Mitochondria Matter: Systemic Aspects of Nonalcoholic Fatty Liver Disease (NAFLD) and Diagnostic Assessment of Liver Function by Stable Isotope Dynamic Breath Tests
Abstract
1. Introduction
2. Mitochondrial Function in the Liver
2.1. General Features of Mitochondria
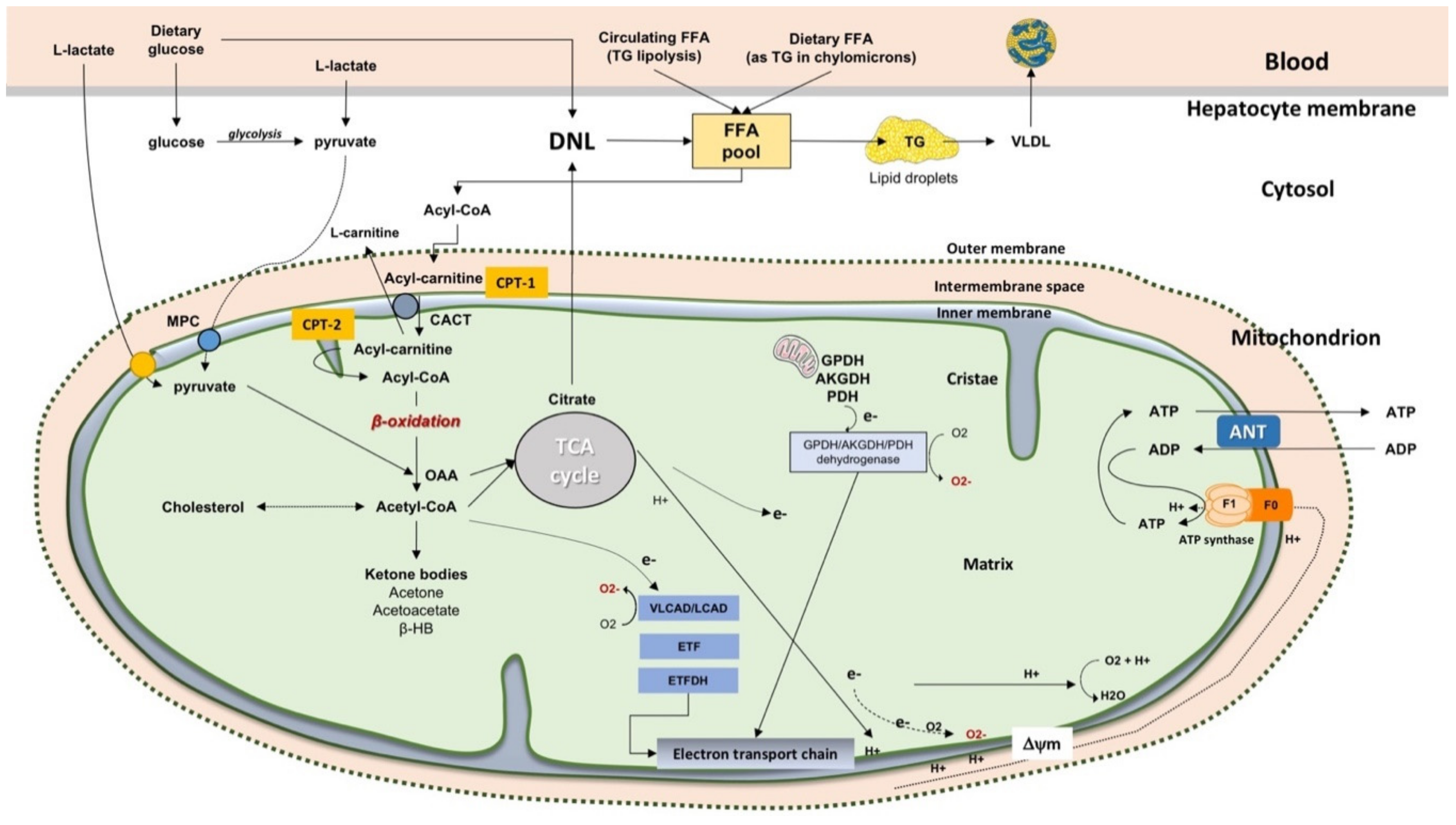
2.2. The Fate of Free Fatty Acids
2.3. β-Oxidation of FFA in Mitochondria
3. General Aspects of NAFLD
3.1. Definition
3.2. Prevalence and Natural History
3.3. Diagnosis
4. Mitochondrial Dysfunction in the Liver
5. Studying Liver Mitochondrial Function at a Translational Level
6. General Features of BT
6.1. Methodology of 13C-BT
6.2. Factors Potentially Affecting the Use of 13C Breath Tests for the Assessment of Liver Function
6.3. Assessing Liver Mitochondrial Function by BT
6.4. Potential Clinical Application
6.4.1. 13C-KICA BT
6.4.2. 13C-Methionine BT
6.4.3. 13C-octanoate BT
7. Why Studying Liver Mitochondrial Function in NAFLD
8. Future Perspectives and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, J.; Park, J.S.; Roh, Y.S. Molecular insights into the role of mitochondria in non-alcoholic fatty liver disease. Arch. Pharm. Res. 2019, 42, 935–946. [Google Scholar] [CrossRef]
- Xiang, L.; Shao, Y.; Chen, Y. Mitochondrial dysfunction and mitochondrion-targeted therapeutics in liver diseases. J. Drug Target. 2021. [Google Scholar] [CrossRef]
- Luangmonkong, T.; Suriguga, S.; Mutsaers, H.A.M.; Groothuis, G.M.M.; Olinga, P.; Boersema, M. Targeting Oxidative Stress for the Treatment of Liver Fibrosis. Rev. Physiol. Biochem. Pharmacol. 2018, 175, 71–102. [Google Scholar] [CrossRef]
- Degli Esposti, D.; Hamelin, J.; Bosselut, N.; Saffroy, R.; Sebagh, M.; Pommier, A.; Martel, C.; Lemoine, A. Mitochondrial roles and cytoprotection in chronic liver injury. Biochem. Res. Int. 2012, 2012, 387626. [Google Scholar] [CrossRef]
- Wei, Y.; Rector, R.S.; Thyfault, J.P.; Ibdah, J.A. Nonalcoholic fatty liver disease and mitochondrial dysfunction. World J. Gastroenterol. WJG 2008, 14, 193–199. [Google Scholar] [CrossRef]
- Guerrieri, F.; Nicoletti, C.; Adorisio, E.; Caraccio, G.; Leonetti, P.; Zanotti, F.; Cantatore, P. Correlation between decreased expression of mitochondrial F0F1-ATP synthase and low regenerating capability of the liver after partial hepatectomy in hypothyroid rats. J. Bioenerg. Biomembr. 2000, 32, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Diogo, C.V.; Grattagliano, I.; Oliveira, P.J.; Bonfrate, L.; Portincasa, P. Re-wiring the circuit: Mitochondria as a pharmacological target in liver disease. Curr. Med. Chem. 2011, 18, 5448–5465. [Google Scholar] [CrossRef] [PubMed]
- Grattagliano, I.; Russmann, S.; Diogo, C.; Bonfrate, L.; Oliveira, P.J.; Wang, D.Q.; Portincasa, P. Mitochondria in chronic liver disease. Curr. Drug. Targets 2011, 12, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Grattagliano, I.; de Bari, O.; Bernardo, T.C.; Oliveira, P.J.; Wang, D.Q.; Portincasa, P. Role of mitochondria in nonalcoholic fatty liver disease--from origin to propagation. Clin. Biochem. 2012, 45, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Grattagliano, I.; De Bari, O.; Di Palo, D.; Montecucco, F.; Carbone, F.; Oliveira, P.; Wang, D.Q.H.; Portincasa, P. Mitochondria in liver diseases. In Mitochondrial Biology and Experimental Therapeutics; Oliveira, P., Ed.; Springer Nature: Cham, Switzerland, 2018; pp. 91–126. [Google Scholar] [CrossRef]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Garruti, G.; Minervini, F.; Bonfrate, L.; Portincasa, P.; Gobbetti, M. The Food-gut Human Axis: The Effects of Diet on Gut Microbiota and Metabolome. Curr. Med. Chem. 2019, 26, 3567–3583. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Garruti, G.; Lunardi Baccetto, R.; Molina-Molina, E.; Bonfrate, L.; Wang, D.Q.; Portincasa, P. Bile Acid Physiology. Ann. Hepatol. 2017, 16, s4–s14. [Google Scholar] [CrossRef] [PubMed]
- Arab, J.P.; Arrese, M.; Trauner, M. Recent Insights into the Pathogenesis of Nonalcoholic Fatty Liver Disease. Annu. Rev. Pathol. 2018, 13, 321–350. [Google Scholar] [CrossRef]
- Bai, L.; Li, H. Innate immune regulatory networks in hepatic lipid metabolism. J. Mol. Med. 2019, 97, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Passarella, S.; Shanmugam, H.; Noviello, M.; Bonfrate, L.; Wang, D.Q.-H.; Portincasa, P. Nonalcoholic Fatty Liver Disease (NAFLD). Mitochondria as Players and Targets of Therapies? Int. J. Mol. Sci. 2021, 22, 5375. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Shulman, G.I. Nonalcoholic fatty liver disease as a nexus of metabolic and hepatic diseases. Cell metab. 2018, 27, 22–41. [Google Scholar] [CrossRef]
- Ono, M.; Okamoto, N.; Saibara, T. The latest idea in NAFLD/NASH pathogenesis. Clin. J. Gastroenterol. 2010, 3, 263–270. [Google Scholar] [CrossRef]
- Paventi, G.; Pizzuto, R.; Passarella, S. The occurrence of l-lactate dehydrogenase in the inner mitochondrial compartment of pig liver. Biochem. Biophys. Res. Commun. 2017, 489, 255–261. [Google Scholar] [CrossRef]
- Passarella, S.; de Bari, L.; Valenti, D.; Pizzuto, R.; Paventi, G.; Atlante, A. Mitochondria and L-lactate metabolism. FEBS Lett. 2008, 582, 3569–3576. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Human fatty liver disease: Old questions and new insights. Science 2011, 332, 1519–1523. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, L.S.; Nurenberg, P.; Leonard, D.; Browning, J.D.; Reingold, J.S.; Grundy, S.; Hobbs, H.H.; Dobbins, R.L. Magnetic resonance spectroscopy to measure hepatic triglyceride content: Prevalence of hepatic steatosis in the general population. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E462–E468. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Singh, S.; Allen, A.M.; Wang, Z.; Prokop, L.J.; Murad, M.H.; Loomba, R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: A systematic review and meta-analysis of paired-biopsy studies. Clin. Gastroenterol. Hepatol. 2015, 13, 643–654.E9. [Google Scholar] [CrossRef]
- Ludwig, J.; Viggiano, T.R.; McGill, D.B.; Oh, B.J. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin. Proc. Mayo Clin. 1980, 55, 434–438. [Google Scholar]
- Caldwell, S.H.; Oelsner, D.H.; Iezzoni, J.C.; Hespenheide, E.E.; Battle, E.H.; Driscoll, C.J. Cryptogenic cirrhosis: Clinical characterization and risk factors for underlying disease. Hepatology 1999, 29, 664–669. [Google Scholar] [CrossRef]
- Browning, J.D.; Kumar, K.S.; Saboorian, M.H.; Thiele, D.L. Ethnic differences in the prevalence of cryptogenic cirrhosis. Am. J. Gastroenterol. 2004, 99, 292–298. [Google Scholar] [CrossRef]
- Nasr, P.; Ignatova, S.; Kechagias, S.; Ekstedt, M. Natural history of nonalcoholic fatty liver disease: A prospective follow-up study with serial biopsies. Hepatol. Commun. 2018, 2, 199–210. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; El-Serag, H.B.; Sada, Y.H.; Kanwal, F.; Duan, Z.; Temple, S.; May, S.B.; Kramer, J.R.; Richardson, P.A.; Davila, J.A. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Sebastiani, G.; Ekstedt, M.; Hagstrom, H.; Nasr, P.; et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Rinella, M.E.; Sanyal, A.J.; Harrison, S.A.; Brunt, E.M.; Goodman, Z.; Cohen, D.E.; Loomba, R. From NAFLD to MAFLD: Implications of a Premature Change in Terminology. Hepatology 2021, 73, 1194–1198. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Baj, J.; Garruti, G.; Celano, G.; De Angelis, M.; Wang, H.H.; Di Palo, D.M.; Bonfrate, L.; Wang, D.Q.-H.; Portincasa, P. Liver Steatosis, Gut-Liver Axis, Microbiome and Environmental Factors. A Never-Ending Bidirectional Cross-Talk. J. Clin. Med. 2020, 9, 2648. [Google Scholar] [CrossRef]
- Di Palo, D.M.; Garruti, G.; Di Ciaula, A.; Molina-Molina, E.; Shanmugam, H.; De Angelis, M.; Portincasa, P. Increased Colonic Permeability and Lifestyles as Contributing Factors to Obesity and Liver Steatosis. Nutrients 2020, 12, 564. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Carbone, F.; Shanmugham, H.; Molina-Molina, E.; Bonfrate, L.; Ministrini, S.; Montecucco, F.; Portincasa, P. Adiponectin involved in portal flow hepatic extraction of 13C-metacethin in obesity and non-alcoholic fatty liver. Eur. J. Intern. Med. 2021. [Google Scholar] [CrossRef]
- Williams, C.D.; Stengel, J.; Asike, M.I.; Torres, D.M.; Shaw, J.; Contreras, M.; Landt, C.L.; Harrison, S.A. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology 2011, 140, 124–131. [Google Scholar] [CrossRef]
- Vernon, G.; Baranova, A.; Younossi, Z.M. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011, 34, 274–285. [Google Scholar] [CrossRef]
- Lazo, M.; Hernaez, R.; Eberhardt, M.S.; Bonekamp, S.; Kamel, I.; Guallar, E.; Koteish, A.; Brancati, F.L.; Clark, J.M. Prevalence of nonalcoholic fatty liver disease in the United States: The Third National Health and Nutrition Examination Survey, 1988–1994. Am. J. Epidemiol. 2013, 178, 38–45. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Afendy, M.; Fang, Y.; Younossi, Y.; Mir, H.; Srishord, M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin. Gastroenterol. Hepatol. 2011, 9, 524–530.e1, quiz e560. [Google Scholar] [CrossRef]
