Androgen/Androgen Receptor Signaling in Ovarian Cancer: Molecular Regulation and Therapeutic Potentials
Abstract
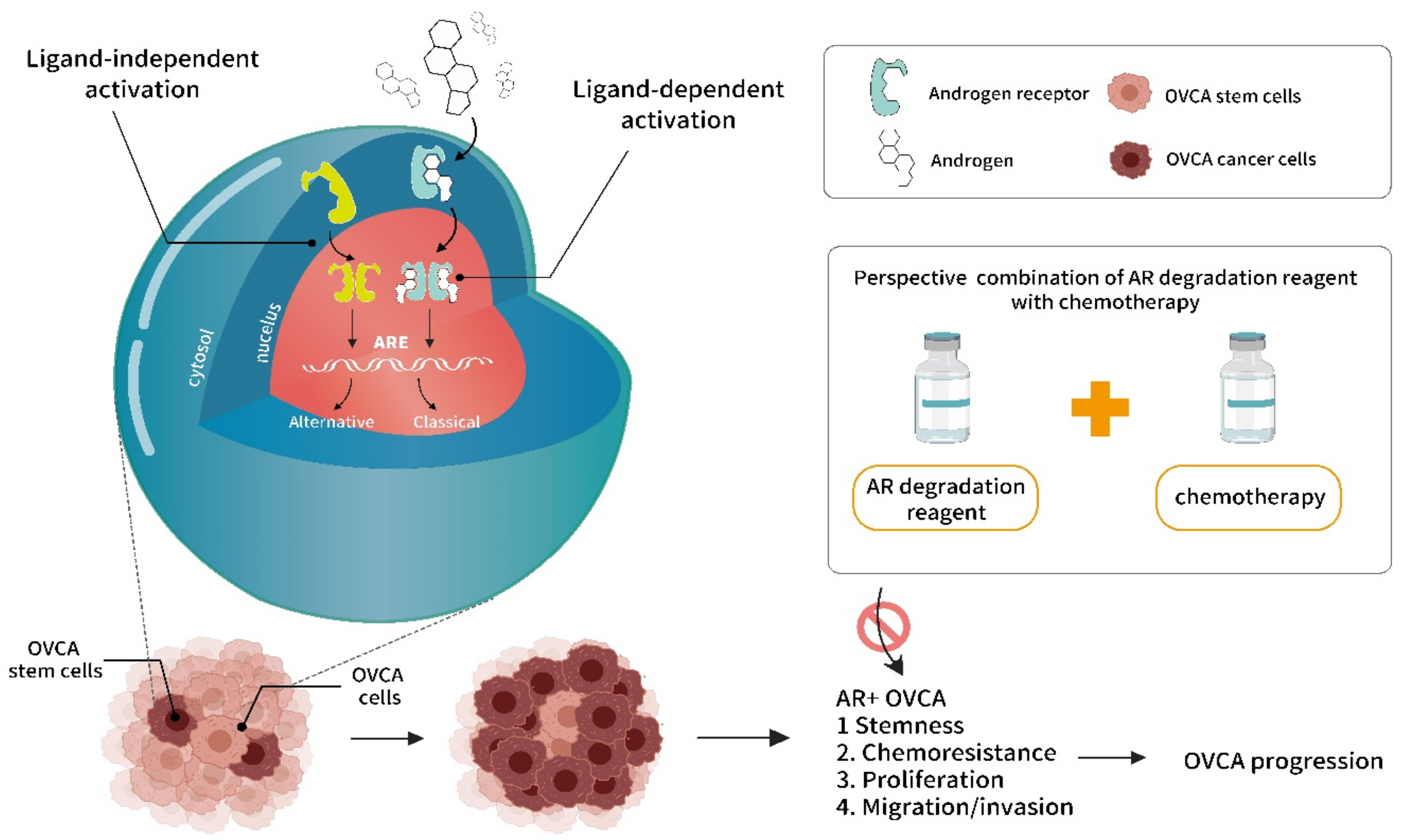
:1. Introduction
2. AR Expression, Genetic Polymorphisms, Function, and Regulation in Human OVCA
2.1. General Description, Classification, and Pathological Features of OVCA
2.2. Biochemical Functions of Androgen/AR Signaling (Classical vs. Non-Classical Androgen/AR Signaling)
2.3. Associations of Androgen Levels, Gene Polymorphisms, and AR Expressions with OVCA Risks
3. Androgen/AR Signaling in OVCA Experimental Models
3.1. Androgen/AR Signaling Function in the Gynecological System
3.2. Androgen/AR Signaling in OVCA Stemness
4. Current Clinical Trials of Targeting Androgen/AR Therapy in OVCA
4.1. Androgen Ablation Therapy in OVCA
4.2. AR Degradation Therapy for OVCA
4.3. Potential for the Use of Anti-Androgen/AR and Anti-PARP Combination Therapy for OVCA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Auersperg, N.; Wong, A.S.; Choi, K.C.; Kang, S.K.; Leung, P.C. Ovarian surface epithelium: Biology, endocrinology, and pathology. Endocr. Rev. 2001, 22, 255–288. [Google Scholar] [CrossRef] [PubMed]
- Varas-Godoy, M.; Rice, G.; Illanes, S.E. The Crosstalk between Ovarian Cancer Stem Cell Niche and the Tumor Microenvironment. Stem Cells Int. 2017, 2017, 5263974. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef]
- Goff, B.A.; Balas, C.; Tenenbaum, C. Ovarian cancer national alliance: A report of the 2012 Consensus Conference on Current Challenges in ovarian cancer. Gynecol. Oncol. 2013, 130, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Gharwan, H.; Bunch, K.P.; Annunziata, C.M. The role of reproductive hormones in epithelial ovarian carcinogenesis. Endocr. Relat. Cancer 2015, 22, R339–R363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizushima, T.; Miyamoto, H. The Role of Androgen Receptor Signaling in Ovarian Cancer. Cells 2019, 8, 176. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.; Kumar, R. Steroid hormone receptors in cancer development: A target for cancer therapeutics. Cancer Lett. 2011, 300, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yu, J.; Mani, R.S.; Cao, Q.; Brenner, C.J.; Cao, X.; Wang, X.; Wu, L.; Li, J.; Hu, M.; et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell 2010, 17, 443–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massie, C.E.; Lynch, A.; Ramos-Montoya, A.; Boren, J.; Stark, R.; Fazli, L.; Warren, A.; Scott, H.; Madhu, B.; Sharma, N.; et al. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J. 2011, 30, 2719–2733. [Google Scholar] [CrossRef] [Green Version]
- Baron, S.; Manin, M.; Beaudoin, C.; Leotoing, L.; Communal, Y.; Veyssiere, G.; Morel, L. Androgen receptor mediates non-genomic activation of phosphatidylinositol 3-OH kinase in androgen-sensitive epithelial cells. J. Biol. Chem. 2004, 279, 14579–14586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonaccorsi, L.; Marchiani, S.; Ferruzzi, P.; Muratori, M.; Crescioli, C.; Forti, G.; Maggi, M.; Baldi, E. Non-genomic effects of the androgen receptor and vitamin D agonist are involved in suppressing invasive phenotype of prostate cancer cells. Steroids 2006, 71, 304–309. [Google Scholar] [CrossRef]
- Vasconsuelo, A.; Pronsato, L.; Ronda, A.C.; Boland, R.; Milanesi, L. Role of 17β-estradiol and testosterone in apoptosis. Steroids 2011, 76, 1223–1231. [Google Scholar] [CrossRef]
