Chitosan-Based Functional Films Integrated with Magnolol: Characterization, Antioxidant and Antimicrobial Activity and Pork Preservation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Film Characterization
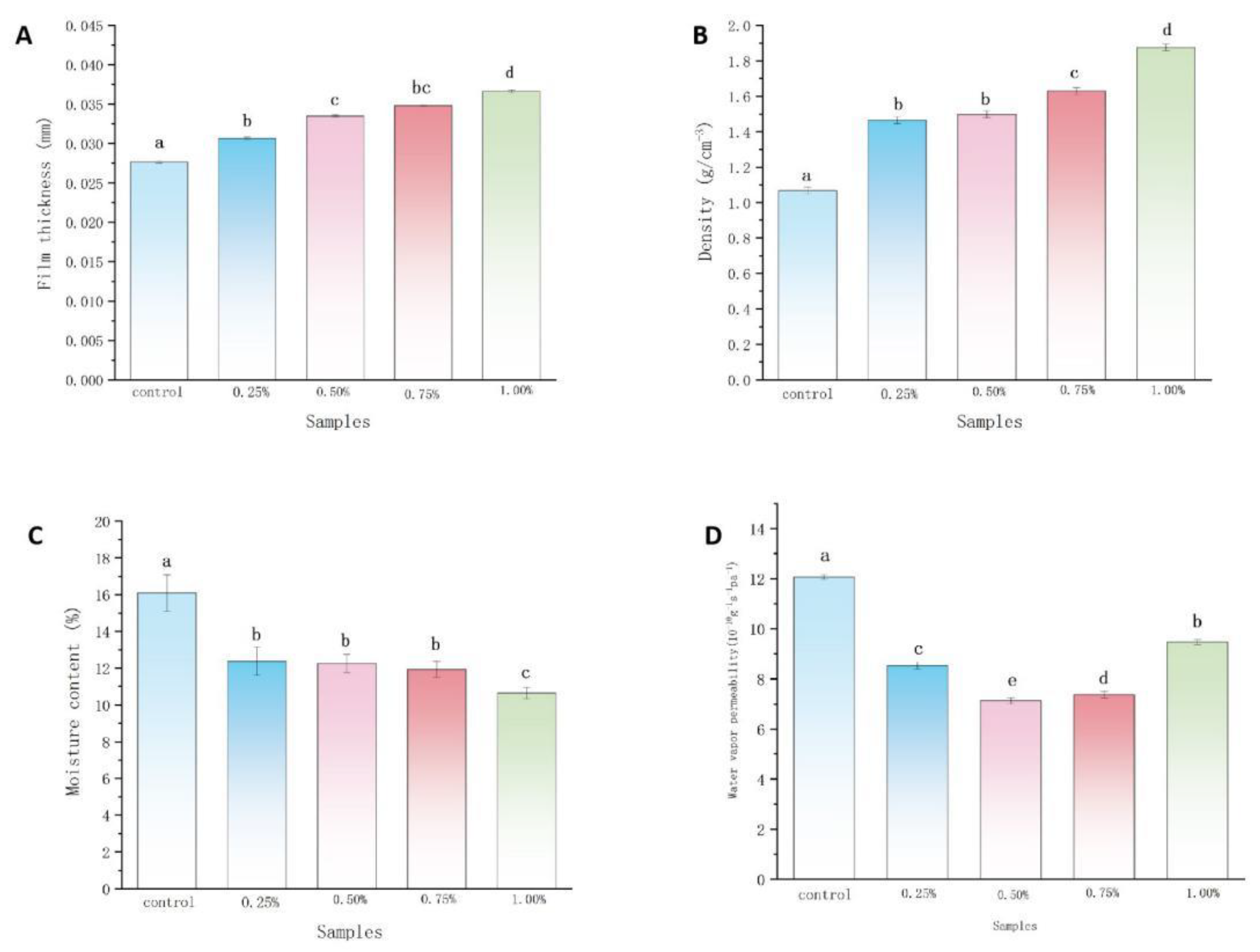
2.1.1. Film Thickness and Density Analysis
2.1.2. Moisture Content and WVP
2.1.3. Color Analysis
2.1.4. Microstructure of Magnolol–Chitosan Films
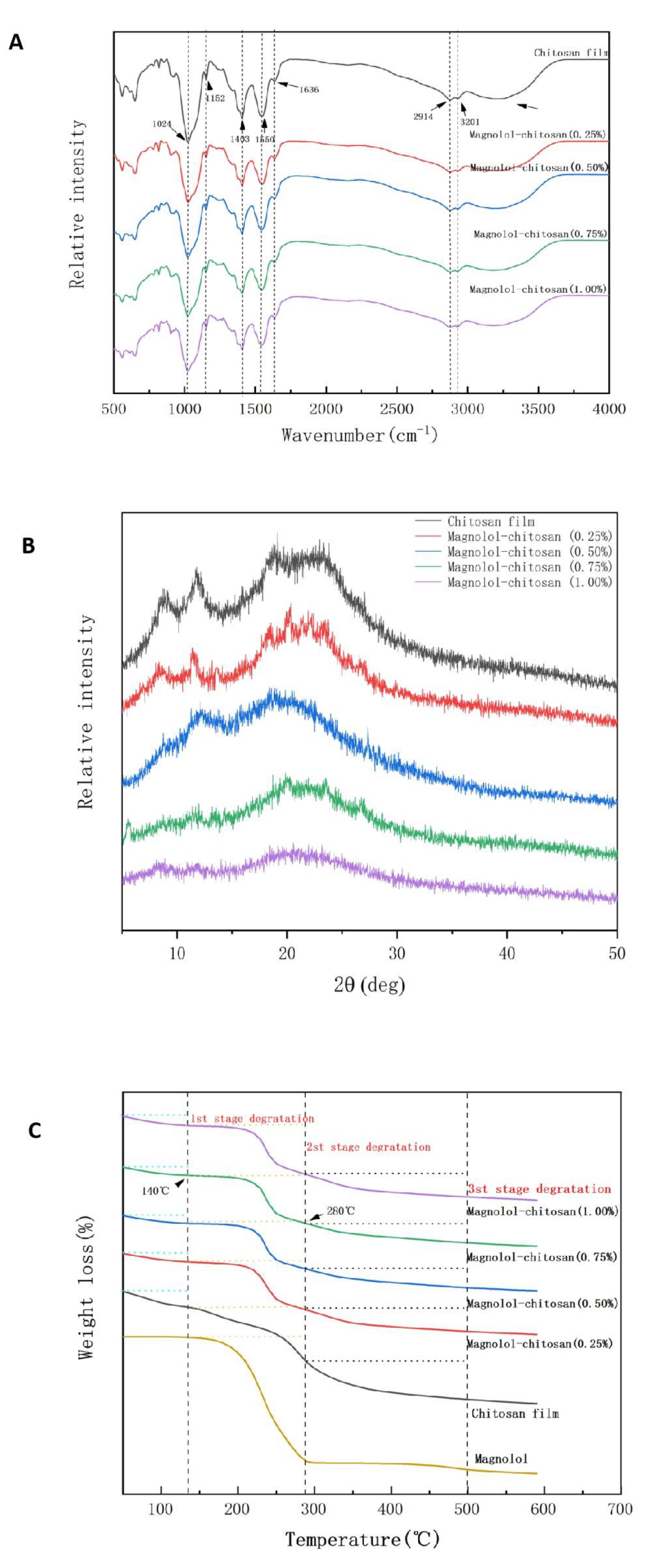
2.1.5. FTIR Spectroscopy Analysis
2.1.6. XRD Analysis
2.1.7. TGA Analysis
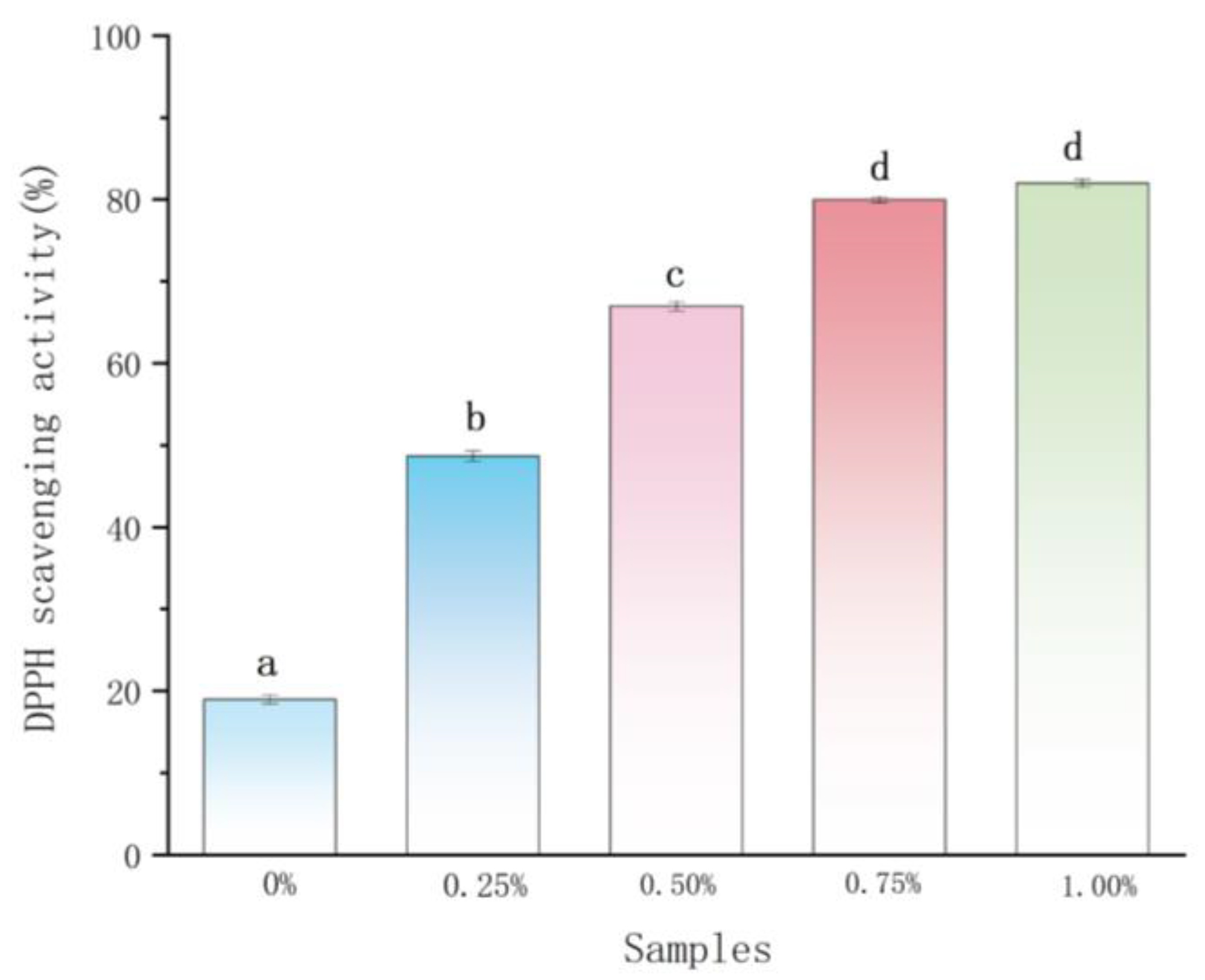
2.2. Antioxidant Activity Analysis
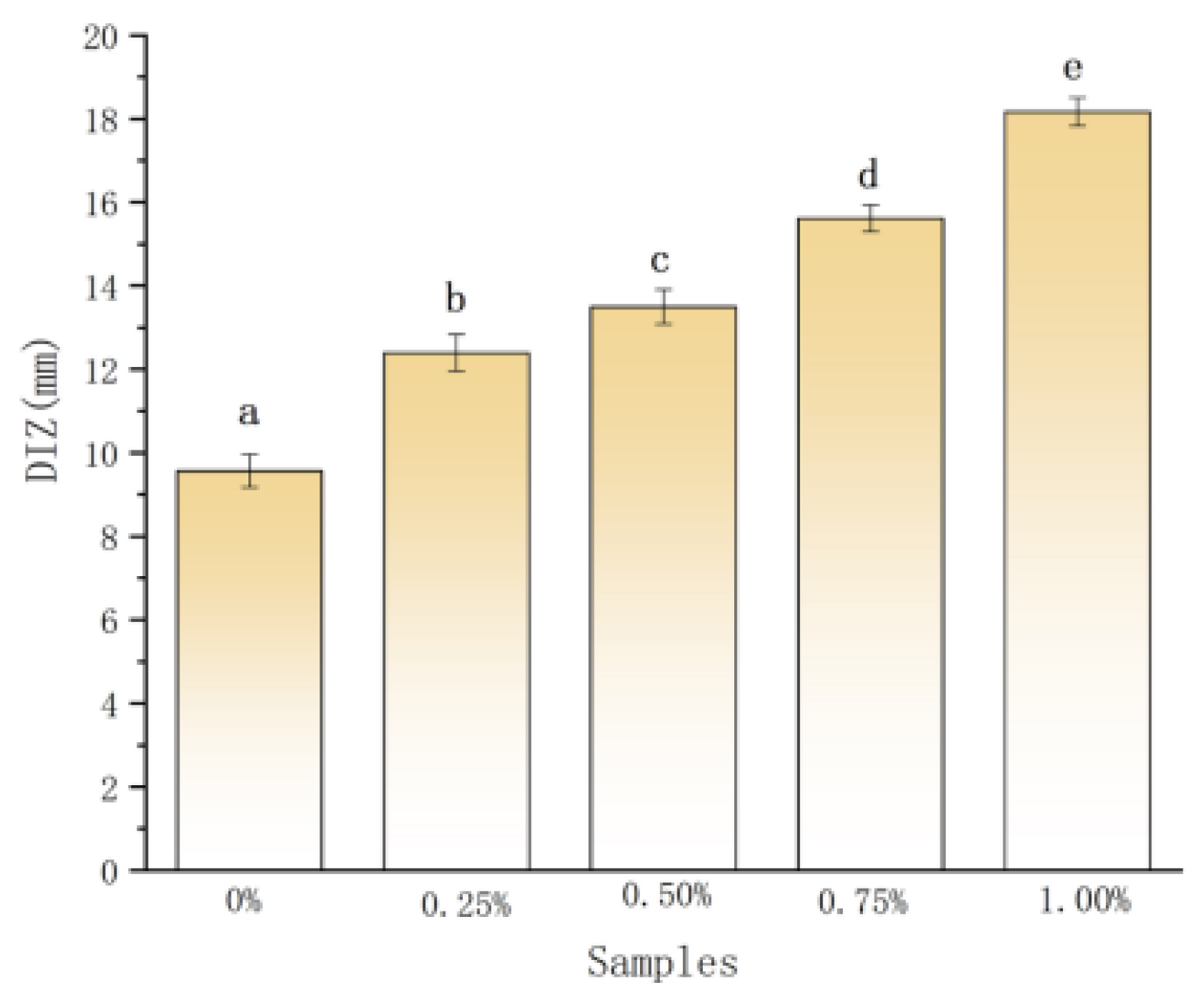
2.3. Antibacterial Activity on Planktonic P. aeruginosa
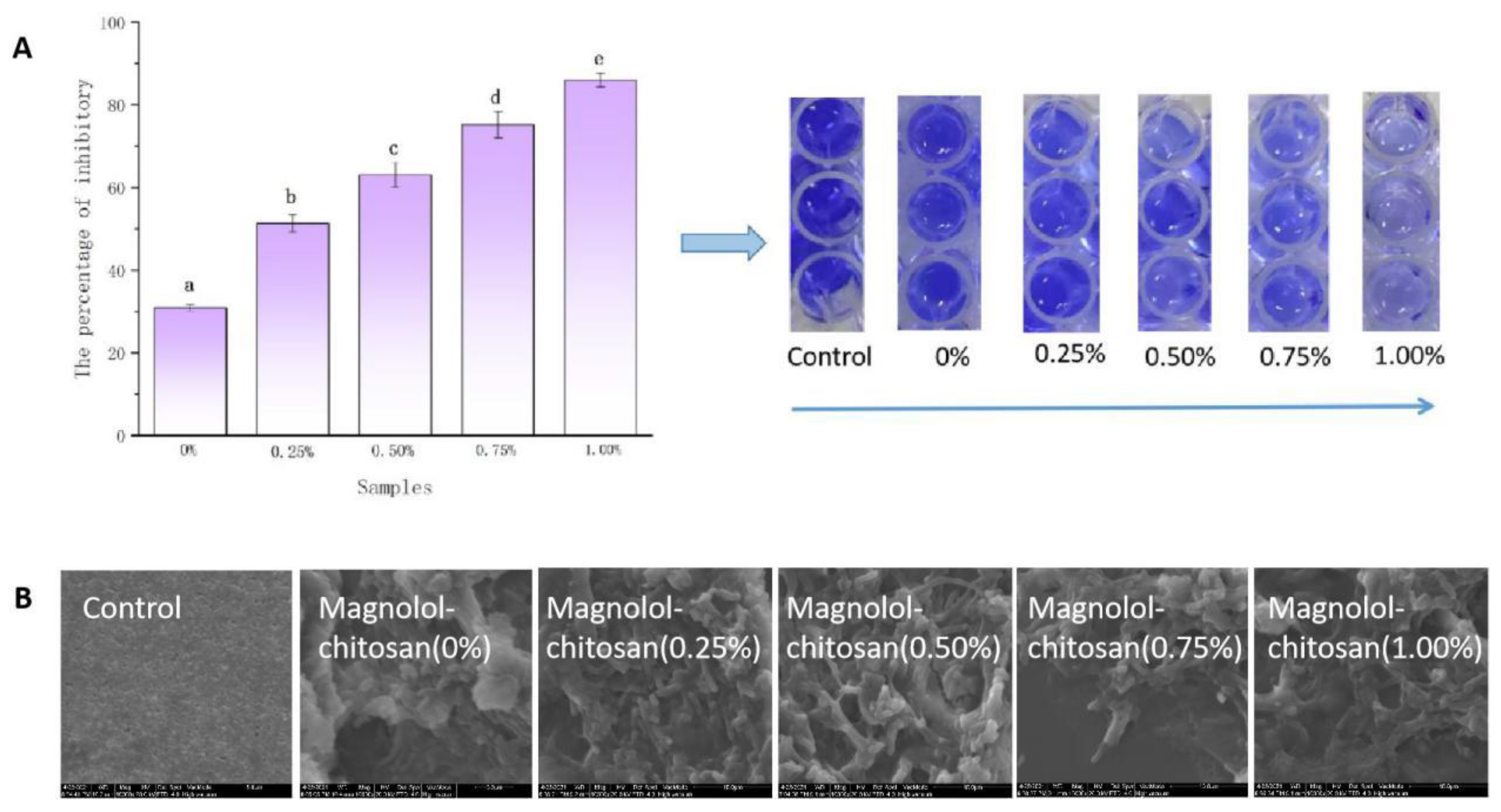
2.4. Inhibition of Biofilm Formation
2.5. Applications in Pork Preservation
3. Materials and Methods
3.1. Materials and Reagents
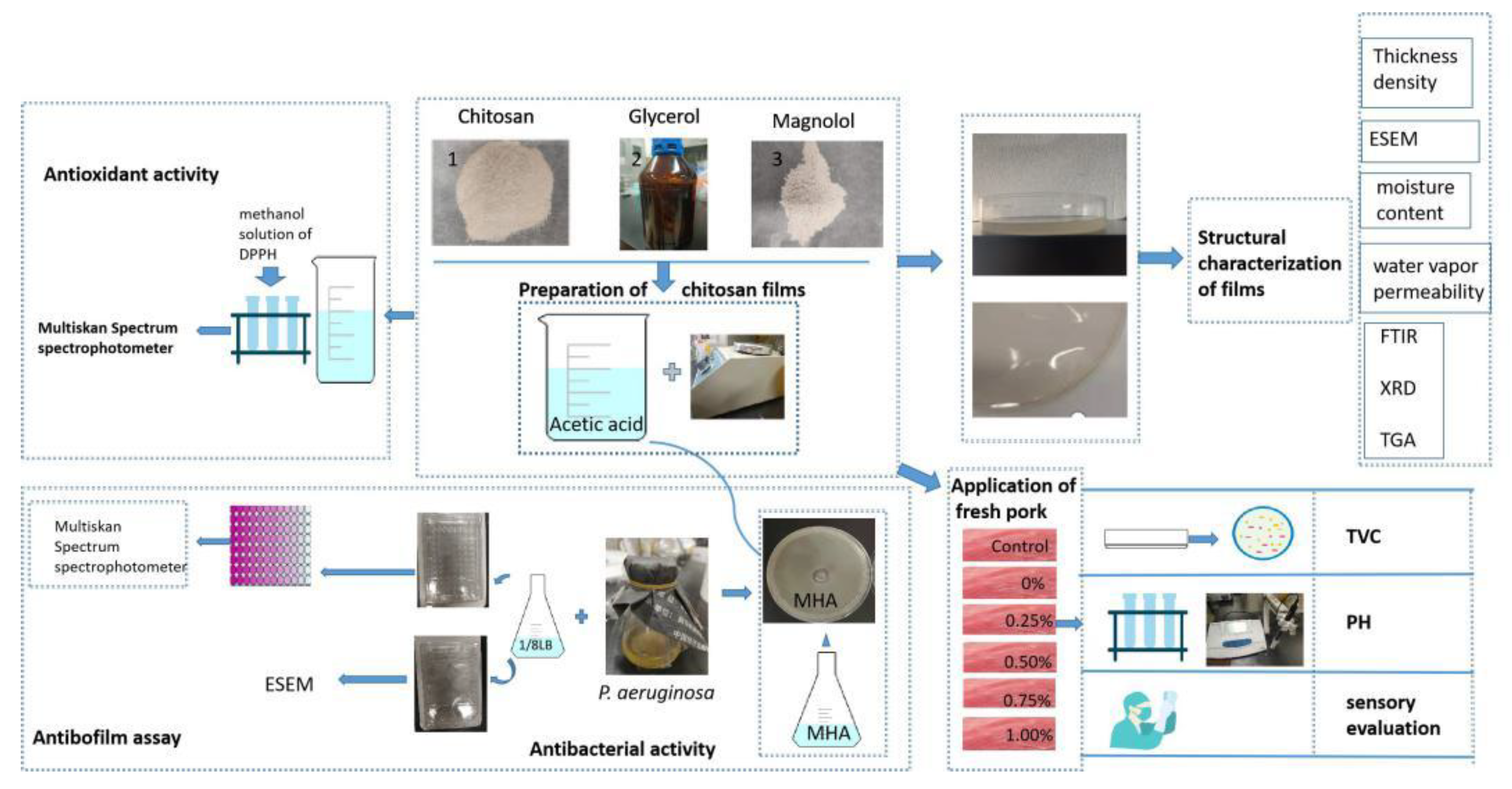
3.2. Preparation of Chitosan Films
3.3. Application of Fresh Pork
3.4. Characterization Methods
3.4.1. Film Thickness and Density Assays
3.4.2. Color Assay
3.4.3. Moisture Content and Water Solubility Assays
3.4.4. WVP Assay
3.5. Structural Characterization of Films
3.5.1. ESEM Assay
3.5.2. FTIR Spectroscopy Assay
3.5.3. XRD Assay
3.5.4. Thermal Property Assay
3.6. Antioxidant Activity Assay
3.7. Antibacterial Activity Assay
3.8. Antibiofilm Assay
3.8.1. Inhibitory Activities on Biofilm Formation
3.8.2. ESEM Analysis of P. aeruginosa Biofilm
