Low-Energy Electron Damage to Condensed-Phase DNA and Its Constituents
Abstract
1. Introduction
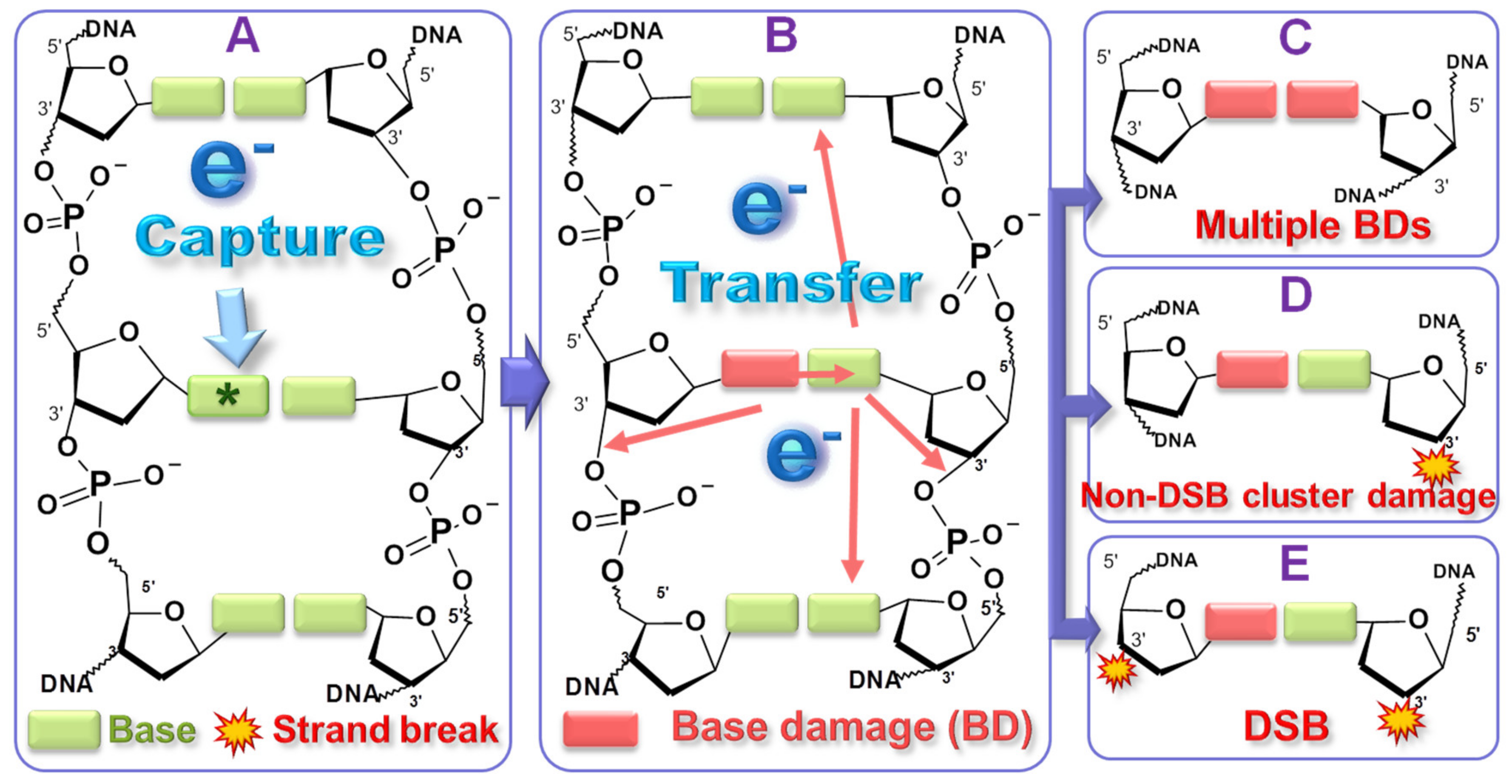
2. Mechanisms of Action of LEEs and Induced DNA Damage
3. Transfer of Knowledge from the Gas to the Condensed Phase
4. LEE Impact on Biomolecular Films in UHV
4.1. Damage Analysed in Vacuo
4.2. Damage Analysed Quantitatively Ex-Vacuo
5. LEE-Biomolecular Films Experiments at Atmospheric Pressure
6. LEE Experiments with Clusters
6.1. LEE Impact on Clusters Containing DNA Constituents or Their Analogs
6.2. LEE Photodetachment from Anions in Binary and Larger Clusters
7. LEE Reactions in Solution
7.1. LEE Reactions Investigated by Pulse Radiolysis
7.2. Secondary LEEs Generated by a Membrane Irradiated by Fast Electrons
7.3. Femtosecond-Laser Induced Cold Low-Density Plasmas
8. Applications to Radiation, Chemoradiation, Targeted-Radionuclide, Gold-Nanoparticle and Laser Therapy
9. Conclusions and Future Challenges
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Adenine |
| BD | base damage |
| C | Cytosine |
| CA | chemotherapeutic agent |
| CL | crosslink |
| CRT | chemoradiation therapy |
| CS | cross section |
| DEA | dissociative electron attachment |
| DNA | deoxyribonucleic acid |
| DSB | double strand break |
| EF | enhancement factor |
| ESD | electron stimulated desorption |
| G GNP | Guanine Gold nanoparticles |
| HER | high energy radiation |
| HPLC | high-performance liquid chromatography |
| IR | infrared |
| LEE | low-energy electron |
| LET | linear energy transfer |
| MC | Monte Carlo |
| MS | mass spectrometry |
| PNA | peptide nucleic acid |
| SAM | self-assembled monolayers |
| SATP | standard ambient temperature and pressure |
| SB | strand break |
| SE | secondary electron |
| SSB | single strand break |
| T | Thymine |
| TA | transient anion |
| U | Uracil |
| UHV | ultra-high vacuum |
| UV | ultraviolet |
| XPS | X-ray photoelectron spectroscopy |
References
- Platzmann, R.L. The physical and chemical basis of mechanisms in radiation biology. In Radiation Biology and Medicine: Selected Reviews in the Life Sciences; Claus, W.D., Ed.; Addison-Wesley: Boston, MA, USA, 1958; pp. 15–72. [Google Scholar]
- Attix, F.H. Introduction to Radiological Physics and Radiation Dosimetry; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2004. [Google Scholar]
- Pimblott, S.M.; La Verne, J.A. Production of low-energy electrons by ionizing radiation. Radiat. Phys. Chem. 2007, 76, 1244–1247. [Google Scholar] [CrossRef]
- Aziz, F.; Rodgers, M.A.J. (Eds.) Radiation Chemistry: Principles and Applications; VCH: New York, NY, USA, 1987. [Google Scholar]
- Bass, A.D.; Sanche, L. Interactions of low-energy electrons with atomic and molecular solids. In Charged Particle and Photon Interactions with Matter; Mozumder, A., Hatano, Y., Eds.; CRC Press: New York, NY, USA, 2004; pp. 207–258. [Google Scholar]
- Baccarelli, I.; Bald, I.; Gianturco, F.A.; Illenberger, E.; Kopyra, J. Electron-induced damage of DNA and its components: Experiments and theoretical models. Phys. Rep. 2011, 508, 1–44. [Google Scholar] [CrossRef]
- Lemelin, V.; Sanche, L. High-resolution electron energy loss spectroscopy: Absolute cross section measurements for low energy electron scattering from biomolecules. In Radiation in Bioanalysis; Pereira, A.S., Tavares, P., Limão-Vieira, P., Eds.; Springer International Publishing: Switzerland, 2019; pp. 3–42. [Google Scholar]
- Pimblott, S.M.; La Verne, J.A. Radiation Damage in DNA: Structure/Function Relationships at Early Times; Fuciarelli, A.F., Zimbrick, J.D., Eds.; Battelle Press: Columbus, OH, USA, 1995; Chapter 1. [Google Scholar]
- Cobut, V.; Frongillo, Y.; Patau, J.P.; Goulet, T.; Fraser, M.J.; Jay-Gerin, J.P. Monte Carlo simulation of fast electron and proton tracks in liquid water—I. Physical and physicochemical aspects. Radiat. Phys. Chem. 1998, 51, 229–243. [Google Scholar]
- Huels, M.A.; Boudaïffa, B.; Cloutier, P.; Hunting, D.; Sanche, L. Single, double, and multiple double strand breaks induced in DNA by 3–100 eV electrons. J. Am. Chem. Soc. 2003, 125, 4467–4477. [Google Scholar] [CrossRef]
- Alizadeh, E.; Sanche, L. Precursors of solvated electrons in radiobiological physics and chemistry. Chem. Rev. 2012, 112, 5578–5602. [Google Scholar] [CrossRef] [PubMed]
- Boudaïffa, B.; Cloutier, P.; Hunting, D.; Huels, M.A.; Sanche, L. Resonant formation of DNA strand breaks by low-energy (3 to 20 eV) electrons. Science 2000, 287, 1658–1660. [Google Scholar]
- Sanche, L. Low-energy electron interaction with DNA: Bond dissociation and formation of transient anions, radicals and radical anions. In Radical and Radical Ion Reactivity in Nucleic Acid Chemistry; Greenberg, M., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 239–294. [Google Scholar]
- Alizadeh, E.; Orlando, T.; Sanche, L. Biomolecular damage induced by ionizing radiation: The direct and indirect effects of low-energy electrons on DNA. Annu. Rev. Phys. Chem. 2015, 66, 379−398. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, Y.; Liu, W.; Gao, T.; Zheng, Y.; Sanche, L. Clustered DNA damage induced by 2−20 eV electrons and transient anions: General mechanism and correlation to cell death. J. Phys. Chem. Lett. 2019, 10, 2985–2990. [Google Scholar] [CrossRef]
- Bukowska, B.; Karwowski, B.T. The clustered DNA lesions-types, pathways of repair and relevance to human health. Curr. Med. Chem. 2018, 25, 2722–2735. [Google Scholar] [CrossRef]
- Dong, Y.; Liao, H.; Gao, Y.; Cloutier, P.; Zheng, Y.; Sanche, L. Early events in radiobiology: Isolated and cluster DNA damage induced by initial cations and non-ionizing secondary electrons. J. Phys. Chem. Lett. 2021, 12, 717–723. [Google Scholar] [CrossRef]
- Sutherland, B.; Bennett, P.V.; Sidorkina, O.; Laval, J. Clustered DNA damages induced in isolated DNA and in human cells by low doses of ionizing radiation. Proc. Natl. Acad. Sci. USA 2000, 97, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Gulston, M.; Fulford, J.; Jenner, T.; de Lara, C.; O’Neill, P. Clustered DNA damage induced by γ radiation in human fibroblasts (HF19), hamster (V79-4) cells and plasmid DNA is revealed as Fpg and Nth sensitive sites. Nucleic Acids Res. 2002, 30, 3464–3472. [Google Scholar] [CrossRef] [PubMed]
- Nikjoo, H.; O’Neill, P.; Goodhead, D.T.; Terrissol, M. Computational modelling of low-energy electron-induced DNA damage by early physical and chemical events. Int. J. Radiat. Biol. 1997, 71, 467–483. [Google Scholar] [CrossRef] [PubMed]
- Semenenko, V.A.; Stewar, R.D. Fast Monte Carlo simulation of DNA damage formed by electrons and light ions. Phys. Med. Biol. 2006, 51, 1693–1706. [Google Scholar] [CrossRef]
- Goodhead, D.T. Initial events in the cellular effects of ionizing radiations: Clustered damage in DNA. Int. J. Radiat. Biol. 1994, 65, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.F. DNA damage produced by ionizing radiation in mammalian cells: Identities, mechanisms of formation and reparability. Prog. Nucleic Acid Res. Mol. Biol. 1988, 35, 95–125. [Google Scholar]
- Sevilla, M.D.; Becker, D.; Kumar, A.; Adhikary, A. Gamma and ion-beam irradiation of DNA: Free radical mechanisms, electron effects, and radiation chemical track structure. Radiat. Phys. Chem. 2016, 128, 60–74. [Google Scholar] [CrossRef]
- Sanche, L.; Michaud, M. Interaction of low-energy electrons (1–30 eV) with condensed molecules: II. Vibrational-librational excitation and shape resonances in thin N2 and CO films. Phys. Rev. B 1984, 30, 6078–6092. [Google Scholar] [CrossRef]
- Michaud, M.; Bazin, M.; Sanche, L. Measurement of inelastic cross sections for low-energy electron scattering from DNA bases. Int. J. Radiat. Biol. 2012, 88, 15–21. [Google Scholar] [CrossRef][Green Version]