- Lee, P.; Rosen, D.G.; Zhu, C.; Silva, E.G.; Liu, J. Expression of progesterone receptor is a favorable prognostic marker in ovarian cancer. Gynecol. Oncol. 2005, 96, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, P.E.; Tindall, D.J. Androgen receptor signaling in prostate cancer development and progression. J. Carcinog. 2011, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Mawji, N.R.; Bruchovsky, N.; Sadar, M.D. Ligand-independent activation of the androgen receptor by interleukin-6 and the role of steroid receptor coactivator-1 in prostate cancer cells. J. Biol. Chem. 2002, 277, 38087–38094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyons, L.S.; Rao, S.; Balkan, W.; Faysal, J.; Maiorino, C.A.; Burnstein, K.L. Ligand-independent activation of androgen receptors by Rho GTPase signaling in prostate cancer. Mol. Endocrinol. 2008, 22, 597–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.; Lee, S.O.; Yeh, S.; Chang, T.M. Androgen receptor (AR) differential roles in hormone-related tumors including prostate, bladder, kidney, lung, breast and liver. Oncogene 2014, 33, 3225–3234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modugno, F.; Laskey, R.; Smith, A.L.; Andersen, C.L.; Haluska, P.; Oesterreich, S. Hormone response in ovarian cancer: Time to reconsider as a clinical target? Endocr. Relat. Cancer 2012, 19, R255–R279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chadha, S.; Rao, B.R.; Slotman, B.J.; van Vroonhoven, C.C.; van der Kwast, T.H. An immunohistochemical evaluation of androgen and progesterone receptors in ovarian tumors. Hum. Pathol. 1993, 24, 90–95. [Google Scholar] [CrossRef]
- Sheach, L.A.; Adeney, E.M.; Kucukmetin, A.; Wilkinson, S.J.; Fisher, A.D.; Elattar, A.; Robson, C.N.; Edmondson, R.J. Androgen-related expression of G-proteins in ovarian cancer. Br. J. Cancer 2009, 101, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Cardillo, M.R.; Petrangeli, E.; Aliotta, N.; Salvatori, L.; Ravenna, L.; Chang, C.; Castagna, G. Androgen receptors in ovarian tumors: Correlation with oestrogen and progesterone receptors in an immunohistochemical and semiquantitative image analysis study. J. Exp. Clin. Cancer Res. 1998, 17, 231–237. [Google Scholar]
- Colombo, N.; Parma, G.; Zanagnolo, V.; Insinga, A. Management of ovarian stromal cell tumors. J. Clin. Oncol. 2007, 25, 2944–2951. [Google Scholar] [CrossRef]
- Horta, M.; Cunha, T.M. Sex cord-stromal tumors of the ovary: A comprehensive review and update for radiologists. Diagn. Interv. Radiol. 2015, 21, 277–286. [Google Scholar] [CrossRef]
- Alexiadis, M.; Eriksson, N.; Jamieson, S.; Davis, M.; Drummond, A.E.; Chu, S.; Clyne, C.D.; Muscat, G.E.; Fuller, P.J. Nuclear receptor profiling of ovarian granulosa cell tumors. Horm. Cancer 2011, 2, 157–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beilby, J.O.; Parkinson, C. Features of prognostic significance in solid ovarian teratoma. Cancer 1975, 36, 2147–2154. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.G.; Dozortsev, D.; Diamond, M.P.; Fracasso, A.; Abdelmassih, S.; Abdelmassih, V.; Goncalves, S.P.; Abdelmassih, R.; Nagy, Z.P. Evidence of parthenogenetic origin of ovarian teratoma: Case report. Hum. Reprod. 2004, 19, 1867–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, L.Y.; Wang, J.J.; Liu, D.R.; Ding, G.P.; Cao, L.P. Management of giant ovarian teratoma: A case series and review of the literature. Oncol. Lett. 2012, 4, 672–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sviracevic, B.; Sedlar, S.; Malobabic, D.; Cuk, D. Mixed malignant germ cell tumor of ovary. Med. Pregl. 2011, 64, 93–95. [Google Scholar] [CrossRef] [Green Version]
