Cuticular Hydrocarbon Plasticity in Three Rice Planthopper Species
Abstract
:1. Introduction
2. Results
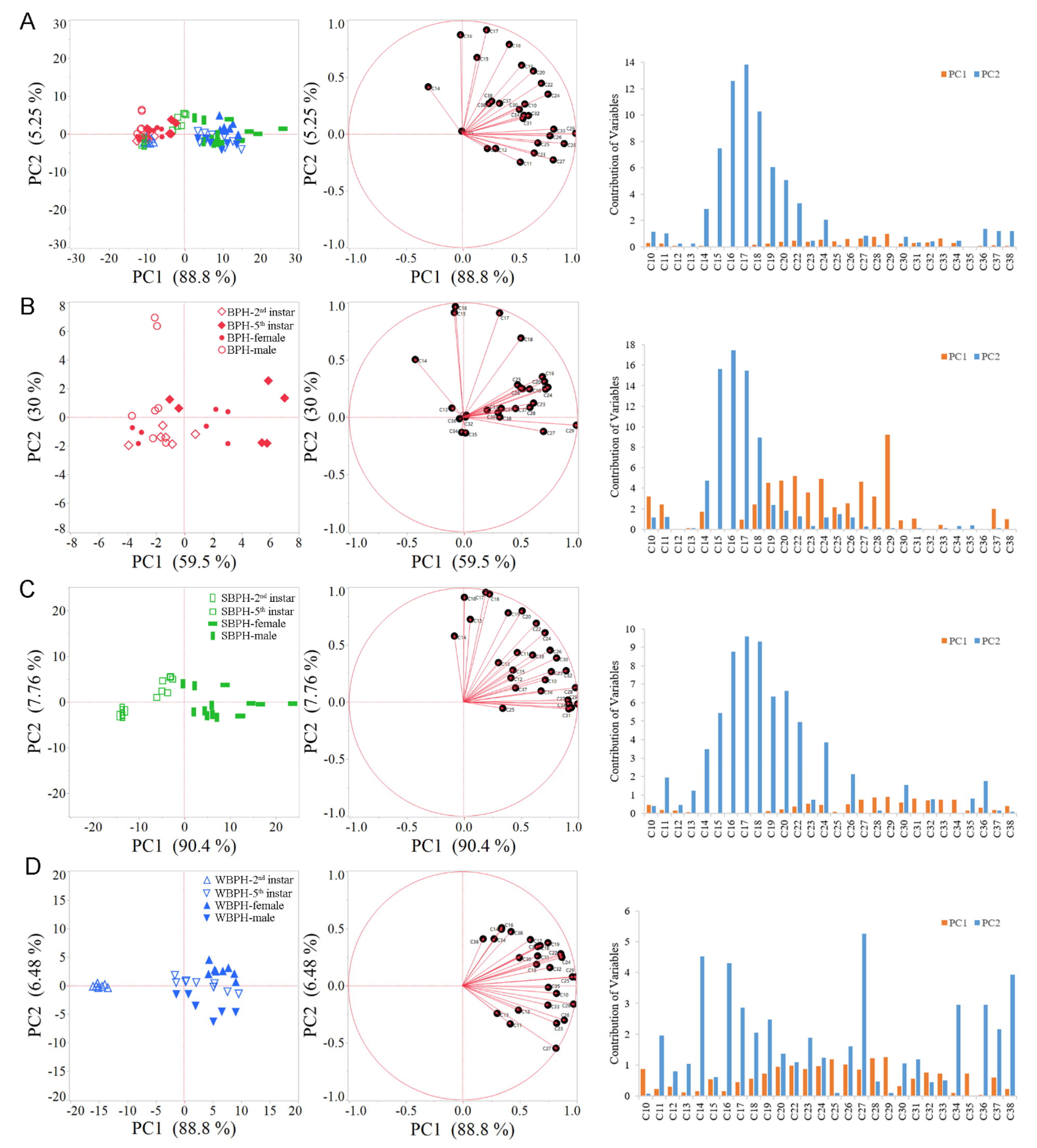
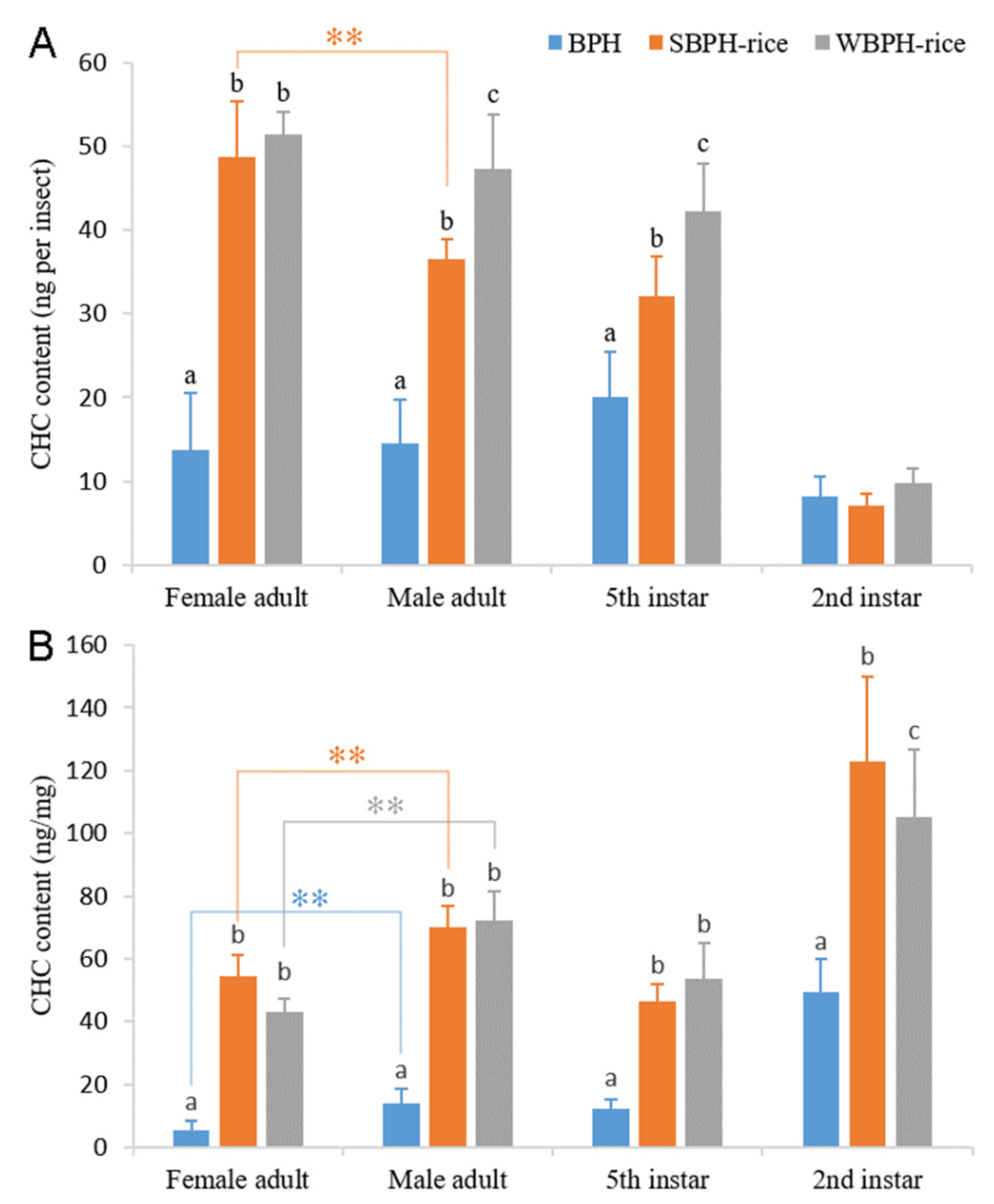
2.1. Effects of Planthopper Species, Developmental Stages and Sexes on Cuticular Hydrocarbon Profiles
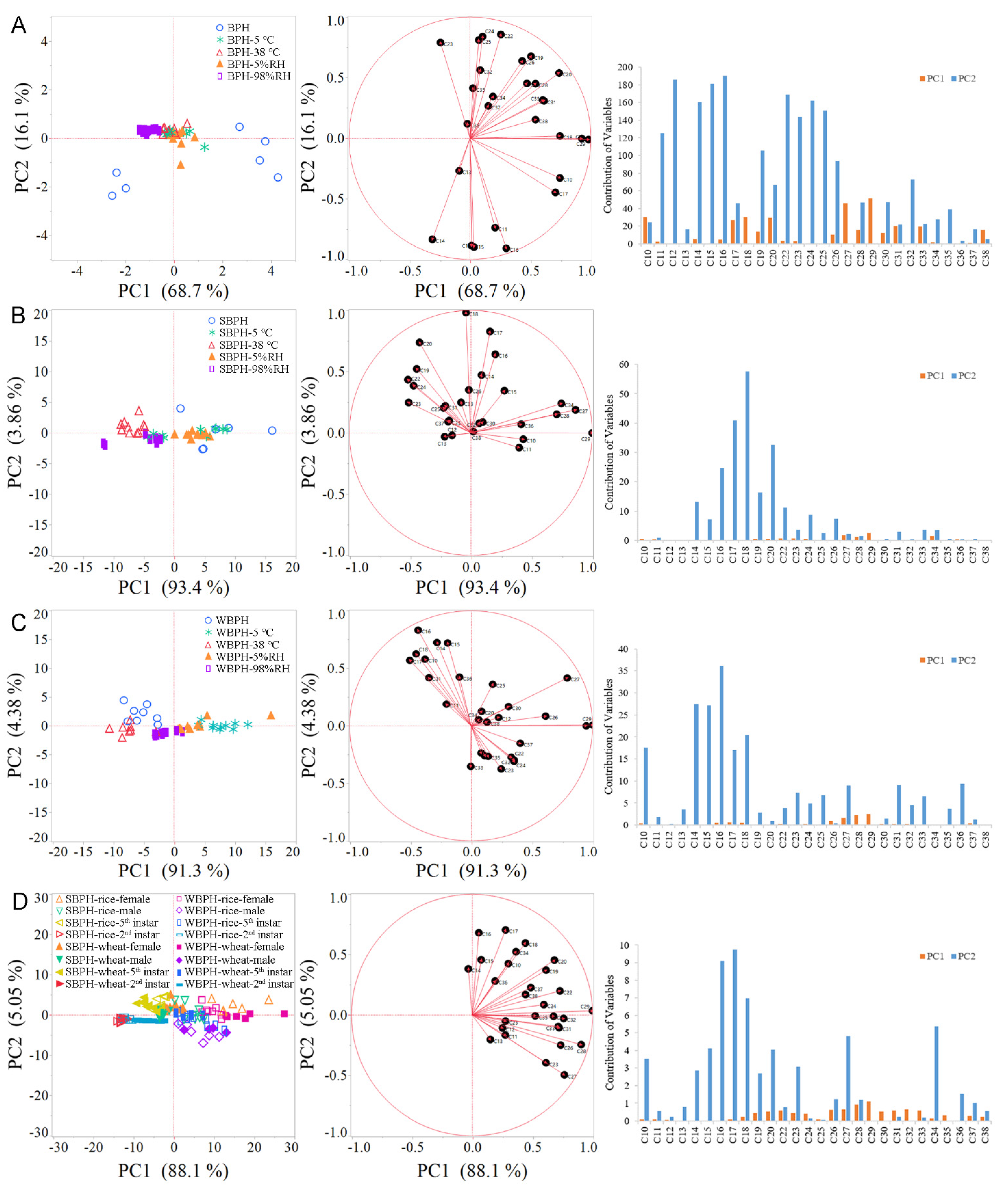
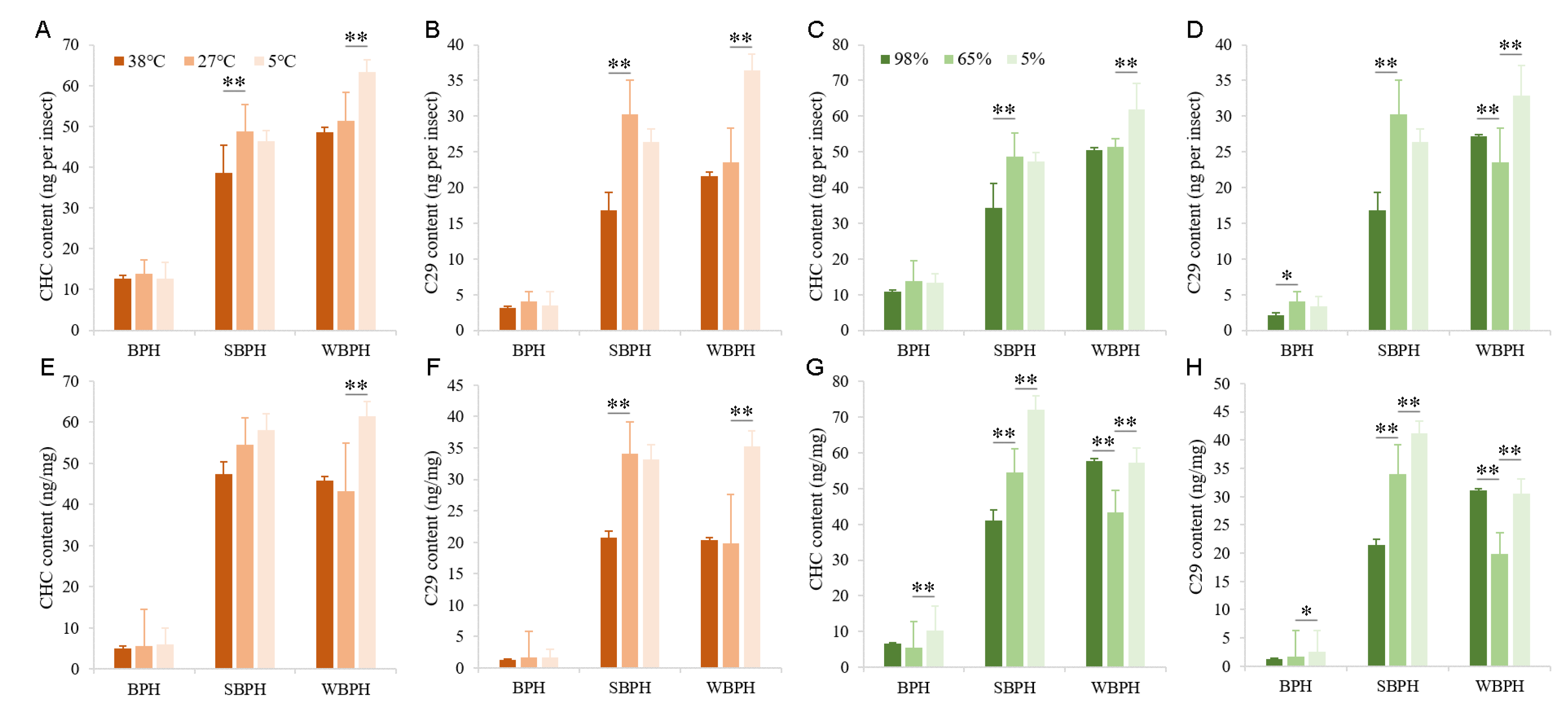
2.2. Effects of Temperature and Relative Humidity on Cuticular Hydrocarbon Profiles
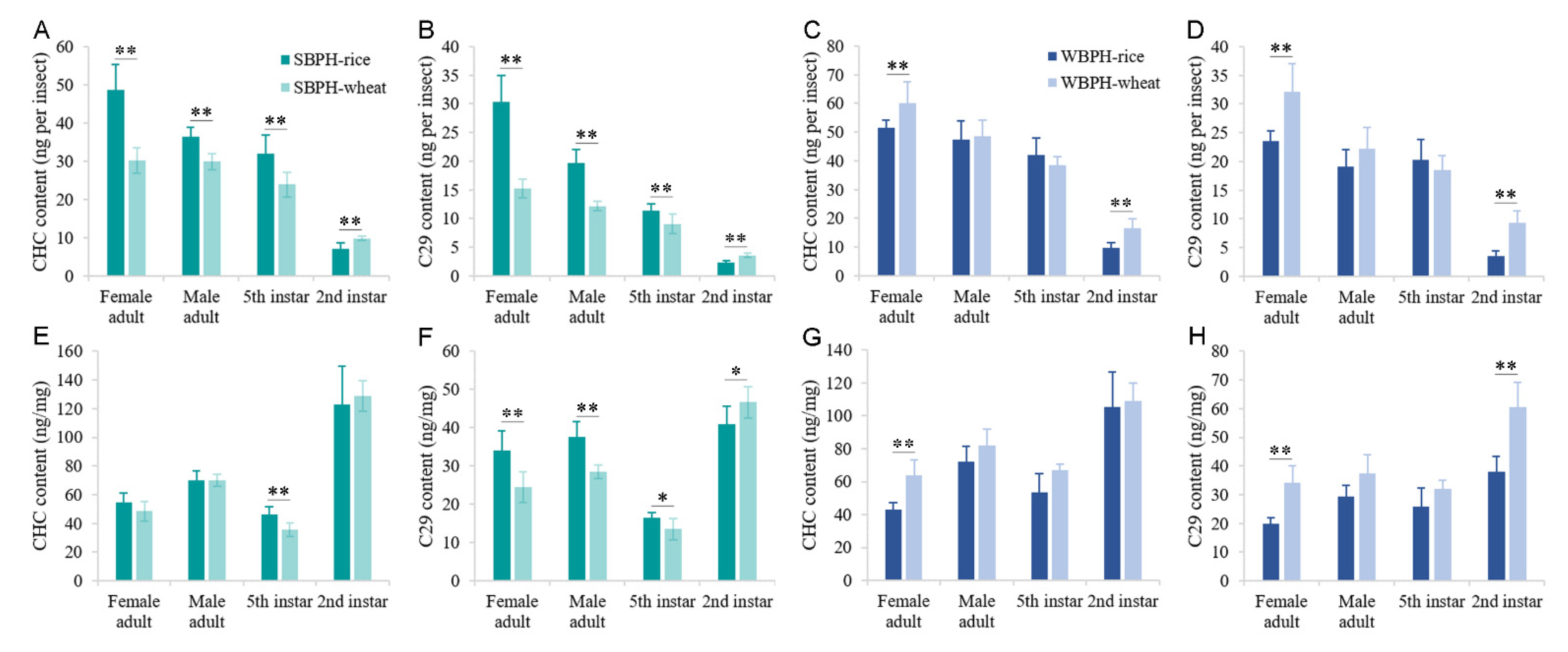
2.3. Effects of Different Host Plants on Cuticular Hydrocarbon Profiles
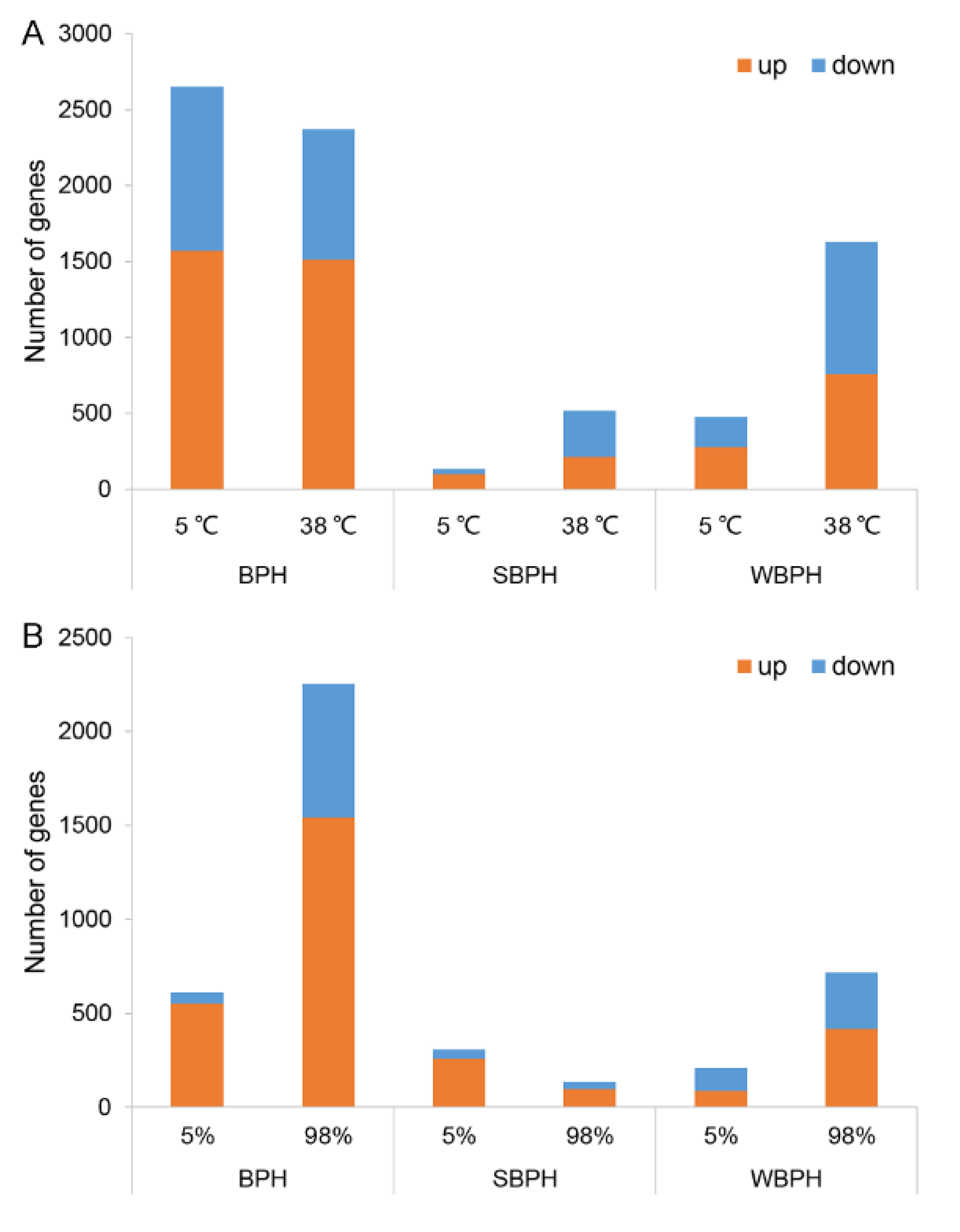
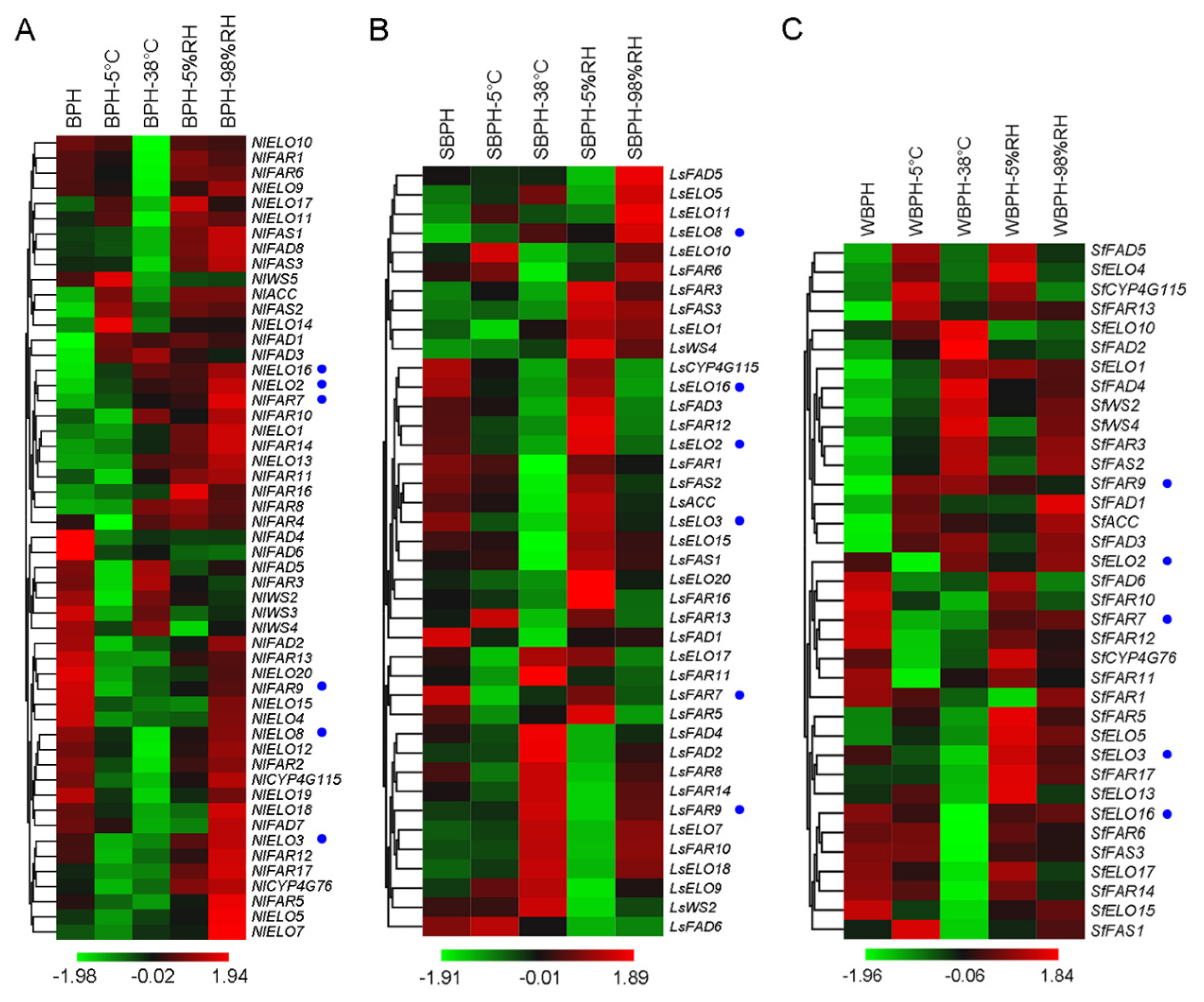
2.4. Effects of Temperature and Relative Humidity on Differential Gene Expression in Three Planthopper Species
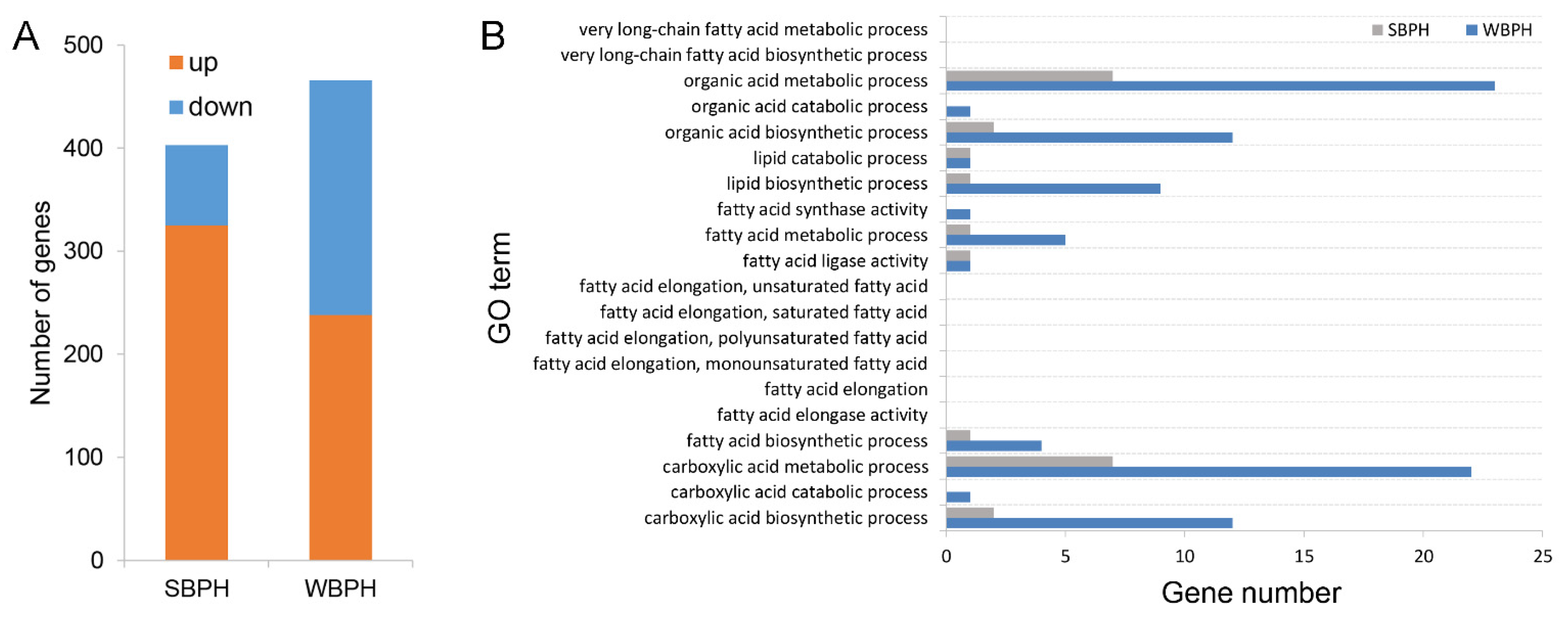
2.5. Different Host Plant Effects on Differential Gene Expression in SBPH and WBPH
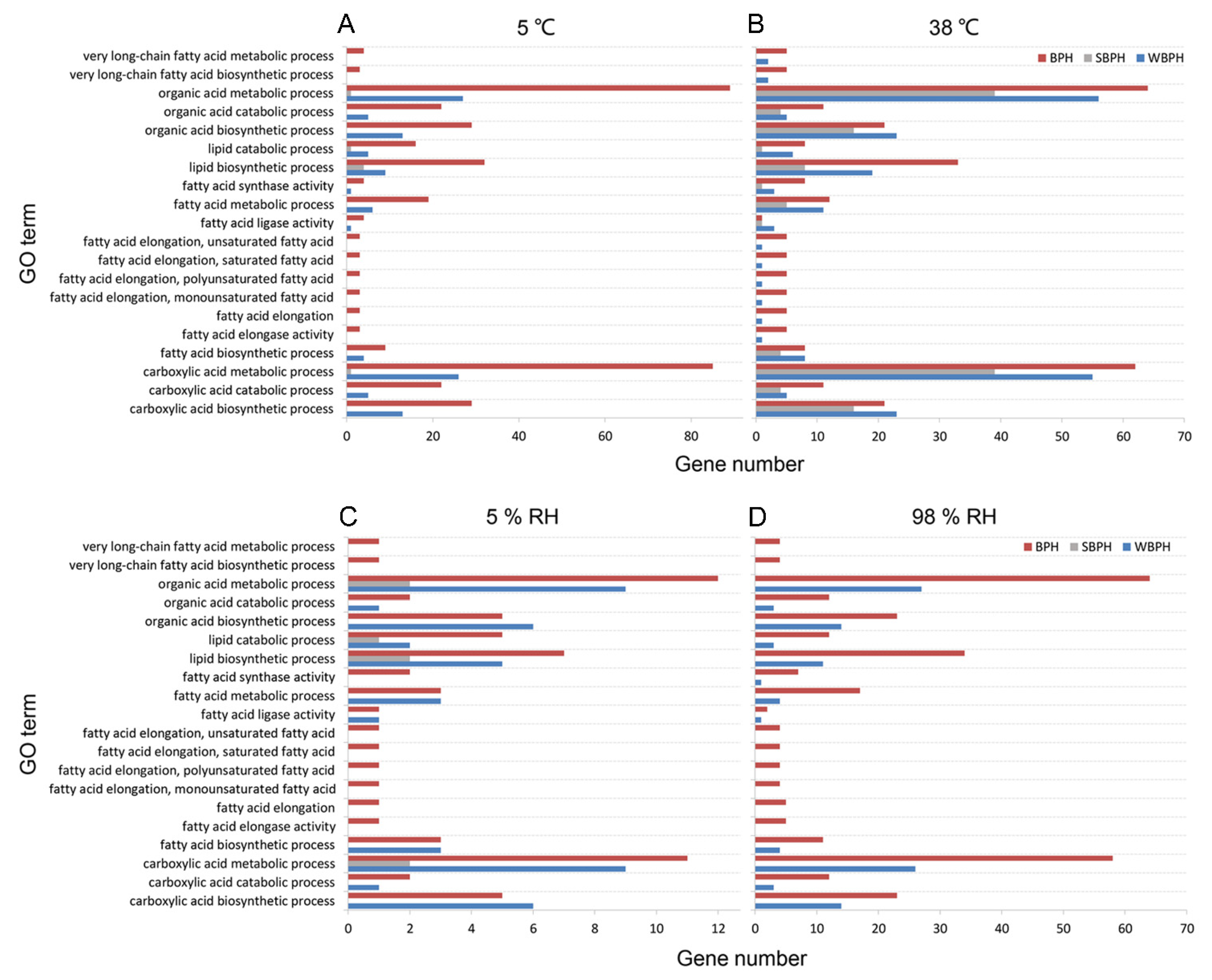
2.6. GO Enrichment Analysis
2.7. Cuticular Lipid Biosynthetic Pathway Gene Expression Profiles in the Three Species
3. Discussion
3.1. Exploring Factors Associated with Variation in CHC Profiles among the Planthopper Species
3.2. Searching for Clues Linking CHC Variation to Different Environmental Factors in the RNA-Seq Analysis
4. Materials and Methods
4.1. Maintenance of Planthopper Strains
4.2. Set-Up of Thermal and Humid Experiments
4.3. Extraction and Quantification of CHCs
4.4. Statistical and Quantitative Analyses
4.5. cDNA Library Preparation and Illumina Sequencing