- Liljequist, D. A model calculation of coherence effects in the elastic backscattering of very low energy electrons (1–20 eV) from amorphous ice. Int. J. Radiat. Biol. 2012, 88, 50–53. [Google Scholar] [CrossRef]
- Sambe, H.; Ramaker, D.E.; Parenteau, L.; Sanche, L. Electron-stimulated desorption enhanced by coherent scattering. Phys. Rev. Lett. 1987, 59, 505–508. [Google Scholar] [CrossRef]
- Michaud, M.; Cloutier, P.; Sanche, L. Phonon excitations in low-energy-electron scattering from solid Ar, Kr, and Xe films: Direct observation of conduction-band density of states. Phys. Rev. B Condens. Matter 1991, 44, 10485–10492. [Google Scholar] [CrossRef]
- Michaud, M.; Cloutier, P.; Sanche, L. Phonon excitations in low-energy electron resonant scattering from solid films of N2. Phys. Rev. B 1994, 49, 8360–8366. [Google Scholar] [CrossRef] [PubMed]
- Michaud, M.; Sanche, L. Low-energy electron-energy-loss spectroscopy of solid film of argon: Surface and bulk valence excitons. Phys. Rev. B 1994, 50, 4725–4732. [Google Scholar] [CrossRef]
- Rowntree, P.; Sambe, H.; Parenteau, L.; Sanche, L. Formation of anionic excitations in the rare-gas solids and their coupling to dissociative states of adsorbed molecules. Phys. Rev. B 1993, 47, 4537–4554. [Google Scholar] [CrossRef]
- Bass, A.D.; Sanche, L. Dissociative electron attachment and charge transfer in condensed matter. Radiat. Phys. Chem. 2003, 68, 3–13. [Google Scholar] [CrossRef]
- Michaud, M.; Wen, A.; Sanche, L. Cross sections for low-energy (1–100 eV) electron elastic and inelastic scattering in amorphous ice. Radiat. Res. 2003, 159, 3–22. [Google Scholar] [CrossRef]
- Caron, L.G.; Sanche, L. Theoretical studies of electron interactions with DNA and its subunits: From tetrahydrofuran to plasmid DNA. In Low-Energy Electron Scattering from Molecules, Biomolecules and Surfaces; Čársky, P., Čurík, R., Eds.; CRC Press (Taylor and Francis Group): Boca Raton, FL, USA, 2012; pp. 161–230. [Google Scholar]
- Fano, U.; Stephens, J.A. Slow electrons in condensed matter. Phys. Rev. B 1986, 34, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Sanche, L. Primary interactions of low-energy electrons in condensed matter. In Excess Electrons in Dielectric Media; Ferradini, C., Jay-Gerin, J.-P., Eds.; CRC Press: Boca Raton, FL, USA, 1991; pp. 1–42. [Google Scholar]
- Michaud, M.; Lepage, M.; Sanche, L. Lifetime of negative ion resonances and the density of free electron states: O2 isolated in an argon matrix. Phys. Rev. Lett. 1998, 81, 2807–2810. [Google Scholar] [CrossRef]
- Alizadeh, E.; Ptasińska, S.; Sanche, L. Transient anions in radiobiology and radiotherapy: From gaseous biomolecules to condensed organic and biomolecular solids. In Radiation Effects in Materials; Monteiro, W.A., Ed.; Intech Open: Rijeka, Croatia, 2016; pp. 179–230. [Google Scholar]
- Kumar, A.; Becker, D.; Adhikary, A.; Sevilla, M.D. Reaction of electrons with DNA: Radiation damage to radiosensitization. Int. J. Mol. Sci. 2019, 20, 3998. [Google Scholar] [CrossRef]
- Zheng, Y.; Wagner, J.R.; Sanche, L. DNA damage induced by low-energy electrons: Electron transfer and diffraction. Phys. Rev. Lett. 2006, 96, 208101. [Google Scholar] [CrossRef] [PubMed]
- Desfrançois, C.; Abdoul-Carime, H.; Schermann, J.-P. Ground-state dipole-bound anions. Int. J. Mod. Phys. B 1996, 10, 1339–1395. [Google Scholar] [CrossRef]
- Jordan, K.D.; Wang, F. Theory of dipole-bound anions. Annu. Rev. Phys. Chem. 2003, 54, 367–396. [Google Scholar] [CrossRef]
- Anusiewicz, I.; Skurski, P.; Simons, J. Fate of dipole-bound anion states when hydrated. J. Phys. Chem. A 2020, 124, 2064–2076. [Google Scholar] [CrossRef]
- Castellani, M.E.; Anstöter, C.S.; Verlet, J.R.R. On the stability of a dipole-bound state in the presence of a molecule. Phys. Chem. Chem. Phys. 2019, 21, 24286–24290. [Google Scholar] [CrossRef] [PubMed]
- Nagesha, K.; Sanche, L. Effects of band structure on electron attachment to adsorbed molecules: Cross section enhancements via coupling to image states. Phys. Rev. Lett. 1998, 81, 5892–5895. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, Y.; Cloutier, P.; Sanche, L.; Wagner, J.R. Low energy electron induced DNA damage: Effects of terminal phosphate and base moieties on the distribution of damage. J. Am. Chem. Soc. 2008, 130, 5612–5613. [Google Scholar] [CrossRef]
- Luo, X.; Zheng, Y.; Sanche, L. DNA strand breaks and crosslinks induced by transient anions in the range 2–20 eV. J. Chem. Phys. 2014, 140, 155101. [Google Scholar] [CrossRef]
- Martin, F.; Burrow, P.D.; Cai, Z.; Cloutier, P.; Hunting, D.; Sanche, L. DNA strand breaks induced by 0–4 eV electrons: The role of shape resonances. Phys. Rev. Lett. 2004, 93, 068101. [Google Scholar] [CrossRef]
- Barrios, R.; Skurski, P.; Simons, J. Mechanism for damage to DNA by low-energy electrons. J. Phys. Chem. B 2002, 106, 7991–7994. [Google Scholar] [CrossRef]
- Pan, X.; Cloutier, P.; Hunting, D.; Sanche, L. Dissociative electron attachment to DNA. Phys. Rev. Lett. 2003, 90, 208102. [Google Scholar] [CrossRef]
- Pan, X.; Sanche, L. Mechanism and site of attack for direct damage to DNA by low-energy electrons. Phys. Rev. Lett. 2005, 94, 198104. [Google Scholar] [CrossRef] [PubMed]
- Simons, J. How do low-energy (0.1–2 eV) electrons cause DNA-strand breaks? Acc. Chem. Res. 2006, 39, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Leszczynski, J.; Schaefer, H.F. Interactions of electrons with bare and hydrated biomolecules: From nucleic acid bases to DNA segments. Chem. Rev. 2012, 112, 5603–5640. [Google Scholar] [CrossRef]
- Kohanoff, J.; McAllister, M.; Tribello, G.A.; Gu, B. Interactions between low energy electrons and DNA: A perspective from first-principles simulations. J. Phys. Condens. Matter 2017, 29, 383001. [Google Scholar] [CrossRef] [PubMed]
- Choy, H. Chemoradiation in Cancer Therapy; Humana Press: Totowa, NJ, USA, 2003. [Google Scholar]
- Von Sonntag, C. Free-Radical-Induced DNA Damage and Its Repair: A Chemical Perspective; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Bald, I.; Langer, J.; Tegeder, P.; Ingólfsson, O. From isolated molecules through clusters and condensates to the building blocks of life. Int. J. Mass Spectrom. 2008, 277, 4–25. [Google Scholar] [CrossRef]
- Bass, A.D.; Sanche, L. Absolute and effective cross-sections for low-energy electron-scattering processes within condensed matter. Radiat. Environ. Biophys. 1998, 37, 243–257. [Google Scholar] [CrossRef]
- Sanche, L. Low-energy electron scattering from molecules on surfaces. J. Phys. B At. Mol. Opt. Phys. 1990, 23, 1597–1624. [Google Scholar] [CrossRef]
- Sanche, L.; Märk, T.D.; Hatano, Y. Low energy electron interactions with condensed matter. In Atomic and Molecular Data for Radiotherapy and Radiation Research; Inokuti, M., Ed.; IAEA Press: Vienna, Austria, 1995; pp. 277–329. [Google Scholar]
- Kühn, A.; Illenberger, E. Low energy (0–10 eV) electron attachment to CF3Cl clusters: Formation of product ions and analysis of excess translational energy. J. Chem. Phys. 1990, 93, 357–364. [Google Scholar] [CrossRef]
- Illenberger, E.; Momigny, J. Gaseous Molecular Ions: An Introduction to Elementary Processes Induced by Ionization; Steinkopff-Verlag: Heidelberg, Germany, 1992. [Google Scholar]
- Ingólfsson, O.; Weik, F.; Illenberger, E. Formation and decay of negative ion resonances in gaseous and condensed molecules. Int. Rev. Phys. Chem. 1996, 15, 133–151. [Google Scholar] [CrossRef]
- Orzol, M.; Martin, I.; Kočišek, J.; Dabkowska, I.; Langer, J.; Illenberger, E. Bond and site selectivity in dissociative electron attachment to gas phase and condensed phase ethanol and trifluoroethanol. Phys. Chem. Chem. Phys. 2007, 9, 3424–3431. [Google Scholar] [CrossRef]