- Moniaga, N.C.; Randall, L.M. Malignant mixed ovarian germ cell tumor with embryonal component. J. Pediatr. Adolesc. Gynecol. 2011, 24, e1–e3. [Google Scholar] [CrossRef] [Green Version]
- Koshy, M.; Vijayananthan, A.; Vadiveloo, V. Malignant ovarian mixed germ cell tumour: A rare combination. Biomed. Imaging Interv. J. 2005, 1, e10. [Google Scholar] [CrossRef] [PubMed]
- Zeuthen, J. Human teratocarcinoma cell lines. A review. Int. J. Androl. 1981, 4, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.C.; Chen, C.A.; Chiang, C.J.; Hsu, T.H.; Lin, M.C.; You, S.L.; Cheng, W.F.; Lai, M.S. Trends in incidence and survival outcome of epithelial ovarian cancer: 30-year national population-based registry in Taiwan. J. Gynecol. Oncol. 2013, 24, 342–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.H.; Chen, Y.C.; Chiang, C.J.; Lu, Y.S.; Kuo, K.T.; Huang, C.S.; Cheng, W.F.; Lai, M.S.; You, S.L.; Cheng, A.L. The emerging epidemic of estrogen-related cancers in young women in a developing Asian country. Int. J. Cancer 2012, 130, 2629–2637. [Google Scholar] [CrossRef] [Green Version]
- Rojas, V.; Hirshfield, K.M.; Ganesan, S.; Rodriguez-Rodriguez, L. Molecular Characterization of Epithelial Ovarian Cancer: Implications for Diagnosis and Treatment. Int. J. Mol. Sci. 2016, 17, 2113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koshiyama, M.; Matsumura, N.; Konishi, I. Recent concepts of ovarian carcinogenesis: Type I and type II. Biomed. Res. Int. 2014, 2014, 934261. [Google Scholar] [CrossRef] [Green Version]
- Soslow, R.A. Histologic subtypes of ovarian carcinoma: An overview. Int. J. Gynecol. Pathol. 2008, 27, 161–174. [Google Scholar] [CrossRef]
- Prat, J. New insights into ovarian cancer pathology. Ann. Oncol. 2012, 23, x111–x117. [Google Scholar] [CrossRef]
- Kim, A.; Ueda, Y.; Naka, T.; Enomoto, T. Therapeutic strategies in epithelial ovarian cancer. J. Exp. Clin. Cancer Res. 2012, 31, 14. [Google Scholar] [CrossRef] [Green Version]
- Guttman, M.; Prieto, J.H.; Croy, J.E.; Komives, E.A. Decoding of lipoprotein-receptor interactions: Properties of ligand binding modules governing interactions with apolipoprotein E. Biochemistry 2010, 49, 1207–1216. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.L.; Lee, M.Y.; Chao, W.R.; Han, C.P. The status of Her2 amplification and Kras mutations in mucinous ovarian carcinoma. Hum. Genom. 2016, 10, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anglesio, M.S.; Kommoss, S.; Tolcher, M.C.; Clarke, B.; Galletta, L.; Porter, H.; Damaraju, S.; Fereday, S.; Winterhoff, B.J.; Kalloger, S.E.; et al. Molecular characterization of mucinous ovarian tumours supports a stratified treatment approach with HER2 targeting in 19% of carcinomas. J. Pathol. 2013, 229, 111–120. [Google Scholar] [CrossRef] [PubMed]
- McCluggage, W.G. Morphological subtypes of ovarian carcinoma: A review with emphasis on new developments and pathogenesis. Pathology 2011, 43, 420–432. [Google Scholar] [CrossRef]
- Garces, A.H.; Dias, M.S.; Paulino, E.; Ferreira, C.G.; de Melo, A.C. Treatment of ovarian cancer beyond chemotherapy: Are we hitting the target? Cancer Chemother. Pharmacol. 2014, 75, 221–234. [Google Scholar] [CrossRef]
- Thigpen, T. First-line therapy for ovarian carcinoma: What’s next? Cancer Investig. 2004, 22, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Martin, A.J. Medical treatment of epithelial ovarian cancer. Expert Rev. Anticancer Ther. 2004, 4, 1125–1143. [Google Scholar] [CrossRef]