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aymerich, T.; Picouet, P.A.; Monfort, J.M. Decontamination technologies for meat products. Meat Sci. 2008, 78, 114–129. [Google Scholar] [CrossRef]
- Wu, M.C.; Li, H.C.; Wu, P.H.; Huang, P.H.; Wang, Y.T. Assessment of oligogalactur onide from citrus pectin as a potential antibacterial agent against foodborne pathogens. J. Food Sci. 2014, 79, M1541–M1544. [Google Scholar] [CrossRef]
- Zhou, G.H.; Xu, X.L.; Liu, Y. Preservation technologies for fresh meat—A review. Meat Sci. 2010, 86, 119–128. [Google Scholar] [CrossRef]
- Liu, X.; Cai, J.; Chen, H.; Zhong, Q.; Hou, Y.; Chen, W.; Chen, W. Antibacterial activity and mechanism of linalool against Pseudomonas aeruginosa. Microb. Pathog. 2020, 141, 103980. [Google Scholar] [CrossRef]
- Liu, P.V. Extracellular toxins of Pseudomonas aeruginosa. J. Infect. Dis. 1974, 130, S94–S99. [Google Scholar] [CrossRef]
- Cendra, M.D.M.; Torrents, E. Pseudomonas aeruginosa biofilms and their partners in crime. Biotechnol. Adv. 2021, 49, 107734. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, D.; Ghigo, J.M.; Beloin, C. Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauch, R.M.; Norregaard, L.L.; Ciofu, O.; Levy, C.E.; Hoiby, N. IgG avidity to Pseudomonas aeruginosa over the course of chronic lung biofilm infection in cystic fibrosis. J. Cyst. Fibros. 2018, 17, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Fu, X.Y. Effects of various plasticizers on mechanical and water vapor barrier properties of gelatin films. Food Hydrocoll. 2009, 23, 729–735. [Google Scholar] [CrossRef]
