Delayed Double Treatment with Adult-Sourced Adipose-Derived Mesenchymal Stem Cells Increases Striatal Medium-Spiny Neuronal Number, Decreases Striatal Microglial Number, and Has No Subventricular Proliferative Effect, after Acute Neonatal Hypoxia-Ischemia in Male Rats
Abstract
:1. Introduction
2. Materials and Methods
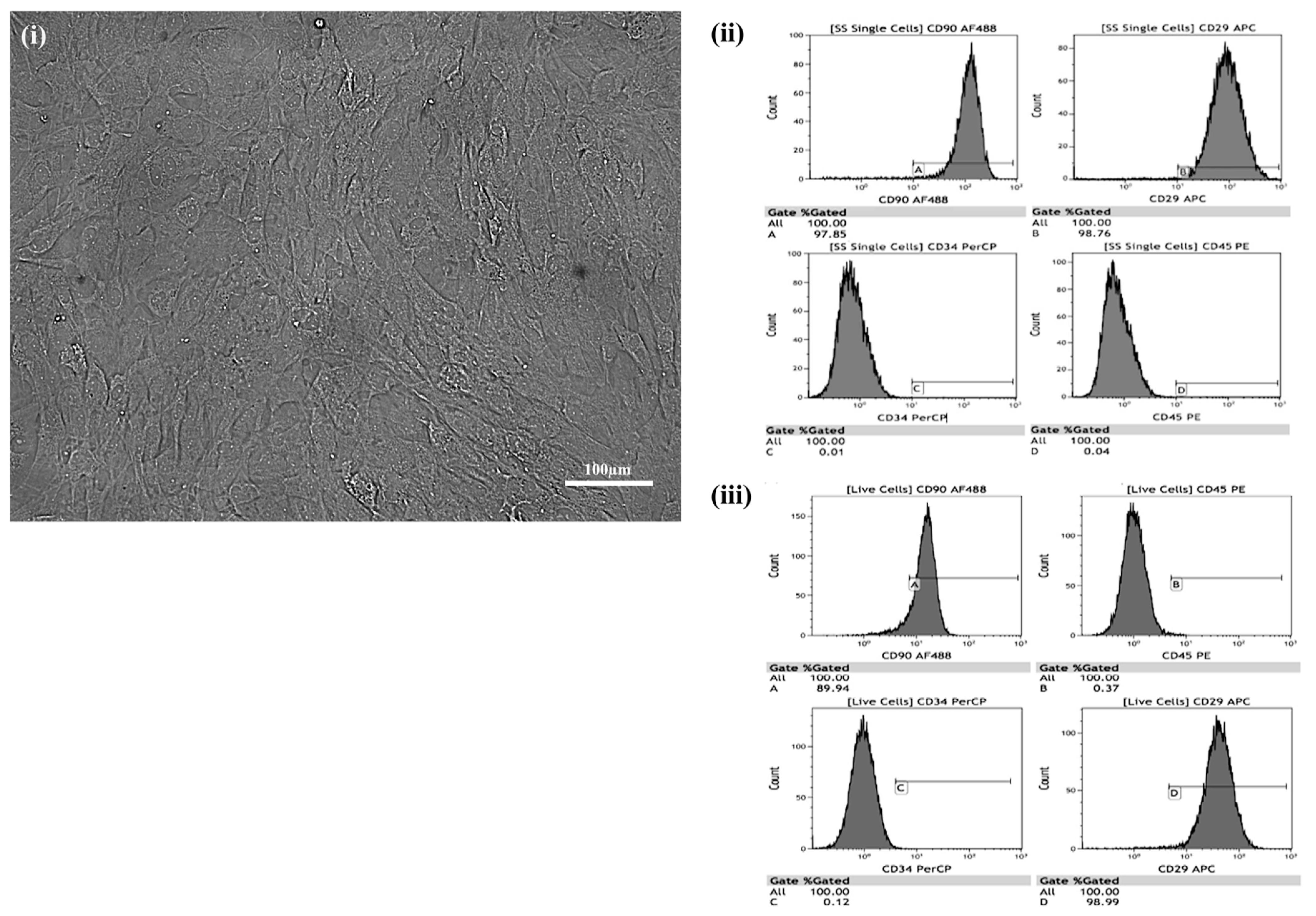
2.1. Culturing of MSCs
2.2. Differentiation of Cultured MSCs
2.3. Staining to Detect Cultured Stem Cell Differentiation
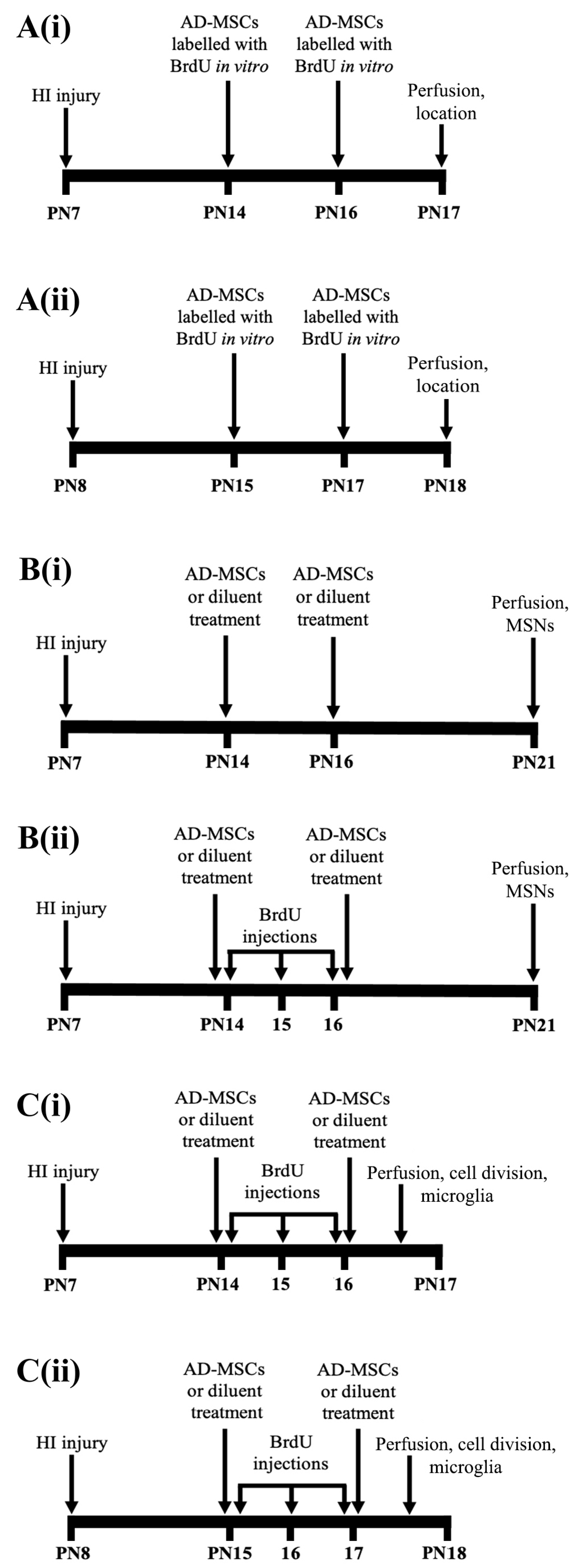
2.4. Neonatal HI and Treatment with AD-MSCs
2.5. Immunohistochemistry
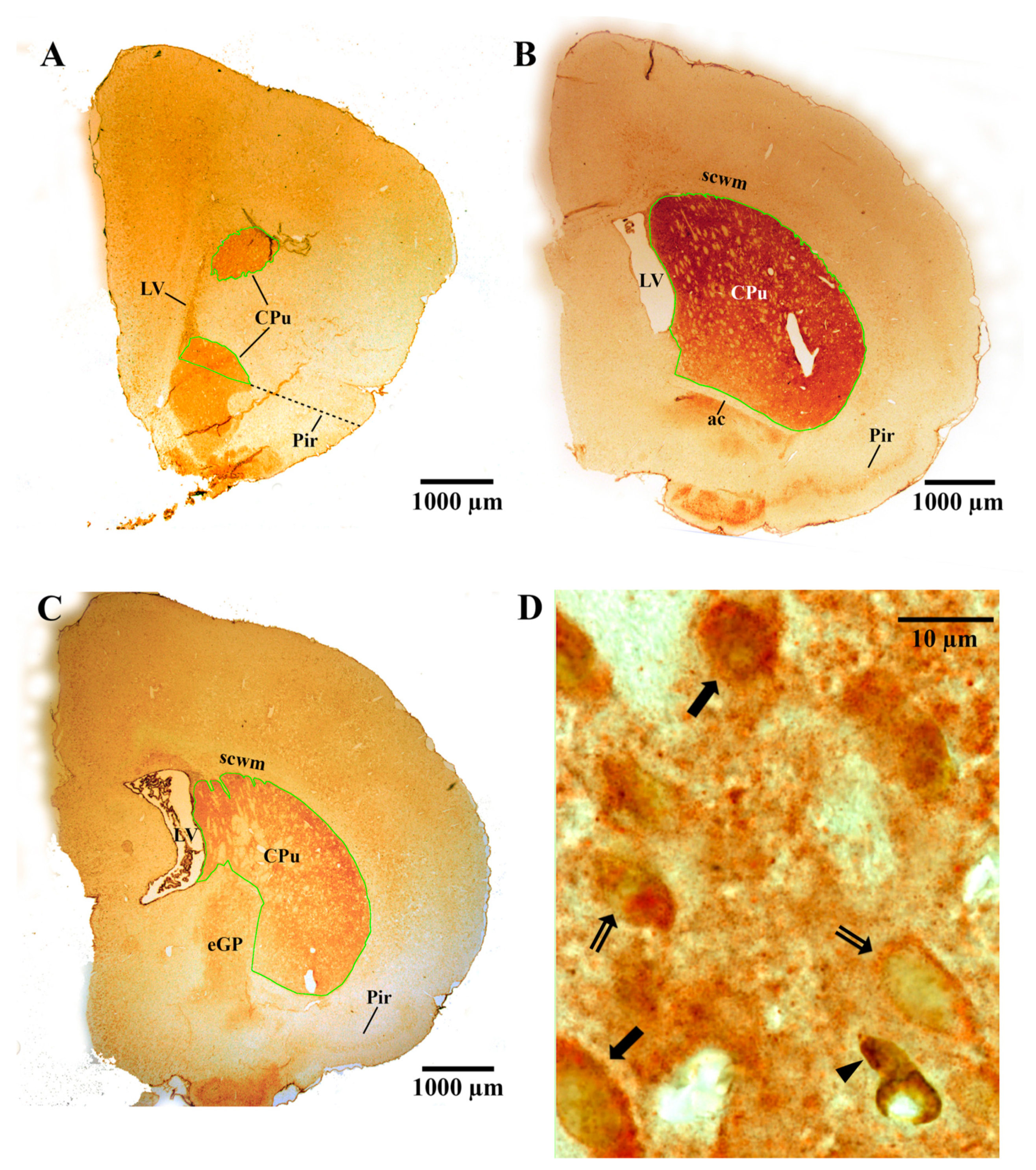
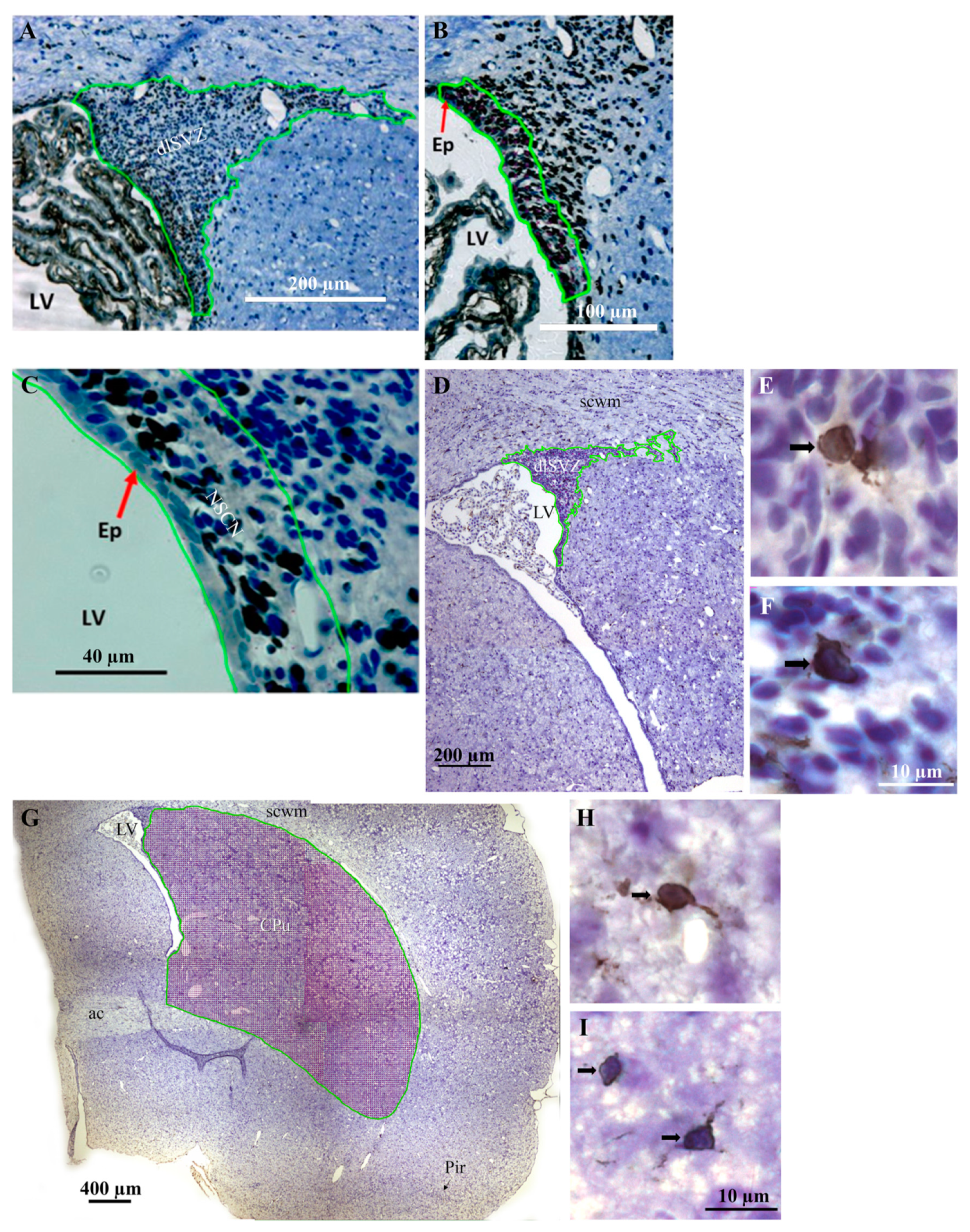
2.6. Stereological Analyses
2.6.1. Measurement of the Vref
2.6.2. Measurement of the Nv
2.6.3. Measurement of the Absolute Number (N)
2.7. Statistical Analyses
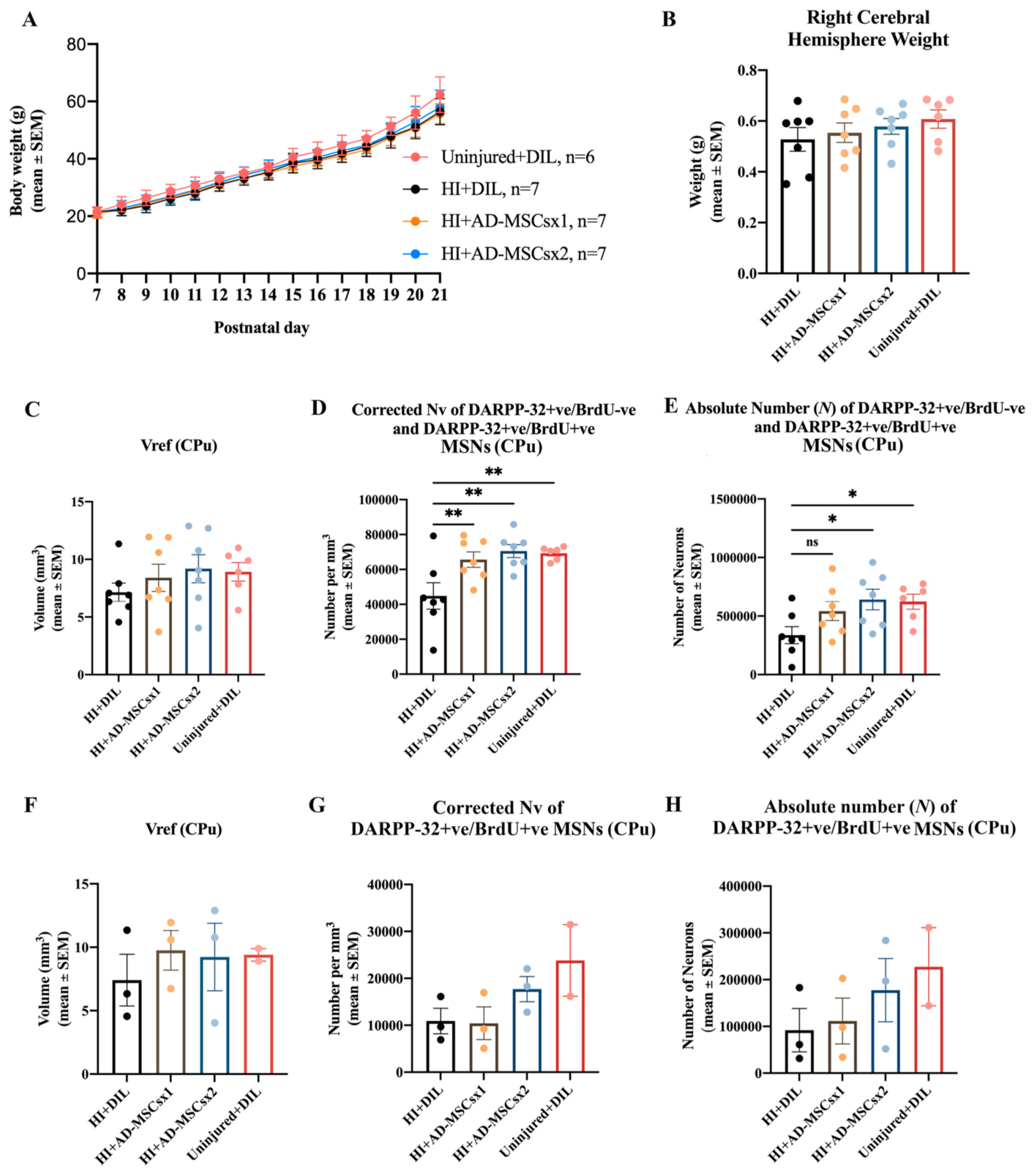
3. Results
4. Discussion
4.1. Methodological Considerations
4.2. Biological Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martinello, K.; Hart, A.R.; Yap, S.; Mitra, S.; Robertson, N.J. Management and investigation of neonatal encephalopathy: 2017 update. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F346–F358. [Google Scholar] [CrossRef]
- Oorschot, D. The percentage of interneurons in the dorsal striatum of the rat, cat, monkey and human: A critique of the evidence. Basal Ganglia 2013, 3, 19–24. [Google Scholar] [CrossRef]
- Rice, J.E.; Vannucci, R.C.; Brierley, J.B. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol. 1981, 9, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Hill, A. Current concepts of hypoxic-ischemic cerebral injury in the term newborn. Pediatrics Neurol. 1991, 7, 317–325. [Google Scholar] [CrossRef]
- Galvin, K.; Oorschot, D. Post-injury magnesium sulfate treatment is not markedly neuroprotective for striatal medium-spiny neurons following perinatal hypoxia/ischemia in the rat. Pediatric Res. 1998, 44, 740–745. [Google Scholar] [CrossRef] [Green Version]
- Cameron, S.H.; Alwakeel, A.J.; Goddard, L.; Hobbs, C.E.; Gowing, E.K.; Barnett, E.R.; Kohe, S.E.; Sizemore, R.J.; Oorschot, D.E. Delayed post-treatment with bone marrow-derived mesenchymal stem cells is neurorestorative of striatal medium-spiny projection neurons and improves motor function after neonatal rat hypoxia–ischemia. Mol. Cell. Neurosci. 2015, 68, 56–72. [Google Scholar] [CrossRef]
- Gunn, A.J.; Gunn, T.R.; Gunning, M.I.; Williams, C.E.; Gluckman, P.D. Neuroprotection With Prolonged Head Cooling Started Before Postischemic Seizures in Fetal Sheep. Pediatrics 1998, 102, 1098–1106. [Google Scholar] [CrossRef]
- Hess, D.C.; Borlongan, C.V. Stem cells and neurological diseases. Cell Prolif. 2007, 41, 94–114. [Google Scholar] [CrossRef]