- Bhoola, S.; Hoskins, W.J. Diagnosis and management of epithelial ovarian cancer. Obstet. Gynecol. 2006, 107, 1399–1410. [Google Scholar] [CrossRef]
- Ko, S.Y.; Naora, H. Therapeutic strategies for targeting the ovarian tumor stroma. World J. Clin. Cases 2014, 2, 194–200. [Google Scholar] [CrossRef]
- Harries, M.; Kaye, S.B. Recent advances in the treatment of epithelial ovarian cancer. Expert Opin. Investig. Drugs 2001, 10, 1715–1724. [Google Scholar] [CrossRef]
- Wang, J.; Li, A.J.; Karlan, B.Y. Chemotherapy in epithelial ovarian cancer. Curr. Women’s Health Rep. 2002, 2, 20–26. [Google Scholar]
- Eltabbakh, G.H. Recent advances in the management of women with ovarian cancer. Minerva Ginecol. 2004, 56, 81–89. [Google Scholar] [PubMed]
- Rizzuto, I.; Stavraka, C.; Chatterjee, J.; Borley, J.; Hopkins, T.G.; Gabra, H.; Ghaem-Maghami, S.; Huson, L.; Blagden, S.P. Risk of Ovarian Cancer Relapse score: A prognostic algorithm to predict relapse following treatment for advanced ovarian cancer. Int. J. Gynecol. Cancer 2015, 25, 416–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elattar, A.; Warburton, K.G.; Mukhopadhyay, A.; Freer, R.M.; Shaheen, F.; Cross, P.; Plummer, E.R.; Robson, C.N.; Edmondson, R.J. Androgen receptor expression is a biological marker for androgen sensitivity in high grade serous epithelial ovarian cancer. Gynecol. Oncol. 2012, 124, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Bulstrode, J.C.; Stratton, I.; Thomas, B.S.; Bulbrook, R.D.; Hayward, J.L. A prospective study of urinary androgen levels and ovarian cancer. Int. J. Cancer 1983, 32, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Helzlsouer, K.J.; Alberg, A.J.; Gordon, G.B.; Longcope, C.; Bush, T.L.; Hoffman, S.C.; Comstock, G.W. Serum gonadotropins and steroid hormones and the development of ovarian cancer. JAMA 1995, 274, 1926–1930. [Google Scholar] [CrossRef]
- Rinaldi, S.; Dossus, L.; Lukanova, A.; Peeters, P.H.; Allen, N.E.; Key, T.; Bingham, S.; Khaw, K.T.; Trichopoulos, D.; Trichopoulou, A.; et al. Endogenous androgens and risk of epithelial ovarian cancer: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC). Cancer Epidemiol. Prev. Biomark. 2007, 16, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ose, J.; Fortner, R.T.; Rinaldi, S.; Schock, H.; Overvad, K.; Tjonneland, A.; Hansen, L.; Dossus, L.; Fournier, A.; Baglietto, L.; et al. Endogenous androgens and risk of epithelial invasive ovarian cancer by tumor characteristics in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2015, 136, 399–410. [Google Scholar] [CrossRef]
- De Toledo, M.C.; Sarian, L.O.; Sallum, L.F.; Andrade, L.L.; Vassallo, J.; de Paiva Silva, G.R.; Pinto, G.A.; Soares, F.A.; Fonseca, C.D.; Derchain, S.F. Analysis of the contribution of immunologically-detectable HER2, steroid receptors and of the “triple-negative” tumor status to disease-free and overall survival of women with epithelial ovarian cancer. Acta Histochem. 2014, 116, 440–447. [Google Scholar] [CrossRef]