- Tharanathan, R.N. Biodegradable films and composite coatings: Past, present and future. Trends Food Sci. Technol. 2003, 14, 71–78. [Google Scholar] [CrossRef]
- Bactericidal, A.; Machado, B.R.; Facchi, S.P.; Oliveira, A.; Martins, A.F. Chitosan/glycerol films for food pack coatings: A critical viewpoint. Int. J. Mol. Sci. 2020, 21, 8663. [Google Scholar]
- Adiletta, G.; Matteo, M.D.; Petriccione, M. Multifunctional role of chitosan edible coatings on antioxidant systems in fruit crops: A review. Int. J. Mol. Sci. 2021, 22, 2633. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Chander, R.; Sharma, A. Chitosan glucose complex—A novel food preservative. Food Chem. 2008, 106, 521–528. [Google Scholar] [CrossRef]
- Dutta, P.K.; Tripathi, S.; Mehrotra, G.K.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Ngo, T.; Nguyen, T.H.; Dang, T.; Tran, T.X.; Rachtanapun, P. Characteristics and antimicrobial properties of active edible films based on pectin and nanochitosan. Int. J. Mol. Sci. 2020, 21, 2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.; Dang, H.; Liu, L.; Hu, X.; Li, X.; Ma, Z.; Ren, T. Effect of syringic acid incorporation on the physical, mechanical, structural and antibacterial properties of chitosan film for quail eggs preservation. Int. J. Biol. Macromol. 2019, 141, 876–884. [Google Scholar] [CrossRef]
- Chiu, K.C.; Shih, Y.H.; Wang, T.H.; Lan, W.C.; Li, P.J.; Huang, H.S.; Shieh, T.M. In vitro antimicrobial and antipro-inflammation potential of honokiol and magnolol against oral pathogens and macrophages. J. Med. Assoc. 2021, 120, 827–837. [Google Scholar]