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander Sharma, B.; Mostafa, I.; Bugianesi, E.; Wai-Sun Wong, V.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef] [PubMed]
- Molina-Molina, E.; Lunardi Baccetto, R.; Wang, D.Q.; de Bari, O.; Krawczyk, M.; Portincasa, P. Exercising the hepatobiliary-gut axis. The impact of physical activity performance. Eur. J. Clin. Investig. 2018, 48, e12958. [Google Scholar] [CrossRef] [PubMed]
- Molina-Molina, E.; Krawczyk, M.; Stachowska, E.; Lammert, F.; Portincasa, P. Non-Alcoholic Fatty Liver Disease in Non-Obese Individuals: Prevalence, Pathogenesis and Treatment. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 638–645. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, F.; Wang, W.; Zhang, X.J.; Ji, Y.X.; Zhang, P.; She, Z.G.; Zhu, L.; Cai, J.; Li, H. Epidemiological Features of NAFLD From 1999 to 2018 in China. Hepatology 2020, 71, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, W.R. Nonobese Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2017, 15, 474–485. [Google Scholar] [CrossRef]
- Yoshitaka, H.; Hamaguchi, M.; Kojima, T.; Fukuda, T.; Ohbora, A.; Fukui, M. Nonoverweight nonalcoholic fatty liver disease and incident cardiovascular disease: A post hoc analysis of a cohort study. Medicine 2017, 96, e6712. [Google Scholar] [CrossRef]
- Adams, L.A.; Lymp, J.F.; St Sauver, J.; Sanderson, S.O.; Lindor, K.D.; Feldstein, A.; Angulo, P. The natural history of nonalcoholic fatty liver disease: A population-based cohort study. Gastroenterology 2005, 129, 113–121. [Google Scholar] [CrossRef]
- Lindenmeyer, C.C.; McCullough, A.J. The Natural History of Nonalcoholic Fatty Liver Disease-An Evolving View. Clin. Liver Dis. 2018, 22, 11–21. [Google Scholar] [CrossRef]
- Rinella, M.E.; Sanyal, A.J. Management of NAFLD: A stage-based approach. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 196–205. [Google Scholar] [CrossRef]
- Caussy, C.; Soni, M.; Cui, J.; Bettencourt, R.; Schork, N.; Chen, C.H.; Ikhwan, M.A.; Bassirian, S.; Cepin, S.; Gonzalez, M.P.; et al. Nonalcoholic fatty liver disease with cirrhosis increases familial risk for advanced fibrosis. J. Clin. Investig. 2017, 127, 2697–2704. [Google Scholar] [CrossRef] [PubMed]
- Stender, S.; Loomba, R. PNPLA3 Genotype and Risk of Liver and All-Cause Mortality. Hepatology 2020, 71, 777–779. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, M.; Portincasa, P.; Lammert, F. PNPLA3-associated steatohepatitis: Toward a gene-based classification of fatty liver disease. Semin. Liver Dis. 2013, 33, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Lim, J.K.; Patton, H.; El-Serag, H.B. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2020, 158, 1822–1830. [Google Scholar] [CrossRef]
- Portincasa, P.; Wang, D.Q.H. Gallstones. In Yamada’s Textbook of Gastroenterology, 6th ed.; Podolsky, K.D., Camilleri, M., Fitz, J.G., Kalloo, A.N., Shanahan, F., Wang, T.C., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 1808–1834. [Google Scholar]
- Portincasa, P.; Moschetta, A.; Palasciano, G. Cholesterol gallstone disease. Lancet 2006, 368, 230–239. [Google Scholar] [CrossRef]
- National Institute of Alcohol Abuse and Alcoholism (NIH). Available online: https://pubs.niaaa.nih.gov/publications/practitioner/pocketguide/pocket_guide2.htm (accessed on 16 March 2021).
- Torres, D.M.; Williams, C.D.; Harrison, S.A. Features, diagnosis, and treatment of nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2012, 10, 837–858. [Google Scholar] [CrossRef]
- Sherlock, S.; Dooley, J. Diseases of the Liver and Biliary System; Blackwell Science: Oxford, UK, 2002; pp. 597–628. [Google Scholar]
- Palmentieri, B.; de Sio, I.; La Mura, V.; Masarone, M.; Vecchione, R.; Bruno, S.; Torella, R.; Persico, M. The role of bright liver echo pattern on ultrasound B-mode examination in the diagnosis of liver steatosis. Dig. Liver Dis. 2006, 38, 485–489. [Google Scholar] [CrossRef]
- Park, Y.S.; Park, S.H.; Lee, S.S.; Kim, D.Y.; Shin, Y.M.; Lee, W.; Lee, S.G.; Yu, E.S. Biopsy-proven nonsteatotic liver in adults: Estimation of reference range for difference in attenuation between the liver and the spleen at nonenhanced CT. Radiology 2011, 258, 760–766. [Google Scholar] [CrossRef]
- Wells, M.M.; Li, Z.; Addeman, B.; McKenzie, C.A.; Mujoomdar, A.; Beaton, M.; Bird, J. Computed Tomography Measurement of Hepatic Steatosis: Prevalence of Hepatic Steatosis in a Canadian Population. Can. J. Gastroenterol. Hepatol. 2016, 2016, 4930987. [Google Scholar] [CrossRef]
- Bohte, A.E.; van Werven, J.R.; Bipat, S.; Stoker, J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: A meta-analysis. Eur. Radiol. 2011, 21, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Fowler, K.J.; Hamilton, G.; Cui, J.Y.; Sy, E.Z.; Balanay, M.; Hooker, J.C.; Szeverenyi, N.; Sirlin, C.B. Liver fat imaging-a clinical overview of ultrasound, CT, and MR imaging. Br. J. Radiol. 2018, 91, 20170959. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, H.; Molina Molina, E.; Di Palo, D.M.; Faienza, M.F.; Di Ciaula, A.; Garruti, G.; Wang, D.Q.H.; Portincasa, P. Physical Activity Modulating Lipid Metabolism in Gallbladder Diseases. J. Gastrointest. Liver Dis. 2020, 29, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, S.H.; Swerdlow, R.H.; Khan, E.M.; Iezzoni, J.C.; Hespenheide, E.E.; Parks, J.K.; Parker, W.D., Jr. Mitochondrial abnormalities in non-alcoholic steatohepatitis. J. Hepatol. 1999, 31, 430–434. [Google Scholar] [CrossRef]
- Simoes, I.C.M.; Karkucinska-Wieckowska, A.; Janikiewicz, J.; Szymanska, S.; Pronicki, M.; Dobrzyn, P.; Dabrowski, M.; Dobrzyn, A.; Oliveira, P.J.; Zischka, H.; et al. Western Diet Causes Obesity-Induced Nonalcoholic Fatty Liver Disease Development by Differentially Compromising the Autophagic Response. Antioxidants 2020, 9, 995. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Meroni, M.; Paolini, E.; Macchi, C.; Dongiovanni, P. Mitochondrial dynamics and nonalcoholic fatty liver disease (NAFLD): New perspectives for a fairy-tale ending? Metab. Clin. Exp. 2021, 117, 154708. [Google Scholar] [CrossRef]
- Ajaz, S.; McPhail, M.J.; Gnudi, L.; Trovato, F.M.; Mujib, S.; Napoli, S.; Carey, I.; Agarwal, K. Mitochondrial dysfunction as a mechanistic biomarker in patients with non-alcoholic fatty liver disease (NAFLD). Mitochondrion 2021, 57, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E.; Ragavan, M.; Palavicini, J.P.; Fourcaudot, M.; Bakewell, T.M.; Valdez, I.A.; Ayala, I.; Jin, E.S.; Madesh, M.; Han, X.; et al. Insulin resistance is mechanistically linked to hepatic mitochondrial remodeling in non-alcoholic fatty liver disease. Mol. Metab. 2021, 45, 101154. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Yang, M.; Wei, L.; Wu, H.; Wang, Q.; Shi, H. Physiological evidence of mitochondrial permeability transition pore opening caused by lipid deposition leading to hepatic steatosis in db/db mice. Free Radic. Biol. Med. 2021, 162, 523–532. [Google Scholar] [CrossRef]
- Yan, C.; Duanmu, X.; Zeng, L.; Liu, B.; Song, Z. Mitochondrial DNA: Distribution, Mutations, and Elimination. Cells 2019, 8, 379. [Google Scholar] [CrossRef]
- Garcia-Martinez, I.; Santoro, N.; Chen, Y.; Hoque, R.; Ouyang, X.; Caprio, S.; Shlomchik, M.J.; Coffman, R.L.; Candia, A.; Mehal, W.Z. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J. Clin. Investig. 2016, 126, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Ou, Z.; Cai, C.; Li, P.; Gong, J.; Ruan, X.Z.; He, K. Fatty acid activates NLRP3 inflammasomes in mouse Kupffer cells through mitochondrial DNA release. Cell Immunol. 2018, 332, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Pirola, C.J.; Garaycoechea, M.; Flichman, D.; Castano, G.O.; Sookoian, S. Liver mitochondrial DNA damage and genetic variability of Cytochrome b—A key component of the respirasome drive the severity of fatty liver disease. J. Intern. Med. 2021, 289, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.N.; Czajka, A. Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction? Mitochondrion 2013, 13, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Begriche, K.; Massart, J.; Robin, M.A.; Bonnet, F.; Fromenty, B. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology 2013, 58, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxidative Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef] [PubMed]
- Seki, S.; Kitada, T.; Yamada, T.; Sakaguchi, H.; Nakatani, K.; Wakasa, K. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. J. Hepatol. 2002, 37, 56–62. [Google Scholar] [CrossRef]
- Weltman, M.D.; Farrell, G.C.; Liddle, C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology 1996, 111, 1645–1653. [Google Scholar] [CrossRef]
- Chalasani, N.; Gorski, J.C.; Asghar, M.S.; Asghar, A.; Foresman, B.; Hall, S.D.; Crabb, D.W. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology 2003, 37, 544–550. [Google Scholar] [CrossRef]