4.6. Transcriptome and Differential Expression Analysis
4.7. GO Enrichment Analysis of Differentially Expressed Genes
4.8. Expression Profile Analysis of Cuticular Lipid Biosynthetic Pathway Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moussian, B. Recent advances in understanding mechanisms of insect cuticle differentiation. Insect. Biochem. Mol. Biol. 2010, 40, 363–375. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Z.; Zhang, J.; Moussian, B. Regionalization of surface lipids in insects. Proc. Biol. Sci. 2016, 283, 20152994. [Google Scholar] [CrossRef] [Green Version]
- Hadley, N.F. Cuticular lipids of terrestrial plants and arthropods-a comparison of their structure, composition, and waterproofing function. Biol. Rev. 1981, 56, 23–47. [Google Scholar] [CrossRef]
- Smith, A.A.; Millar, J.G.; Suarez, A.V. Comparative analysis of fertility signals and sex-specific cuticular chemical profiles of Odontomachus trap-jaw ants. J. Exp. Biol. 2016, 219, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Menzel, F.; Schmitt, T.; Blaimer, B.B. The evolution of a complex trait: Cuticular hydrocarbons in ants evolve independent from phylogenetic constraints. J. Evol. Biol. 2017, 30, 1372–1385. [Google Scholar] [CrossRef]
- Rouault, J.D.; Marican, C.; Wicker-Thomas, C.; Jallon, J.M. Relations between cuticular hydrocarbon (HC) polymorphism, resistance against desiccation and breeding temperature; a model for HC evolution in D. melanogaster and D. Simulans. Genet 2004, 120, 195–212. [Google Scholar] [CrossRef]
