Cold Atmospheric Plasma Changes the Amino Acid Composition of Solutions and Influences the Anti-Tumor Effect on Melanoma Cells
Abstract
:1. Introduction
2. Results
2.1. Reactive Species in DMEM + FBS PTS
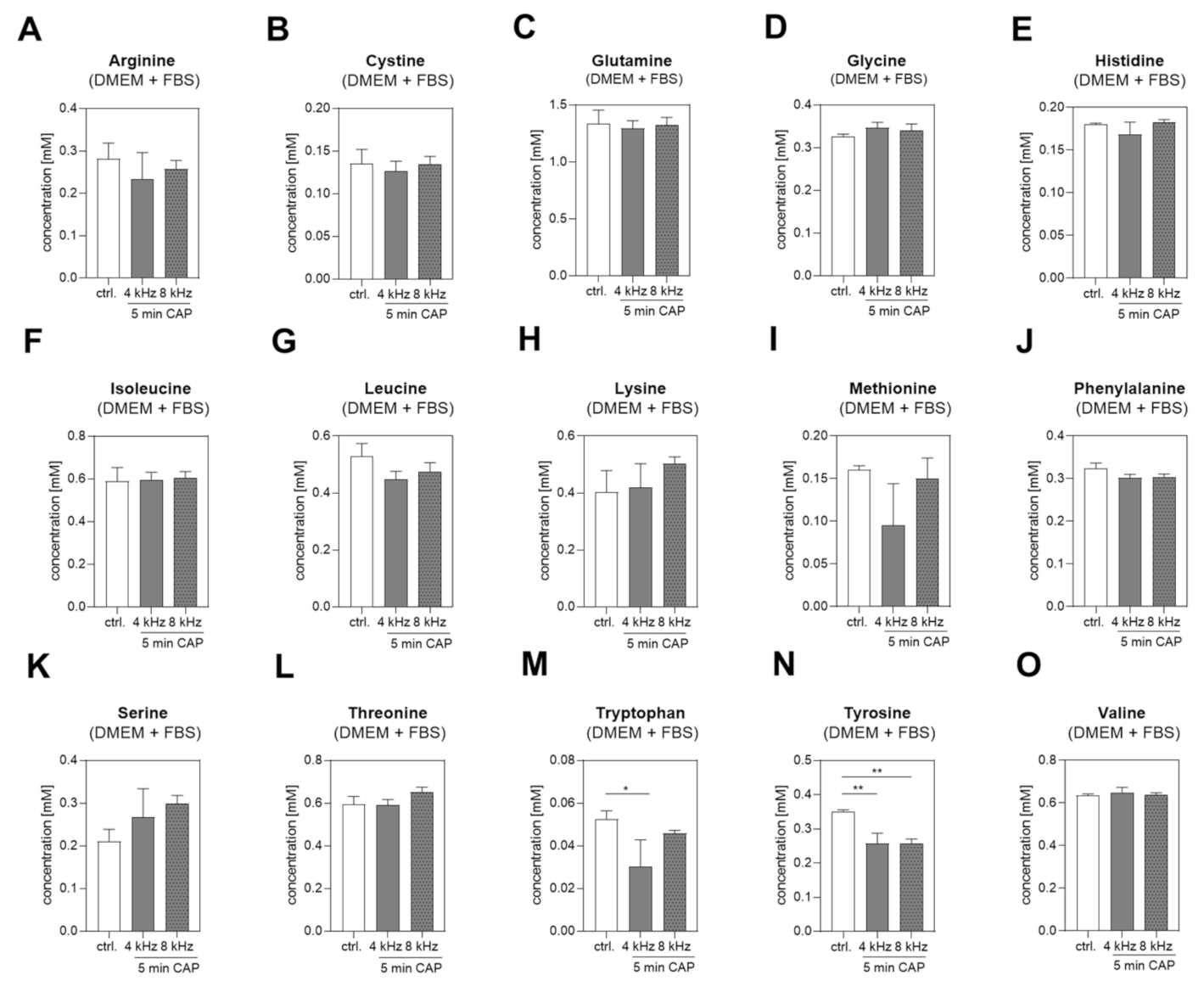
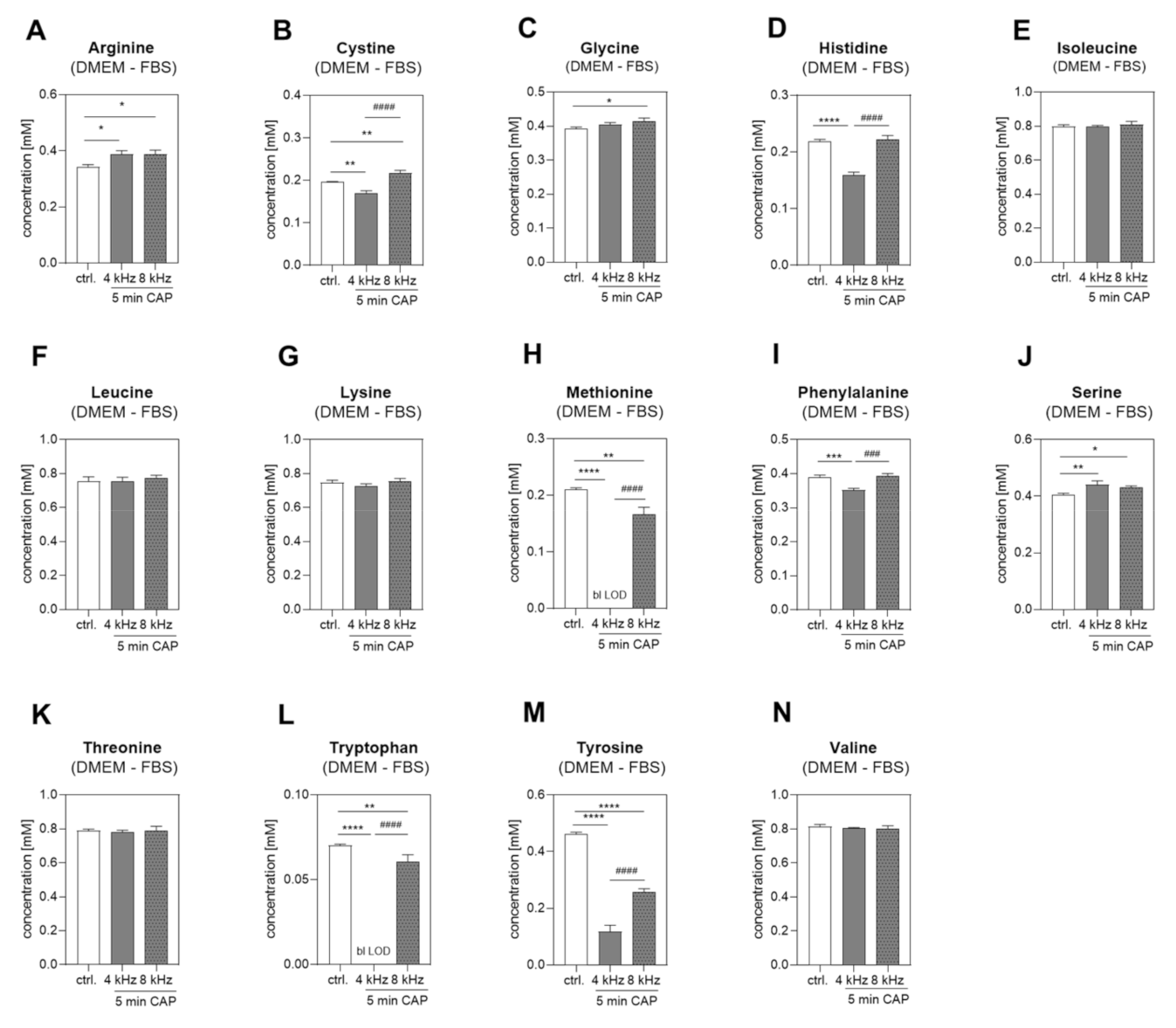
2.2. Amino Acid Concentrations in DMEM +/− FBS PTS
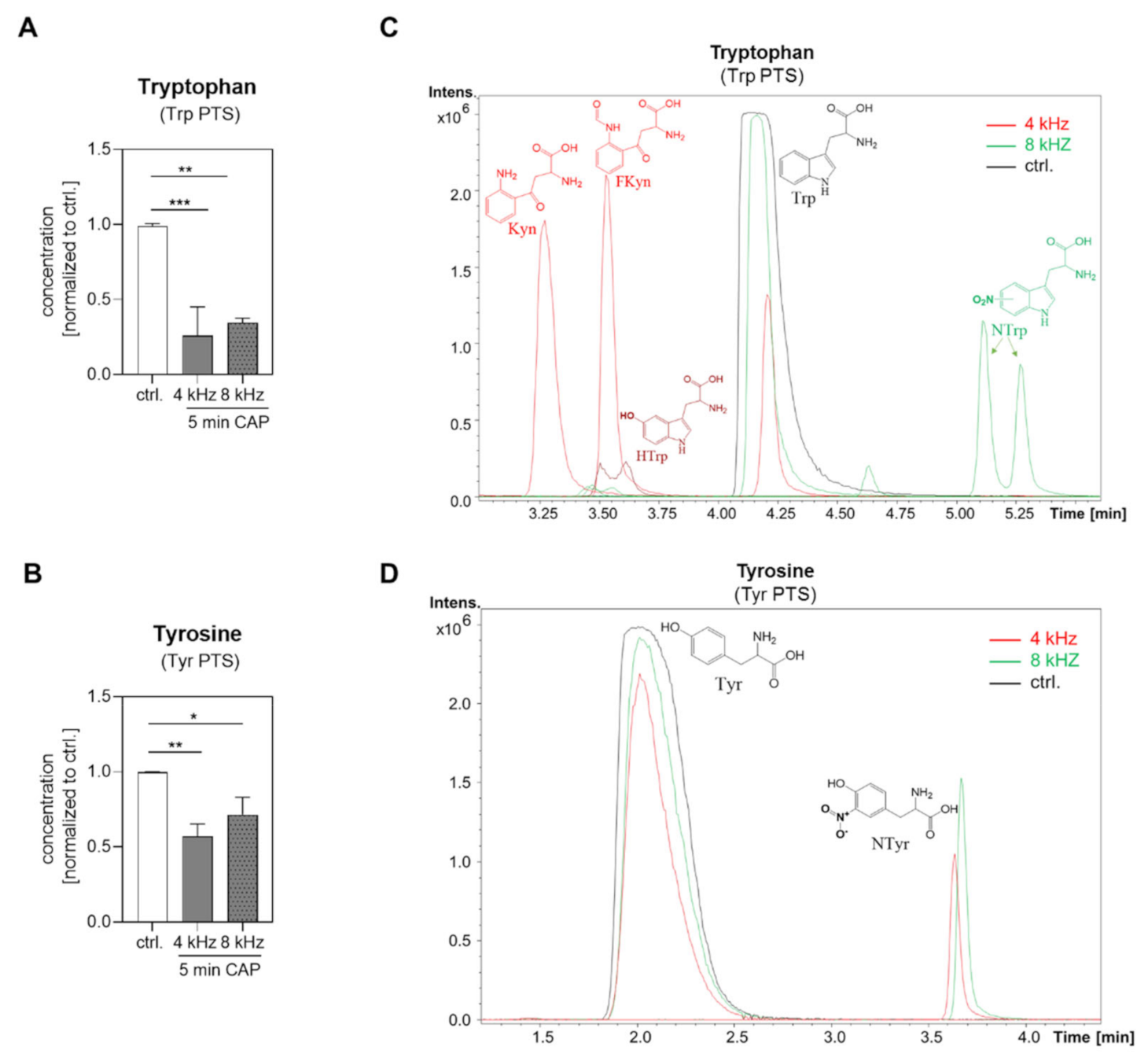
2.3. Amino Acid Modifications in Trp and Tyr PTS
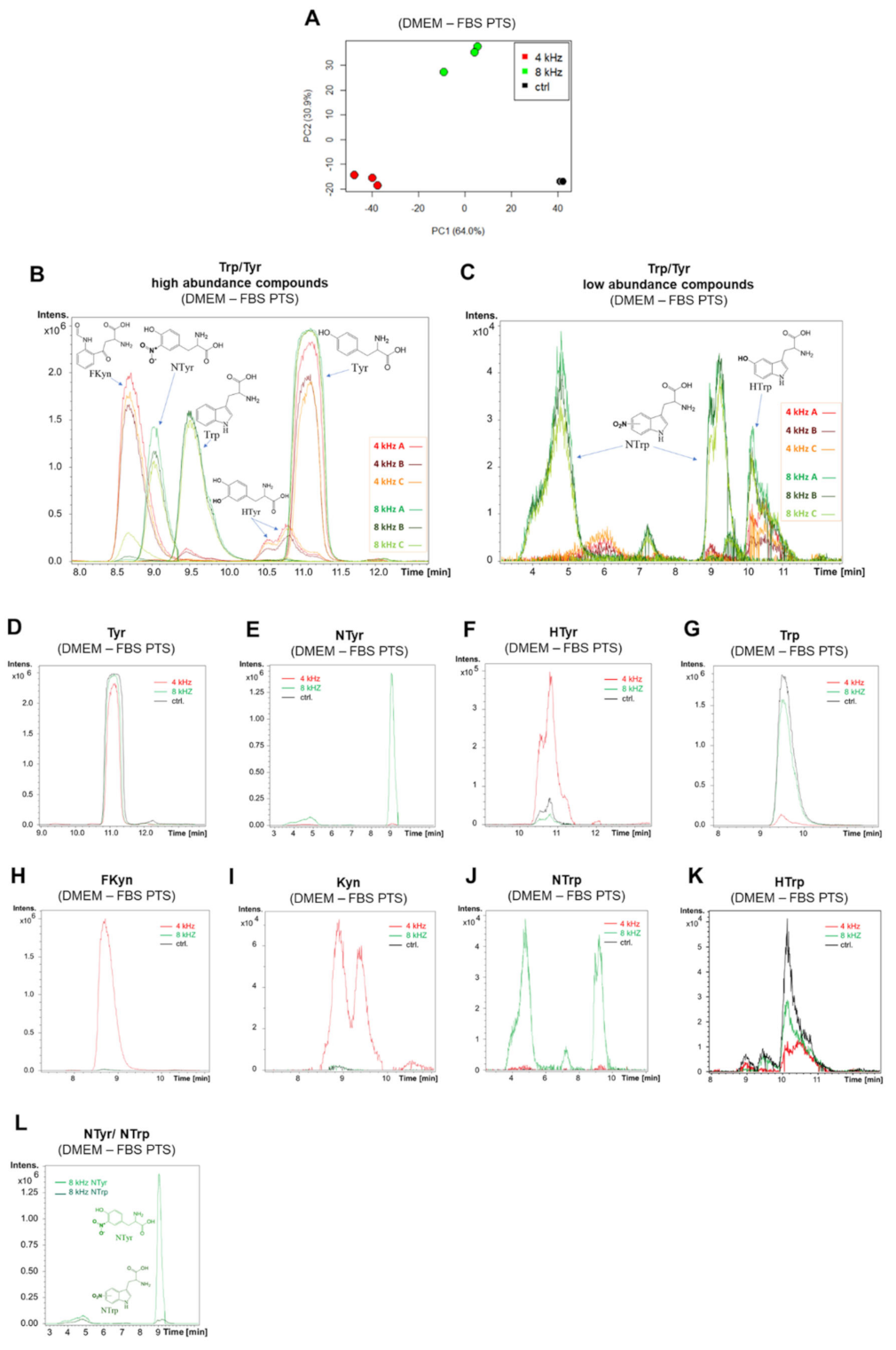
2.4. Amino Acid Modifications in DMEM–FBS PTS
2.5. RONS in Tyr and Trp PTS
2.6. pH-Value and Color Analysis of PTS
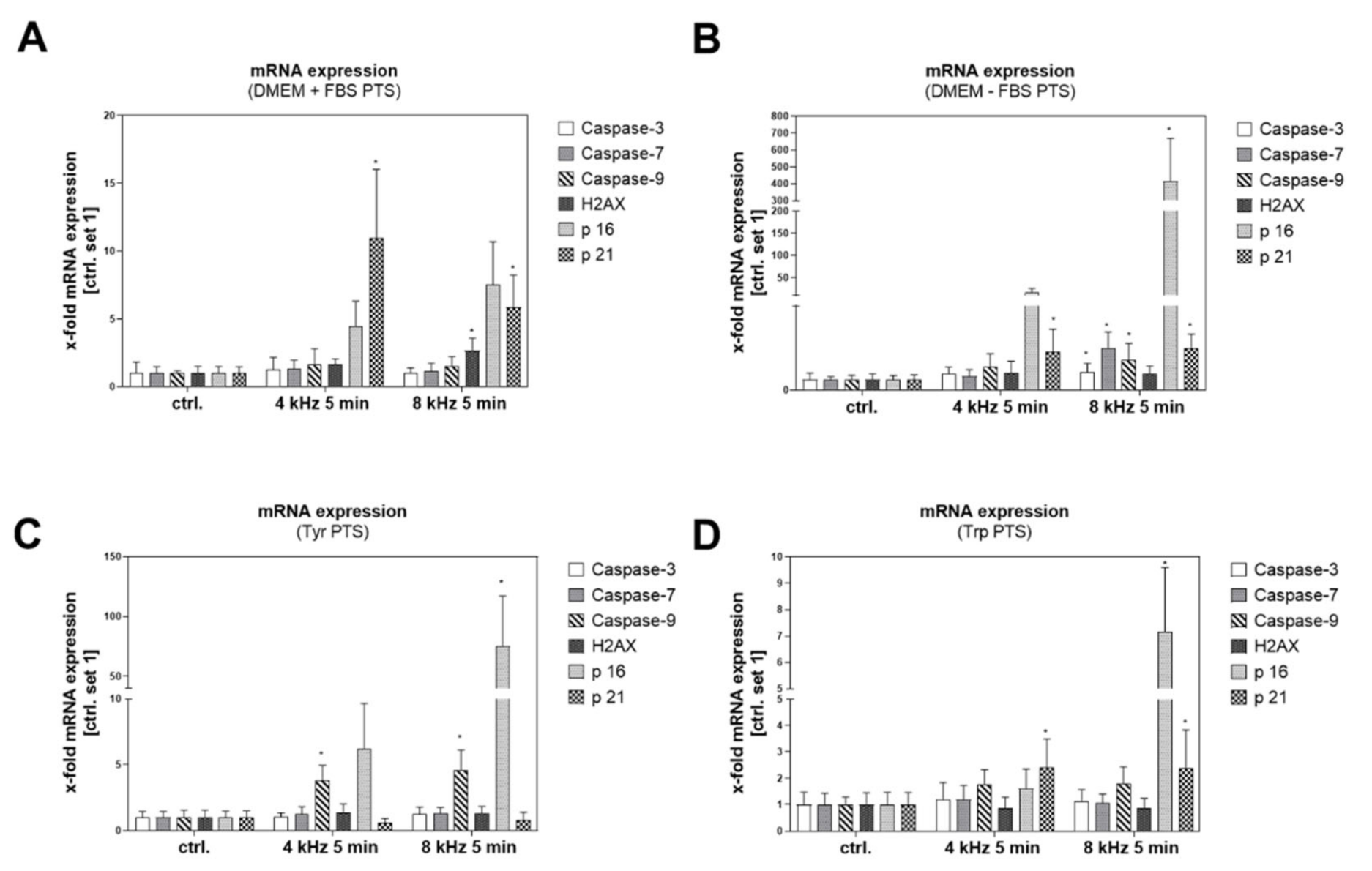
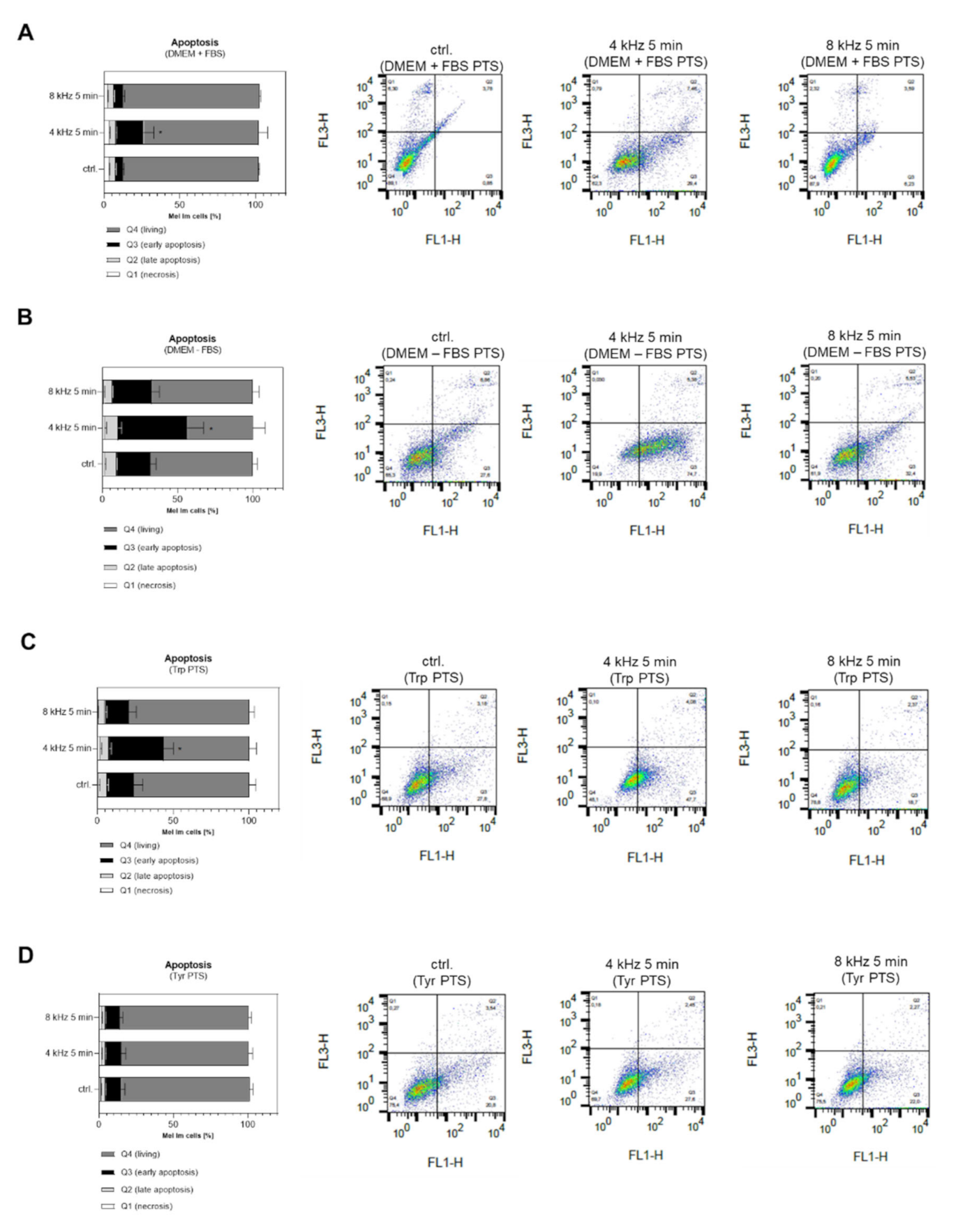
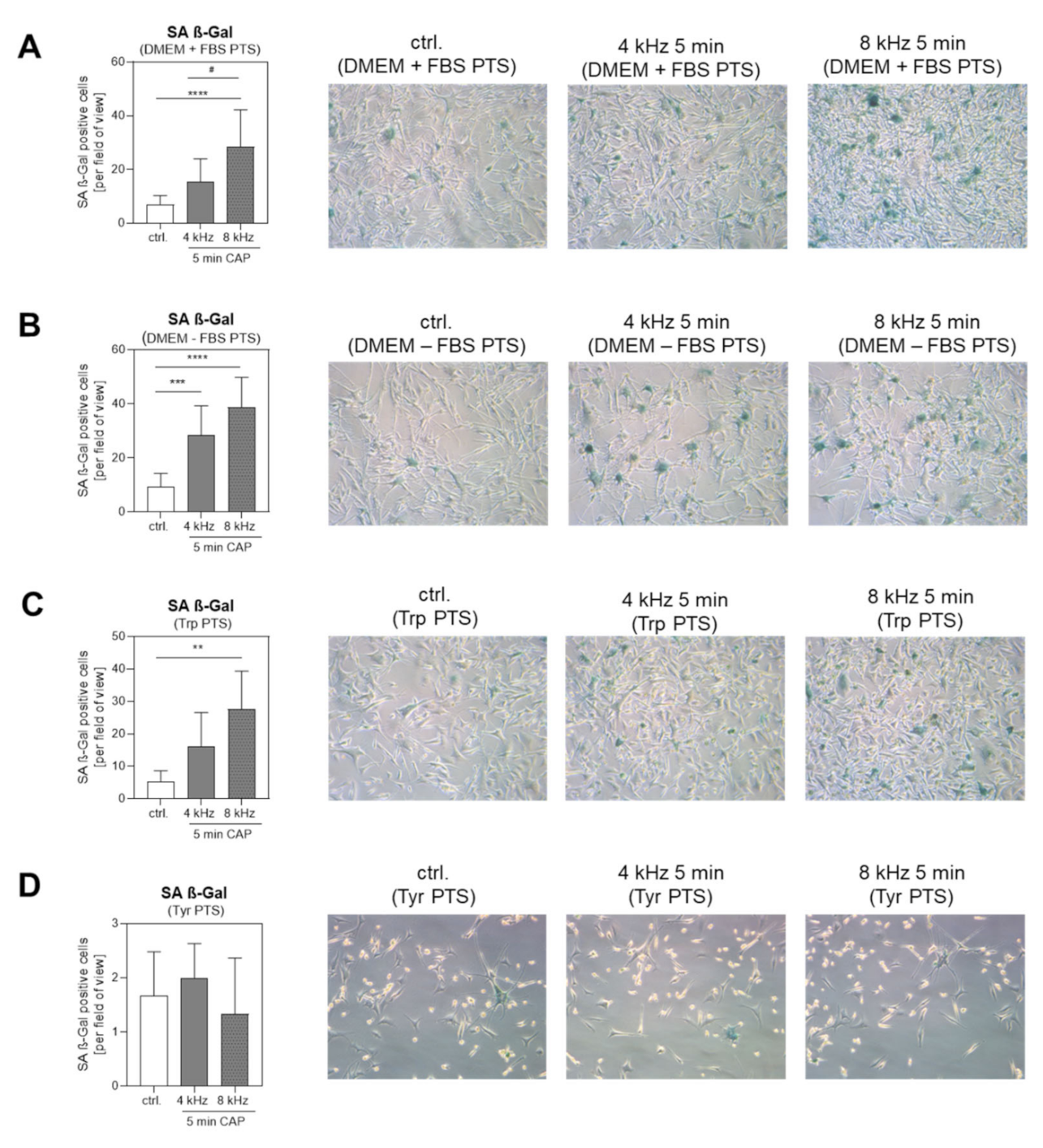
2.7. Molecular and Cellular Anti-Tumor Effects of PTS on Melanoma Cells
2.7.1. Molecular Anti-Tumor Analysis of PTS
2.7.2. Cellular Anti-Tumor Analysis of PTS
3. Discussion
4. Materials and Methods
4.1. Plasma Source
4.2. Preparation of the Plasma-Treated Solutions (PTS)
4.3. Assessment of the ROS, H2O2 and NO2−/NO3− in PTS
4.4. pH-Value Measurement of the PTS
4.5. Color Analysis of the PTS
4.6. Determination of the Amino Acids in the PTS by NMR Spectroscopy
4.7. Metabolite Quantification from the NMR Spectra
4.8. Analysis of the Amino Acid Degradation in the PTS by HPLC Time of Flight Mass-Spectrometry (HPLC-TOFMS)
4.9. Fingerprinting Analysis
4.10. Cell Culture
4.11. Treatment of the Cells with PTS
4.12. Measurement of the Cell Apoptosis
4.13. Measurement of Cellular Senescence
4.14. RNA Isolation and Reverse Transcription
4.15. Quantitative Real-Time PCR Analysis
4.16. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Niedźwiedź, I.; Waśko, A.; Pawłat, J.; Polak-Berecka, M. The State of Research on Antimicrobial Activity of Cold Plasma. Pol. J. Microbiol. 2019, 68, 153–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, L.; Zhang, S.; Poorun, D.; Liu, D.; Lu, X.; He, M.; Duan, X.; Chen, H. Medical applications of nonthermal atmospheric pressure plasma in dermatology. J. Der Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. JDDG 2018, 16, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinlin, J.; Isbary, G.; Stolz, W.; Morfill, G.; Landthaler, M.; Shimizu, T.; Steffes, B.; Nosenko, T.; Zimmermann, J.; Karrer, S. Plasma applications in medicine with a special focus on dermatology. J. Eur. Acad. Dermatol. Venereol. JEADV 2011, 25, 1–11. [Google Scholar] [CrossRef]
- Guo, L.; Xu, R.; Gou, L.; Liu, Z.; Zhao, Y.; Liu, D.; Zhang, L.; Chen, H.; Kong, M.G. Mechanism of Virus Inactivation by Cold Atmospheric-Pressure Plasma and Plasma-Activated Water. Appl. Environ. Microbiol. 2018, 84, 17. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget 2017, 8, 15977–15995. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, A.; Bekeschus, S.; Jablonowski, H.; Barton, A.; Weltmann, K.D.; Wende, K. Role of Ambient Gas Composition on Cold Physical Plasma-Elicited Cell Signaling in Keratinocytes. Biophys. J. 2017, 112, 2397–2407. [Google Scholar] [CrossRef] [Green Version]
