 2012, 120, e69–e80. [Google Scholar] [CrossRef]
- Marelja, Z.; Simons, M. Filling the Gap: Drosophila Nephrocytes as Model System in Kidney Research. J. Am. Soc. Nephrol. 2019, 30, 719–720. [Google Scholar] [CrossRef]
- Bechtel, W.; Helmstädter, M.; Balica, J.; Hartleben, B.; Kiefer, B.; Hrnjic, F.; Schell, C.; Kretz, O.; Liu, S.; Geist, F.; et al. Vps34 deficiency reveals the importance of endocytosis for podocyte homeostasis. J. Am. Soc. Nephrol. 2013, 24, 727–743. [Google Scholar] [CrossRef]
- Chen, J.; Chen, M.X.; Fogo, A.B.; Harris, R.C.; Chen, J.K. mVps34 deletion in podocytes causes glomerulosclerosis by disrupting intracellular vesicle trafficking. J. Am. Soc. Nephrol. 2013, 24, 198–207. [Google Scholar] [CrossRef]
- Hochapfel, F.; Denk, L.; Mendl, G.; Schulze, U.; Maaßen, C.; Zaytseva, Y.; Pavenstädt, H.; Weide, T.; Rachel, R.; Witzgall, R.; et al. Distinct functions of Crumbs regulating slit diaphragms and endocytosis in Drosophila nephrocytes. Cell. Mol. Life Sci. 2017, 74, 4573–4586. [Google Scholar] [CrossRef] [PubMed]
- Mallipattu, S.K.; Liu, R.; Zheng, F.; Narla, G.; Ma’ayan, A.; Dikman, S.; Jain, M.K.; Saleem, M.; D’Agati, V.; Klotman, P.; et al. Kruppel-like factor 15 (KLF15) is a key regulator of podocyte differentiation. J. Biol. Chem. 2012, 287, 19122–19135. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Shao, H.; Guo, F.; Trimble, R.; Pearce, E.; Abmayr, S.M. Sns and Kirre, the Drosophila orthologs of Nephrin and Neph1, direct adhesion, fusion and formation of a slit diaphragm-like structure in insect nephrocytes. Development 2009, 136, 2335–2344. [Google Scholar] [CrossRef] [PubMed]
- Muraleedharan, S.; Sam, A.; Skaer, H.; Inamdar, M.S. Networks that link cytoskeletal regulators and diaphragm proteins underpin filtration function in Drosophila nephrocytes. Exp. Cell Res. 2018, 364, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Hermle, T.; Braun, D.A.; Helmstädter, M.; Huber, T.B.; Hildebrandt, F. Modeling Monogenic Human Nephrotic Syndrome in the Drosophila Garland Cell Nephrocyte. J. Am. Soc. Nephrol. 2017, 28, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhao, Y.; Chao, Y.; Muir, K.; Han, Z. Cubilin and amnionless mediate protein reabsorption in Drosophila nephrocytes. J. Am. Soc. Nephrol. 2013, 24, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Arroyo, O.; Ortega, A.; Perez-Hernandez, J.; Chaves, F.J.; Redon, J.; Cortes, R. The Rab-Rabphilin system in injured human podocytes stressed by glucose overload and angiotensin II. Am. J. Physiol. Renal Physiol. 2020, 319, F178–F191. [Google Scholar] [CrossRef] [PubMed]
- Selma-Soriano, E.; Casillas-Serra, C.; Artero, R.; Llamosi, B.; Navarro, J.A.; Redon, J. Rabphilin silencing causes dilated cardiomyopathy in a Drosophila model of nephrocyte damage. Sci. Rep. 2021, in press. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Arroyo, O.; Selma-Soriano, E.; Ortega, A.; Cortes, R.; Redon, J. Small Rab GTPases in Intracellular Vesicle Trafficking: The Case of Rab3A/Raphillin-3A Complex in the Kidney. Int. J. Mol. Sci. 2021, 22, 7679. https://doi.org/10.3390/ijms22147679
Martinez-Arroyo O, Selma-Soriano E, Ortega A, Cortes R, Redon J. Small Rab GTPases in Intracellular Vesicle Trafficking: The Case of Rab3A/Raphillin-3A Complex in the Kidney. International Journal of Molecular Sciences. 2021; 22(14):7679. https://doi.org/10.3390/ijms22147679
Chicago/Turabian StyleMartinez-Arroyo, Olga, Estela Selma-Soriano, Ana Ortega, Raquel Cortes, and Josep Redon. 2021. "Small Rab GTPases in Intracellular Vesicle Trafficking: The Case of Rab3A/Raphillin-3A Complex in the Kidney" International Journal of Molecular Sciences 22, no. 14: 7679. https://doi.org/10.3390/ijms22147679
APA StyleMartinez-Arroyo, O., Selma-Soriano, E., Ortega, A., Cortes, R., & Redon, J. (2021). Small Rab GTPases in Intracellular Vesicle Trafficking: The Case of Rab3A/Raphillin-3A Complex in the Kidney. International Journal of Molecular Sciences, 22(14), 7679. https://doi.org/10.3390/ijms22147679