- Menzel, F.; Blaimer, B.B.; Schmitt, T. How do cuticular hydrocarbons evolve? Physiological constraints and climatic and biotic selection pressures act on a complex functional trait. Proc. R. Soc. B Biol. Sci. 2017, 284, 20161727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menzel, F.; Schmitt, T. Tolerance requires the right smell: First evidence for interspecific selection on chemical recognition cues. Evolution 2012, 66, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, S.D.; Menzel, F.; Nehring, V.; Schmitt, T. Ecology and evolution of communication in social insects. Cell 2016, 164, 1277–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, H.; Carroll, S.B. Wax, sex and the origin of species: Dual roles of insect cuticular hydrocarbons in adaptation and mating. Bioessays 2015, 37, 822–830. [Google Scholar] [CrossRef]
- Butterworth, N.J.; Wallman, J.F.; Drijfhout, F.P.; Johnston, N.P.; Keller, P.A.; Byrne, P.G. The evolution of sexually dimorphic cuticular hydrocarbons in blowflies (Diptera: Calliphoridae). J. Evol. Biol. 2020, 33, 1468–1486. [Google Scholar] [CrossRef]
- Guillem, R.M.; Drijfhout, F.P.; Martin, S.J. Species-specific cuticular hydrocarbon stability within European Myrmica Ants. J. Chem. Ecol. 2016, 42, 1052–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buellesbach, J.; Gadau, J.; Beukeboom, L.W.; Echinger, F.; Raychoudhury, R.; Werren, J.H.; Schmitt, T. Cuticular hydrocarbon divergence in the jewel wasp Nasonia: Evolutionary shifts in chemical communication