- Yadav, D.K.; Adhikari, M.; Kumar, S.; Ghimire, B.; Han, I.; Kim, M.-H.; Choi, E.-H.J.S.R. Cold atmospheric plasma generated reactive species aided inhibitory effects on human melanoma cells: An in vitro and in silico study. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Boxhammer, V.; Li, Y.F.; Koritzer, J.; Shimizu, T.; Maisch, T.; Thomas, H.M.; Schlegel, J.; Morfill, G.E.; Zimmermann, J.L. Investigation of the mutagenic potential of cold atmospheric plasma at bactericidal dosages. Mutat. Res. 2013, 753, 23–28. [Google Scholar] [CrossRef]
- Kluge, S.; Bekeschus, S.; Bender, C.; Benkhai, H.; Sckell, A.; Below, H.; Stope, M.B.; Kramer, A. Investigating the Mutagenicity of a Cold Argon-Plasma Jet in an HET-MN Model. PLoS ONE 2016, 11, e0160667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maisch, T.; Bosserhoff, A.K.; Unger, P.; Heider, J.; Shimizu, T.; Zimmermann, J.L.; Morfill, G.E.; Landthaler, M.; Karrer, S. Investigation of toxicity and mutagenicity of cold atmospheric argon plasma. Environ. Mol. Mutagenesis 2017, 58, 172–177. [Google Scholar] [CrossRef]
- Wende, K.; Bekeschus, S.; Schmidt, A.; Jatsch, L.; Hasse, S.; Weltmann, K.; Masur, K.; Von Woedtke, T. Risk assessment of a cold argon plasma jet in respect to its mutagenicity. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2016, 798, 48–54. [Google Scholar] [CrossRef]
- Wende, K.; Reuter, S.; Von Woedtke, T.; Weltmann, K.; Masur, K. Redox-Based Assay for Assessment of Biological Impact of Plasma Treatment. Plasma Process Polym. 2014, 11, 655–663. [Google Scholar] [CrossRef]
- Chang, J.W.; Kang, S.U.; Shin, Y.S.; Kim, K.I.; Seo, S.J.; Yang, S.S.; Lee, J.S.; Moon, E.; Baek, S.J.; Lee, K.; et al. Non-thermal atmospheric pressure plasma induces apoptosis in oral cavity squamous cell carcinoma: Involvement of DNA-damage-triggering sub-G(1) arrest via the ATM/p53 pathway. Arch. Biochem. Biophys. 2014, 545, 133–140. [Google Scholar] [CrossRef]
- Han, D.; Cho, J.H.; Lee, R.H.; Bang, W.; Park, K.; Kim, M.S.; Shim, J.H.; Chae, J.I.; Moon, S.Y. Antitumorigenic effect of atmospheric-pressure dielectric barrier discharge on human colorectal cancer cells via regulation of Sp1 transcription factor. Sci. Rep. 2017, 7, 43081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushik, N.K.; Kaushik, N.; Park, D.; Choi, E.H. Altered antioxidant system stimulates dielectric barrier discharge plasma-induced cell death for solid tumor cell treatment. PLoS ONE 2014, 9, e103349. [Google Scholar]