- Aubert, J.; Begriche, K.; Knockaert, L.; Robin, M.A.; Fromenty, B. Increased expression of cytochrome P450 2E1 in nonalcoholic fatty liver disease: Mechanisms and pathophysiological role. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 630–637. [Google Scholar] [CrossRef]
- Caballero, F.; Fernandez, A.; Matias, N.; Martinez, L.; Fucho, R.; Elena, M.; Caballeria, J.; Morales, A.; Fernandez-Checa, J.C.; Garcia-Ruiz, C. Specific contribution of methionine and choline in nutritional nonalcoholic steatohepatitis: Impact on mitochondrial S-adenosyl-L-methionine and glutathione. J. Biol. Chem. 2010, 285, 18528–18536. [Google Scholar] [CrossRef]
- Gao, D.; Wei, C.; Chen, L.; Huang, J.; Yang, S.; Diehl, A.M. Oxidative DNA damage and DNA repair enzyme expression are inversely related in murine models of fatty liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G1070–G1077. [Google Scholar] [CrossRef] [PubMed]
- Tesse, A.; Grossini, E.; Tamma, G.; Brenner, C.; Portincasa, P.; Marinelli, R.A.; Calamita, G. Aquaporins as targets of dietary bioactive phytocompounds. Front. Mol. Biosci. 2018, 5, 30. [Google Scholar] [CrossRef]
- Calamita, G.; Portincasa, P. The power of science diplomacy, a lesson from the Nobel laureate Peter Agre. Eur. J. Clin. Investig. 2016, 46, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Palasciano, G.; Svelto, M.; Calamita, G. Aquaporins in the hepatobiliary tract. Which, where and what they do in health and disease. Eur. J. Clin. Investig. 2008, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ferri, D.; Mazzone, A.; Liquori, G.E.; Cassano, G.; Svelto, M.; Calamita, G. Ontogeny, distribution, and possible functional implications of an unusual aquaporin, AQP8, in mouse liver. Hepatology 2003, 38, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Marchissio, M.J.; Frances, D.E.; Carnovale, C.E.; Marinelli, R.A. Mitochondrial aquaporin-8 knockdown in human hepatoma HepG2 cells causes ROS-induced mitochondrial depolarization and loss of viability. Toxicol. Appl. Pharmacol. 2012, 264, 246–254. [Google Scholar] [CrossRef]
- Busanello, E.N.B.; Figueira, T.R.; Marques, A.C.; Navarro, C.D.C.; Oliveira, H.C.F.; Vercesi, A.E. Facilitation of Ca(2+) -induced opening of the mitochondrial permeability transition pore either by nicotinamide nucleotide transhydrogenase deficiency or statins treatment. Cell Biol. Int. 2018, 42, 742–746. [Google Scholar] [CrossRef]
- Slater, T.F. Free radical mechanisms in tissue injury. Cell Funct. Dis. 1988, 222, 1–15. [Google Scholar]
- Petrosillo, G.; Portincasa, P.; Grattagliano, I.; Casanova, G.; Matera, M.; Ruggiero, F.M.; Ferri, D.; Paradies, G. Mitochondrial dysfunction in rat with nonalcoholic fatty liver Involvement of complex I, reactive oxygen species and cardiolipin. Biochim. Biophys. Acta 2007, 1767, 1260–1267. [Google Scholar] [CrossRef]
- Grattagliano, I.; Caraceni, P.; Calamita, G.; Ferri, D.; Gargano, I.; Palasciano, G.; Portincasa, P. Severe liver steatosis correlates with nitrosative and oxidative stress in rats. Eur. J. Clin. Investig. 2008, 38, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Grattagliano, I.; Lauterburg, B.H.; Palasciano, G.; Portincasa, P. 13C-breath tests for clinical investigation of liver mitochondrial function. Eur. J. Clin. Investig. 2010, 40, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Sunny, N.E.; Parks, E.J.; Browning, J.D.; Burgess, S.C. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 2011, 14, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.I.; Szendroedi, J.; Chmelik, M.; Krssak, M.; Moser, E.; Roden, M. Liver ATP synthesis is lower and relates to insulin sensitivity in patients with type 2 diabetes. Diabetes Care 2011, 34, 448–453. [Google Scholar] [CrossRef]
- Peng, K.Y.; Watt, M.J.; Rensen, S.; Greve, J.W.; Huynh, K.; Jayawardana, K.S.; Meikle, P.J.; Meex, R.C.R. Mitochondrial dysfunction-related lipid changes occur in nonalcoholic fatty liver disease progression. J. Lipid Res. 2018, 59, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Szendroedi, J.; Kaul, K.; Jelenik, T.; Nowotny, P.; Jankowiak, F.; Herder, C.; Carstensen, M.; Krausch, M.; Knoefel, W.T. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015, 21, 739–746. [Google Scholar] [CrossRef]
- Fletcher, J.A.; Deja, S.; Satapati, S.; Fu, X.; Burgess, S.C.; Browning, J.D. Impaired ketogenesis and increased acetyl-CoA oxidation promote hyperglycemia in human fatty liver. JCI Insight 2019, 5. [Google Scholar] [CrossRef]
- Grasselli, E.; Baldini, F.; Vecchione, G.; Oliveira, P.J.; Sardao, V.A.; Voci, A.; Portincasa, P.; Vergani, L. Excess fructose and fatty acids trigger a model of nonalcoholic fatty liver disease progression in vitro: Protective effect of the flavonoid silybin. Int. J. Mol. Med. 2019, 44, 705–712. [Google Scholar] [CrossRef]
- An, P.; Wei, L.L.; Zhao, S.; Sverdlov, D.Y.; Vaid, K.A.; Miyamoto, M.; Kuramitsu, K.; Lai, M.; Popov, Y.V. Hepatocyte mitochondria-derived danger signals directly activate hepatic stellate cells and drive progression of liver fibrosis. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef]
- Grossini, E.; Garhwal, D.P.; Calamita, G.; Romito, R.; Rigamonti, C.; Minisini, R.; Smirne, C.; Surico, D.; Bellan, M.; Pirisi, M. Exposure to Plasma From Non-alcoholic Fatty Liver Disease Patients Affects Hepatocyte Viability, Generates Mitochondrial Dysfunction, and Modulates Pathways Involved in Fat Accumulation and Inflammation. Front. Med. 2021, 8. [Google Scholar] [CrossRef]
- Reddy, J.K. Nonalcoholic steatosis and steatohepatitis. III. Peroxisomal beta-oxidation, PPAR alpha, and steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G1333–G1339. [Google Scholar] [CrossRef]
- Camporez, J.P.; Wang, Y.; Faarkrog, K.; Chukijrungroat, N.; Petersen, K.F.; Shulman, G.I. Mechanism by which arylamine N-acetyltransferase 1 ablation causes insulin resistance in mice. Proc. Natl. Acad. Sci. USA 2017, 114, E11285–E11292. [Google Scholar] [CrossRef] [PubMed]
- Chennamsetty, I.; Coronado, M.; Contrepois, K.; Keller, M.P.; Carcamo-Orive, I.; Sandin, J.; Fajardo, G.; Whittle, A.J.; Fathzadeh, M.; Snyder, M.; et al. Nat1 Deficiency Is Associated with Mitochondrial Dysfunction and Exercise Intolerance in Mice. Cell Rep. 2016, 17, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Gijón, M.A.; Riekhof, W.R.; Zarini, S.; Murphy, R.C.; Voelker, D.R. Lysophospholipid Acyltransferases and Arachidonate Recycling in Human Neutrophils*. J. Biol. Chem. 2008, 283, 30235–30245. [Google Scholar] [CrossRef] [PubMed]
- Mancina, R.M.; Dongiovanni, P.; Petta, S.; Pingitore, P.; Meroni, M.; Rametta, R.; Boren, J.; Montalcini, T.; Pujia, A.; Wiklund, O.; et al. The MBOAT7-TMC4 Variant rs641738 Increases Risk of Nonalcoholic Fatty Liver Disease in Individuals of European Descent. Gastroenterology 2016, 150, 1219–1230. [Google Scholar] [CrossRef]
- Luukkonen, P.K.; Zhou, Y.; Hyötyläinen, T.; Leivonen, M.; Arola, J.; Orho-Melander, M.; Orešič, M.; Yki-Järvinen, H. The MBOAT7 variant rs641738 alters hepatic phosphatidylinositols and increases severity of non-alcoholic fatty liver disease in humans. J. Hepatol. 2016, 65, 1263–1265. [Google Scholar] [CrossRef]
- Buch, S.; Stickel, F.; Trépo, E.; Way, M.; Herrmann, A.; Nischalke, H.D.; Brosch, M.; Rosendahl, J.; Berg, T.; Ridinger, M.; et al. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat. Genet. 2015, 47, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Thabet, K.; Asimakopoulos, A.; Shojaei, M.; Romero-Gomez, M.; Mangia, A.; Irving, W.L.; Berg, T.; Dore, G.J.; Grønbæk, H.; Sheridan, D.; et al. MBOAT7 rs641738 increases risk of liver inflammation and transition to fibrosis in chronic hepatitis C. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- Thangapandi, V.R.; Knittelfelder, O.; Brosch, M.; Patsenker, E.; Vvedenskaya, O.; Buch, S.; Hinz, S.; Hendricks, A.; Nati, M.; Herrmann, A.; et al. Loss of hepatic Mboat7 leads to liver fibrosis. Gut 2021, 70, 940–950. [Google Scholar] [CrossRef]
- Helsley, R.N.; Venkateshwari, V.; Brown, A.L.; Gromovsky, A.D.; Schugar, R.C.; Ramachandiran, I.; Fung, K.; Kabbany, M.N.; Banerjee, R.; Neumann, C.K. Obesity-linked suppression of membrane-bound Oacyltransferase 7 (MBOAT7) drives non-alcoholic fatty liver disease. Elife 2019, 8, e49882. [Google Scholar] [CrossRef]
- Fondevila, M.F.; Fernandez, U.; Gonzalez-Rellan, M.J.; Da Silva Lima, N.; Buque, X.; Gonzalez-Rodriguez, A.; Alonso, C.; Iruarrizaga-Lejarreta, M.; Delgado, T.C.; Varela-Rey, M.; et al. The L-α-Lysophosphatidylinositol/G Protein–Coupled Receptor 55 System Induces the Development of Nonalcoholic Steatosis and Steatohepatitis. Hepatology 2021, 73, 606–624. [Google Scholar] [CrossRef]
- Petersen, K.F.; Befroy, D.; Dufour, S.; Dziura, J.; Ariyan, C.; Rothman, D.L.; DiPietro, L.; Cline, G.W.; Shulman, G.I. Mitochondrial dysfunction in the elderly: Possible role in insulin resistance. Science 2003, 300, 1140–1142. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Choi, C.S.; Birkenfeld, A.L.; Alves, T.C.; Jornayvaz, F.R.; Jurczak, M.J.; Zhang, D.; Woo, D.K.; Shadel, G.S.; Ladiges, W.; et al. Targeted Expression of Catalase to Mitochondria Prevents Age-Associated Reductions in Mitochondrial Function and Insulin Resistance. Cell Metab. 2010, 12, 668–674. [Google Scholar] [CrossRef]