channels? J. Evol. Biol. 2013, 26, 2467–2478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kather, R.; Martin, S.J. Evolution of cuticular hydrocarbons in the hymenoptera: A meta-analysis. J. Chem. Ecol. 2015, 41, 871–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buellesbach, J.; Vetter, S.G.; Schmitt, T. Differences in the reliance on cuticular hydrocarbons as sexual signaling and species discrimination cues in parasitoid wasps. Front. Zool. 2018, 15, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira, C.C.; Manfrin, M.H.; Sene, F.D.; Jackson, L.L.; Etges, W.J. Variations on a theme: Diversification of cuticular hydrocarbons in a clade of cactophilic. Drosophila. Bmc. Evol. Biol. 2011, 11, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Symonds, M.R.E.; Wertheim, B. The mode of evolution of aggregation pheromones in Drosophila species. J. Evol. Biol. 2005, 18, 1253–1263. [Google Scholar] [CrossRef]
- Martin, S.J.; Helantera, H.; Drijfhout, F.P. Evolution of species-specific cuticular hydrocarbon patterns in Formica ants. Biol. J. Linn. Soc. 2008, 95, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Schwander, T.; Arbuthnott, D.; Gries, R.; Gries, G.; Nosil, P.; Crespi, B.J. Hydrocarbon divergence and reproductive isolation in Timema stick insects. BMC Evol. Biol. 2013, 13, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Otte, T.; Hilker, M.; Geiselhardt, S. Phenotypic plasticity of cuticular hydrocarbon profiles in insects. J. Chem. Ecol. 2018, 44, 235–247. [Google Scholar] [CrossRef]