- Kumar, N.; Attri, P.; Yadav, D.K.; Choi, J.; Choi, E.H.; Uhm, H.S. Induced apoptosis in melanocytes cancer cell and oxidation in biomolecules through deuterium oxide generated from atmospheric pressure non-thermal plasma jet. Sci. Rep. 2014, 4, 7589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liedtke, K.R.; Bekeschus, S.; Kaeding, A.; Hackbarth, C.; Kuehn, J.P.; Heidecke, C.D.; Von Bernstorff, W.; Von Woedtke, T.; Partecke, L.I. Non-thermal plasma-treated solution demonstrates antitumor activity against pancreatic cancer cells in vitro and in vivo. Sci. Rep. 2017, 7, 8319. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Arndt, S.; Zimmermann, J.L.; Li, Y.; Karrer, S.; Bosserhoff, A.K. Cold atmospheric plasma treatment inhibits growth in colorectal cancer cells. Biol. Chem. 2018, 400, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Metelmann, H.R.; Nedrelow, D.S.; Seebauer, C.; Schuster, M.; Von Woedtke, T.; Weltmann, K.-D.; Kindler, S.; Metelmann, P.H.; Finkelstein, S.E.; Von Hoff, D.D.; et al. Head and neck cancer treatment and physical plasma. Clin. Plasma Med. 2015, 3, 17–23. [Google Scholar] [CrossRef]
- Metelmann, H.R.; Seebauer, C.; Miller, V.; Fridman, A.; Bauer, G.; Graves, D.B.; Pouvesle, J.-M.; Rutkowskia, R.; Schuster, M.; Bekeschus, S.; et al. Clinical experience with cold plasma in the treatment of locally advanced head and neck cancer. Clin. Plasma Med. 2018, 9, 6–13. [Google Scholar] [CrossRef]
- Schuster, M.; Seebauer, C.; Rutkowski, R.; Hauschild, A.; Podmelle, F.; Metelmann, C.; Metelmann, B.; Von Woedtke, T.; Hasse, S.; Weltmann, K.D.; et al. Visible tumor surface response to physical plasma and apoptotic cell kill in head and neck cancer. J. Cranio-Maxillo-Facial Surg. 2016, 44, 1445–1452. [Google Scholar] [CrossRef]
- Azzariti, A.; Iacobazzi, R.M.; Di Fonte, R.; Porcelli, L.; Gristina, R.; Favia, P.; Fracassi, F.; Trizio, I.; Silvestris, N.; Guida, G.; et al. Plasma-activated medium triggers cell death and the presentation of immune activating danger signals in melanoma and pancreatic cancer cells. Sci. Rep. 2019, 9, 4099. [Google Scholar] [CrossRef]
- Bauer, G.; Sersenová, D.; Graves, D.B.; Machala, Z. Cold Atmospheric Plasma and Plasma-Activated Medium Trigger RONS-Based Tumor Cell Apoptosis. Sci. Rep. 2019, 9, 14210. [Google Scholar] [CrossRef]
- Ikeda, J.I.; Tanaka, H.; Ishikawa, K.; Sakakita, H.; Ikehara, Y.; Hori, M. Plasma-activated medium (PAM) kills human cancer-initiating cells. Pathol. Int. 2018, 68, 23–30. [Google Scholar] [CrossRef]
- Wang, L.; Yang, X.; Yang, C.; Gao, J.; Zhao, Y.; Cheng, C.; Zhao, G.; Liu, S. The inhibition effect of cold atmospheric plasma-activated media in cutaneous squamous carcinoma cells. Future Oncol. 2019, 15, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Sherman, J.H.; Keidar, M. The Application of the Cold Atmospheric Plasma-Activated Solutions in Cancer Treatment. Anti-Cancer Agents Med. Chem. 2018, 18, 769–775. [Google Scholar] [CrossRef]