- Illenberger, E.; Scheunemann, H.-U.; Baumgrtel, H. Negative ion formation in CF2Cl2, CF3Cl and CFCl3 following low energy (0–10 eV) impact with near monoenergetic electrons. Chem. Phys. 1979, 37, 21–31. [Google Scholar] [CrossRef]
- Huels, M.A.; Hahndorf, I.; Illenberger, E.; Sanche, L. Resonant dissociation of DNA bases by subionization electrons. J. Chem. Phys. 1998, 108, 1309–1312. [Google Scholar] [CrossRef]
- Sulzer, P.; Ptasińska, S.; Zappa, F.; Mielewska, B.; Milosavljevic, A.R.; Scheier, P.; Mark, T.D.; Bald, I.; Gohlke, S.; Huels, M.A.; et al. Dissociative electron attachment to furan, tetrahydrofuran, and fructose. J. Chem. Phys. 2006, 125, 044304. [Google Scholar] [CrossRef]
- König, C.; Kopyra, J.; Bald, I.; Illenberger, E. Dissociative electron attachment to phosphoric acid esters: The direct mechanism for single strand breaks in DNA. Phys. Rev. Lett. 2006, 97, 018105. [Google Scholar] [CrossRef] [PubMed]
- Bald, I.; Dąbkowska, I.; Illenberger, E. Probing biomolecules by laser-induced acoustic desorption: Electrons at near zero electron volts trigger sugar–phosphate cleavage. Angew. Chem. Int. Ed. Engl. 2008, 47, 8518–8520. [Google Scholar] [CrossRef]
- Ptasińska, S.; Denifl, S.; Scheier, P.; Illenberger, E.; Märk, T.D. Bond- and site-selective loss of H atom from nucleobases by very-low-energy electrons (<3 eV) . Angew. Chem. Int. Ed. 2005, 44, 6941–6943. [Google Scholar] [CrossRef] [PubMed]
- Vizcaino, V.; Bartl, P.; Gschliesser, D.; Huber, S.E.; Probst, M.; Märk, T.D.; Scheier, P.; Denifl, S. Dissociative electron attachment to β-alanine. Chem. Phys. Chem. 2011, 12, 1272–1279. [Google Scholar] [CrossRef]
- Ptasińska, S.; Denifl, S.; Grill, V.; Märk, T.D.; Illenberger, E.; Scheier, P. Bond- and site-selective loss of H− from pyrimidine bases. Phys. Rev. Lett. 2005, 95, 093201. [Google Scholar] [CrossRef] [PubMed]
- Illenberger, E. Electron-attachment reactions in molecular clusters. Chem. Rev. 1992, 92, 1589–1609. [Google Scholar] [CrossRef]
- Zheng, Y.; Sanche, L. Effective and absolute cross sections for low-energy (1–30 eV) electron interactions with condensed biomolecules. Appl. Phys. Rev. 2018, 5, 021302. [Google Scholar] [CrossRef]
- Antic, D.; Parenteau, L.; Lepage, M.; Sanche, L. Low energy electron damage to condensed phase deoxyribose analogues investigated by electron stimulated desorption of H− and electron energy loss spectroscopy. J. Phys. Chem. B 1999, 103, 6611–6619. [Google Scholar] [CrossRef]
- Antic, D.; Parenteau, L.; Sanche, L. Electron stimulated desorption of H− from condensed phase deoxyribose analogues: Dissociative electron attachment versus resonance decay into dipolar dissociation. J. Phys. Chem. B 2000, 104, 4711–4716. [Google Scholar] [CrossRef]
- Abdoul-Carime, H.; Cloutier, P.; Sanche, L. Low-energy (5–40 eV) electron-stimulated desorption of anions from physisorbed DNA bases. Radiat. Res. 2001, 155, 625–633. [Google Scholar] [CrossRef]
- Hervé du Penhoat, M.-A.; Huels, M.A.; Cloutier, P.; Jay-Gerin, J.-P.; Sanche, L. Electron stimulated desorption of H− from thin films of thymine and uracil. J. Chem. Phys. 2001, 114, 5755–5764. [Google Scholar] [CrossRef]
- Abdoul-Carime, H.; Huels, M.A.; Illenberger, E.; Sanche, L. Sensitizing DNA to secondary electron damage: Resonant formation of oxidative radicals from 5-halouracils. J. Am. Chem. Soc. 2001, 123, 5354–5355. [Google Scholar] [CrossRef]
- Pan, X.; Sanche, L. Dissociative electron attachment to DNA basic constituents: The phosphate group. Chem. Phys. Lett. 2006, 421, 404–408. [Google Scholar] [CrossRef]
- Dugal, P.-C.; Huels, M.A.; Sanche, L. Low-energy (5–25 eV) electron damage to homo-oligonucleotides. Radiat. Res. 1999, 151, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Abdoul-Carime, H.; Dugal, P.-C.; Sanche, L. Damage induced by 1–30 eV electrons on thymine and bromouracil-substituted oligonucleotides. Radiat. Res. 2000, 153, 23–28. [Google Scholar] [CrossRef]
- Abdoul-Carime, H.; Dugal, P.C.; Sanche, L. Desorption induced by electronic transitions (DIET) of neutral fragments from chemisorbed biological molecular systems. Surf. Sci. 2000, 451, 102–107. [Google Scholar] [CrossRef]
- Dugal, P.-C.; Abdoul-Carime, H.; Sanche, L. Mechanisms for low energy (0.5–30 eV) electron-induced pyrimidine ring fragmentation within thymine and halogen-substituted single strands of DNA. J. Phys. Chem. B 2000, 104, 5610–5617. [Google Scholar] [CrossRef]
- Abdoul-Carime, H.; Sanche, L. Sequence-specific damage induced by the impact of 3–30 eV electrons on oligonucleotides. Radiat. Res. 2001, 156, 151–157. [Google Scholar] [CrossRef]
- Abdoul-Carime, H.; Sanche, L. Fragmentation of short single DNA strands by 1–30 eV electrons: Dependence on base identity and sequence. Int. J. Radiat. Biol. 2002, 78, 89–99. [Google Scholar] [CrossRef]
- Ptasińska, S.; Sanche, L. On the mechanism of anion desorption from DNA induced by low energy electrons. J. Chem. Phys. 2006, 125, 144713. [Google Scholar] [CrossRef] [PubMed]
- Ptasińska, S.; Sanche, L. Dissociative electron attachment to abasic DNA. Phys. Chem. Chem. Phys. 2007, 9, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Ptasińska, S.; Sanche, L. Dissociative electron attachment to hydrated single DNA strands. Phys. Rev. E 2007, 75, 031915. [Google Scholar] [CrossRef]
- Mirsaleh-Kohan, N.; Bass, A.D.; Cloutier, P.; Sanche, L. Electron stimulated desorption of anions containing oxygen and nitrogen from self-assembled monolayers of DNA. J. Phys. Conf. Ser. 2010, 204, 012005. [Google Scholar] [CrossRef]
- Mirsaleh-Kohan, N.; Bass, A.D.; Cloutier, P.; Massey, S.; Sanche, L. Low energy electron stimulated desorption from DNA films dosed with oxygen. J. Chem. Phys. 2012, 136, 235104. [Google Scholar] [CrossRef]
- Mirsaleh-Kohan, N.; Bass, A.D.; Sanche, L. Effect of morphology of thin DNA films on the electron stimulated desorption of anions. J. Chem. Phys. 2011, 134, 015102. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.J.; Giaccia, A.J. Oxygen effect and reoxygenation. In Radiobiology for the Radiologist; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006. [Google Scholar]
- Boulanouar, O.; Fromm, M.; Mavon, C.; Cloutier, P.; Sanche, L. Dissociative electron attachment to DNA-diamine thin films: Impact of the DNA close environment on the OH− and O− decay channels. J. Chem. Phys. 2013, 139, 055101. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Bald, I.; Rotaru, A.; Cauët, E.; Gothelf, K.V.; Besenbacher, F. Probing electron-induced bond cleavage at the single-molecule level using DNA origami templates. ACS Nano 2012, 6, 4392–4399. [Google Scholar] [CrossRef] [PubMed]
- Schürmann, R.; Tsering, T.; Tanzer, K.; Denifl, S.; Kumar, S.V.K.; Bald, I. Resonant formation of strand breaks in sensitized oligonucleotides induced by low-energy electrons (0.5–9 eV). Angew. Chem. Int. Ed. 2017, 56, 10952–10955. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.; Rackwitz, J.; Schürman, R.; Prinz, J.; Milosavljevic, A.R.; Refregiers, M.; Giuliani, A.; Bald, I. Using DNA origami nanostructures to determine absolute cross sections for UV photon-induced DNA strand breakage. J. Phys. Chem. Lett. 2015, 6, 4589–4593. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.; Ebel, K.; Schürmann, R.M.; Heck, C.; Meiling, T.; Milosavljevic, A.R.; Giuliani, A.; Bald, I. Vacuum-UV and low-energy electron-induced DNA strand breaks—Influence of the DNA sequence and substrate. Chem. Phys. Chem. 2019, 20, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Ebel, K.; Bald, I. Length and energy dependence of low-energy electron-induced strand breaks in poly(A) DNA. Int. J. Mol. Sci. 2020, 21, 111. [Google Scholar] [CrossRef]