- Behbehani, J.; Shreaz, S.; Irshad, M.; Karched, M. The natural compound magnolol affects growth, biofilm formation, and ultrastructure of oral Candida isolates. Microb. Pathog. 2017, 113, 209–217. [Google Scholar] [CrossRef]
- Sun, L.; Liao, K. Effects of magnolol and honokiol on adhesion, yeast-hyphal transition, and formation of biofilm by Candida albicans. PLoS ONE 2015, 10, e0117695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, H.; Qiu, J.Z.; Wang, D.C.; Jiang, Y.S.; Xia, L.J.; Deng, X.M. Influence of magnolol on the secretion of alpha-toxin by Staphylococcus Aureus. Molecules 2010, 15, 1679–1689. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Wu, Y.; Li, Y. Development of tea extracts and chitosan composite films for active packaging materials. Int. J. Biol. Macromol. 2013, 59, 282–289. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Tang, K.; Hu, X.; Zou, G. Physicochemical characterization and antioxidant activity of quercetin-loaded chitosan nanoparticles. J. Appl. Polym. Sci. 2008, 107, 891–897. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and characterization of chitosan film incorporated with thinned young apple polyphenols as an active packaging material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siripatrawan, U.; Harte, B.R. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Liu, T.; Liu, L.; Gong, X.; Chi, F.; Ma, Z. Fabrication and comparison of active films from chitosan incorporating different spice extracts for shelf life extension of refrigerated pork. LWT 2021, 135, 110181. [Google Scholar] [CrossRef]