- Saadati, F.; Mahdikia, H.; Abbaszadeh, H.A.; Abdollahifar, M.A.; Khoramgah, M.S.; Shokri, B. Comparison of Direct and Indirect cold atmospheric-pressure plasma methods in the B(16)F(10) melanoma cancer cells treatment. Sci. Rep. 2018, 8, 7689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauvin, J.; Judée, F.; Yousfi, M.; Vicendo, P.; Merbahi, N. Analysis of reactive oxygen and nitrogen species generated in three liquid media by low temperature helium plasma jet. Sci. Rep. 2017, 7, 4562. [Google Scholar] [CrossRef] [PubMed]
- Gorbanev, Y.; Privat-Maldonado, A.; Bogaerts, A. Analysis of Short-Lived Reactive Species in Plasma-Air-Water Systems: The Dos and the Do Nots. Anal. Chem. 2018, 90, 13151–13158. [Google Scholar] [CrossRef] [Green Version]
- Ishaq, M.; Kumar, S.; Varinli, H.; Han, Z.J.; Rider, A.E.; Evans, M.D.; Murphy, A.B.; Ostrikov, K. Atmospheric gas plasma-induced ROS production activates TNF-ASK1 pathway for the induction of melanoma cancer cell apoptosis. Mol. Biol. Cell 2014, 25, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.J.; Kim, K.I.; Kim, G.; Moon, E.; Yang, S.S.; Lee, J.S. Atmospheric-pressure plasma jet induces apoptosis involving mitochondria via generation of free radicals. PLoS ONE 2011, 6, e28154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takai, E.; Kitamura, T.; Kuwabara, J.; Ikawa, S.; Yoshizawa, S.; Shiraki, K.; Kawasaki, H.; Arakawa, R.; Kitano, K. Chemical modification of amino acids by atmospheric-pressure cold plasma in aqueous solution. J. Phys. D Appl. Phys. 2014, 47, 28. [Google Scholar] [CrossRef]
- Uchida, G.; Mino, Y.; Suzuki, T.; Ikeda, J.I.; Suzuki, T.; Takenaka, K.; Setsuhara, Y. Decomposition and oxidation of methionine and tryptophan following irradiation with a nonequilibrium plasma jet and applications for killing cancer cells. Sci. Rep. 2019, 9, 6625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Feng, Z.; Pu, S.; Yang, Y.; Shi, X.; Xu, Z. Cold atmospheric Plasma Jet-Generated Oxidized Derivatives of Tryptophan and Their Selective Effects on Murine Melanoma and Fibroblast Cells. Plasma Chem. Plasma Process. 2018, 38, 919–936. [Google Scholar] [CrossRef]
- Zimmermann, T.; Gebhardt, L.A.; Kreiss, L.; Schneider, C.; Arndt, S.; Karrer, S.; Friedrich, O.; Fischer, M.J.; Bosserhoff, A.-K. Acidified Nitrite Contributes to the Antitumor Effect of Cold Atmospheric Plasma on Melanoma Cells. Int. J. Mol. Sci. 2021, 22, 3757. [Google Scholar] [CrossRef]