- Choi, Y.; Schmidt, C.; Tinnefeld, P.; Bald, I.; Rödiger, S. A new reporter design based on DNA origami nanostructures for quantification of short oligonucleotides using microbeads. Sci. Rep. 2019, 9, 4769. [Google Scholar] [CrossRef]
- Du, Y.; Dong, S. Nucleic acid biosensors: Recent advances and perspectives. Anal. Chem. 2017, 89, 189–215. [Google Scholar] [CrossRef]
- Rackwitz, J.; Kopyra, J.; Dabkowska, I.; Ebel, K.; Ranković, M.L.; Milosavljević, A.R.; Bald, I. Sensitizing DNA towards low-energy electrons with 2-fluoroadenine. Angew. Chem. Int. Ed. 2016, 55, 10248–10252. [Google Scholar] [CrossRef] [PubMed]
- Rackwitz, J.; Ranković, M.L.; Milosavljević, A.R.; Bald, I. A novel setup for the determination of absolute cross sections for low-energy electron induced strand breaks in oligonucleotides- The effect of the radiosensitizer 5-fluorouracil. Eur. Phys. J. D 2017, 71, 32. [Google Scholar] [CrossRef]
- Ray, S.G.; Daube, S.S.; Cohen, H.; Naaman, R. Electron capturing by DNA. Isr. J. Chem. 2007, 47, 149–159. [Google Scholar] [CrossRef]
- Markus, T.Z.; Daube, S.S.; Naaman, R. Cooperative effect in the electronic properties of human telomere sequence. J. Phys. Chem. B 2010, 114, 13897–13903. [Google Scholar] [CrossRef] [PubMed]
- Markus, T.Z.; de Leon, A.R.; Reid, D.; Achim, C.; Naaman, R. The capture of low-energy electrons by PNA versus DNA. J. Phys. Chem. Lett. 2013, 4, 3298–3302. [Google Scholar] [CrossRef]
- Naaman, R.; Waldeck, D.H. Spintronics and chirality: Spin selectivity in electron transport through chiral molecules. Annu. Rev. Phys. Chem. 2015, 66, 263–281. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Markus, T.Z.; Cohen, S.R.; Vager, Z.; Gutierrez, R.; Naaman, R. Spin specific electron conduction through DNA oligomers. Nano Lett. 2011, 11, 4652–4655. [Google Scholar] [CrossRef]
- Kumar, K.S.; Kantor-Uriel, N.; Mathew, S.P.; Guliamov, R.; Naaman, R. A device for measuring spin selectivity in electron transfer. Phys. Chem. Chem. Phys. 2013, 15, 18357–18362. [Google Scholar] [CrossRef] [PubMed]
- Carmeli, I.; Kumar, K.S.; Heifler, O.; Carmeli, C.; Naaman, R. Spin selectivity in electron transfer in photosystem I. Angew. Chem. Int. Ed. 2014, 53, 8953–8958. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, R.A.; Mishra, D.; Naaman, R. Chiral selective chemistry induced by natural selection of spin-polarized electrons. Angew. Chem. Int. Ed. 2015, 54, 7295–7298. [Google Scholar] [CrossRef]
- Kumar, K.S.; Naaman, R. Quantitative analysis and characterization of self-assembled DNA on a silver surface. Langmuir 2012, 28, 14514–14517. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.D.; Schaible, M.J.; Rosenberg, R.A.; Kundu, S.; Orlando, T.M. Low energy secondary electron induced damage of condensed nucleotides. J. Chem. Phys. 2019, 150, 204709. [Google Scholar] [CrossRef]
- Klyachko, D.V.; Huels, M.A.; Sanche, L. Halogen anion formation in 5-halo-uracil films: X-rays compared to subionization electrons. Radiat. Res. 1999, 151, 177–187. [Google Scholar] [CrossRef]
- Klyachko, D.V.; Gantchev, T.; Huels, M.A.; Sanche, L. An X-ray photoelectron investigation of the effects of low-energy electrons in DNA bases. In Microdosimetry: An Interdisciplinary Approach; Goodhead, D.T., O’Neill, P., Menzel, H.G., Eds.; The Royal Society of Chemistry: Cambridge, UK, 1997; pp. 89–92. [Google Scholar]
- Kundu, S.; Schaible, M.J.; McKee, A.D.; Orlando, T.M. Direct damage of deoxyadenosine monophosphate by low-energy electrons probed by X-ray photoelectron spectroscopy. J. Phys. Chem. B 2020, 124, 1585–1591. [Google Scholar] [CrossRef]
- Sidorov, A.N.; Orlando, T.M. Monolayer graphene platform for the study of DNA damage by low-energy electron irradiation. J. Phys. Chem. Lett. 2013, 4, 2328–2333. [Google Scholar] [CrossRef]
- Zheng, Y.; Cloutier, P.; Wagner, J.R.; Sanche, L. Irradiator to study damage induced to large nonvolatile molecules by low-energy electrons. Rev. Sci. Instrum. 2004, 75, 4534–4540. [Google Scholar] [CrossRef]
- Zheng, Y.; Cloutier, P.; Hunting, D.J.; Wagner, J.R.; Sanche, L. Glycosidic bond cleavage of thymidine by low-energy electrons. J. Am. Chem. Soc. 2004, 126, 1002–1003. [Google Scholar] [CrossRef]
- Zheng, Y.; Cloutier, P.; Hunting, D.J.; Sanche, L.; Wagner, J.R. Chemical basis of DNA sugar-phosphate cleavage by low-energy electrons. J. Am. Chem. Soc. 2005, 127, 16592–16598. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cloutier, P.; Hunting, D.J.; Wagner, J.R.; Sanche, L. Phosphodiester and N-glycosidic bond cleavage in DNA induced by 4–15 eV electrons. J. Chem. Phys. 2006, 124, 064710. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Li, Z.; Cloutier, P.; Sanche, L.; Wagner, J.R. DNA damage induced by low-energy electrons: Conversion of thymine to 5,6-dihydrothymine in the oligonucleotide trimer TpTpT. Radiat. Res. 2011, 175, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cloutier, P.; Sanche, L.; Wagner, J.R. Low-energy electron-induced damage in a trinucleotide containing 5-bromouracil. J. Phys. Chem. B 2011, 115, 13668–13673. [Google Scholar] [CrossRef] [PubMed]
- Madugundu, G.S.; Park, Y.; Sanche, L.; Wagner, J.R. Radiation-induced formation of 2′, 3′-dideoxyribonucleosides in DNA: A potential signature of low-energy electrons. J. Am. Chem. Soc. 2012, 134, 17366–17368. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Z.; Cloutier, P.; Sanche, L.; Wagner, J.R. Low energy electron induced DNA damage: Effect of base sequence in oligonucleotide trimers. J. Am. Chem. Soc. 2010, 132, 5422–5427. [Google Scholar] [CrossRef]
- Park, Y.; Polska, K.; Rak, J.; Wagner, J.R.; Sanche, L. Fundamental mechanisms of DNA radiosensitization: Damage induced by low-energy electrons in brominated oligonucleotide trimers. J. Phys. Chem. B 2012, 116, 9676–9682. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Peoples, A.R.; Madugundu, G.S.; Sanche, L.; Wagner, J.R. Side-by-side comparison of DNA damage induced by low-energy electrons and high-energy photons with solid TpTpT trinucleotide. J. Phys. Chem. B 2013, 117, 10122–10131. [Google Scholar] [CrossRef] [PubMed]
- Choofong, S.; Cloutier, P.; Sanche, L.; Wagner, J.R. Base release and modification in solid-phase DNA exposed to low-energy electrons. Radiat. Res. 2016, 186, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Khorsandgolchin, G.; Sanche, L.; Cloutier, P.; Wagner, J.R. Strand breaks induced by very low energy electrons: Product analysis and mechanistic insight into the reaction with TpT. J. Am. Chem. Soc. 2019, 141, 10315–10323. [Google Scholar] [CrossRef] [PubMed]
- Ptasińska, S.; Li, Z.; Mason, N.J.; Sanche, L. Damage to amino acid-nucleotide pairs induced by 1 eV electrons. Phys. Chem. Chem. Phys. 2010, 12, 9367–9372. [Google Scholar] [CrossRef] [PubMed]
- Solomun, T.; Sturm, H. Bringing electrons and microarray technology together. J. Phys. Chem. B 2007, 111, 10636–10638. [Google Scholar] [CrossRef]
- Solomun, T.; Seitz, H.; Sturm, H. DNA damage by low-energy electron impact: Dependence on guanine content. J. Phys. Chem. B 2009, 113, 11557–11559. [Google Scholar] [CrossRef]
- Solomun, T.; Mix, R.; Sturm, H. Immobilization of silanized DNA on glass: Influence of the silane tether on the DNA hybridization. ACS Appl. Mater. Interfaces 2010, 2, 2171–2174. [Google Scholar] [CrossRef] [PubMed]
- Solomun, T.; Sturm, H.; Wellhausen, R.; Seitz, H. Interaction of a single-stranded DNA-binding protein g5p with DNA oligonucleotides immobilised on a gold surface. Chem. Phys. Lett. 2012, 533, 92–94. [Google Scholar] [CrossRef]
- Boulanouar, O.; Khatyr, A.; Herlem, G.; Palmino, F.; Sanche, L.; Fromm, M. Soft adsorption of densely packed layers of DNA-plasmid·1,3-diaminopropane complexes onto highly oriented pyrolitic graphite designed to erode in water. J. Phys. Chem. C 2011, 115, 21291–21298. [Google Scholar] [CrossRef]