- Martin, S.; Drijfhout, F. A review of ant cuticular hydrocarbons. J. Chem. Ecol. 2009, 35, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, A.G. Water-proofing properties of cuticular lipids. Am. Zool. 1998, 38, 471–482. [Google Scholar] [CrossRef]
- Heuskin, S.; Vanderplanck, M.; Bacquet, P.; Holveck, M.J.; Kaltenpoth, M.; Engl, T.; Pels, C.; Taverne, C.; Lognay, G.; Nieberding, C.M. The composition of cuticular compounds indicates body parts, sex and age in the model butterfly. Bicyclus anynana (Lepidoptera). Front. Ecol. Evol. 2014, 2, 37. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Bai, Y.; Fan, Y.L.; Liu, T.X. Solid-phase microextraction-based cuticular hydrocarbon profiling for intraspecific delimitation in Acyrthosiphon pisum. PLoS ONE 2017, 12, e0184243. [Google Scholar] [CrossRef] [Green Version]
- Chown, S.L.; Sorensen, J.G.; Terblanche, J.S. Water loss in insects: An environmental change perspective. J. Insect Physiol. 2011, 57, 1070–1084. [Google Scholar] [CrossRef]
- Stinziano, J.R.; Sove, R.J.; Rundle, H.D.; Sinclair, B.J. Rapid desiccation hardening changes the cuticular hydrocarbon profile of Drosophila melanogaster. Comp. Biochem. Phys. Part A 2015, 180, 38–42. [Google Scholar] [CrossRef] [Green Version]
- Dembeck, L.M.; Boroczky, K.; Huang, W.; Schal, C.; Anholt, R.R.H.; Mackay, T.F.C. Genetic architecture of natural variation in cuticular hydrocarbon composition in Drosophila melanogaster. eLife 2015, 4, e09861. [Google Scholar] [CrossRef]