- Schneider, C.; Gebhardt, L.; Arndt, S.; Karrer, S.; Zimmermann, J.L.; Fischer, M.J.; Bosserhoff, A.-K. Acidification is an essential process of cold atmospheric plasma and promotes the anti-cancer effect on malignant melanoma cells. Cancers 2019, 11, 671. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Preston, R.; Ogawa, T.; Uemura, M.; Shumulinsky, G.; Valle, B.L.; Pirini, F.; Ravi, R.; Sidransky, D.; Keidar, M.; Trink, B. Cold atmospheric plasma treatment selectively targets head and neck squamous cell carcinoma cells. Int. J. Mol. Med. 2014, 34, 941–946. [Google Scholar] [CrossRef] [Green Version]
- Keidar, M.; Walk, R.; Shashurin, A.; Srinivasan, P.; Sandler, A.; Dasgupta, S.; Ravi, R.; Guerrero-Preston, R.; Trink, B. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br. J. Cancer 2011, 105, 1295–1301. [Google Scholar] [CrossRef]
- Bauer, G. Targeting Protective Catalase of Tumor Cells with Cold Atmospheric Plasma- Activated Medium (PAM). Anti-Cancer Agents Med. Chem. 2018, 18, 784–804. [Google Scholar] [CrossRef]
- Adachi, T.; Tanaka, H.; Nonomura, S.; Hara, H.; Kondo, S.; Hori, M. Plasma-activated medium induces A549 cell injury via a spiral apoptotic cascade involving the mitochondrial-nuclear network. Free Radic. Biol. Med. 2015, 79, 28–44. [Google Scholar] [CrossRef]
- Griseti, E.; Merbahi, N.; Golzio, M. Anti-Cancer Potential of Two Plasma-Activated Liquids: Implication of Long-Lived Reactive Oxygen and Nitrogen Species. Cancers 2020, 12, 721. [Google Scholar] [CrossRef] [Green Version]
- Girard, P.M.; Arbabian, A.; Fleury, M.; Bauville, G.; Puech, V.; Dutreix, M.; Sousa, J.S. Synergistic Effect of H2O2 and NO2 in Cell Death Induced by Cold Atmospheric He Plasma. Sci. Rep. 2016, 6, 29098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurake, N.; Tanaka, H.; Ishikawa, K.; Kondo, T.; Sekine, M.; Nakamura, K.; Kajiyama, H.; Kikkawa, F.; Mizuno, M.; Hori, M. Cell survival of glioblastoma grown in medium containing hydrogen peroxide and/or nitrite, or in plasma-activated medium. Arch. Biochem. Biophys. 2016, 605, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Kolata, J.; Winterbourn, C.; Kramer, A.; Turner, R.; Weltmann, K.D.; Bröker, B.; Masur, K. Hydrogen peroxide: A central player in physical plasma-induced oxidative stress in human blood cells. Free Radic. Res. 2014, 48, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Tresp, H.; Hammer, M.U.; Iseni, S.; Kupsch, S.; Schmidt-Bleker, A.; Wende, K.; Dünnbier, M.; Masur, K.; Weltmann, K.D.; et al. Tracking plasma generated H2O2from gas into liquid phase and revealing its dominant impact on human skin cells. J. Phys. D Appl. Phys. 2014, 47, 285401. [Google Scholar] [CrossRef]
- Bellmaine, S.; Schnellbaecher, A.; Zimmer, A. Medicine, Reactivity and degradation products of tryptophan in solution and proteins. Free Radic. Biol. 2020, 160, 696–718. [Google Scholar] [CrossRef]