- Zarandona, I.; Puertas, A.I.; Dueñas, M.T.; Guerrero, P.; de la Caba, K. Assessment of active chitosan films incorporated with gallic acid. Food Hydrocoll. 2020, 101, 105486. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Wu, Q.; Gu, Y.; Kan, J.; Jin, C. Effect of protocatechuic acid incorporation on the physical, mechanical, structural and antioxidant properties of chitosan film. Food Hydrocoll. 2017, 73, 90–100. [Google Scholar] [CrossRef]
- Rubilar, J.F.; Cruz, R.M.S.; Silva, H.D.; Vicente, A.A.; Khmelinskii, I.; Vieira, M.C. Physico-mechanical properties of chitosan films with carvacrol and grape seed extract. J. Food Eng. 2013, 115, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Kalaycıoglu, Z.; Torlak, E.; Akın-Evingür, G.; Ozen, I.; Erim, F.B. Antimicrobial and physical properties of chitosan films incorporated with turmeric extract. Int. J. Biol. Macromol. 2017, 101, 882–888. [Google Scholar] [CrossRef]
- Talon, E.; Trifkovic, K.T.; Nedovic, V.A.; Bugarski, B.M.; Vargas, M.; Chiralt, A.; Gonzalez-Martinez, C. Antioxidant edible films based on chitosan and starch containing polyphenols from thyme extracts. Carbohydr Polym. 2017, 157, 1153–1161. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Y.; Jiang, X.; Wu, J.; Le, X. Molecular interactions, characterization and antimicrobial activity of curcumin–chitosan blend films. Food Hydrocoll. 2016, 52, 564–572. [Google Scholar] [CrossRef]