- Wicker-Thomas, C.; Garrido, D.; Bontonou, G.; Napal, L.; Mazuras, N.; Denis, B.; Rubin, T.; Parvy, J.P.; Montagne, J. Flexible origin of hydrocarbon/pheromone precursors in Drosophila melanogaster. J. Lipid. Res. 2015, 56, 2094–2101. [Google Scholar] [CrossRef] [Green Version]
- Pei, X.J.; Chen, N.; Bai, Y.; Qiao, J.W.; Li, S.; Fan, Y.L.; Liu, T.X. BgFas1: A fatty acid synthase gene required for both hydrocarbon and cuticular fatty acid biosynthesis in the German cockroach, Blattella germanica (L.). Insect Biochem. Mol. Biol. 2019, 112, 103203. [Google Scholar] [CrossRef]
- Li, D.T.; Chen, X.; Wang, X.Q.; Moussian, B.; Zhang, C.X. The fatty acid elongase gene family in the brown planthopper, Nilaparvata lugens. Insect Biochem. Mol. Biol. 2019, 108, 32–43. [Google Scholar] [CrossRef]
- Li, D.T.; Dai, Y.T.; Chen, X.; Wang, X.Q.; Li, Z.D.; Moussian, B.; Zhang, C.X. Ten fatty acyl-CoA reductase family genes were essential for the survival of the destructive rice pest, Nilaparvata lugens. Pest. Manag. Sci. 2020, 76, 2304–2315. [Google Scholar] [CrossRef]
- Chen, N.; Fan, Y.L.; Bai, Y.; Li, X.D.; Zhang, Z.F.; Liu, T.X. Cytochrome P450 gene, CYP4G51, modulates hydrocarbon production in the pea aphid, Acyrthosiphon pisum. Insect Biochem. Mol. Biol. 2016, 76, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.T.; Zhang, X.Y.; Wang, Y.W.; Moussian, B.; Zhu, K.Y.; Li, S.; Ma, E.B.; Zhang, J.Z. LmCYP4G102: An oenocyte-specific cytochrome P450 gene required for cuticular waterproofing in the migratory locust, Locusta migratoria. Sci. Rep. 2016, 6, 1–11. [Google Scholar]