- Taylor, L.M.; Aquilina, J.A.; Jamie, J.F.; Truscott, R.J. UV filter instability: Consequences for the human lens. J Exp. Eye Res. 2002, 75, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Tokuyama, T.; Senoh, S.; Sakan, T.; Brown, K.; Witkop, B. The photoreduction of kynurenic acid to kynurenine yellow and the occurrence of 3-hydroxy-L-kynurenine in butterflies. J. Am. Chem. Soc. 1967, 89, 1017–1021. [Google Scholar] [CrossRef]
- Tsentalovich, Y.P.; Snytnikova, O.A.; Forbes, M.D.; Chernyak, E.I.; Morozov, S.V. Photochemical and thermal reactivity of kynurenine. Exp. Eye Res. 2006, 83, 1439–1445. [Google Scholar] [CrossRef]
- Wenske, S.; Lackmann, J.W.; Bekeschus, S.; Weltmann, K.D.; Von Woedtke, T.; Wende, K. Nonenzymatic post-translational modifications in peptides by cold plasma-derived reactive oxygen and nitrogen species. Biointerphases 2020, 15, 061008. [Google Scholar] [CrossRef]
- Tanaka, H.; Mizuno, M.; Ishikawa, K.; Nakamura, K.; Utsumi, F.; Kajiyama, H.; Kano, H.; Maruyama, S.; Kikkawa, F.; Hori, M. Cell survival and proliferation signaling pathways are downregulated by plasma-activated medium in glioblastoma brain tumor cells. Plasma Med. 2012, 2, 4. [Google Scholar] [CrossRef] [Green Version]
- Arndt, S.; Wacker, E.; Li, Y.F.; Shimizu, T.; Thomas, H.M.; Morfill, G.E.; Karrer, S.; Zimmermann, J.L.; Bosserhoff, A.K. Cold atmospheric plasma, a new strategy to induce senescence in melanoma cells. Exp. Dermatol. 2013, 22, 284–289. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Ghimire, B.; Li, Y.; Adhikari, M.; Veerana, M.; Kaushik, N.; Jha, N.; Adhikari, B.; Lee, S.-J.; Masur, K.J.B.C. Biological and medical applications of plasma-activated media, water and solutions. Biol. Chem. 2019, 400, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Ohene, Y.; Marinov, I.; De Laulanié, L.; Dupuy, C.; Wattelier, B.; Starikovskaia, S. Phase imaging microscopy for the diagnostics of plasma-cell interaction. Appl. Phys. Lett. 2015, 106, 233703. [Google Scholar] [CrossRef]
- Slikboer, E.; Sobota, A.; Garcia-Caurel, E.; Guaitella, O.J.S.R. In-situ monitoring of an organic sample with electric field determination during cold plasma jet exposure. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Wang, Q.; Adhikari, M.; Malyavko, A.; Lin, L.; Zolotukhin, D.B.; Yao, X.; Kirschner, M.; Sherman, J.H.; Keidar, M. Interfaces, A physically triggered cell death via transbarrier cold atmospheric plasma cancer treatment. ACS Appl. Mater. Interfaces 2020, 12, 34548–34563. [Google Scholar] [CrossRef]
- Judee, F.; Fongia, C.; Ducommun, B.; Yousfi, M.; Lobjois, V.; Merbahi, N. Short and long time effects of low temperature Plasma Activated Media on 3D multicellular tumor spheroids. Sci. Rep. 2016, 6, 21421. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Gumbel, D.; Gelbrich, N.; Brandenburg, L.O.; Mandelkow, R.; Zimmermann, U.; Ziegler, P.; Burchardt, M.; Stope, M.B. Inhibition of Cell Growth of the Prostate Cancer Cell Model LNCaP by Cold Atmospheric Plasma. In Vivo 2015, 29, 611–616. [Google Scholar]