- Butler, M.S.; Ricciardelli, C.; Tilley, W.D.; Hickey, T.E. Androgen receptor protein levels are significantly reduced in serous ovarian carcinomas compared with benign or borderline disease but are not altered by cancer stage or metastatic progression. Horm. Cancer 2013, 4, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Nodin, B.; Zendehrokh, N.; Brandstedt, J.; Nilsson, E.; Manjer, J.; Brennan, D.J.; Jirstrom, K. Increased androgen receptor expression in serous carcinoma of the ovary is associated with an improved survival. J. Ovarian Res. 2010, 3, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Zhu, X.; Zheng, L.; Hu, X.; Sun, L.; Zhu, X. The role of the androgen receptor in ovarian cancer carcinogenesis and its clinical implications. Oncotarget 2017, 8, 29395–29405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spurdle, A.B.; Webb, P.M.; Chen, X.; Martin, N.G.; Giles, G.G.; Hopper, J.L.; Chenevix-Trench, G. Androgen receptor exon 1 CAG repeat length and risk of ovarian cancer. Int. J. Cancer 2000, 87, 637–643. [Google Scholar] [CrossRef]
- Dagan, E.; Friedman, E.; Paperna, T.; Carmi, N.; Gershoni-Baruch, R. Androgen receptor CAG repeat length in Jewish Israeli women who are BRCA1/2 mutation carriers: Association with breast/ovarian cancer phenotype. Eur. J. Hum. Genet. 2002, 10, 724–728. [Google Scholar] [CrossRef]
- Santarosa, M.; Bidoli, E.; Gallo, A.; Steffan, A.; Boiocchi, M.; Viel, A. Polymorphic CAG repeat length within the androgen receptor gene: Identification of a subgroup of patients with increased risk of ovarian cancer. Oncol. Rep. 2002, 9, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Terry, K.L.; De Vivo, I.; Titus-Ernstoff, L.; Shih, M.C.; Cramer, D.W. Androgen receptor cytosine, adenine, guanine repeats, and haplotypes in relation to ovarian cancer risk. Cancer Res. 2005, 65, 5974–5981. [Google Scholar] [CrossRef] [Green Version]
- Schildkraut, J.M.; Murphy, S.K.; Palmieri, R.T.; Iversen, E.; Moorman, P.G.; Huang, Z.; Halabi, S.; Calingaert, B.; Gusberg, A.; Marks, J.R.; et al. Trinucleotide repeat polymorphisms in the androgen receptor gene and risk of ovarian cancer. Cancer Epidemiol. Prev. Biomark. 2007, 16, 473–480. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Yuan, J.; Xie, Y.; Li, H.; Wang, Y. Association of androgen receptor CAG repeat polymorphism and risk of epithelial ovarian cancer. Gene 2016, 575, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Lu, P.; Chu, Z.; Fan, Q. The androgen receptor cytosine-adenine-guanine repeat length contributes to the development of epithelial ovarian cancer. Oncotarget 2016, 7, 2105–2112. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Wang, J.; Wang, L.; Du, Y. Androgen receptor gene CAG repeat polymorphism and ovarian cancer risk: A meta-analysis. Biosci. Trends 2017, 11, 193–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engehausen, D.G.; Tong, X.W.; Oehler, M.K.; Freund, C.T.; Schrott, K.M.; Kieback, D.G. Androgen receptor gene mutations do not occur in ovarian cancer. Anticancer Res. 2000, 20, 815–819. [Google Scholar]
- Syed, V.; Ulinski, G.; Mok, S.C.; Yiu, G.K.; Ho, S.M. Expression of gonadotropin receptor and growth responses to key reproductive hormones in normal and malignant human ovarian surface epithelial cells. Cancer Res. 2001, 61, 6768–6776. [Google Scholar] [PubMed]
- Edmondson, R.J.; Monaghan, J.M.; Davies, B.R. The human ovarian surface epithelium is an androgen responsive tissue. Br. J. Cancer 2002, 86, 879–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohan-Ivani, K.; Gabler, F.; Selman, A.; Vega, M.; Romero, C. Role of dihydrotestosterone (DHT) on TGF-beta1 signaling pathway in epithelial ovarian cancer cells. J. Cancer Res. Clin. Oncol. 2016, 142, 47–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evangelou, A.; Jindal, S.K.; Brown, T.J.; Letarte, M. Down-regulation of transforming growth factor beta receptors by androgen in ovarian cancer cells. Cancer Res. 2000, 60, 929–935. [Google Scholar] [PubMed]