- Kouass Sahbani, S.; Cloutier, P.; Bass, A.D.; Hunting, D.J.; Sanche, L. Electron resonance decay into a biological function: Decrease in viability of E. coli transformed by plasmid DNA irradiated with 0.5–18 eV electrons. J. Phys. Chem. Lett. 2015, 6, 3911–3914. [Google Scholar] [CrossRef]
- Kouass-Sahbani, S.; Sanche, L.; Cloutier, P.; Bass, A.D.; Hunting, D.J. Loss of cellular transformation efficiency induced by DNA irradiation with low-energy (10 eV) electrons. J. Phys. Chem. B 2014, 118, 13123–13131. [Google Scholar] [CrossRef]
- Kouass-Sahbani, S.; Girouard, S.; Cloutier, P.; Sanche, L.; Hunting, D.J. The relative contributions of DNA strand breaks, base damage and clustered lesions to the loss of DNA functionality induced by ionizing radiation. Radiat. Res. 2014, 181, 99–110. [Google Scholar] [CrossRef]
- Folkard, M.; Prise, K.; Vojnovic, B.; Davies, S.; Roper, M.; Michael, B. Measurement of DNA damage by electrons with energies between 25 and 4000 eV. Int. J. Radiat. Biol. 1993, 64, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Dong, Y.; Hunting, D.J.; Zheng, Y.; Sanche, L. Unified mechanism for the generation of isolated and clustered DNA damages by a single low energy (5–10 eV) electron. J. Phys. Chem. C 2017, 121, 2466–2472. [Google Scholar] [CrossRef]
- Orlando, T.M.; Oh, D.; Chen, Y. Aleksandrov, A.B. Low-energy electron diffraction and induced damage in hydrated DNA. J. Chem. Phys. 2008, 128, 195102. [Google Scholar] [CrossRef]
- Cai, Z.; Cloutier, P.; Hunting, D.; Sanche, L. Comparison between X-ray photon and secondary electron damage to DNA in vacuum. J. Phys. Chem. B 2005, 109, 4796–4800. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Cloutier, P.; Hunting, D.; Sanche, L. Enhanced DNA damage induced by secondary electron emission from a tantalum surface exposed to soft X rays. Radiat. Res. 2006, 165, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Swarts, S.G.; Sevilla, M.D.; Becker, D.; Tokar, C.J.; Wheeler, K.T. Radiation-induced DNA damage as a function of hydration. I. Release of unaltered bases. Radiat. Res. 1992, 129, 333–344. [Google Scholar] [CrossRef]
- Ito, T.; Baker, S.C.; Stickley, C.D.; Peak, J.G.; Peak, M.J. Dependence of the yield of strand breaks induced by γ-rays in DNA on the physical conditions of exposure: Water content and temperature. Int. J. Radiat. Biol. 1993, 63, 289–296. [Google Scholar] [CrossRef]
- Brun, É.; Cloutier, P.; Sicard-Roselli, C.; Fromm, M.; Sanche, L. Damage induced to DNA by low-energy (0–30 eV) electrons under vacuum and atmospheric conditions. J. Phys. Chem. B 2009, 113, 10008–10013. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, E.; Cloutier, P.; Hunting, D.; Sanche, L. Soft X-ray and low energy electron-induced damage to DNA under N2 and O2 atmospheres. J. Phys. Chem. B 2011, 115, 4523–4531. [Google Scholar] [CrossRef]
- Alizadeh, E.; Sanche, L. Low-energy-electron interactions with DNA: Approaching cellular conditions with atmospheric experiments. Eur. Phys. J. D 2014, 68, 97. [Google Scholar] [CrossRef]
- Alizadeh, E.; Sanz, A.G.; Garcia, G.; Sanche, L. Radiation damage to DNA: The indirect effect of low-energy electrons. J. Phys. Chem. Lett. 2013, 4, 820–825. [Google Scholar] [CrossRef]
- Alizadeh, E.; Sanche, L. Role of humidity and oxygen level on damage to DNA induced by soft X-rays and low-energy electrons. J. Phys. Chem. C 2013, 117, 22445–22453. [Google Scholar] [CrossRef]
- Alizadeh, E.; Sanche, L. Induction of strand breaks in DNA films by low energy electrons and soft X-ray under nitrous oxide atmosphere. Radiat. Phys. Chem. 2012, 81, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yao, X.; Cloutier, P.; Zheng, Y.; Sanche, L. DNA strand breaks induced by 0–1.5 eV UV photoelectrons under atmospheric pressure. J. Phys. Chem. C 2016, 120, 487–495. [Google Scholar] [CrossRef]
- Hahn, M.B.; Dietrich, P.M.; Radnik, J. In situ monitoring of the influence of water on DNA radiation damage by near-ambient pressure X-ray photoelectron spectroscopy. Commun. Chem. 2021, 4, 50. [Google Scholar] [CrossRef]
- Ptasińska, S.; Denifl, S.; Gohlke, S.; Scheier, P.; Illenberger, E.; Märk, T.D. Decomposition of thymidine by low-energy electrons: Implications for the molecular mechanisms of single-strand breaks in DNA. Angew. Chem. Int. Ed. 2006, 45, 1893–1896. [Google Scholar] [CrossRef] [PubMed]
- Förstel, M.; Neustetter, M.; Denifl, S.; Lelievre, F.; Hergenhahn, U. A source for microhydrated biomolecules. Rev. Sci. Instrum. 2015, 86, 073103. [Google Scholar] [CrossRef]
- Kočišek, J.; Sedmidubská, B.; Indrajith, S.; Fárník, M.; Fedor, J. Electron attachment to microhydrated deoxycytidine monophosphate. J. Phys. Chem. B 2018, 122, 5212–5217. [Google Scholar] [CrossRef]
- Maddern, T.M.; Jamier, V.; Brunton, J.R.; Brunger, M.J.; Papamicaël, C.; Smith, S.V.; Buckman, S.J. A technique to determine the thermal stability of uracil and uracil derivatives in a molecular beam. Int. J. Mass Spectrom. 2016, 409, 73–80. [Google Scholar] [CrossRef]
- Prell, J.S.; O’Brien, J.T.; Holm, A.I.S.; Leib, R.D.; Donald, W.A.; Williams, E.R. Electron capture by a hydrated gaseous peptide: Effects of water on fragmentation and molecular survival. J. Am. Chem. Soc. 2008, 130, 12680–12689. [Google Scholar] [CrossRef]
- Feketeová, L.; O’Hair, R.A.J. Electron-induced dissociation of doubly protonated betaine clusters: Controlling fragmentation chemistry through electron energy. Rapid Commun. Mass Spectrom. 2009, 23, 3259–3263. [Google Scholar] [CrossRef]
- Tiefenthaler, L.; Kočišek, J.; Scheier, P. Cluster ion polymerization of serine and tryptophan, the water loss channel. Eur. Phys. J. D 2020, 74, 85. [Google Scholar] [CrossRef]
- Neustetter, M.; Aysina, J.; da Silva, F.; Denifl, S. The effect of solvation on electron attachment to pure and hydrated pyrimidine clusters. Angew. Chem. Int. Ed. 2015, 54, 9124–9126. [Google Scholar] [CrossRef] [PubMed]
- Kočišek, J.; Pysanenko, A.; Fárník, M.; Fedor, J. Microhydration prevents fragmentation of uracil and thymine by low-energy electrons. J. Phys. Chem. Lett. 2016, 7, 3401–3405. [Google Scholar] [CrossRef] [PubMed]
- Luxford, T.F.M.; Pshenichnyuk, S.A.; Asfandiarov, N.L.; Perečko, T.; Falk, M.; Kočišek, J. 5-Nitro-2,4-dichloropyrimidine as an universal model for low-energy electron processes relevant for radiosensitization. Int. J. Mol. Sci. 2020, 21, 8173. [Google Scholar] [CrossRef]
- Fabrikant, I.I. Electron attachment to molecules in a cluster environment: Suppression and enhancement effects. Eur. Phys. J. D 2018, 72, 96. [Google Scholar] [CrossRef]
- Dessent, C.E.H.; Bailey, C.G.; Johnson, M.A. On the vibrational fine structure in the near-threshold photofragmentation spectrum of the I-CH3I complex: Spectroscopic observation of nonadiabatic effects in electron molecule scattering. J. Chem. Phys. 1996, 105, 10416–10423. [Google Scholar] [CrossRef]
- Dessent, C.E.H.; Kim, J.; Johnson, M.A. Spectroscopic observation of vibrational Feshbach resonances in near-threshold photoexcitation of X− •CH3NO2 (X−= I− and Br−). Faraday Discuss. 2000, 115, 395–406. [Google Scholar] [CrossRef]
- Mbaiwa, F.; Van Duzor, M.; Wei, J.; Mabbs, R. Direct and indirect detachment in the iodide-pyrrole cluster anion: The role of dipole bound and neutral cluster states. J. Phys. Chem. A 2010, 114, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Yandell, M.A.; King, S.B.; Neumark, D.M. Time-resolved radiation chemistry: Photoelectron imaging of transient negative ions of nucleobases. J. Am. Chem. Soc. 2013, 135, 2128–2131. [Google Scholar] [CrossRef]
- King, S.B.; Yandell, M.A.; Neumark, D.M. Time-resolved photoelectron imaging of the iodide-thymine and iodide-uracil binary cluster systems. Faraday Discuss. 2013, 163, 59–72. [Google Scholar] [CrossRef]