- Liggett, W.H., Jr.; Sidransky, D. Role of the p16 tumor suppressor gene in cancer. J. Clin. Oncol. 1998, 16, 1197–1206. [Google Scholar] [CrossRef]
- Rayess, H.; Wang, M.B.; Srivatsan, E. Cellular senescence and tumor suppressor gene p16. Int. J. Cancer 2012, 130, 1715–1725. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Nourmohammadi, N.; Bian, K.; Murad, F.; Sherman, J.H.; Keidar, M. Stabilizing the cold plasma-stimulated medium by regulating medium’s composition. Sci. Rep. 2016, 6, 26016. [Google Scholar] [CrossRef] [PubMed]
- Biscop, E.; Lin, A.; Boxem, W.V.; Loenhout, J.V.; Backer, J.; Deben, C.; Dewilde, S.; Smits, E.; Bogaerts, A.A. Influence of Cell Type and Culture Medium on Determining Cancer Selectivity of Cold Atmospheric Plasma Treatment. Cancers 2019, 11, 1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupke, L.S.; Arndt, S.; Lenzer, S.; Metz, S.; Unger, P.; Zimmermann, J.L.; Bosserhoff, A.-K.; Gruber, M.; Karrer, S.J.B. Cold Atmospheric Plasma Promotes the Immunoreactivity of Granulocytes In Vitro. Biomolecules 2021, 11, 902. [Google Scholar] [CrossRef]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Oresic, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, K.; Bosserhoff, A.K.; Wach, F.; Knüchel, R.; Klein, E.C.; Hein, R.; Buettner, R. Characterization of selected strongly and weakly invasive sublines of a primary human melanoma cell line and isolation of subtractive cDNA clones. Int. J. Cancer 1995, 60, 668–675. [Google Scholar] [CrossRef]
- Arndt, S.; Unger, P.; Berneburg, M.; Bosserhoff, A.K.; Karrer, S. Cold atmospheric plasma (CAP) activates angiogenesis-related molecules in skin keratinocytes, fibroblasts and endothelial cells and improves wound angiogenesis in an autocrine and paracrine mode. J. Dermatol. Sci. 2018, 89, 181–190. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]
- Dettmer, K.; Vogl, F.C.; Ritter, A.P.; Zhu, W.; Nürnberger, N.; Kreutz, M.; Oefner, P.J.; Gronwald, W.; Gottfried, E.J.E. Distinct metabolic differences between various human cancer and primary cells. Electrophoresis 2013, 34, 2836–2847. [Google Scholar] [CrossRef]
- Smyth, G.K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004, 3, 3. [Google Scholar] [CrossRef] [PubMed]


| Solution Number | Solution Name | pH-Value 1 (mean +/− SD) |
|---|---|---|
| 1 | DMEM+FBS (ctrl.) | 7.23 +/− 0.32 |
| 2 | DMEM+FBS (4 kHz 1 min) | 7.65 +/− 0.14 |
| 3 | DMEM+FBS (4 kHz 2 min) | 7.76 +/− 0.12 |
| 4 | DMEM+FBS (4 kHz 5 min) | 7.77 +/− 0.09 |
| 5 | DMEM+FBS (ctrl.) | 7.23 +/− 0.32 |
| 6 | DMEM+FBS (8 kHz 1 min) | 7.69 +/− 0.34 |
| 7 | DMEM+FBS (8 kHz 2 min) | 7.79 +/− 0.26 |
| 8 | DMEM+FBS (8 kHz 5 min) | 7.91 +/− 0.29 |