- Altiok, D.; Altiok, E.; Tihminlioglu, F. Physical, antibacterial and antioxidant properties of chitosan films incorporated with thyme oil for potential wound healing applications. J. Mater. Sci. Mater. Med. 2010, 21, 2227–2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; He, Y.; Liu, F.; Liao, L.; Huang, X.; Li, R.; Li, J. Carboxymethyl chitosan-pullulan edible films enriched with galangal essential oil: Characterization and application in mango preservation. Carbohydr. Polym. 2021, 256, 117579. [Google Scholar] [CrossRef] [PubMed]
- Ogata, M.; Hoshi, M.; Shimotohno, K.; Urano, S.; Endo, T. Antioxidant activity of magnolol, honokiol, and related phenolic compounds. J. Am. Oil Chem. Soc. 1997, 74, 557–562. [Google Scholar] [CrossRef]
- Kang, J.; Jin, W.; Wang, J.; Sun, Y.; Wu, X.; Liu, L. Antibacterial and anti-biofilm activities of peppermint essential oil against staphylococcus aureus. LWT 2019, 101, 639–645. [Google Scholar] [CrossRef]
- Sakaue, Y.; Domon, H.; Oda, M.; Takenaka, S.; Kubo, M.; Fukuyama, Y.; Terao, Y. Anti-biofilm and bactericidal effects of magnolia bark-derived magnolol and honokiol on Streptococcus mutans. Microbiol. Immunol. 2016, 60, 10–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khadke, S.K.; Lee, J.H.; Woo, J.T.; Lee, J. Inhibitory effects of honokiol and magnolol on biofilm formation by Acinetobacter baumannii. Biotechnol. Bioprocess Eng. 2019, 24, 359–365. [Google Scholar] [CrossRef]