- Hobbs, C.E.; Oorschot, D.E. Neonatal Rat Hypoxia-Ischemia: Long-Term Rescue of Striatal Neurons and Motor Skills by Combined Antioxidant-Hypothermia Treatment. Brain Pathol. 2008, 18, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, C.E.; Thoresen, M.; Tucker, A.; Aquilina, K.; Chakkarapani, E.; Dingley, J. Xenon and hypothermia combine additively, offering long-term functional and histopathologic neuroprotection after neonatal hypoxia/ischemia. Stroke 2008, 39, 1307–1313. [Google Scholar] [CrossRef] [Green Version]
- Van Velthoven, C.T.; Kavelaars, A.; van Bel, F.; Heijnen, C.J. Mesenchymal stem cell treatment after neonatal hypoxic-ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain Behav. Immunity 2010, 24, 387–393. [Google Scholar] [CrossRef]
- van Velthoven, C.T.; Kavelaars, A.; van Bel, F.; Heijnen, C.J. Repeated mesenchymal stem cell treatment after neonatal hypoxia–ischemia has distinct effects on formation and maturation of new neurons and oligodendrocytes leading to restoration of damage, corticospinal motor tract activity, and sensorimotor function. J. Neurosci. 2010, 30, 9603–9611. [Google Scholar] [CrossRef] [Green Version]
- Ophelders, D.R.M.G.; Gussenhoven, R.; Klein, L.; Jellema, R.K.; Westerlaken, R.J.; Hütten, M.C.; Vermeulen, J.; Wassink, G.; Gunn, A.J.; Wolfs, T.G. Preterm brain injury, antenatal triggers, and therapeutics: Timing is key. Cells 2020, 9, 1871. [Google Scholar] [CrossRef]
- Archambault, J.; Moreira, A.; McDaniel, D.; Winter, L.; Sun, L.; Hornsby, P. Therapeutic potential of mesenchymal stromal cells for hypoxic ischemic encephalopathy: A systematic review and meta-analysis of preclinical studies. PLoS ONE 2017, 12, e0189895. [Google Scholar] [CrossRef]
- Mastrolia, I.; Foppiani, E.M.; Murgia, A.; Candini, O.; Samarelli, A.V.; Grisendi, G.; Veronesi, E.; Horwitz, E.M.; Dominici, M. Challenges in Clinical Development of Mesenchymal Stromal/Stem Cells: Concise Review. STEM CELLS Transl. Med. 2019, 8, 1135–1148. [Google Scholar] [CrossRef] [Green Version]
- Ikegame, Y.; Yamashita, K.; Hayashi, S.-I.; Mizuno, H.; Tawada, M.; You, F.; Yamada, K.; Tanaka, Y.; Egashira, Y.; Nakashima, S.; et al. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy 2011, 13, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Strioga, M.; Viswanathan, S.; Darinskas, A.; Slaby, O.; Michalek, J. Same or Not the Same? Comparison of Adipose Tissue-Derived Versus Bone Marrow-Derived Mesenchymal Stem and Stromal Cells. Stem Cells Dev. 2012, 21, 2724–2752. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Piatetzky-Shapiro, I.I.; Petrakova, K.V. Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 1966, 16, 381–390. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, D.; Shah, J.; Srivastava, A.S. Therapeutic Potential of Mesenchymal Stem Cells in Regenerative Medicine. Stem Cells Int. 2013, 2013, 496218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leibacher, J.; Henschler, R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res. Ther. 2016, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Donega, V.; Nijboer, C.H.; van Tilborg, G.; Dijkhuizen, R.; Kavelaars, A.; Heijnen, C.J. Intranasally administered mesenchymal stem cells promote a regenerative niche for repair of neonatal ischemic brain injury. Exp. Neurol. 2014, 261, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Li, Y.; Katakowski, M.; Chen, X.; Wang, L.; Lu, D.; Lu, M.; Gautam, S.C.; Chopp, M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J. Neurosci. Res. 2003, 73, 778–786. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Zhang, C.L.; Wang, L.; Lu, D.; Katakowski, M.; Gao, Q.; Shen, L.H.; Zhang, J.; Lu, M.; et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia 2005, 49, 407–417. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Chen, J.; Gao, Q.; Zacharek, A.; Kapke, A.; Chopp, M. Bone marrow stromal cells upregulate expression of bone morphogenetic proteins 2 and 4, gap junction protein connexin-43 and synaptophysin after stroke in rats. Neuroscience 2006, 141, 687–695. [Google Scholar] [CrossRef]
- Yoo, S.-W.; Kim, S.-S.; Lee, S.-Y.; Lee, H.-S.; Kim, H.-S.; Lee, Y.-D.; Suh-Kim, H. Mesenchymal stem cells promote proliferation of endogenous neural stem cells and survival of newborn cells in a rat stroke model. Exp. Mol. Med. 2008, 40, 387–397. [Google Scholar] [CrossRef] [Green Version]
- Van Velthoven, C.T.; Kavelaars, A.; van Bel, F.; Heijnen, C.J. Mesenchymal stem cell transplantation changes the gene expression profile of the neonatal ischemic brain. Brain Behav. Immunity 2011, 25, 1342–1348. [Google Scholar] [CrossRef]
- Van Velthoven, C.T.; Kavelaars, A.; Heijnen, C.J. Mesenchymal stem cells as a treatment for neonatal ischemic brain damage. Pediatr. Res. 2012, 71, 474–481. [Google Scholar] [CrossRef]
- Van Velthoven, C.T.; Kavelaars, A.; van Bel, F.; Heijnen, C.J. Nasal administration of stem cells: A promising novel route to treat neonatal ischemic brain damage. Pediatric Res. 2010, 68, 419–422. [Google Scholar] [CrossRef]
- Bruschettini, M.; Romantsik, O.; Moreira, A.; Ley, D.; Thébaud, B. Stem cell-based interventions for the prevention of morbidity and mortality following hypoxic-ischaemic encephalopathy in newborn infants. Cochrane Database Syst. Rev. 2018, 8. [Google Scholar] [CrossRef]
- Lin, G.; Huang, Y.-C.; Shindel, A.W.; Banie, L.; Wang, G.; Lue, T.F.; Lin, C.-S. Labeling and tracking of mesenchymal stromal cells with EdU. Cytotherapy 2009, 11, 864–873. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.A.; Kim, B.I.; Jo, C.H.; Choi, C.W.; Kim, E.-K.; Kim, H.-S.; Yoon, K.-S.; Choi, J.-H. Mesenchymal Stem-Cell Transplantation for Hypoxic-Ischemic Brain Injury in Neonatal Rat Model. Pediatr. Res. 2010, 67, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Leu, S.; Lin, Y.-C.; Yuen, C.-M.; Yen, C.-H.; Kao, Y.-H.; Sun, C.-K.; Yip, H.-K. Adipose-derived mesenchymal stem cells markedly attenuate brain infarct size and improve neurological function in rats. J. Transl. Med. 2010, 8, 63. [Google Scholar] [CrossRef] [Green Version]