| 9 | DMEM–FBS (ctrl.) | 7.84 +/− 0.31 |
| 10 | DMEM–FBS (4 kHz 5 min) | 7.87 +/− 0.27 |
| 11 | DMEM–FBS (8 kHz 5 min) | 7.93 +/− 0.26 |
| 12 | Tyrosine (ctrl.) | 8.86 +/− 0.09 |
| 13 | Tyrosine (4 kHz 5 min) | 3.66 +/− 0.20 **** |
| 14 | Tyrosine (8 kHz 5min) | 3.32 +/− 0.10 **** |
| 15 | Tryptophan (ctrl.) | 6.70 +/− 0.24 |
| 16 | Tryptophan (4 kHz 5 min) | 3.52 +/− 0.19 **** |
| 17 | Tryptophan (8 kHz 5min) | 3.37 +/− 0.12 **** |
| CAP Treatment Mode | PTS | 4 kHz (Oxygen Mode) | 8 kHz (Nitrogen Mode) |
|---|---|---|---|
| Duration | 5 min | 5 min | |
| RONS production | DMEM+FBS DMEM–FBS Trp Tyr | ROS ++, H2O2 ++ ROS +++, H2O2 +++ ROS ++, H2O2 +++ ROS ++, H2O2 --- | NO2 ++, NO3 ++ NO2 +++, NO3 +++ NO2 +++, NO3 ++ NO2 +++, NO3 +++ |
| amino acid degradation | DMEM+FBS DMEM–FBS Trp Tyr | Tyr, Trp Cys, His, Met, Phe, Tyr, Trp Trp Tyr | Tyr Tyr, Trp Trp Tyr |
| amino acid modification | DMEM–FBS Trp Tyr | HTyr, FKyn, Kyn HTrp, FKyn, Kyn NTyr | NTyr, NTrp NTrp NTyr |
| PTS coloration | DMEM+FBS DMEM–FBS Trp Tyr | ++ ++ + | ++ ++ |
| p16 gene expression | all PTS | + | +++ |
| apoptosis induction | DMEM+FBS DMEM–FBS Trp Tyr | ++ +++ +++ | |
| senescence induction | DMEM+FBS DMEM–FBS Trp Tyr | + + + | +++ +++ ++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arndt, S.; Fadil, F.; Dettmer, K.; Unger, P.; Boskovic, M.; Samol, C.; Bosserhoff, A.-K.; Zimmermann, J.L.; Gruber, M.; Gronwald, W.; et al. Cold Atmospheric Plasma Changes the Amino Acid Composition of Solutions and Influences the Anti-Tumor Effect on Melanoma Cells. Int. J. Mol. Sci. 2021, 22, 7886. https://doi.org/10.3390/ijms22157886
Arndt S, Fadil F, Dettmer K, Unger P, Boskovic M, Samol C, Bosserhoff A-K, Zimmermann JL, Gruber M, Gronwald W, et al. Cold Atmospheric Plasma Changes the Amino Acid Composition of Solutions and Influences the Anti-Tumor Effect on Melanoma Cells. International Journal of Molecular Sciences. 2021; 22(15):7886. https://doi.org/10.3390/ijms22157886
Chicago/Turabian StyleArndt, Stephanie, Fadi Fadil, Katja Dettmer, Petra Unger, Marko Boskovic, Claudia Samol, Anja-Katrin Bosserhoff, Julia L. Zimmermann, Michael Gruber, Wolfram Gronwald, and et al. 2021. "Cold Atmospheric Plasma Changes the Amino Acid Composition of Solutions and Influences the Anti-Tumor Effect on Melanoma Cells" International Journal of Molecular Sciences 22, no. 15: 7886. https://doi.org/10.3390/ijms22157886
APA StyleArndt, S., Fadil, F., Dettmer, K., Unger, P., Boskovic, M., Samol, C., Bosserhoff, A.-K., Zimmermann, J. L., Gruber, M., Gronwald, W., & Karrer, S. (2021). Cold Atmospheric Plasma Changes the Amino Acid Composition of Solutions and Influences the Anti-Tumor Effect on Melanoma Cells. International Journal of Molecular Sciences, 22(15), 7886. https://doi.org/10.3390/ijms22157886