- Palmer, A.K.; Kirkland, J.L. Aging and adipose tissue: Potential interventions for diabetes and regenerative medicine. Exp. Gerontol. 2016, 86, 97–105. [Google Scholar] [CrossRef]
- Lyu, K.; Zhang, Y.; Zhang, D.; Kahn, M.; ter Horst, K.W.; Rodrigues, M.R.S.; Gaspar, R.C.; Hirabara, S.M.; Luukkonen, P.K.; Lee, S.; et al. A Membrane-Bound Diacylglycerol Species Induces PKCϵ-Mediated Hepatic Insulin Resistance. Cell Metab. 2020, 32, 654–664.e655. [Google Scholar] [CrossRef]
- Piccinin, E.; Villani, G.; Moschetta, A. Metabolic aspects in NAFLD, NASH and hepatocellular carcinoma: The role of PGC1 coactivators. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, C.; Lu, L.; Guo, W.; VanWagner, L.B.; Shikany, J.M.; Zhang, S.; Kahe, K. Cadmium Exposure in Young Adulthood Is Associated with Risk of Nonalcoholic Fatty Liver Disease in Midlife. Dig. Dis. Sci. 2021. [Google Scholar] [CrossRef]
- He, X.; Gao, J.; Hou, H.; Qi, Z.; Chen, H.; Zhang, X.X. Inhibition of Mitochondrial Fatty Acid Oxidation Contributes to Development of Nonalcoholic Fatty Liver Disease Induced by Environmental Cadmium Exposure. Environ. Sci. Technol. 2019, 53, 13992–14000. [Google Scholar] [CrossRef] [PubMed]
- Frediani, J.K.; Naioti, E.A.; Vos, M.B.; Figueroa, J.; Marsit, C.J.; Welsh, J.A. Arsenic exposure and risk of nonalcoholic fatty liver disease (NAFLD) among U.S. adolescents and adults: An association modified by race/ethnicity, NHANES 2005–2014. Environ. Health Glob. Access Sci. Source 2018, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, F.; Dehghani, M.A.; Vanani, A.R.; Mahdavinia, M. Protective Effects of Alpha Lipoic Acid Against Arsenic Induced Oxidative Stress in Isolated Rat Liver Mitochondria. Biol. Trace Elem. Res. 2021. [Google Scholar] [CrossRef]
- Chen, R.; Xu, Y.; Xu, C.; Shu, Y.; Ma, S.; Lu, C.; Mo, X. Associations between mercury exposure and the risk of nonalcoholic fatty liver disease (NAFLD) in US adolescents. Environ. Sci. Pollut. Res. Int. 2019, 26, 31384–31391. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.C.; de Paula, E.S.; Pazin, M.; Carneiro, M.F.H.; Grotto, D.; Barbosa, F., Jr.; Dorta, D.J. Niacin prevents mitochondrial oxidative stress caused by sub-chronic exposure to methylmercury. Drug Chem. Toxicol. 2020, 43, 64–70. [Google Scholar] [CrossRef]
- Qiu, Y.N.; Wang, G.H.; Zhou, F.; Hao, J.J.; Tian, L.; Guan, L.F.; Geng, X.K.; Ding, Y.C.; Wu, H.W.; Zhang, K.Z. PM2.5 induces liver fibrosis via triggering ROS-mediated mitophagy. Ecotoxicol. Environ. Saf. 2019, 167, 178–187. [Google Scholar] [CrossRef]
- Breton, C.V.; Song, A.Y.; Xiao, J.; Kim, S.J.; Mehta, H.H.; Wan, J.; Yen, K.; Sioutas, C.; Lurmann, F.; Xue, S.; et al. Effects of air pollution on mitochondrial function, mitochondrial DNA methylation, and mitochondrial peptide expression. Mitochondrion 2019, 46, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, L.; Yang, G.; Zhang, C.; Liu, X.; Sun, X.; Chen, X.; Wang, N. The influence of PM2.5 exposure on non-alcoholic fatty liver disease. Life Sci. 2021, 270, 119135. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Beigh, S.; Chaudhari, B.P.; Sharma, S.; Aliul Hasan Abdi, S.; Ahmad, S.; Ahmad, F.; Parvez, S.; Raisuddin, S. Mitochondrial dysfunction induced by Bisphenol A is a factor of its hepatotoxicity in rats. Environ. Toxicol. 2016, 31, 1922–1934. [Google Scholar] [CrossRef]
- Huc, L.; Lemarie, A.; Gueraud, F.; Helies-Toussaint, C. Low concentrations of bisphenol A induce lipid accumulation mediated by the production of reactive oxygen species in the mitochondria of HepG2 cells. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2012, 26, 709–717. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Q.; Xu, C.; Shao, W.; Zhang, C.; Liu, H.; Jiang, Z.; Gu, A. Organochloride pesticides impaired mitochondrial function in hepatocytes and aggravated disorders of fatty acid metabolism. Sci. Rep. 2017, 7, 46339. [Google Scholar] [CrossRef]
- Wahlang, B.; Appana, S.; Falkner, K.C.; McClain, C.J.; Brock, G.; Cave, M.C. Insecticide and metal exposures are associated with a surrogate biomarker for non-alcoholic fatty liver disease in the National Health and Nutrition Examination Survey 2003-2004. Environ. Sci. Pollut. Res. Int. 2020, 27, 6476–6487. [Google Scholar] [CrossRef]
- Miranda, C.A.; Guimaraes, A.; Bizerra, P.F.V.; Mingatto, F.E. Diazinon impairs bioenergetics and induces membrane permeability transition on mitochondria isolated from rat liver. J. Toxicol. Environ. Health A 2020, 83, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Rives, C.; Fougerat, A.; Ellero-Simatos, S.; Loiseau, N.; Guillou, H.; Gamet-Payrastre, L.; Wahli, W. Oxidative Stress in NAFLD: Role of Nutrients and Food Contaminants. Biomolecules 2020, 10, 1702. [Google Scholar] [CrossRef]
- Vecchione, G.; Grasselli, E.; Voci, A.; Baldini, F.; Grattagliano, I.; Wang, D.Q.; Portincasa, P.; Vergani, L. Silybin counteracts lipid excess and oxidative stress in cultured steatotic hepatic cells. World J. Gastroenterol. WJG 2016, 22, 6016–6026. [Google Scholar] [CrossRef] [PubMed]
- Nadanaciva, S.; Will, Y. New insights in drug-induced mitochondrial toxicity. Curr. Pharm. Des. 2011, 17, 2100–2112. [Google Scholar] [CrossRef]
- Pereira, C.V.; Nadanaciva, S.; Oliveira, P.J.; Will, Y. The contribution of oxidative stress to drug-induced organ toxicity and its detection in vitro and in vivo. Expert Opin. Drug Metab. Toxicol. 2012, 8, 219–237. [Google Scholar] [CrossRef]
- Masuo, Y.; Imai, T.; Shibato, J.; Hirano, M.; Jones, O.A.; Maguire, M.L.; Satoh, K.; Kikuchi, S.; Rakwal, R. Omic analyses unravels global molecular changes in the brain and liver of a rat model for chronic Sake (Japanese alcoholic beverage) intake. Electrophoresis 2009, 30, 1259–1275. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.L.; Nicholls, A.W. Metabolomics as a functional genomic tool for understanding lipid dysfunction in diabetes, obesity and related disorders. Pharmacogenomics 2006, 7, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.N.; Simoes, I.C.M.; Rosa, H.S.; Khan, S.; Karkucinska-Wieckowska, A.; Wieckowski, M.R. A Diet Induced Maladaptive Increase in Hepatic Mitochondrial DNA Precedes OXPHOS Defects and May Contribute to Non-Alcoholic Fatty Liver Disease. Cells 2019, 8, 1222. [Google Scholar] [CrossRef]
- Molina-Molina, E.; Shanmugam, H.; Di Ciaula, A.; Grattagliano, I.; Di Palo, D.M.; Palmieri, V.O.; Portincasa, P. ((13)C)-Methacetin breath test provides evidence of subclinical liver dysfunction linked to fat storage but not lifestyle. JHEP Rep. 2021, 3, 100203. [Google Scholar] [CrossRef]
- Bonfrate, L.; Grattagliano, I.; Palasciano, G.; Portincasa, P. Dynamic carbon 13 breath tests for the study of liver function and gastric emptying. Gastroenterol. Rep. 2015, 3, 12–21. [Google Scholar] [CrossRef]
- Grattagliano, I.; Bonfrate, L.; Lorusso, M.; Castorani, L.; de Bari, O.; Portincasa, P. Exploring liver mitochondrial function by (1)(3)C-stable isotope breath tests: Implications in clinical biochemistry. Methods Mol. Biol. 2015, 1241, 137–152. [Google Scholar] [CrossRef]
- Grattagliano, I.; Bonfrate, L.; Oliveira, P.J.; Castorani, L.; Ruggiero, V.; Valenzano, A.T.; Ascensao, A.; Buzoianu, A.; Portincasa, P. Breath tests with novel 13C-substrates for clinical studies of liver mitochondrial function in health and disease. Eur. Rev. Med Pharmacol. Sci. 2013, 17 (Suppl. 2), 72–81. [Google Scholar] [PubMed]
- Perri, F.; Bellini, M.; Portincasa, P.; Parodi, A.; Bonazzi, P.; Marzio, L.; Galeazzi, F.; Usai, P.; Citrino, A.; Usai-Satta, P. (13)C-octanoic acid breath test (OBT) with a new test meal (EXPIROGer): Toward standardization for testing gastric emptying of solids. Dig. Liver Dis. 2010, 42, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Grattagliano, I.; Lauterburg, B.H.; Palmieri, V.O.; Palasciano, G.; Stellaard, F. Liver breath tests non-invasively predict higher stages of non-alcoholic steatohepatitis. Clin. Sci. 2006, 111, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Festi, D.; Capodicasa, S.; Sandri, L.; Colaiocco-Ferrante, L.; Staniscia, T.; Vitacolonna, E.; Vestito, A.; Simoni, P.; Mazzella, G.; Portincasa, P.; et al. Measurement of hepatic functional mass by means of 13C-methacetin and 13C-phenylalanine breath tests in chronic liver disease: Comparison with Child-Pugh score and serum bile acid levels. World J. Gastroenterol. WJG 2005, 11, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Moschetta, A.; Palasciano, G. Nuovi breath test per lo studio dello svuotamento gastrico e del transito intestinale. In Rilevanza in Pazienti Con Stipsi Funzionale; UCP News: Johar Town, Pakistan, 2001; in press. [Google Scholar]
- Gasbarrini, A.; Corazza, G.R.; Gasbarrini, G.; Montalto, M.; Di Stefano, M.; Basilisco, G.; Parodi, A.; Usai-Satta, P.; Vernia, P.; Anania, C.; et al. Methodology and indications of H2-breath testing in gastrointestinal diseases: The Rome Consensus Conference. Aliment. Pharmacol. Ther. 2009, 29 (Suppl. 1), 1–49. [Google Scholar] [CrossRef]