- Marin-Bañasco, C.; García, M.S.; Guerrero, I.H.; Sánchez, R.M.; Estivill-Torrús, G.; Fernández, L.L.; Fernández, O.F. Mesenchymal properties of SJL mice-stem cells and their efficacy as autologous therapy in a relapsing–remitting multiple sclerosis model. Stem Cell Res. Ther. 2014, 5, 134. [Google Scholar] [CrossRef] [Green Version]
- Covey, M.V.; Oorschot, D.E. Effect of hypothermic post-treatment on hypoxic-ischemic striatal injury, and normal striatal development, in neonatal rats: A stereological study. Pediatric Res. 2007, 62, 646–651. [Google Scholar] [CrossRef] [Green Version]
- Felling, R.J.; Snyder, M.J.; Romanko, M.J.; Rothstein, R.P.; Ziegler, A.N.; Yang, Z.; Givogri, M.I.; Bongarzone, E.R.; Levison, S.W. Neural Stem/Progenitor Cells Participate in the Regenerative Response to Perinatal Hypoxia/Ischemia. J. Neurosci. 2006, 26, 4359–4369. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Levison, S. Hypoxia/ischemia expands the regenerative capacity of progenitors in the perinatal subventricular zone. Neuroscience 2006, 139, 555–564. [Google Scholar] [CrossRef]
- Yang, Z.; Covey, M.V.; Bitel, C.L.; Ni, L.; Jonakait, G.M.; Levison, S.W. Sustained neocortical neurogenesis after neonatal hypoxic/ischemic injury. Ann. Neurol. 2007, 61, 199–208. [Google Scholar] [CrossRef]
- Yoshikawa, G.; Momiyama, T.; Oya, S.; Takai, K.; Tanaka, J.-I.; Higashiyama, S.; Saito, N.; Kirino, T.; Kawahara, N. Induction of striatal neurogenesis and generation of region-specific functional mature neurons after ischemia by growth factors. J. Neurosurg. 2010, 113, 835–850. [Google Scholar] [CrossRef]
- Ong, J.; Plane, J.M.; Parent, J.M.; Silverstein, F.S. Hypoxic-ischemic injury stimulates subventricular zone proliferation and neurogenesis in the neonatal rat. Pediatric Res. 2005, 58, 600–606. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Levison, S.W. Perinatal hypoxic/ischemic brain injury induces persistent production of striatal neurons from subventricular zone progenitors. Dev. Neurosci. 2007, 29, 331–340. [Google Scholar] [CrossRef]
- McRae, A.; Gilland, A.; Bona, E.; Hagberg, H. Microglia activation after neonatal hypoxia-ischemia. Dev. Brain Res. 1995, 84, 245–252. [Google Scholar] [CrossRef]
- Bona, E.; Andersson, A.-L.; Blomgren, K.; Gilland, E.; Puka-Sundvall, M.; Gustafson, K.; Hagberg, H. Chemokine and inflammatory cell response to hypoxia-ischemia in immature rats. Pediatric Res. 1999, 45, 500–509. [Google Scholar] [CrossRef] [Green Version]
- Luskin, M.B. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron 1993, 11, 173–189. [Google Scholar] [CrossRef]