- Wang, J.H.; Shih, K.S.; Liou, J.P.; Wu, Y.W.; Chang, A.S.Y.; Wang, K.L.; Tsai, C.L.; Yang, C.R. Anti-arthritic effects of magnolol in human interleukin 1β-stimulated fibroblast-like synoviocytes and in a rat arthritis model. PLoS ONE 2012, 7, 31368. [Google Scholar] [CrossRef]
- Miao, J.; Peng, W.; Liu, G.; Chen, Y.; Chen, F.; Cao, Y. Biopreservative effect of the natural antimicrobial substance from Lactobacillus paracasei subsp. tolerans FX-6 on fresh pork during chilled storage. Food Control 2015, 56, 53–56. [Google Scholar]
- Cao, Y.; Warner, R.D.; Fang, Z. Effect of chitosan/nisin/gallic acid coating on preservation of pork loin in high oxygen modified atmosphere packaging. Food Control 2019, 101, 9–16. [Google Scholar] [CrossRef]
- Ruan, C.; Zhang, Y.; Sun, Y.; Gao, X.; Liang, J. Effect of sodium alginate and carboxymethyl cellulose edible coating with epigallocatechin gallate on quality and shelf life of fresh pork. Int. J. Biol. Macromol. 2019, 141, 178–184. [Google Scholar] [CrossRef] [PubMed]


| Color Parameters | Films | ||||
|---|---|---|---|---|---|
| Magnolol–Chitosan (0%) | Magnolol–Chitosan (0.25%) | Magnolol–Chitosan (0.50%) | Magnolol–Chitosan (0.75%) | Magnolol–Chitosan (1.00%) | |
| a* | −0.18 ± 0.01 d | −0.12 ± 0.01 c | 0.11 ± 0.03 b | 0.25 ± 0.04 a | 0.28 ± 0.01 a |
| b* | 1.99 ± 0.05 e | 3.73 ± 0.04 d | 9.71 ± 0.01 c | 12.19 ± 0.06 b | 13.09 ± 0.02 a |
| L* | 67.40 ± 0.02 d | 62.22 ± 0.03 d | 60.11 ± 0.01 c | 57.21 ± 0.04 b | 51.79 ± 0.04 a |
| ΔE | 30.65 ± 0.02 e | 35.90 ± 0.02 d | 38.96 ± 0.01 c | 42.34 ± 0.06 b | 47.84 ± 0.01 a |
| WI | 67.39 ± 0.02 e | 62.02 ± 0.02 d | 58.94 ± 0.01 c | 55.54 ± 0.01 b | 50.04 ± 0.01 a |