- Vitellio, P.; Celano, G.; Bonfrate, L.; Gobbetti, M.; Portincasa, P.; De Angelis, M. Effects of Bifidobacterium longum and Lactobacillus rhamnosus on Gut Microbiota in Patients with Lactose Intolerance and Persisting Functional Gastrointestinal Symptoms: A Randomised, Double-Blind, Cross-Over Study. Nutrients 2019, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Di Ciaula, A.; Vacca, M.; Montelli, R.; Wang, D.Q.; Palasciano, G. Beneficial effects of oral tilactase on patients with hypolactasia. Eur. J. Clin. Investig. 2008, 38, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, M.; Wolska, M.; Schwartz, S.; Gruenhage, F.; Terjung, B.; Portincasa, P.; Sauerbruch, T.; Lammert, F. Concordance of genetic and breath tests for lactose intolerance in a tertiary referral centre. J. Gastrointest. Liver Dis. JGLD 2008, 17, 135–139. [Google Scholar]
- Bonfrate, L.; Krawczyk, M.; Lembo, A.; Grattagliano, I.; Lammert, F.; Portincasa, P. Effects of dietary education, followed by a tailored fructose-restricted diet in adults with fructose malabsorption. Eur. J. Gastroenterol. Hepatol. 2015, 27, 785–796. [Google Scholar] [CrossRef]
- Merkel, C.; Bolognesi, M.; Bellon, S.; Bianco, S.; Honisch, B.; Lampe, H.; Angeli, P.; Gatta, A. Aminopyrine breath test in the prognostic evaluation of patients with cirrhosis. Gut 1992, 33, 836–842. [Google Scholar] [CrossRef]
- Armuzzi, A.; Candelli, M.; Zocco, M.A.; Andreoli, A.; De Lorenzo, A.; Nista, E.C.; Miele, L.; Cremonini, F.; Cazzato, I.A.; Grieco, A.; et al. Review article: Breath testing for human liver function assessment. Aliment. Pharmacol. Ther. 2002, 16, 1977–1996. [Google Scholar] [CrossRef]
- Michaletz, P.A.; Cap, L.; Alpert, E.; Lauterburg, B.H. Assessment of mitochondrial function in vivo with a breath test utilizing alpha-ketoisocaproic acid. Hepatology 1989, 10, 829–832. [Google Scholar] [CrossRef]
- Palmieri, V.O.; Grattagliano, I.; Minerva, F.; Pollice, S.; Palasciano, G.; Portincasa, P. Liver function as assessed by breath tests in patients with hepatocellular carcinoma. J. Surg. Res. 2009, 157, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Dawson, B.; Trapp, R.G. Basic & Clinical Biostatistics; McGraw-Hill: New York, NY, USA, 2001; Volume 3. [Google Scholar]
- Hintze, J. NCSS 2020 Statistical Software; Number Cruncher Statistical System (NCSS): Kaysville, UT, USA, 2020. [Google Scholar]
- Gorowska-Kowolik, K.; Chobot, A.; Kwiecien, J. (13)C Methacetin Breath Test for Assessment of Microsomal Liver Function: Methodology and Clinical Application. Gastroenterol. Res. Pract. 2017, 2017, 7397840. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, P.; Wright, M.; Wootton, S.A.; Jackson, A.A. Clinical utility of 13C-liver-function breath tests for assessment of hepatic function. Dig. Dis. Sci. 2013, 58, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, M.; Lock, J.F.; Riecke, B.; Heyne, K.; Martus, P.; Fricke, M.; Lehmann, S.; Niehues, S.M.; Schwabe, M.; Lemke, A.J.; et al. Prediction of postoperative outcome after hepatectomy with a new bedside test for maximal liver function capacity. Ann. Surg. 2009, 250, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Buechter, M.; Kersting, S.; Gerken, G.; Kahraman, A. Enzymatic liver function measured by LiMAx—A reliable diagnostic and prognostic tool in chronic liver disease. Sci. Rep. 2019, 9, 13577. [Google Scholar] [CrossRef] [PubMed]
- Holzhutter, H.G.; Wuensch, T.; Gajowski, R.; Berndt, N.; Bulik, S.; Meierhofer, D.; Stockmann, M. A novel variant of the (13)C-methacetin liver function breath test that eliminates the confounding effect of individual differences in systemic CO2 kinetics. Arch. Toxicol. 2020, 94, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, S.M.; Kroh, A.; Ulmer, T.F.; Andruszkow, J.; Luedde, T.; Brozat, J.F.; Neumann, U.P.; Alizai, P.H. Evaluation of NAFLD and fibrosis in obese patients—A comparison of histological and clinical scoring systems. BMC Gastroenterol. 2020, 20, 254. [Google Scholar] [CrossRef]
- Afolabi, P.R.; Scorletti, E.; Smith, D.E.; Almehmadi, A.A.; Calder, P.C.; Byrne, C.D. The characterisation of hepatic mitochondrial function in patients with non-alcoholic fatty liver disease (NAFLD) using the 13C-ketoisocaproate breath test. J. Breath Res. 2018, 12, 046002. [Google Scholar] [CrossRef] [PubMed]
- Krahenbuhl, L.; Ledermann, M.; Lang, C.; Krahenbuhl, S. Relationship between hepatic mitochondrial functions in vivo and in vitro in rats with carbon tetrachloride-induced liver cirrhosis. J. Hepatol. 2000, 33, 216–223. [Google Scholar] [CrossRef]
- Miele, L.; Marrone, G.; Cefalo, C.; D’Achille, S.; Rapaccini, G.L.; Gasbarrini, A.; Grieco, A. Potential use of liver function breath tests in the clinical practice. Eur. Rev. Med Pharmacol. Sci. 2013, 17 (Suppl. 2), 82–89. [Google Scholar]
- Giannini, E.; Fasoli, A.; Chiarbonello, B.; Malfatti, F.; Romagnoli, P.; Botta, F.; Testa, E.; Polegato, S.; Fumagalli, A.; Testa, R. 13C-aminopyrine breath test to evaluate severity of disease in patients with chronic hepatitis C virus infection. Aliment. Pharmacol. Ther. 2002, 16, 717–725. [Google Scholar] [CrossRef]
- Lauterburg, B.H.; Grattagliano, I.; Gmur, R.; Stalder, M.; Hildebrand, P. Noninvasive assessment of the effect of xenobiotics on mitochondrial function in human beings: Studies with acetylsalicylic acid and ethanol with the use of the carbon 13-labeled ketoisocaproate breath test. J. Lab. Clin. Med. 1995, 125, 378–383. [Google Scholar]
- Molina-Molina, E.; Shanmugam, H.; Di Palo, D.; Grattagliano, I.; Portincasa, P. Exploring Liver Mitochondrial Function by (13)C-Stable Isotope Breath Tests: Implications in Clinical Biochemistry. Methods Mol. Biol. 2021, 2310, 179–199. [Google Scholar] [CrossRef]
- Lauterburg, B.H.; Liang, D.; Schwarzenbach, F.A.; Breen, K.J. Mitochondrial dysfunction in alcoholic patients as assessed by breath analysis. Hepatology 1993, 17, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Armuzzi, A.; Marcoccia, S.; Zocco, M.A.; De Lorenzo, A.; Grieco, A.; Tondi, P.; Pola, P.; Gasbarrini, G.; Gasbarrini, A. Non-Invasive assessment of human hepatic mitochondrial function through the 13C-methionine breath test. Scand. J. Gastroenterol. 2000, 35, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Russmann, S.; Junker, E.; Lauterburg, B.H. Remethylation and transsulfuration of methionine in cirrhosis: Studies with L-[H3-methyl-1-C]methionine. Hepatology 2002, 36, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Banasch, M.; Ellrichmann, M.; Tannapfel, A.; Schmidt, W.E.; Goetze, O. The non-invasive (13)C-methionine breath test detects hepatic mitochondrial dysfunction as a marker of disease activity in non-alcoholic steatohepatitis. Eur. J. Med. Res. 2011, 16, 258–264. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Banasch, M.; Emminghaus, R.; Ellrichmann, M.; Schmidt, W.E.; Goetze, O. Longitudinal effects of hepatitis C virus treatment on hepatic mitochondrial dysfunction assessed by C-methionine breath test. Aliment. Pharmacol. Ther. 2008, 28, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Spahr, L.; Negro, F.; Leandro, G.; Marinescu, O.; Goodman, K.J.; Rubbia-Brandt, L.; Jordan, M.; Hadengue, A. Impaired hepatic mitochondrial oxidation using the 13C-methionine breath test in patients with macrovesicular steatosis and patients with cirrhosis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2003, 9, CR6–CR11. [Google Scholar]
- Stuwe, S.H.; Goetze, O.; Arning, L.; Banasch, M.; Schmidt, W.E.; Schols, L.; Saft, C. Hepatic mitochondrial dysfunction in Friedreich ataxia. BMC Neurol. 2011, 11, 145. [Google Scholar] [CrossRef] [PubMed]
- Miele, L.; Grieco, A.; Armuzzi, A.; Candelli, M.; Forgione, A.; Gasbarrini, A.; Gasbarrini, G. Hepatic mitochondrial beta-oxidation in patients with nonalcoholic steatohepatitis assessed by 13 C-octanoate breath test. Am. J. Gastroenterol. 2003, 98, 2335. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.R.J.; Kraut, C.; Lindenthal, B.; Braden, B.; Caspary, W.F.; Stein, J. Total body metabolism of 13C-octanoic acid is preserved in patients with non-alcoholic steatohepatitis, but differs between women and men. Eur. J. Gastroenterol. Hepatol. 2005, 17, 1181–1184. [Google Scholar] [CrossRef]
- Grattagliano, I.; Vendemiale, G.; Lauterburg, B.H. Reperfusion injury of the liver: Role of mitochondria and protection by glutathione ester. J. Surg. Res. 1999, 86, 2–8. [Google Scholar] [CrossRef]
- Berthold, H.K.; Giesen, T.A.; Gouni-Berthold, I. The stable isotope ketoisocaproic acid breath test as a measure of hepatic decarboxylation capacity: A quantitative analysis in normal subjects after oral and intravenous administration. Liver Int. 2009, 29, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Witschi, A.; Mossi, S.; Meyer, B.; Junker, E.; Lauterburg, B.H. Mitochondrial function reflected by the decarboxylation of [13C]ketoisocaproate is impaired in alcoholics. Alcohol. Clin. Exp. Res. 1994, 18, 951–955. [Google Scholar] [CrossRef]