- King, S.B.; Yandell, M.A.; Stephansen, A.B.; Neumark, D.M. Time-resolved radiation chemistry: Dynamics of electron attachment to uracil following UV excitation of iodide-uracil complexes. J. Chem. Phys. 2014, 141, 224310. [Google Scholar] [CrossRef] [PubMed]
- King, S.B.; Stephansen, A.B.; Yokoi, Y.; Yandell, M.A.; Kunin, A.; Takayanagi, T.; Neumark, D.M. Electron accommodation dynamics in the DNA base thymine. J. Chem. Phys. 2015, 143, 024312. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-L.; Kunin, A.; Matthews, E.; Yoshikawa, N.; Dessent, C.E.H.; Neumark, D.M. Photodissociation dynamics of the iodide-uracil (I−U) complex. J. Chem. Phys. 2016, 145, 044319. [Google Scholar] [CrossRef] [PubMed]
- Matthews, E.; Cercola, R.; Mensa-Bonsu, G.; Neumark, D.M.; Dessent, C.E.H. Photoexcitation of iodide ion-pyrimidine clusters above the electron detachment threshold: Intracluster electron transfer versus nucleobase-centred excitations. J. Chem. Phys. 2018, 148, 084304. [Google Scholar] [CrossRef]
- Kunin, A.; Neumark, D.M. Time-resolved radiation chemistry: Femtosecond photoelectron spectroscopy of electron attachment and photodissociation dynamics in iodide-nucleobase clusters. Phys. Chem. Chem. Phys. 2019, 21, 7239–7255. [Google Scholar] [CrossRef] [PubMed]
- Stephansen, A.B.; King, S.B.; Yokoi, Y.; Minoshima, Y.; Li, W.-L.; Kunin, A.; Takayanagi, T.; Neumark, D.M. Dynamics of dipole- and valence bound anions in iodide-adenine binary complexes: A time-resolved photoelectron imaging and quantum mechanical investigation. J. Chem. Phys. 2015, 143, 104308. [Google Scholar] [CrossRef] [PubMed]
- Kunin, A.; Li, W.-L.; Neumark, D.M. Dynamics of electron attachment and photodissociation in iodide-uracil-water clusters via time-resolved photoelectron imaging. J. Chem. Phys. 2018, 149, 084301. [Google Scholar] [CrossRef]
- Kunin, A.; McGraw, V.S.; Lunny, K.G.; Neumark, D.M. Time-resolved dynamics in iodide-uracil-water clusters upon excitation of the nucleobase. J. Chem. Phys. 2019, 151, 154304. [Google Scholar] [CrossRef]
- Osterwalder, A.; Nee, M.J.; Zhou, J.; Neumark, D.M. High resolution photodetachment spectroscopy of negative ions via slow photoelectron imaging. J. Chem. Phys. 2004, 121, 6317–6322. [Google Scholar] [CrossRef]
- Parsons, B.F.; Sheehan, S.M.; Yen, T.A.; Neumark, D.M.; Wehres, N.; Weinkauf, R. Anion photoelectron imaging of deprotonated thymine and cytosine. Phys. Chem. Chem. Phys. 2007, 9, 3291–3297. [Google Scholar] [CrossRef] [PubMed]
- Siefermann, K.R.; Liu, Y.; Lugovoy, E.; Link, O.; Faubel, M.; Buck, U.; Winter, B.; Abel, B. Binding energies, lifetimes and implications of bulk and interface solvated electrons in water. Nat. Chem. 2010, 2, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Neumark, D.M. Ejecting electrons from water. Nat. Chem. 2010, 2, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Ehrler, O.T.; Neumark, D.M. Dynamics of electron solvation in molecular clusters. Acc. Chem. Res. 2009, 42, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Neumark, D.M. Slow electron velocity-map imaging of negative ions: Applications to spectroscopy and dynamics. J. Phys. Chem. A 2008, 112, 13287–13301. [Google Scholar] [CrossRef]
- Ma, J.; Kumar, A.; Muroya, Y.; Yamashita, S.; Sakurai, T.; Denisov, S.; Sevilla, M.; Adhikary, A.; Seki, S.; Mostafavi, M. Observation of dissociative quasi-free electron attachment to nucleoside via excited anion radical in solution. Nat. Commun. 2019, 10, 102. [Google Scholar] [CrossRef]
- Ma, J.; Denisov, S.A.; Adhikary, A.; Mostafavi, M. Ultrafast processes occurring in radiolysis of highly concentrated solutions of nucleosides/tides. Int. J. Mol. Sci. 2019, 20, 4963. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-R.; Lu, Q.-B. Real-time observation of a molecular reaction mechanism of aqueous 5-halo-2′-deoxyuridines under UV/ionizing radiation. Angew. Chem. Int. Ed. 2007, 46, 6316–6320. [Google Scholar] [CrossRef]
- Lu, Q.-B. Effects of ultrashort-lived prehydrated electrons in radiation biology and their applications for radiotherapy of cancer. Mutat. Res. 2010, 704, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.; Ma, Y.; Luo, T.; Bristow, R.G.; Jaffray, D.A.; Lu, Q.-B. Direct observation of ultrafast-electron-transfer reactions unravels high effectiveness of reductive DNA damage. Proc. Natl. Acad. Sci. USA 2011, 108, 11778–11783. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, F.; Denisov, S.A.; Adhikary, A.; Mostafavi, M. Reactivity of prehydrated electrons toward nucleobases and nucleotides in aqueous solution. Sci. Adv. 2017, 3, e1701669. [Google Scholar] [CrossRef]
- Ma, J.; Marignier, J.-L.; Pernot, P.; Houee-Levin, C.; Kumar, A.; Sevilla, M.D.; Adhikary, A.; Mostafavi, M. Direct observation of the oxidation of DNA bases by phosphate radicals formed under radiation: A model of the backbone-to-base hole transfer. Phys. Chem. Chem. Phys. 2018, 20, 14927–14937. [Google Scholar] [CrossRef]
- Ma, J.; Denisov, S.; Marignier, J.; Pernot, P.; Adhikary, A.; Seki, S.; Mostafavi, M. Ultrafast electron attachment and hole transfer following ionizing radiation of aqueous uridine monophosphate. J. Phys. Chem. Lett. 2018, 9, 5105–5109. [Google Scholar] [CrossRef] [PubMed]
- Solomun, T.; Seitz, H.; Sturm, H. Electron irradiation of immobilized DNA in solution through a silicon nano-membrane. Radiat. Phys. Chem. 2013, 88, 70–73. [Google Scholar] [CrossRef]
- Hahn, M.; Meyer, S.; Schroter, M.; Seitz, H.; Kunte, H.; Solomun, T.; Sturm, H. Direct electron irradiation of DNA in a fully aqueous environment. Damage determination in combination with Monte Carlo simulations. Phys. Chem. Chem. Phys. 2017, 19, 1798–1805. [Google Scholar] [CrossRef]
- Hahn, M.B.; Meyer, S.; Kunte, H.-J.; Solomun, T.; Sturm, H. Measurements and simulations of microscopic damage to DNA in water by 30 keV electrons: A general approach applicable to other radiation sources and biological targets. Phys. Rev. E 2017, 95, 052419. [Google Scholar] [CrossRef]
- Nikjoo, H.; Martin, R.; Charlton, D.; Terrissol, M.; Kandaiya, S.; Lobachevsky, P. Modelling auger-induced DNA damage by incorporated 125I. Acta Oncol. 1996, 35, 849–856. [Google Scholar] [CrossRef]
- Schürmann, R.; Vogel, S.; Ebel, K.; Bald, I. The physico-chemical basis of DNA radiosensitization: Implications for cancer radiation therapy. Chem. Eur. J. 2018, 24, 10271–100279. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.B.; Smales, G.J.; Seitz, H.; Solomun, T.; Sturm, H. Ectoine interaction with DNA: Influence on ultraviolet radiation damage. Phys. Chem. Chem. Phys. 2020, 22, 6984–6992. [Google Scholar] [CrossRef]
- Schröter, M.-A.; Meyer, S.; Hahn, M.B.; Solomun, T.; Sturm, H.; Kunte, H.J. Ectoine protects DNA from damage by ionizing radiation. Sci. Rep. 2017, 7, 15272. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.B.; Meyer, S.; Schröter, M.-A.; Kunte, H.-J.; Solomun, T.; Sturm, H. DNA protection by ectoine from ionizing radiation: Molecular mechanisms. Phys. Chem. Chem. Phys. 2017, 19, 25717–25722. [Google Scholar] [CrossRef] [PubMed]
- Solomun, T.; Hahn, M.B.; Smiatek, J. Raman spectroscopic signature of ectoine conformations in bulk solution and crystalline state. Chem. Phys. Chem. 2020, 21, 1945–1950. [Google Scholar] [CrossRef]
- Hahn, M.B.; Solomun, T.; Wellhausen, R.; Hermann, S.; Seitz, H.; Meyer, S.; Kunte, H.-J.; Zeman, J.; Uhlig, F.; Smiatek, J.; et al. Influence of the compatible solute ectoine on the local water structure: Implications for the binding of the protein G5P to DNA. J. Phys. Chem. B 2015, 119, 15212–15220. [Google Scholar] [CrossRef]