- Tattersfield, A.; Croon, R.; Liu, Y.; Kells, A.; Faull, R.; Connor, B. Neurogenesis in the striatum of the quinolinic acid lesion model of Huntington’s disease. Neuroscience 2004, 127, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Oorschot, D.E.; Lin, N.; Cooper, B.H.; Reynolds, J.N.; Sun, H.; Wickens, J.R. Synaptic connectivity between rat striatal spiny projection neurons in vivo: Unexpected multiple somatic innervation in the context of overall sparse proximal connectivity. Basal Ganglia 2013, 3, 93–108. [Google Scholar] [CrossRef]
- Ouimet, C.C.; Langley-Gullion, K.C.; Greengard, P. Quantitative immunocytochemistry of DARPP-32-expressing neurons in the rat caudatoputamen. Brain Res. 1998, 808, 8–12. [Google Scholar] [CrossRef]
- Gundersen, H.J. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J. Microsc. 1986, 143, 3–45. [Google Scholar] [CrossRef]
- Oorschot, D.E. Total number of neurons in the neostriatal, pallidal, subthalamic, and substantia nigral nuclei of the rat basal ganglia: A stereological study using the cavalieri and optical disector methods. J. Comp. Neurol. 1996, 366, 580–599. [Google Scholar] [CrossRef]
- Aghoghovwia, B.E.; Oorschot, D.E. Absolute number of parvicellular and magnocellular neurons in the red nucleus of the rat midbrain: A stereological study. J. Anat. 2016, 229, 406–415. [Google Scholar] [CrossRef]
- Aghoghovwia, B.E.; Goddard, L.; Oorschot, D.E. Long-Term Fine Motor Capability on the Staircase Test Correlates with the Absolute Number, but Not the Density, of DARPP-Positive Neurons in the Caudate-Putamen. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2019, 302, 2040–2048. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C.R.; Emson, P.C. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J. Neurosci. Methods 1980, 3, 129–149. [Google Scholar] [CrossRef]
- Gundersen, H.J.G. Notes on the estimation of the numerical density of arbitrary profiles: The edge effect. J. Microsc. 1977, 111, 219–223. [Google Scholar] [CrossRef]
- Bedi, K. Effects of undernutrition on brain morphology: A critical review of methods and results. In Current Topics in Research on Synapses; Jones, D.G., Ed.; Alan R. Liss, Inc.: New York, NY, USA, 1984; Volume 2, pp. 93–163. [Google Scholar]
- Oorschot, D. Are you using neuronal densities, synaptic densities or neurochemical densities as your definitive data? there is a better way to go. Prog. Neurobiol. 1994, 44, 233–247. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oorschot, D. Indirect measures of neuroprotection are parameters to avoid: Examples from research on neonatal rat hypoxia-ischemia. Image Anal. Ster. 2011, 30, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Afonso, F.D.A.; Siapati, E.K.; Bonnet, D. In vivo contribution of murine mesenchymal stem cells into multiple cell-types under minimal damage conditions. J. Cell Sci. 2004, 117, 5655–5664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willing, A.E.; Foran, E.A. Cord blood as a treatment for stroke. In Cellular Therapy for Stroke and CNS Injuries; Zhao, L.-R., Zhang, J.H., Eds.; Springer: Cham, Switzerland, 2015; pp. 71–107. [Google Scholar]