- Bendtsen, P.; Hannestad, U.; Pahlsson, P. Evaluation of the carbon 13-labeled Ketoisocaproate breath test to assess mitochondrial dysfunction in patients with high alcohol consumption. Alcohol. Clin. Exp. Res. 1998, 22, 1792–1795. [Google Scholar] [CrossRef]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef]
- Pessayre, D.; Mansouri, A.; Haouzi, D.; Fromenty, B. Hepatotoxicity due to mitochondrial dysfunction. Cell Biol. Toxicol. 1999, 15, 367–373. [Google Scholar] [CrossRef]
- Kass, G.E.; Price, S.C. Role of mitochondria in drug-induced cholestatic injury. Clin. Liver Dis. 2008, 12, 27–51, vii. [Google Scholar] [CrossRef]
- Milazzo, L.; Piazza, M.; Sangaletti, O.; Gatti, N.; Cappelletti, A.; Adorni, F.; Antinori, S.; Galli, M.; Moroni, M.; Riva, A. [13C]Methionine breath test: A novel method to detect antiretroviral drug-related mitochondrial toxicity. J. Antimicrob. Chemother. 2005, 55, 84–89. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Boehning, D.F.; Qian, T.; Popov, V.L.; Weinman, S.A. Hepatitis C virus core protein increases mitochondrial ROS production by stimulation of Ca2+ uniporter activity. FASEB J. 2007, 21, 2474–2485. [Google Scholar] [CrossRef]
- Jaeschke, H.; McGill, M.R.; Ramachandran, A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: Lessons learned from acetaminophen hepatotoxicity. Drug Metab. Rev. 2012, 44, 88–106. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Suzuki, T.; Kamimura, H.; Nagai, F. Role of mitochondrial membrane permeability transition in N-nitrosofenfluramine-induced cell injury in rat hepatocytes. Eur. J. Pharmacol. 2006, 529, 33–39. [Google Scholar] [CrossRef]
- Trost, L.C.; Lemasters, J.J. Role of the mitochondrial permeability transition in salicylate toxicity to cultured rat hepatocytes: Implications for the pathogenesis of Reye’s syndrome. Toxicol. Appl. Pharmacol. 1997, 147, 431–441. [Google Scholar] [CrossRef]
- Mingatto, F.E.; dos Santos, A.C.; Rodrigues, T.; Pigoso, A.A.; Uyemura, S.A.; Curti, C. Effects of nimesulide and its reduced metabolite on mitochondria. Br. J. Pharmacol. 2000, 131, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Danicke, S.; Diers, S. Effects of ergot alkaloids on liver function of piglets as evaluated by the (13)C-methacetin and (13)C-alpha-ketoisocaproic acid breath test. Toxins 2013, 5, 139–161. [Google Scholar] [CrossRef]
- Storch, K.J.; Wagner, D.A.; Burke, J.F.; Young, V.R. Quantitative study in vivo of methionine cycle in humans using [methyl-2H3]- and [1-13C]methionine. Am. J. Physiol. 1988, 255, E322–E331. [Google Scholar] [CrossRef] [PubMed]
- Candelli, M.; Miele, L.; Armuzzi, A.; Nista, E.C.; Pignataro, G.; Fini, L.; Cazzato, I.A.; Zocco, M.A.; Bartolozzi, F.; Gasbarrini, G.; et al. 13C-methionine breath tests for mitochondrial liver function assessment. Eur. Rev. Med Pharmacol. Sci. 2008, 12, 245–249. [Google Scholar] [PubMed]
- Duro, D.; Duggan, C.; Valim, C.; Bechard, L.; Fitzgibbons, S.; Jaksic, T.; Yu, Y.M. Novel intravenous (13)C-methionine breath test as a measure of liver function in children with short bowel syndrome. J. Pediatric Surg. 2009, 44, 236–240, Discussion 240. [Google Scholar] [CrossRef]
- Durr, A.; Cossee, M.; Agid, Y.; Campuzano, V.; Mignard, C.; Penet, C.; Mandel, J.L.; Brice, A.; Koenig, M. Clinical and genetic abnormalities in patients with Friedreich’s ataxia. N. Engl. J. Med. 1996, 335, 1169–1175. [Google Scholar] [CrossRef]
- Walton, M.E.; Ebert, D.; Haller, R.G. Octanoate oxidation measured by 13C-NMR spectroscopy in rat skeletal muscle, heart, and liver. J. Appl. Physiol. 2003, 95, 1908–1916. [Google Scholar] [CrossRef][Green Version]
- Shalev, T.; Aeed, H.; Sorin, V.; Shahmurov, M.; Didkovsky, E.; Ilan, Y.; Avni, Y.; Shirin, H. Evaluation of the 13C-octanoate breath test as a surrogate marker of liver damage in animal models. Dig. Dis. Sci. 2010, 55, 1589–1598. [Google Scholar] [CrossRef]
- Van de Casteele, M.; Luypaerts, A.; Geypens, B.; Fevery, J.; Ghoos, Y.; Nevens, F. Oxidative breakdown of octanoic acid is maintained in patients with cirrhosis despite advanced disease. Neurogastroenterol. Motil. 2003, 15, 113–120. [Google Scholar] [CrossRef]
- Ghoos, Y.F.; Maes, B.D.; Geypens, B.J.; Mys, G.; Hiele, M.I.; Rutgeerts, P.J.; Vantrappen, G. Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology 1993, 104, 1640–1647. [Google Scholar] [CrossRef]
- Promrat, K.; Kleiner, D.E.; Niemeier, H.M.; Jackvony, E.; Kearns, M.; Wands, J.R.; Fava, J.L.; Wing, R.R. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010, 51, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Keating, S.E.; Hackett, D.A.; George, J.; Johnson, N.A. Exercise and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Hepatol. 2012, 57, 157–166. [Google Scholar] [CrossRef]
- Keating, S.E.; Hackett, D.A.; Parker, H.M.; O’Connor, H.T.; Gerofi, J.A.; Sainsbury, A.; Baker, M.K.; Chuter, V.H.; Caterson, I.D.; George, J.; et al. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J. Hepatol. 2015, 63, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e365, quiz e314–e365. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.F.; Dufour, S.; Befroy, D.; Lehrke, M.; Hendler, R.E.; Shulman, G.I. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 2005, 54, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Cassader, M.; Rosina, F.; Gambino, R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of randomised trials. Diabetologia 2012, 55, 885–904. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, F.M.; Cunha, F.M.d.; Silva, C.C.; Chausse, B.; Romano, R.L.; Garcia, C.; Colepicolo, P.; Medeiros, M.H.G.d.; Kowaltowski, A.J. Redox state, insulin sensitivity and aging. Resumos 2011, 37, 1333–1345. [Google Scholar]
- Kowaltowski, A.J. Caloric restriction and redox state: Does this diet increase or decrease oxidant production? Redox Rep. 2011, 16, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.E.; Shi, Y.; Van Remmen, H. The effects of dietary restriction on oxidative stress in rodents. Free Radic. Biol. Med. 2014, 66, 88–99. [Google Scholar] [CrossRef]
- Bower, G.; Toma, T.; Harling, L.; Jiao, L.R.; Efthimiou, E.; Darzi, A.; Athanasiou, T.; Ashrafian, H. Bariatric Surgery and Non-Alcoholic Fatty Liver Disease: A Systematic Review of Liver Biochemistry and Histology. Obes. Surg. 2015, 25, 2280–2289. [Google Scholar] [CrossRef]
- Mathurin, P.; Hollebecque, A.; Arnalsteen, L.; Buob, D.; Leteurtre, E.; Caiazzo, R.; Pigeyre, M.; Verkindt, H.; Dharancy, S.; Louvet, A. Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology 2009, 137, 532–540. [Google Scholar] [CrossRef]
- Ekstedt, M.; Franzen, L.E.; Holmqvist, M.; Bendtsen, P.; Mathiesen, U.L.; Bodemar, G.; Kechagias, S. Alcohol consumption is associated with progression of hepatic fibrosis in non-alcoholic fatty liver disease. Scand. J. Gastroenterol. 2009, 44, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Pessayre, D.; Berson, A.; Fromenty, B.; Mansouri, A. Mitochondria in steatohepatitis. Semin. Liver Dis. 2001, 21, 57–69. [Google Scholar] [CrossRef]
- Thoma, C.; Day, C.P.; Trenell, M.I. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: A systematic review. J. Hepatol. 2012, 56, 255–266. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Zhang, H.X.; Guo, J.R.; Lam, C.W.K.; Wang, C.Y.; Zhang, W. Mitochondria-Mediated Pathogenesis and Therapeutics for Non-Alcoholic Fatty Liver Disease. Mol. Nutr. Food Res. 2019, 63, e1900043. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, J.; Beleza, J.; Coxito, P.; Ascensao, A.; Magalhaes, J. Physical exercise and liver “fitness”: Role of mitochondrial function and epigenetics-related mechanisms in non-alcoholic fatty liver disease. Mol. Metab. 2020, 32, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Venditti, P.; Di Meo, S. Antioxidants, tissue damage, and endurance in trained and untrained young male rats. Arch. Biochem. Biophys. 1996, 331, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Ascensao, A.; Martins, M.J.; Santos-Alves, E.; Goncalves, I.O.; Portincasa, P.; Oliveira, P.J.; Magalhaes, J. Modulation of hepatic redox status and mitochondrial metabolism by exercise: Therapeutic strategy for liver diseases. Mitochondrion 2013, 13, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Shulman, G.I. Mechanisms for insulin resistance: Common threads and missing links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A.; Loomba, R.; Sanyal, A.J.; Lavine, J.E.; Van Natta, M.L.; Abdelmalek, M.F.; Chalasani, N.; Dasarathy, S.; Diehl, A.M.; Hameed, B.; et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): A multicentre, randomised, placebo-controlled trial. Lancet 2015, 385, 956–965. [Google Scholar] [CrossRef]
