Polymeric Microspheres/Cells/Extracellular Matrix Constructs Produced by Auto-Assembly for Bone Modular Tissue Engineering
Abstract
:1. Introduction
2. Results and Discussion
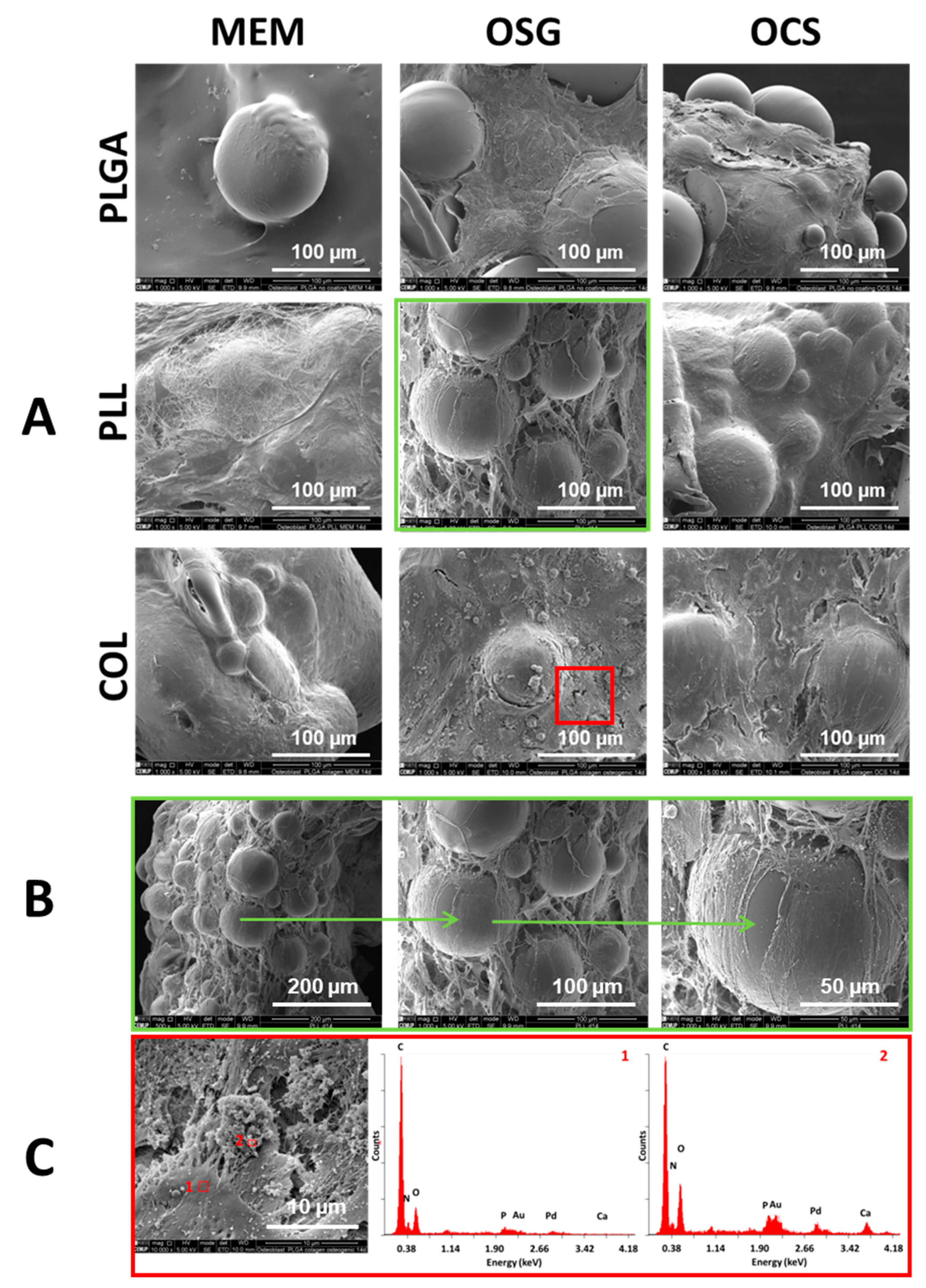