- Decarolis, N.A.; Kirby, E.; Wyss-Coray, T.; Palmer, T.D. The Role of the Microenvironmental Niche in Declining Stem-Cell Functions Associated with Biological Aging. Cold Spring Harb. Perspect. Med. 2015, 5, a025874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.S.; Lee, K.-H.; Sung, D.K.; Shim, J.W.; Kim, M.J.; Jeon, G.W.; Chang, Y.S.; Park, W.S. Erythropoietin Attenuates Brain Injury, Subventricular Zone Expansion, and Sensorimotor Deficits in Hypoxic-Ischemic Neonatal Rats. J. Korean Med. Sci. 2008, 23, 484–491. [Google Scholar] [CrossRef] [Green Version]
- Park, D.; Lee, S.H.; Bae, D.K.; Yang, Y.-H.; Yang, G.; Kyung, J.; Kim, D.; Choi, E.-K.; Hong, J.T.; Shin, I.S.; et al. Transplantation of Human Adipose Tissue-Derived Mesenchymal Stem Cells Restores the Neurobehavioral Disorders of Rats with Neonatal Hypoxic-Ischemic Encephalopathy. Cell Med. 2013, 5, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Donega, V.; Nijboer, C.H.; Braccioli, L.; Slaper-Cortenbach, I.; Kavelaars, A.; Van Bel, F.; Heijnen, C.J. Intranasal Administration of Human MSC for Ischemic Brain Injury in the Mouse: In Vitro and In Vivo Neuroregenerative Functions. PLoS ONE 2014, 9, e112339. [Google Scholar] [CrossRef] [Green Version]
- McKay, S.M.; Brooks, D.J.; Hu, P.; McLachlan, E.M. Distinct types of microglia activation in white and grey matter of rat lumbosacral cord after mid-thoracic spinal transection. J. Neuropathol. Exp. Neurol. 2007, 66, 698–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braendgaard, H.; Evans, S.M.; Howard, C.V.; Gundersen, H.J.G. The total number of neurons in the human neocortex unbiasedly estimated using optical disectors. J. Microsc. 1990, 157, 285–304. [Google Scholar] [CrossRef] [PubMed]
- Slomianka, L.; West, M. Estimators of the precision of stereological estimates: An example based on the CA1 pyramidal cell layer of rats. Neuroscience 2005, 136, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Frassoni, F.; Ball, L.; Locatelli, F.; Roelofs, H.; Lewis, I.; Lanino, E.; Sundberg, B.; Bernardo, M.E.; Remberger, M.; et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet 2008, 371, 1579–1586. [Google Scholar] [CrossRef]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Donega, V.; van Velthoven, C.; Nijboer, C.H.; Kavelaars, A.; Heijnen, C.J. The Endogenous Regenerative Capacity of the Damaged Newborn Brain: Boosting Neurogenesis with Mesenchymal Stem Cell Treatment. Br. J. Pharmacol. 2013, 33, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Williams, C.G.; Kim, T.K.; Taboas, A.; Malik, A.; Manson, P.; Elisseeff, J. In Vitro Chondrogenesis of Bone Marrow-Derived Mesenchymal Stem Cells in a Photopolymerizing Hydrogel. Tissue Eng. 2003, 9, 679–688. [Google Scholar] [CrossRef]