- Mudaliar, S.; Henry, R.R.; Sanyal, A.J.; Morrow, L.; Marschall, H.U.; Kipnes, M.; Adorini, L.; Sciacca, C.I.; Clopton, P.; Castelloe, E.; et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 2013, 145, 574–582.e1. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Sanyal, A.J.; Loomba, R.; Rinella, M.; Harrison, S.; Anstee, Q.M.; Goodman, Z.; Bedossa, P.; MacConell, L.; Shringarpure, R.; et al. REGENERATE: Design of a pivotal, randomised, phase 3 study evaluating the safety and efficacy of obeticholic acid in patients with fibrosis due to nonalcoholic steatohepatitis. Contemp. Clin. Trials 2019, 84, 105803. [Google Scholar] [CrossRef]
- Chen, Y.S.; Liu, H.M.; Lee, T.Y. Ursodeoxycholic Acid Regulates Hepatic Energy Homeostasis and White Adipose Tissue Macrophages Polarization in Leptin-Deficiency Obese Mice. Cells 2019, 8, 253. [Google Scholar] [CrossRef]
- Xie, C.; Jiang, C.; Shi, J.; Gao, X.; Sun, D.; Sun, L.; Wang, T.; Takahashi, S.; Anitha, M.; Krausz, K.W.; et al. An Intestinal Farnesoid X Receptor-Ceramide Signaling Axis Modulates Hepatic Gluconeogenesis in Mice. Diabetes 2017, 66, 613–626. [Google Scholar] [CrossRef]
- Ferramosca, A.; Di Giacomo, M.; Zara, V. Antioxidant dietary approach in treatment of fatty liver: New insights and updates. World J. Gastroenterol. WJG 2017, 23, 4146–4157. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Jiang, J.; Zhang, G.; Bu, Y.; Zhang, G.; Zhao, X. Resveratrol and caloric restriction prevent hepatic steatosis by regulating SIRT1-autophagy pathway and alleviating endoplasmic reticulum stress in high-fat diet-fed rats. PLoS ONE 2017, 12, e0183541. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Ma, J.; Wang, W.; Zhang, L.; Xu, J.; Wang, K.; Li, D. Resveratrol supplement inhibited the NF-κB inflammation pathway through activating AMPKα-SIRT1 pathway in mice with fatty liver. Mol. Cell. Biochem. 2016, 422, 75–84. [Google Scholar] [CrossRef]
- Shang, J.; Chen, L.L.; Xiao, F.X.; Sun, H.; Ding, H.C.; Xiao, H. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol. Sin. 2008, 29, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Asin-Cayuela, J.; Manas, A.R.; James, A.M.; Smith, R.A.; Murphy, M.P. Fine-tuning the hydrophobicity of a mitochondria-targeted antioxidant. FEBS Lett. 2004, 571, 9–16. [Google Scholar] [CrossRef]
- Rokitskaya, T.I.; Klishin, S.S.; Severina, I.I.; Skulachev, V.P.; Antonenko, Y.N. Kinetic analysis of permeation of mitochondria-targeted antioxidants across bilayer lipid membranes. J. Membr. Biol. 2008, 224, 9–19. [Google Scholar] [CrossRef]
- Smith, R.A.; Porteous, C.M.; Gane, A.M.; Murphy, M.P. Delivery of bioactive molecules to mitochondria in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 5407–5412. [Google Scholar] [CrossRef]
- Grattagliano, I.; Diogo, C.V.; Mastrodonato, M.; de Bari, O.; Persichella, M.; Wang, D.Q.; Liquori, A.; Ferri, D.; Carratu, M.R.; Oliveira, P.J.; et al. A silybin-phospholipids complex counteracts rat fatty liver degeneration and mitochondrial oxidative changes. World J. Gastroenterol. WJG 2013, 19, 3007–3017. [Google Scholar] [CrossRef]
- Vecchione, G.; Grasselli, E.; Cioffi, F.; Baldini, F.; Oliveira, P.J.; Sardao, V.A.; Cortese, K.; Lanni, A.; Voci, A.; Portincasa, P.; et al. The Nutraceutic Silybin Counteracts Excess Lipid Accumulation and Ongoing Oxidative Stress in an In Vitro Model of Non-Alcoholic Fatty Liver Disease Progression. Front. Nutr. 2017, 4, 42. [Google Scholar] [CrossRef]
- Wu, N.; Zu, Y.; Fu, Y.; Kong, Y.; Zhao, J.; Li, X.; Li, J.; Wink, M.; Efferth, T. Antioxidant activities and xanthine oxidase inhibitory effects of extracts and main polyphenolic compounds obtained from Geranium sibiricum L. J. Agric. Food Chem. 2010, 58, 4737–4743. [Google Scholar] [CrossRef]
- Ling, W.H.; Shen, T.R.; Tang, X.L.; Jiang, X.W. Anthocyanins Improved Mitochondrial Dysfunction in Mice of Non-alcoholic Fatty Liver Disease Induced by High Fat Diet. Faseb J. 2016, 30, 915. [Google Scholar]
- Tang, X.; Shen, T.; Jiang, X.; Xia, M.; Sun, X.; Guo, H.; Ling, W. Purified anthocyanins from bilberry and black currant attenuate hepatic mitochondrial dysfunction and steatohepatitis in mice with methionine and choline deficiency. J. Agric. Food Chem. 2015, 63, 552–561. [Google Scholar] [CrossRef]
- Zeng, X.; Yang, J.; Hu, O.; Huang, J.; Ran, L.; Chen, M.; Zhang, Y.; Zhou, X.; Zhu, J.; Zhang, Q. Dihydromyricetin ameliorates nonalcoholic fatty liver disease by improving mitochondrial respiratory capacity and redox homeostasis through modulation of SIRT3 signaling. Antioxid. Redox Signal. 2019, 30, 163–183. [Google Scholar] [CrossRef]
- Teodoro, J.S.; Duarte, F.V.; Gomes, A.P.; Varela, A.T.; Peixoto, F.M.; Rolo, A.P.; Palmeira, C.M. Berberine reverts hepatic mitochondrial dysfunction in high-fat fed rats: A possible role for SirT3 activation. Mitochondrion 2013, 13, 637–646. [Google Scholar] [CrossRef]
- Echeverria, F.; Valenzuela, R.; Bustamante, A.; Alvarez, D.; Ortiz, M.; Espinosa, A.; Illesca, P.; Gonzalez-Manan, D.; Videla, L.A. High-fat diet induces mouse liver steatosis with a concomitant decline in energy metabolism: Attenuation by eicosapentaenoic acid (EPA) or hydroxytyrosol (HT) supplementation and the additive effects upon EPA and HT co-administration. Food Funct. 2019, 10, 6170–6183. [Google Scholar] [CrossRef] [PubMed]
- Schwimmer, J.B.; Lavine, J.E.; Wilson, L.A.; Neuschwander-Tetri, B.A.; Xanthakos, S.A.; Kohli, R.; Barlow, S.E.; Vos, M.B.; Karpen, S.J.; Molleston, J.P. In children with nonalcoholic fatty liver disease, cysteamine bitartrate delayed release improves liver enzymes but does not reduce disease activity scores. Gastroenterology 2016, 151, 1141–1154.e1149. [Google Scholar] [CrossRef] [PubMed]
- Dohil, R.; Schmeltzer, S.; Cabrera, B.L.; Wang, T.; Durelle, J.; Duke, K.B.; Schwimmer, J.B.; Lavine, J.E. Enteric-coated cysteamine for the treatment of paediatric non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2011, 33, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.H.; Chao, J.; Chang, M.L.; Peng, W.H.; Cheng, H.Y.; Liao, J.W.; Pao, L.H. Pentoxifylline ameliorates non-alcoholic fatty liver disease in hyperglycaemic and dyslipidaemic mice by upregulating fatty acid beta-oxidation. Sci. Rep. 2016, 6, 33102. [Google Scholar] [CrossRef]
- Zein, C.O.; Lopez, R.; Yerian, L.; Anderson, K.A.; McCullough, A.J.; Rinella, M.E. 932 Pentoxifylline Improves Non-Invasive Serum Markers of Fibrosis: Combined Results From 2 Randomized, Placebo-Controlled Trials. Gastroenterology 2012, 142, S936. [Google Scholar] [CrossRef]
- Zein, C.O.; Yerian, L.M.; Gogate, P.; Lopez, R.; Kirwan, J.P.; Feldstein, A.E.; McCullough, A.J. Pentoxifylline improves nonalcoholic steatohepatitis: A randomized placebo-controlled trial. Hepatology 2011, 54, 1610–1619. [Google Scholar] [CrossRef]
- Rendon, D.A. Letter to the Editor: The bioenergetics of hepatic mitochondria isolated from avocado oil-treated rats: Typical experimental errors in the study of the bioenergetics of isolated mitochondria. J. Bioenerg. Biomembr. 2015, 47, 451–453. [Google Scholar] [CrossRef][Green Version]
- Ortiz-Avila, O.; Gallegos-Corona, M.A.; Sanchez-Briones, L.A.; Calderon-Cortes, E.; Montoya-Perez, R.; Rodriguez-Orozco, A.R.; Campos-Garcia, J.; Saavedra-Molina, A.; Mejia-Zepeda, R.; Cortes-Rojo, C. Protective effects of dietary avocado oil on impaired electron transport chain function and exacerbated oxidative stress in liver mitochondria from diabetic rats. J. Bioenerg. Biomembr. 2015, 47, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Berumen, C.I.; Olmos-Orizaba, B.E.; Marquez-Ramirez, C.A.; Orozco, A.R.R.; Gonzalez-Cortez, A.; Saavedra-Molina, A.; Montoya-Perez, R.; Cortes-Rojo, C. Avocado Oil Ameliorates Non-Alcoholic Fatty Liver Disease by Down-Regulating Inflammatory Cytokines and Improving Mitochondrial Dynamics. FASEB J. 2019, 33, 660–666. [Google Scholar] [CrossRef]
- Sanyal, A.; Charles, E.D.; Neuschwander-Tetri, B.A.; Loomba, R.; Harrison, S.A.; Abdelmalek, M.F.; Lawitz, E.J.; Halegoua-DeMarzio, D.; Kundu, S.; Noviello, S.; et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: A randomised, double-blind, placebo-controlled, phase 2a trial. Lancet 2019, 392, 2705–2717. [Google Scholar] [CrossRef]
- Staels, B.; Rubenstrunk, A.; Noel, B.; Rigou, G.; Delataille, P.; Millatt, L.J.; Baron, M.; Lucas, A.; Tailleux, A.; Hum, D.W.; et al. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 2013, 58, 1941–1952. [Google Scholar] [CrossRef]
- Ratziu, V.; Harrison, S.A.; Francque, S.; Bedossa, P.; Lehert, P.; Serfaty, L.; Romero-Gomez, M.; Boursier, J.; Abdelmalek, M.; Caldwell, S.; et al. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-alpha and -delta, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology 2016, 150, 1147–1159. [Google Scholar] [CrossRef]
- Tong, W.; Ju, L.; Qiu, M.; Xie, Q.; Chen, Y.; Shen, W.; Sun, W.; Wang, W.; Tian, J. Liraglutide ameliorates non-alcoholic fatty liver disease by enhancing mitochondrial architecture and promoting autophagy through the SIRT1/SIRT3-FOXO3a pathway. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2016, 46, 933–943. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Sabet, A.; Djedjos, S.; Miller, R.; Sun, X.; Hussain, M.A.; Radovick, S.; Wondisford, F.E. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell 2009, 137, 635–646. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, Q.; Xu, T.; Yao, L.; Feng, J.; Ma, J.; Wang, L.; Lu, C.; Wang, D. Pioglitazone Improves Mitochondrial Function in the Remnant Kidney and Protects against Renal Fibrosis in 5/6 Nephrectomized Rats. Front. Pharmacol. 2017, 8, 545. [Google Scholar] [CrossRef]