- Qiu, Y.; Tittiger, C.; Wicker-Thomas, C.; Le Goff, G.; Young, S.; Wajnberg, E.; Fricaux, T.; Taquet, N.; Blomquist, G.J.; Feyereisen, R. An insect-specific P450 oxidative decarbonylase for cuticular hydrocarbon biosynthesis. Proc. Natl. Acad. Sci. USA 2012, 109, 14858–14863. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Pei, X.J.; Li, S.; Fan, Y.L.; Liu, T.X. Involvement of integument-rich CYP4G19 in hydrocarbon biosynthesis and cuticular penetration resistance in Blattella germanica (L.). Pest. Manag. Sci. 2020, 76, 215–226. [Google Scholar] [CrossRef]
- Wang, S.; Li, B.; Zhang, D. NlCYP4G76 and NlCYP4G115 Modulate susceptibility to desiccation and insecticide penetration through affecting cuticular hydrocarbon biosynthesis in Nilaparvata lugens (Hemiptera: Delphacidae). Front. Physiol. 2019, 10, 913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, R.W.; Blomquist, G.J. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 2005, 50, 371–393. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.H.; Ye, G.Y.; Hu, C.; Xu, X.H.; Li, K. Development changes of cuticular hydrocarbons in Chrysomya rufifacies larvae: Potential for determining larval age. Med. Vet. Entomol. 2006, 20, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Duarte, B.F.; Michelutti, K.B.; Antonialli, W.F.; Cardoso, C.A.L. Effect of temperature on survival and cuticular composition of three different ant species. J. Therm. Biol. 2019, 80, 178–189. [Google Scholar] [CrossRef]