- Wilson, T.; Stark, C.; Holmbom, J.; Rosling, A.; Kuusilehto, A.; Tirri, T.; Penttinen, R.; Ekholm, E. Fate of Bone Marrow-Derived Stromal Cells after Intraperitoneal Infusion or Implantation into Femoral Bone Defects in the Host Animal. J. Tissue Eng. 2010, 1, 345806. [Google Scholar] [CrossRef]
- Gordon, D.; Pavlovska, G.; Glover, C.P.; Uney, J.B.; Wraith, D.; Scolding, N.J. Human mesenchymal stem cells abrogate experimental allergic encephalomyelitis after intraperitoneal injection, and with sparse CNS infiltration. Neurosci. Lett. 2008, 448, 71–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, J.Y.; Kim, T.W.; Jeong, H.J.; Lee, H.J.; Ryu, J.S.; Wee, W.R.; Heo, J.W.; Kim, M.K. Intraperitoneal Infusion of Mesenchymal Stem/Stromal Cells Prevents Experimental Autoimmune Uveitis in Mice. Mediat. Inflamm. 2014, 2014, 624640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borlongan, C.V.; Hadman, M.; Sanberg, C.D.; Sanberg, P.R. Central Nervous System Entry of Peripherally Injected Umbilical Cord Blood Cells Is Not Required for Neuroprotection in Stroke. Stroke 2004, 35, 2385–2389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; You, Y.; Levison, S. Neonatal hypoxic/ischemic brain injury induces production of calretinin-expressing interneurons in the striatum. J. Comp. Neurol. 2008, 511, 19–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoehn, B.D.; Palmer, T.; Steinberg, G.K. Neurogenesis in Rats After Focal Cerebral Ischemia is Enhanced by Indomethacin. Stroke 2005, 36, 2718–2724. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Fernández, M.; Rodríguez-Frutos, B.; Ramos-Cejudo, J.; Teresa Vallejo-Cremades, M.; Fuentes, B.; Cerdán, S.; Díez-Tejedor, E. Effects of intravenous administration of allogenic bone marrow- and adipose tissue-derived mesenchymal stem cells on functional recovery and brain repair markers in experimental ischemic stroke. Stem Cell Res. Ther. 2013, 4, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Experiment | Section Sampling Interval | Real Area of Each Point, a(p) | Number of Sections Analysed per Brain |
|---|---|---|---|
| CPu Vref, DARRP-32 MSN experiment, PN21 | Every 30th or 40th | 200 µm × 200 µm = 40,000 µm2 | 6–17 |
| NSCN, PN17/18 | Every 30th | 10 µm × 10 µm = 100 µm2 | 11–18 |
| dlSVZ Vref, NSCN experiment, PN17/18 | Every 30th | 50 µm × 50 µm = 2500 µm2 | 11–18 |
| dlSVZ Vref, microglial experiment, PN17/18 | Every 60th | 20 µm × 20 µm = 400 µm2 | 5–9 |
| CPu Vref, microglial experiment, PN17/18 | Every 60th | 40 µm × 40 µm = 1600 µm2 | 5–9 |
| Experiment | Size of Unbiased Sampling Frame | Sampling Interval |
|---|---|---|