- Gui, T.; Shen, K. The epidermal growth factor receptor as a therapeutic target in epithelial ovarian cancer. Cancer Epidemiol. 2012, 36, 490–496. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Gao, Y.; Dong, L.J.; Liu, S.; Yao, Z. Reciprocal regulation of 5α-dihydrotestosterone, interleukin-6 and interleukin-8 during proliferation of epithelial ovarian carcinoma. Cancer Biol. Ther. 2007, 6, 864–871. [Google Scholar] [CrossRef] [Green Version]
- Ligr, M.; Patwa, R.R.; Daniels, G.; Pan, L.; Wu, X.; Li, Y.; Tian, L.; Wang, Z.; Xu, R.; Wu, J.; et al. Expression and function of androgen receptor coactivator p44/Mep50/WDR77 in ovarian cancer. PLoS ONE 2011, 6, e26250. [Google Scholar] [CrossRef]
- Du, F.; Li, Y.; Zhang, W.; Kale, S.P.; McFerrin, H.; Davenport, I.; Wang, G.; Skripnikova, E.; Li, X.L.; Bowen, N.J.; et al. Highly and moderately aggressive mouse ovarian cancer cell lines exhibit differential gene expression. Tumor Biol. 2016, 37, 11147–11162. [Google Scholar] [CrossRef] [Green Version]
- Meacham, C.E.; Morrison, S.J. Tumour heterogeneity and cancer cell plasticity. Nature 2013, 501, 328–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, J.M.; Joseph, S.; Sudhahar, C.G.; Cowden Dahl, K.D. Enrichment for chemoresistant ovarian cancer stem cells from human cell lines. J. Vis. Exp. 2014. [Google Scholar] [CrossRef] [Green Version]
- Lam, S.S.; Mak, A.S.; Yam, J.W.; Cheung, A.N.; Ngan, H.Y.; Wong, A.S. Targeting estrogen-related receptor alpha inhibits epithelial-to-mesenchymal transition and stem cell properties of ovarian cancer cells. Mol. Ther. 2014, 22, 743–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, M.M.; Landen, C.N. Ovarian cancer stem cells: Are they real and why are they important? Gynecol. Oncol. 2014, 132, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Chung, W.M.; Chang, W.C.; Chen, L.; Lin, T.Y.; Chen, L.C.; Hung, Y.C.; Ma, W.L. Ligand-independent androgen receptors promote ovarian teratocarcinoma cell growth by stimulating self-renewal of cancer stem/progenitor cells. Stem Cell Res. 2014, 13, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Chang, W.C.; Hung, Y.C.; Chang, Y.Y.; Bao, B.Y.; Huang, H.C.; Chung, W.M.; Shyr, C.R.; Ma, W.L. Androgen receptor increases CD133 expression and progenitor-like population that associate with cisplatin resistance in endometrial cancer cell line. Reprod. Sci. 2014, 21, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.; Jiang, L.; Liang, S.; Kwong, J.; Yang, L.; Li, Y.; Yin, P.; Deng, Q.; Liang, Z. Nanog interaction with the androgen receptor signaling axis induce ovarian cancer stem cell regulation: Studies based on the CRISPR/Cas9 system. J. Ovarian Res. 2018, 11, 36. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, E.; Montgomery, R.B.; Mostaghel, E.A. Minireview: SLCO and ABC transporters: A role for steroid transport in prostate cancer progression. Endocrinology 2014, 155, 4124–4132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scotto, K.W. Transcriptional regulation of ABC drug transporters. Oncogene 2003, 22, 7496–7511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Liu, C.; Armstrong, C.; Lou, W.; Sandher, A.; Gao, A.C. Antiandrogens Inhibit ABCB1 Efflux and ATPase Activity and Reverse Docetaxel Resistance in Advanced Prostate Cancer. Clin. Cancer Res. 2015, 21, 4133–4142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huisman, M.T.; Chhatta, A.A.; van Tellingen, O.; Beijnen, J.H.; Schinkel, A.H. MRP2 (ABCC2) transports taxanes and confers paclitaxel resistance and both processes are stimulated by probenecid. Int. J. Cancer 2005, 116, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.; Ross, D.D. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene 2003, 22, 7340–7358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, W.M.; Ho, Y.P.; Chang, W.C.; Dai, Y.C.; Chen, L.; Hung, Y.C.; Ma, W.L. Increase Paclitaxel Sensitivity to Better Suppress Serous Epithelial Ovarian Cancer via Ablating Androgen Receptor/Aryl Hydrocarbon Receptor-ABCG2 Axis. Cancers 2019, 11, 463. [Google Scholar] [CrossRef] [Green Version]
- Gruessner, C.; Gruessner, A.; Glaser, K.; AbuShahin, N.; Zhou, Y.; Laughren, C.; Wright, H.; Pinkerton, S.; Yi, X.; Stoffer, J.; et al. Flutamide and biomarkers in women at high risk for ovarian cancer: Preclinical and clinical evidence. Cancer Prev. Res. 2014, 7, 896–905. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, H.; Messing, E.M.; Chang, C. Androgen deprivation therapy for prostate cancer: Current status and future prospects. Prostate 2004, 61, 332–353. [Google Scholar] [CrossRef]
- Tumolo, S.; Rao, B.R.; van der Burg, M.E.; Guastalla, J.P.; Renard, J.; Vermorken, J.B. Phase II trial of flutamide in advanced ovarian cancer: An EORTC Gynaecological Cancer Cooperative Group study. Eur. J. Cancer 1994, 30, 911–914. [Google Scholar] [CrossRef]
- Vassilomanolakis, M.; Koumakis, G.; Barbounis, V.; Hajichristou, H.; Tsousis, S.; Efremidis, A. A phase II study of flutamide in ovarian cancer. Oncology 1997, 54, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.; Park, K.; Juretzka, M.; Esch, J.; Hensley, M.; Aghajanian, C.; Lewin, S.; Konner, J.; Derosa, F.; Spriggs, D.; et al. A phase II evaluation of goserelin and bicalutamide in patients with ovarian cancer in second or higher complete clinical disease remission. Cancer 2007, 110, 2448–2456. [Google Scholar] [CrossRef] [PubMed]
- Makar, A.P. Hormone therapy in epithelial ovarian cancer. Endocr. Relat. Cancer 2000, 7, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, Y.; Mizunuma, H. Recurrent epithelial ovarian cancer and hormone therapy. World J. Clin. Cases 2013, 1, 187–190. [Google Scholar] [CrossRef]
- Hirakawa, H.; Yokoyama, Y.; Yoshida, H.; Mizunuma, H. Inhibitory effects of aromatase inhibitor on estrogen receptor-alpha positive ovarian cancer in mice. J. Ovarian Res. 2014, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Swisher, E.M.; Lin, K.K.; Oza, A.M.; Scott, C.L.; Giordano, H.; Sun, J.; Konecny, G.E.; Coleman, R.L.; Tinker, A.V.; O’Malley, D.M.; et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): An international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Ang, Y.L.E.; Tan, D.S.P. Development of PARP inhibitors in gynecological malignancies. Curr. Probl. Cancer 2017, 41, 273–286. [Google Scholar] [CrossRef]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N. Engl. J. Med. 2012, 366, 1382–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lheureux, S.; Lai, Z.; Dougherty, B.A.; Runswick, S.; Hodgson, D.R.; Timms, K.M.; Lanchbury, J.S.; Kaye, S.; Gourley, C.; Bowtell, D.; et al. Long-Term Responders on Olaparib Maintenance in High-Grade Serous Ovarian Cancer: Clinical and Molecular Characterization. Clin. Cancer Res. 2017, 23, 4086–4094. [Google Scholar] [CrossRef] [Green Version]