- Meesat, R.; Belmouaddine, H.; Allard, J.-F.; Tanguay-Renaud, C.; Lemay, R.; Brastaviceanu, T.; Tremblay, L.; Paquette, B.; Wagner, J.R.; Jay-Gerin, J.P.; et al. Cancer radiotherapy based on femtosecond IR laser-beam filamentation yielding ultra-high dose rates and zero entrance dose. Proc. Natl. Acad. Sci. USA 2012, 109, E2508–E2513. [Google Scholar] [CrossRef]
- Belmouaddine, H.; Madugundu, G.S.; Wagner, J.R.; Couairon, A.; Houde, D.; Sanche, L. DNA base modifications mediated by femtosecond laser-induced cold low-density plasma in aqueous solutions. J. Phys. Chem. Lett. 2019, 10, 2753–2760. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, Z.; Vogel, A. Multi-rate-equation modeling of the energy spectrum of laser-induced conduction band electrons in water. Opt. Express 2019, 27, 4672–4693. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, L.; Ruther, R.; Hamers, R. Photo-illuminated diamond as a solid-state source of solvated electrons in water for nitrogen reduction. Nat. Mater. 2013, 12, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Szczerbiński, J.; Gyr, L.; Kaeslin, J.; Zenobi, R. Plasmon-driven photocatalysis leads to products known from e-beam and X-ray-induced surface chemistry. Nano Lett. 2018, 18, 6740–6749. [Google Scholar] [CrossRef]
- Dong, Y.; Parobek, D.; Rossi, D.; Son, D. Photoemission of energetic hot electrons produced via up-conversion in doped quantum dots. Nano Lett. 2016, 16, 7270–7275. [Google Scholar] [CrossRef] [PubMed]
- Simoncelli, S.; Pensa, E.; Brick, T.; Gargiulo, J.; Lauri, A.; Cambiasso, J.; Li, Y.; Maier, S.; Cortés, E. Monitoring plasmonic hot-carrier chemical reactions at the single particle level. Faraday Discuss. 2019, 214, 73–87. [Google Scholar] [CrossRef]
- Tang, H.; Chen, C.-J.; Huang, Z.; Bright, J.; Meng, G.; Liu, R.-S.; Wu, N. Plasmonic hot electrons for sensing, photodetection, and solar energy applications: A perspective. J. Chem. Phys. 2020, 152, 220901. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, S.; Belmouaddine, H.; Morris, D.; Houde, D. Phase control algorithms and filamentation of ultrashort laser pulses in a scattering medium. Appl. Phys. B 2018, 124, 210. [Google Scholar] [CrossRef]
- Belmouaddine, H.; Shi, M.; Sanche, L.; Houde, D. Tuning the size of gold nanoparticles produced by multiple filamentation of femtosecond laser pulses in aqueous solutions. Phys. Chem. Chem. Phys. 2018, 20, 23403–23413. [Google Scholar] [CrossRef]
- Belmouaddine, H.; Shi, M.; Karsenti, P.-L.; Meesat, R.; Sanche, L.; Houde, D. Dense ionization and subsequent non-homogeneous radical-mediated chemistry of femtosecond laser-induced low density plasma in aqueous solutions: Synthesis of colloidal gold. Phys. Chem. Chem. Phys. 2017, 19, 7897–7909. [Google Scholar] [CrossRef] [PubMed]
- Meesat, R.; Allard, J.-F.; Belmouaddine, H.; Brastaviceanu, T.; Tremblay, L.; Paquette, B.; Jay-Gerin, J.-P.; Wagner, J.R.; Lepage, M.; Houde, D. Filamentation of femtosecond laser pulses as a source for radiotherapy. In Proceedings of the Society of Photo-Optical Instrumentation Engineers (SPIE), Photonics North 2011, Ottawa, ON, Canada, 16–18 May 2011; p. 800708. [Google Scholar]
- Yanik, M.F.; Cinar, H.; Cinar, H.N.; Chisholm, A.D.; Jin, Y.; Ben-Yakar, A. Neurosurgery: Functional regeneration after laser axotomy. Nature 2004, 432, 822. [Google Scholar] [CrossRef]
- Vogel, A.; Noack, J.; Hüttman, G.; Paltauf, G. Mechanisms of femtosecond laser nanosurgery of cells and tissues. Appl. Phys. B 2005, 81, 1015–1047. [Google Scholar] [CrossRef]
- Chung, S.H.; Mazur, E. Surgical applications of femtosecond lasers. J. Biophoton. 2009, 2, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Palanker, D.V.; Blumenkranz, M.S.; Andersen, D.; Wiltberger, M.; Marcellino, G.; Gooding, P.; Angeley, D.; Schuele, G.; Woodley, B.; Simoneau, M.; et al. Femtosecond laser-assisted cataract surgery with integrated optical coherence tomography. Sci. Transl. Med. 2010, 2, 58ra85. [Google Scholar] [CrossRef]
- Hoy, C.L.; Ferhanoglu, O.; Yildirim, M.; Kim, K.H.; Karajanagi, S.S.; Chan, K.M.C.; Kobler, J.B.; Zeitels, S.M.; Ben-Yakar, A. Clinical ultrafast laser surgery: Recent advances and future directions. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 7100814. [Google Scholar] [CrossRef]
- Tirlapur, U.K.; König, K. Targeted transfection by femtosecond laser. Nature 2002, 418, 290–291. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gonon, G.; Buonanno, M.; Autsavapromporn, N.; de Toledo, S.M.; Pain, D.; Azzam, E.I. Health risks of space exploration: Targeted and nontargeted oxidative injury by high-charge and high-energy particles. Antioxid. Redox Signal. 2014, 20, 1501–1523. [Google Scholar] [CrossRef]
- Rezaee, M.; Hill, R.P.; Jaffray, D.A. The exploitation of low-energy electrons in cancer treatment. Radiat. Res. 2017, 188, 123–143. [Google Scholar] [CrossRef]
- Mortezaee, K.; Najafi, M.; Farhood, B.; Ahmadi, A.; Shabeeb, D.; Musa, A.E. Genomic instability and carcinogenesis of heavy charged particles radiation: Clinical and environmental implications. Medicina 2019, 55, 591. [Google Scholar] [CrossRef] [PubMed]
- Nikjoo, H.; Emfietzoglou, D.; Liamsuwan, T.; Taleei, R.; Liljequist, D.; Uehara, S. Radiation track, DNA damage and response—A review. Rep. Prog. Phys. 2016, 79, 116601. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, M.; Cloutier, P.; Bass, A.D.; Michaud, M.; Hunting, D.J.; Sanche, L. Absolute cross section for low energy electron damage to condensed macromolecules: A case study of DNA. Phys. Rev. E 2012, 86, 031913. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Hunting, D.J.; Ayotte, P.; Sanche, L. Role of secondary low-energy electrons in the concomitant chemoradiation therapy of cancer. Phys. Rev. Lett. 2008, 100, 198101. [Google Scholar] [CrossRef] [PubMed]
- Tippayamontri, T.; Kotb, R.; Paquette, B.; Sanche, L. Synergism in concomitant chemoradiotherapy of cisplatin and oxaliplatin and their liposomal formulation in the human colorectal cancer HCT116 model. Anticancer Res. 2012, 32, 4395–4404. [Google Scholar]
- Tippayamontri, T.; Kotb, R.; Paquette, B.; Sanche, L. Efficacy of cisplatin and lipoplatin™ in combined treatment with radiation of a colorectal tumor in nude mouse. Anticancer Res. 2013, 33, 3005–3014. [Google Scholar]
- Tippayamontri, T.; Kotb, R.; Sanche, L.; Paquette, B. New therapeutic possibilities of combined treatment of radiotherapy with oxaliplatin and its liposomal formulations, Lipoxal™, in rectal cancer using xenograft in nude mice. Anticancer Res. 2014, 34, 5303–5312. [Google Scholar] [PubMed]
- Charest, G.; Sanche, L.; Fortin, D.; Mathieu, D.; Paquette, B. Optimization of the route of platinum drugs administration to optimize the concomitant treatment with radiotherapy for glioblastoma implanted in the Fischer rat brain. J. Neurooncol. 2013, 115, 365–373. [Google Scholar] [CrossRef]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, M.; Hunting, D.J.; Sanche, L. New insights into the mechanism underlying the synergistic action of ionizing radiation with platinum chemotherapeutic drugs: The role of low-energy electrons. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 847–853. [Google Scholar] [CrossRef]
- Bao, Q.; Chen, Y.; Zheng, Y.; Sanche, L. Cisplatin radiosensitization of DNA irradiated with 2–20 eV electrons: Role of transient anions. J. Phys. Chem. C 2014, 118, 15516–15524. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, L.; Tian, Q.; Zheng, Y.; Sanche, L. Chemoradiation cancer therapy: Molecular mechanisms of cisplatin radiosensitization. J. Phys. Chem. C 2017, 121, 17505–17513. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, Y.; Zhou, L.; Shao, Y.; Fu, X.; Zheng, Y. Molecular efficacy of radio- and chemotherapy sequences from direct DNA damage measurements. Int. J. Radiat. Biol. 2017, 93, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, W.; Brodeur, N.; Cloutier, P.; Zheng, Y.; Sanche, L. Absolute cross sections for chemoradiation therapy: Damages to cisplatin-DNA complexes induced by 10 eV electrons. J. Chem. Phys. 2019, 150, 195101. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Sanche, L. Low energy electrons in nanoscale radiation physics: Relationship to radiosensitization and chemoradiation therapy. Rev. Nanosci. Nanotechnol. 2013, 2, 1–28. [Google Scholar] [CrossRef]