| CPu Vref DARRP-32 MSN experiment, PN21 | 60 µm × 60 µm = 3600 µm2 | Every 10th |
| NSCN, PN17/18 | Cells in entire NSCN measured | Not applicable |
| dlSVZ Vref, NSCN experiment, PN17/18 | 20 µm × 20 µm = 400 µm2 | Every 3rd |
| dlSVZ Vref, microglial experiment, PN17/18 | 40 µm × 40 µm = 1600 µm2 | Every 30th |
| CPu Vref, microglial experiment, PN17/18 | 60 µm × 60 µm = 3600 µm2 | Every 30th |
| PN21 MSN Experiments | BrdU-Positive/DARPP-Positive Neurons in CPu | BrdU-Negative/DARPP-Positive Neurons in CPu | All DARPP-Positive Neurons in CPu |
| HI-DIL | 8.69 ± 0.56 | 9.12 ± 0.56 | 8.91 ± 0.55 |
| HI-MSC×1 | 9.29 ± 0.55 | 9.46 ± 0.55 | 9.38 ± 0.55 |
| HI-MSC×2 | 9.24 ± 0.89 | 9.30 ± 0.39 | 9.27 ± 0.87 |
| Uninjured | 9.18 ± 0.24 (SD) | 9.12 ± 0.17 (SD) | 9.15 ± 0.04 (SD) |
| PN17/18 Mechanism(s) Experiments | BrdU-Positive Cells in NSCN | IBA-1-Positive Cells in dlSVZ | IBA-1-Positive Cells in CPu |
| HI-DIL | 6.29 ± 0.20 | 5.02 ± 0.32 | 5.87 ± 0.37 |
| HI-MSC×2 | 6.02 ± 0.26 | 5.04 ± 0.22 | 5.23 ± 0.31 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basham, H.K.; Aghoghovwia, B.E.; Papaioannou, P.; Seo, S.; Oorschot, D.E. Delayed Double Treatment with Adult-Sourced Adipose-Derived Mesenchymal Stem Cells Increases Striatal Medium-Spiny Neuronal Number, Decreases Striatal Microglial Number, and Has No Subventricular Proliferative Effect, after Acute Neonatal Hypoxia-Ischemia in Male Rats. Int. J. Mol. Sci. 2021, 22, 7862. https://doi.org/10.3390/ijms22157862
Basham HK, Aghoghovwia BE, Papaioannou P, Seo S, Oorschot DE. Delayed Double Treatment with Adult-Sourced Adipose-Derived Mesenchymal Stem Cells Increases Striatal Medium-Spiny Neuronal Number, Decreases Striatal Microglial Number, and Has No Subventricular Proliferative Effect, after Acute Neonatal Hypoxia-Ischemia in Male Rats. International Journal of Molecular Sciences. 2021; 22(15):7862. https://doi.org/10.3390/ijms22157862
Chicago/Turabian StyleBasham, Haylee K., Benjamin E. Aghoghovwia, Panagiotis Papaioannou, Steve Seo, and Dorothy E. Oorschot. 2021. "Delayed Double Treatment with Adult-Sourced Adipose-Derived Mesenchymal Stem Cells Increases Striatal Medium-Spiny Neuronal Number, Decreases Striatal Microglial Number, and Has No Subventricular Proliferative Effect, after Acute Neonatal Hypoxia-Ischemia in Male Rats" International Journal of Molecular Sciences 22, no. 15: 7862. https://doi.org/10.3390/ijms22157862
APA StyleBasham, H. K., Aghoghovwia, B. E., Papaioannou, P., Seo, S., & Oorschot, D. E. (2021). Delayed Double Treatment with Adult-Sourced Adipose-Derived Mesenchymal Stem Cells Increases Striatal Medium-Spiny Neuronal Number, Decreases Striatal Microglial Number, and Has No Subventricular Proliferative Effect, after Acute Neonatal Hypoxia-Ischemia in Male Rats. International Journal of Molecular Sciences, 22(15), 7862. https://doi.org/10.3390/ijms22157862