- Geiselhardt, S.; Otte, T.; Hilker, M. Looking for a similar partner: Host plants shape mating preferences of herbivorous insects by altering their contact pheromones. Ecol. Lett. 2012, 15, 971–977. [Google Scholar] [CrossRef]
- Huang, H.J.; Xue, J.; Zhuo, J.C.; Cheng, R.L.; Xu, H.J.; Zhang, C.X. Comparative analysis of the transcriptional responses to low and high temperatures in three rice planthopper species. Mol. Ecol. 2017, 26, 2726–2737. [Google Scholar] [CrossRef] [PubMed]
- Li, D.T.; Chen, X.; Wang, X.Q.; Zhang, C.X. FAR gene enables the brown planthopper to walk and jump on water in paddy field. Sci. China Life Sci. 2019, 62, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.-T.; Pei, X.-J.; Ye, Y.-X.; Wang, X.-Q.; Wang, Z.-C.; Chen, N.; Liu, T.-X.; Fan, Y.-L.; Zhang, C.-X. Cuticular Hydrocarbon Plasticity in Three Rice Planthopper Species. Int. J. Mol. Sci. 2021, 22, 7733. https://doi.org/10.3390/ijms22147733
Li D-T, Pei X-J, Ye Y-X, Wang X-Q, Wang Z-C, Chen N, Liu T-X, Fan Y-L, Zhang C-X. Cuticular Hydrocarbon Plasticity in Three Rice Planthopper Species. International Journal of Molecular Sciences. 2021; 22(14):7733. https://doi.org/10.3390/ijms22147733
Chicago/Turabian StyleLi, Dan-Ting, Xiao-Jin Pei, Yu-Xuan Ye, Xin-Qiu Wang, Zhe-Chao Wang, Nan Chen, Tong-Xian Liu, Yong-Liang Fan, and Chuan-Xi Zhang. 2021. "Cuticular Hydrocarbon Plasticity in Three Rice Planthopper Species" International Journal of Molecular Sciences 22, no. 14: 7733. https://doi.org/10.3390/ijms22147733