| Sample Code | Magnolol Content | ||||
|---|---|---|---|---|---|
| 0% | 0.25% | 0.5% | 0.75% | 1% | |
| Chitosan (g) | 1 g | 1 g | 1 g | 1 g | 1 g |
| 1% Acetic acid (mL) | 50 mL | 50 mL | 50 mL | 50 mL | 50 mL |
| Glycerol (mL) | 0.3 mL | 0.3 mL | 0.3 mL | 0.3 mL | 0.3 mL |
| Magnolol (g) | 0 g | 0.25 g | 0.5 g | 0.75 g | 1 g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Liu, L.; Wu, X.; Liu, Y.; Yuan, J. Chitosan-Based Functional Films Integrated with Magnolol: Characterization, Antioxidant and Antimicrobial Activity and Pork Preservation. Int. J. Mol. Sci. 2021, 22, 7769. https://doi.org/10.3390/ijms22157769
Song X, Liu L, Wu X, Liu Y, Yuan J. Chitosan-Based Functional Films Integrated with Magnolol: Characterization, Antioxidant and Antimicrobial Activity and Pork Preservation. International Journal of Molecular Sciences. 2021; 22(15):7769. https://doi.org/10.3390/ijms22157769
Chicago/Turabian StyleSong, Xueying, Liu Liu, Xiaoxia Wu, Yongfeng Liu, and Jialu Yuan. 2021. "Chitosan-Based Functional Films Integrated with Magnolol: Characterization, Antioxidant and Antimicrobial Activity and Pork Preservation" International Journal of Molecular Sciences 22, no. 15: 7769. https://doi.org/10.3390/ijms22157769
APA StyleSong, X., Liu, L., Wu, X., Liu, Y., & Yuan, J. (2021). Chitosan-Based Functional Films Integrated with Magnolol: Characterization, Antioxidant and Antimicrobial Activity and Pork Preservation. International Journal of Molecular Sciences, 22(15), 7769. https://doi.org/10.3390/ijms22157769