- Asim, M.; Tarish, F.; Zecchini, H.I.; Sanjiv, K.; Gelali, E.; Massie, C.E.; Baridi, A.; Warren, A.Y.; Zhao, W.; Ogris, C.; et al. Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat. Commun. 2017, 8, 374. [Google Scholar] [CrossRef] [Green Version]
- Pezaro, C. PARP inhibitor combinations in prostate cancer. Ther. Adv. Med. Oncol. 2020, 12. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Karanika, S.; Yang, G.; Wang, J.; Park, S.; Broom, B.M.; Manyam, G.C.; Wu, W.; Luo, Y.; Basourakos, S.; et al. Androgen receptor inhibitor-induced “BRCAness” and PARP inhibition are synthetically lethal for castration-resistant prostate cancer. Sci. Signal. 2017, 10, eaam7479. [Google Scholar] [CrossRef] [Green Version]
| Study | No. of Patients | Clinical Trial | Treatment | Key Findings |
|---|---|---|---|---|
| Thompson et al. (1991) | 62 | NR | cyproterone acetate | 6.8% patients partially responded. |
| Tumolo et al. (1994) | 32/68 | phase II | oral flutamide treatment (750 mg/day) | Combined chemotherapy was invalid with side effects. |
| Van der Vange et al. (1995) | 33 | Phase II | flutamide (500 mg i.m) | 1 patients showed stable disease for 8 months. |
| Vassilomanolakis et al. (1997) | 43 | phase II | flutamide (300 mg/day) | Partial response (4.3%) and disease stabilization (8.7%) in 23 patients. |
| Levine et al. (2007) | 35 | phase II | bicalutamide (50 mg/daily) and goserelin (3.6 mg/mon) | Failed to prolong PFS in EOC patients |
| Rachel et al. (2017) | 59 | phase II | Enzalutamide (160mg by mouth QD) | Currently recruiting patients (NCT01974765) |
| Susana et al. (2020) | 42 | Phase II | abiraterone (1000mg daily) | Closured. |
| Study | Cell Lines | Treatment | MOA | Key Findings |
|---|---|---|---|---|
| Park et al. (2016) | OVCAR-3 | Enzalutamide | NR | Showed efficacy in the ovarian cancer with AR expression. |
| Lin et al. (2018) | A2780 and SKOV3 | ASC-J9 | Suppressing AR→Nanog axis | AR promotes Nanog expression which contribute to CSPCs stemness in EOC cells |
| Chung et al. (2019) | HeyA8, SKOV3ip1, OVCAR-3 | Paclitaxel/ASC-J9 | Suppressing AR→ABCG2 axis | Degradation of AR is effective in suppressing OCSO subtype. |
| Addie et al. (2019) | A2780, OV90, OVCAR3, OVCAR8, SKOV3, COV362.4 | Metformin/enzalutamide | AR→PI3K pathway | Anti-androgen failed to suppress AR+ EOC cells, implicating an ligand-independent pathway of AR. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, W.-M.; Chen, L.; Chang, W.-C.; Su, S.-Y.; Hung, Y.-C.; Ma, W.-L. Androgen/Androgen Receptor Signaling in Ovarian Cancer: Molecular Regulation and Therapeutic Potentials. Int. J. Mol. Sci. 2021, 22, 7748. https://doi.org/10.3390/ijms22147748
Chung W-M, Chen L, Chang W-C, Su S-Y, Hung Y-C, Ma W-L. Androgen/Androgen Receptor Signaling in Ovarian Cancer: Molecular Regulation and Therapeutic Potentials. International Journal of Molecular Sciences. 2021; 22(14):7748. https://doi.org/10.3390/ijms22147748
Chicago/Turabian StyleChung, Wei-Min, Lumin Chen, Wei-Chun Chang, Sheng-Yuan Su, Yao-Ching Hung, and Wen-Lung Ma. 2021. "Androgen/Androgen Receptor Signaling in Ovarian Cancer: Molecular Regulation and Therapeutic Potentials" International Journal of Molecular Sciences 22, no. 14: 7748. https://doi.org/10.3390/ijms22147748
APA StyleChung, W.-M., Chen, L., Chang, W.-C., Su, S.-Y., Hung, Y.-C., & Ma, W.-L. (2021). Androgen/Androgen Receptor Signaling in Ovarian Cancer: Molecular Regulation and Therapeutic Potentials. International Journal of Molecular Sciences, 22(14), 7748. https://doi.org/10.3390/ijms22147748