- Harrison, S.A.; Alkhouri, N.; Davison, B.A.; Sanyal, A.; Edwards, C.; Colca, J.R.; Lee, B.H.; Loomba, R.; Cusi, K.; Kolterman, O.; et al. Insulin sensitizer MSDC-0602K in non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled phase IIb study. J. Hepatol. 2020, 72, 613–626. [Google Scholar] [CrossRef]
- Iruarrizaga-Lejarreta, M.; Varela-Rey, M.; Fernandez-Ramos, D.; Martinez-Arranz, I.; Delgado, T.C.; Simon, J.; Juan, V.G.; delaCruz-Villar, L.; Azkargorta, M.; Lavin, J.L.; et al. Role of Aramchol in steatohepatitis and fibrosis in mice. Hepatol. Commun. 2017, 1, 911–927. [Google Scholar] [CrossRef]
- Safadi, R.; Konikoff, F.M.; Mahamid, M.; Zelber-Sagi, S.; Halpern, M.; Gilat, T.; Oren, R.; Group, F. The fatty acid-bile acid conjugate Aramchol reduces liver fat content in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2014, 12, 2085–2091.e1. [Google Scholar] [CrossRef]
- Dai, J.; Liang, K.; Zhao, S.; Jia, W.; Liu, Y.; Wu, H.; Lv, J.; Cao, C.; Chen, T.; Zhuang, S.; et al. Chemoproteomics reveals baicalin activates hepatic CPT1 to ameliorate diet-induced obesity and hepatic steatosis. Proc. Natl. Acad. Sci. USA 2018, 115, E5896–E5905. [Google Scholar] [CrossRef]
- Fazzari, M.; Chartoumpekis, D.; Li, L.; Guimaraes, D.A.; Shiva, S.; Freeman, B.A.; Khoo, N. Nitro-oleic Acid Protects Mice from Diet-Induced Hepatic Steatosis and Insulin Resistance without the Adverse Side Effects of Thiazolidinediones. Free Radic. Biol. Med. 2017, 112, 152. [Google Scholar] [CrossRef]
- Cho, J.; Zhang, Y.; Park, S.-Y.; Joseph, A.-M.; Han, C.; Park, H.-J.; Kalavalapalli, S.; Chun, S.-K.; Morgan, D.; Kim, J.-S. Mitochondrial ATP transporter depletion protects mice against liver steatosis and insulin resistance. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Amanat, S.; Eftekhari, M.H.; Fararouei, M.; Bagheri Lankarani, K.; Massoumi, S.J. Genistein supplementation improves insulin resistance and inflammatory state in non-alcoholic fatty liver patients: A randomized, controlled trial. Clin. Nutr. 2018, 37, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Lawitz, E.J.; Neff, G.; Ruane, P.J.; Younes, Z.; Zhang, J.; Jia, C.; Chuang, J.; Huss, R.; Chung, C.; Subramanian, M. Fenofibrate mitigates increases in serum triglycerides due to the ACC inhibitor firsocostat in patients with advanced fibrosis due to NASH: A phase 2 randomized trial. Hepatology 2019, 70, 1489–1490. [Google Scholar]
- Fu, A.; Shi, X.; Zhang, H.; Fu, B. Mitotherapy for Fatty Liver by Intravenous Administration of Exogenous Mitochondria in Male Mice. Front. Pharmacol. 2017, 8, 241. [Google Scholar] [CrossRef] [PubMed]
- Ajith, T.A. Role of mitochondria and mitochondria-targeted agents in non-alcoholic fatty liver disease. Clin. Exp. Pharmacol. Physiol. 2018, 45, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Pessayre, D.; Fromenty, B. NASH: A mitochondrial disease. J. Hepatol. 2005, 42, 928–940. [Google Scholar] [CrossRef]
- Winchell, H.S.; Wiley, K. Considerations in analysis of breath 14CO2 data. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1970, 11, 708–710. [Google Scholar]





| Pharmacokinetic and metabolic aspects |
| Rapidly and consistently absorbed by oral route |
| Primary liver metabolization |
| Low hepatic extraction ratio (20–30%) (i.e., metabolism independent from liver blood flow) |
| Clear metabolic pathway Simple pharmacokinetic Short elimination half-life |
| Minimal compartmentalization of generated 13CO2 Early appearance of 13CO2 in breath |
| Methodological aspects |
| Safe, without side effects |
| Test simple to prepare and administer |
| No (or minimal) interaction with extra-hepatic tissues (i.e., adipose tissue, muscle) |
| Reproducible over time Repeatable (useful for follow-up) |
| Costs |
| Affordable |
| Increased Total Amount of CO2 Production. |
| Elderly Increased physical activity Consumed meal Sparkling beverage Respiratory diseases Fever |
| Altered liver perfusion |
| Anemia Chronic heart failure Transjugular portosystemic shunt |
| Altered gastrointestinal function |
| Delayed gastric emptying. Altered gastrointestinal absorption. |
| Induction of CYP450 1A2 |
| Chronic cigarette smoking Marijuana Brussel spouts Cabbage Caffeine Carbamezepine Cauliflower Charbroiled foods Clarithromycin Erythromycin Esomeprazole Griseifulvin Insulin Lansoprazole Moricizine Omeprazole Phenobarbital Phenytoin Rifampin Ritonavir |
| Inhibition of CYP450 1A2 |
| Anastrazole Caffeine Cimetidine Ciprofloxacin Enoxacin Fluphenazine Flutamide Fluvoxamine Grapefruit juice Grepafloxacin Isoniazid Lidocaine Lomeflozacin Mexiletine Mibefradil Nelfinavir Norfloxacin Ofloxacin Oral contraceptives Perphenazine Phenacetin Propafenone Ranitidine Rifampin Ropinirole Sparfloxacin Tacrine Ticlopidine Verapamil Zafirlukast |
| Substrate/Clinical Applications | Information |
|---|---|
| KICA | |
| ALD | Effect of acute alcohol consumption (even low-moderate doses) [171] |
| ALD | Discrimination between chronic alcohol consumption and nonalcoholic chronic liver disease [173] |
| ALD | Monitoring and ascertaining of alcohol withdrawal [173] |
| NAFLD | Discrimination between simple steatosis and steatohepatitis (NASH) and between low-grade and high-grade fibrosis [147] (Figure 6) |
| HCC | Effect of treatment (thermoablation, chemoembolization) and prediction of tumour recurrence after local treatment. Comparison with methacetin [158] |
| Drugs | Evaluation of acute drug toxicity [171] |
| Methionine | |
| Healthy subjects | Validation studies with 2 mg/kg body weight (methyl-13C)-methionine. Breath 13CO2 enrichment measured at base line and every 15 min thereafter for 180 min. [174] |
| Healthy subjects | Effect of alcohol consumption (30 min after the ingestion of ethanol 0.3 g/kg body weight). Decreased excretion with ethanol, due to impaired mitochondrial oxidation [175]. |
| Liver cirrhosis | Discrimination between different degree of chronic liver damage [175] |
| ALD | Diagnosis of acute alcohol ingestion [174] |
| NAFLD | Discrimination between simple steatosis and NASH [176] |
| HCV | Discrimination between HCV infected patients and healthy subjects and toxicity of pegylated interferon plus ribavirin treatment [177] |
| Drugs | Evaluation of chronic drug toxicity [178] |
| Friedriech’s ataxia | Diagnosis of neurological disorders [179] |
| Octanoate | |
| NAFLD | Evaluation of altered lipid metabolism [180] |
| NASH | Total β-oxidation of octanoic acid remained normal between controls and NASH patients, although cumulative 13CO2 recovery was higher in women than men [181] |
| Increasing Scientific Interest About the Role of Mitochondria in NAFLD |
| Impaired liver mitochondrial function may occur early during the onset and progression of NAFLD. |
| General measures for NAFLD can be beneficial to liver mitochondria as well. |
| Few medications show some beneficial effects on liver mitochondria. |
| Improved mitochondrial function can contribute to ameliorate other liver dysfunctions in NAFLD patients. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Ciaula, A.; Calamita, G.; Shanmugam, H.; Khalil, M.; Bonfrate, L.; Wang, D.Q.-H.; Baffy, G.; Portincasa, P. Mitochondria Matter: Systemic Aspects of Nonalcoholic Fatty Liver Disease (NAFLD) and Diagnostic Assessment of Liver Function by Stable Isotope Dynamic Breath Tests. Int. J. Mol. Sci. 2021, 22, 7702. https://doi.org/10.3390/ijms22147702
Di Ciaula A, Calamita G, Shanmugam H, Khalil M, Bonfrate L, Wang DQ-H, Baffy G, Portincasa P. Mitochondria Matter: Systemic Aspects of Nonalcoholic Fatty Liver Disease (NAFLD) and Diagnostic Assessment of Liver Function by Stable Isotope Dynamic Breath Tests. International Journal of Molecular Sciences. 2021; 22(14):7702. https://doi.org/10.3390/ijms22147702
Chicago/Turabian StyleDi Ciaula, Agostino, Giuseppe Calamita, Harshitha Shanmugam, Mohamad Khalil, Leonilde Bonfrate, David Q.-H. Wang, Gyorgy Baffy, and Piero Portincasa. 2021. "Mitochondria Matter: Systemic Aspects of Nonalcoholic Fatty Liver Disease (NAFLD) and Diagnostic Assessment of Liver Function by Stable Isotope Dynamic Breath Tests" International Journal of Molecular Sciences 22, no. 14: 7702. https://doi.org/10.3390/ijms22147702
APA StyleDi Ciaula, A., Calamita, G., Shanmugam, H., Khalil, M., Bonfrate, L., Wang, D. Q.-H., Baffy, G., & Portincasa, P. (2021). Mitochondria Matter: Systemic Aspects of Nonalcoholic Fatty Liver Disease (NAFLD) and Diagnostic Assessment of Liver Function by Stable Isotope Dynamic Breath Tests. International Journal of Molecular Sciences, 22(14), 7702. https://doi.org/10.3390/ijms22147702