- Sanche, L. Interaction of low energy electrons with DNA: Applications to cancer radiation therapy. Radiat. Phys. Chem. 2016, 128, 36–43. [Google Scholar] [CrossRef]
- McGinn, C.J.; Shewach, D.S.; Lawrence, T.S. Radiosensitizing nucleosides. J. Natl. Cancer Inst. 1996, 88, 1193–1203. [Google Scholar] [CrossRef]
- Modelli, A.; Bolognesi, P.; Avaldi, L. Temporary anion states of pyrimidine and halopyrimidines. J. Phys. Chem. A. 2011, 115, 10775–10782. [Google Scholar] [CrossRef]
- Sosnowska, M.; Makurat, S.; Zdrowowicz, M.; Rak, J. 5-Selenocyanatouracil: A potential hypoxic radiosensitizer. Electron attachment induced formation of selenium centered radical. J. Phys. Chem. B 2017, 121, 6139–6147. [Google Scholar] [CrossRef]
- Makurat, S.; Zdrowowicz, M.; Chomicz-Manka, L.; Kozak, W.; Serdiuk, I.E.; Wityk, P.; Kawecka, A.; Sosnowska, M.; Rak, J. 5-Selenocyanato and 5-trifluoromethanesulfonyl derivatives of 2′-deoxyuridine: Synthesis, radiation and computational chemistry as well as cytotoxicity. RSC Adv. 2018, 8, 21378–21388. [Google Scholar] [CrossRef]
- Meißner, R.; Makurat, S.; Kozak, W.; Limão-Vieira, P.; Rak, J.; Denifl, S. Electron-induced dissociation of the potential radiosensitizer 5-selenocyanato-2′-deoxyuridine. J. Phys. Chem. B 2019, 123, 1274–1282. [Google Scholar] [CrossRef]
- Zdrowowicz, M.; Chomicz, L.; Zyndul, M.; Wityk, P.; Rak, J.; Wiegand, T.J.; Hanson, C.G.; Adhikary, A.; Sevilla, M.D. 5-Thiocyanato-2′-deoxyuridine as a possible radiosensitizer: Electron-induced formation of uracil-C5-thiyl radical and its dimerization. Phys. Chem. Chem. Phys. 2015, 17, 16907–16916. [Google Scholar] [CrossRef]
- Ameixa, J.; Arthur-Baidoo, E.; Meißner, R.; Makurat, S.; Kozak, W.; Butowska, K.; da Silva, F.; Rak, J.; Denifl, S. Low-energy electron-induced decomposition of 5-trifluoromethanesulfonyl-uracil: A potential radiosensitizer. J. Chem. Phys. 2018, 149, 164307. [Google Scholar] [CrossRef]
- Her, S.; Jaffray, D.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Schuemann, J.; Bagley, A.F.; Berbeco, R.; Bromma, K.; Butterworth, K.T.; Byrne, H.L.; Chithrani, B.D.; Cho, S.H.; Cook, J.R.; Favaudon, V.; et al. Roadmap for metal nanoparticles in radiation therapy: Current status, translational challenges, and future directions. Phys. Med. Biol. 2020, 65, 21RM02. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Sanche, L. Mechanisms of low energy electron interactions with biomolecules: Relationship to gold nanoparticle radiosensitization. In Nanoparticle Enhanced Radiation Therapy: Principles, Methods and Applications; Sajo, E., Zygmanski, P., Eds.; IOP Series in Global Health and Radiation Oncology; IOP Publishing Ltd.: London, UK, 2020. [Google Scholar]
- Ghandi, K.; Wang, F.; Landry, C.; Mostafavi, M. Naked gold nanoparticles and hot electrons in water. Sci. Rep. 2018, 8, 7258. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Sanche, L. Gold nanoparticles enhance DNA damage induced by anti-cancer drugs and radiation. Radiat. Res. 2009, 172, 114–119. [Google Scholar] [CrossRef]
- Shi, M.; Anantha, M.; Wehbe, M.; Bally, M.; Fortin, D.; Roy, L.-O.; Charest, G.; Richer, M.; Paquette, B.; Sanche, L. Liposomal formulations of carboplatin injected by convection-enhanced delivery increases the median survival time of F98 glioma bearing rats. J. Nanobiotechnol. 2018, 16, 77–89. [Google Scholar] [CrossRef]
- Charest, G.; Paquette, B.; Fortin, D.; Mathieu, D.; Sanche, L. Concomittant treatment of F98 glioma cells with new liposomal platinum compounds and ionizing radiation. J. Neuro Oncol. 2010, 97, 187–193. [Google Scholar] [CrossRef]
- Charest, G.; Tippayamontri, T.; Shi, M.; Wehbe, M.; Anantha, M.; Bally, M.; Sanche, L. Concomitant chemoradiation therapy with gold nanoparticles and platinum drugs co-encapsulated in liposomes. Int. J. Mol. Sci. 2020, 21, 4848. [Google Scholar] [CrossRef] [PubMed]
- Pavliuk, M.; Álvarez, S.; Hattori, Y.; Messing, M.; Czapla-Masztafiak, J.; Szlachetko, J.; Silva, J.; Araujo, C.; Fernandes, D.; Lu, L.; et al. Hydrated electron generation by excitation of copper localized surface plasmon resonance. J. Phys. Chem. Lett. 2019, 10, 1743–1749. [Google Scholar] [CrossRef]
- Cadet, J.; Wagner, J.R.; Angelov, D. Biphotonic ionization of DNA: From model studies to cell. Photochem. Photobiol. 2019, 95, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Pouget, J.-P.; Frelon, S.; Ravanat, J.-L.; Testard, I.; Odin, F.; Cadet, J. Formation of modified DNA bases in cells exposed either to gamma radiation or to high-LET particles. Radiat. Res. 2002, 157, 589–595. [Google Scholar] [CrossRef]
- Douki, T.; Ravanat, J.-L.; Pouget, J.-P.; Testard, I.; Cadet, J. Minor contribution of direct ionization to DNA base damage induced by heavy ions. Int. J. Radiat. Biol. 2006, 82, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Madugundu, G.S.; Cadet, J.; Wagner, J.R. Hydroxyl-radical-induced oxidation of 5-methylcytosine in isolated and cellular DNA. Nucleic Acids Res. 2014, 42, 7450–7460. [Google Scholar] [CrossRef]
- Cadet, J.; Wagner, J.R. Radiation-induced damage to cellular DNA: Chemical nature and mechanisms of lesion formation. Radiat. Phys. Chem. 2016, 128, 54–59. [Google Scholar] [CrossRef]


| References | Photon Energy (eV) | Average LEE Energy (eV) | GLEE (× nmol/J) | Hydration Level a | Environment | ||
|---|---|---|---|---|---|---|---|
| GSSB | GLS | GDSB | |||||
| Brun et al. [147] | 1486 | 4.0 | 400 ± 200 | Г = 2.5 | vacuum | ||
| 1486 | 4.0 | 600 ± 200 | Г ≈ 10 | Air | |||
| Alizadeh et al. [149,150] | 1486 | 5.85 | 248 ± 65 | 260 ± 50 | Г = 2.5 | N2, SATP b | |
| 1486 | 5.85 | 226 ± 59 | 247 ± 64 | - | Г = 5 ± 1 | N2, SATP | |
| 1486 | 5.85 | 223 ± 57 | 309 ± 80 | - | Г = 10 ± 1 | N2, SATP | |
| 1486 | 5.85 | 268 ± 70 | 412 ± 107 | 21 ± 5 | Г = 20 ± 1 | N2, SATP | |
| 1486 | 5.85 | 1545 ± 403 | 1852 ± 482 | 21 ± 5 | Г = 33 ± 1 | N2, SATP | |
| Alizadeh et al. [148] | 1486 | 5.85 | 227 ± 15 | Г = 2.5 | N2, SATP | ||
| 415 ± 15 | Г = 2.5 | O2, SATP | |||||
| Alizadeh et al. [148,151] | 1486 | 5.85 | 206 ± 54 | 288 ± 75 | 10 ± 3 | Г = 2.5 | N2 + O2, SATP |
| 1486 | 5.85 | 432 ± 112 | 473 ± 123 | - | Г = 2.5 | O2, SATP | |
| Alizadeh et al. [152] | 1486 | 5.85 | 540 ± 80 | 737 ± 110 | 46 ± 66 | Г = 2.5 | N2O, SATP |
| Liu et al. [153] | 3.1–5.2 c | 0.75 | 47 ± 37 | 49 ± 38 | - | Г = 2.5 | N2, SATP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Zheng, Y.; Sanche, L. Low-Energy Electron Damage to Condensed-Phase DNA and Its Constituents. Int. J. Mol. Sci. 2021, 22, 7879. https://doi.org/10.3390/ijms22157879
Gao Y, Zheng Y, Sanche L. Low-Energy Electron Damage to Condensed-Phase DNA and Its Constituents. International Journal of Molecular Sciences. 2021; 22(15):7879. https://doi.org/10.3390/ijms22157879
Chicago/Turabian StyleGao, Yingxia, Yi Zheng, and Léon Sanche. 2021. "Low-Energy Electron Damage to Condensed-Phase DNA and Its Constituents" International Journal of Molecular Sciences 22, no. 15: 7879. https://doi.org/10.3390/ijms22157879
APA StyleGao, Y., Zheng, Y., & Sanche, L. (2021). Low-Energy Electron Damage to Condensed-Phase DNA and Its Constituents. International Journal of Molecular Sciences, 22(15), 7879. https://doi.org/10.3390/ijms22157879