2.1. Microstructure and Surface Composition of MS
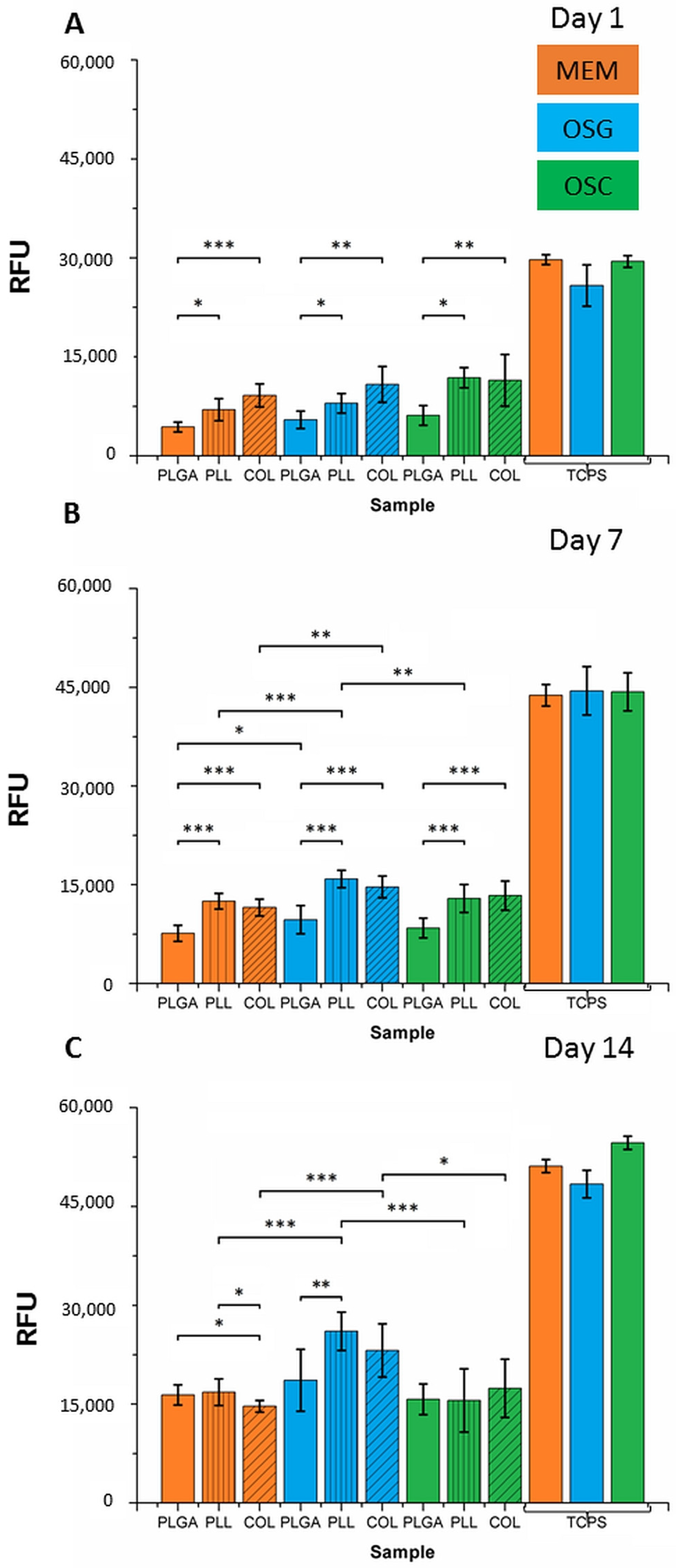
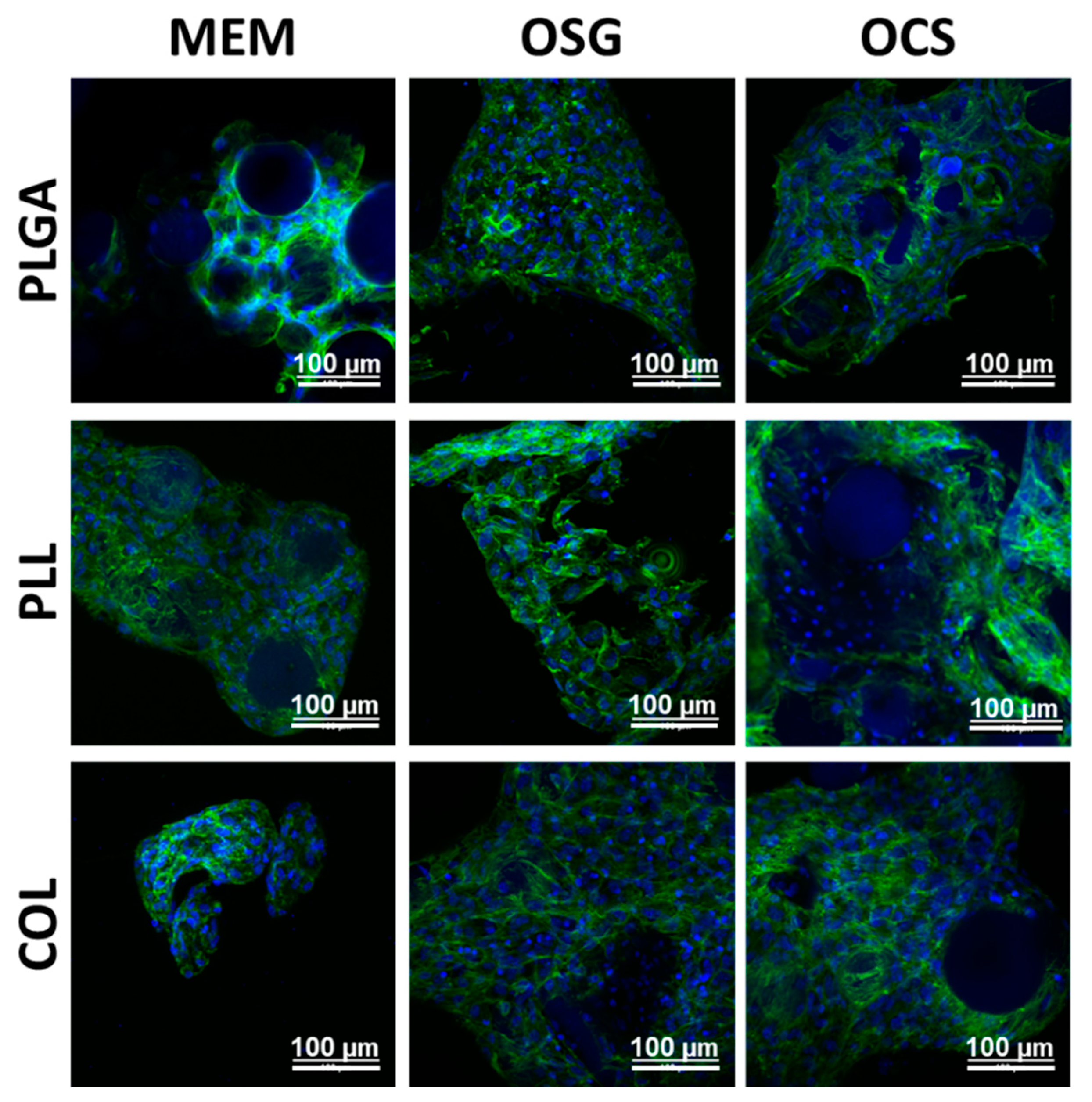
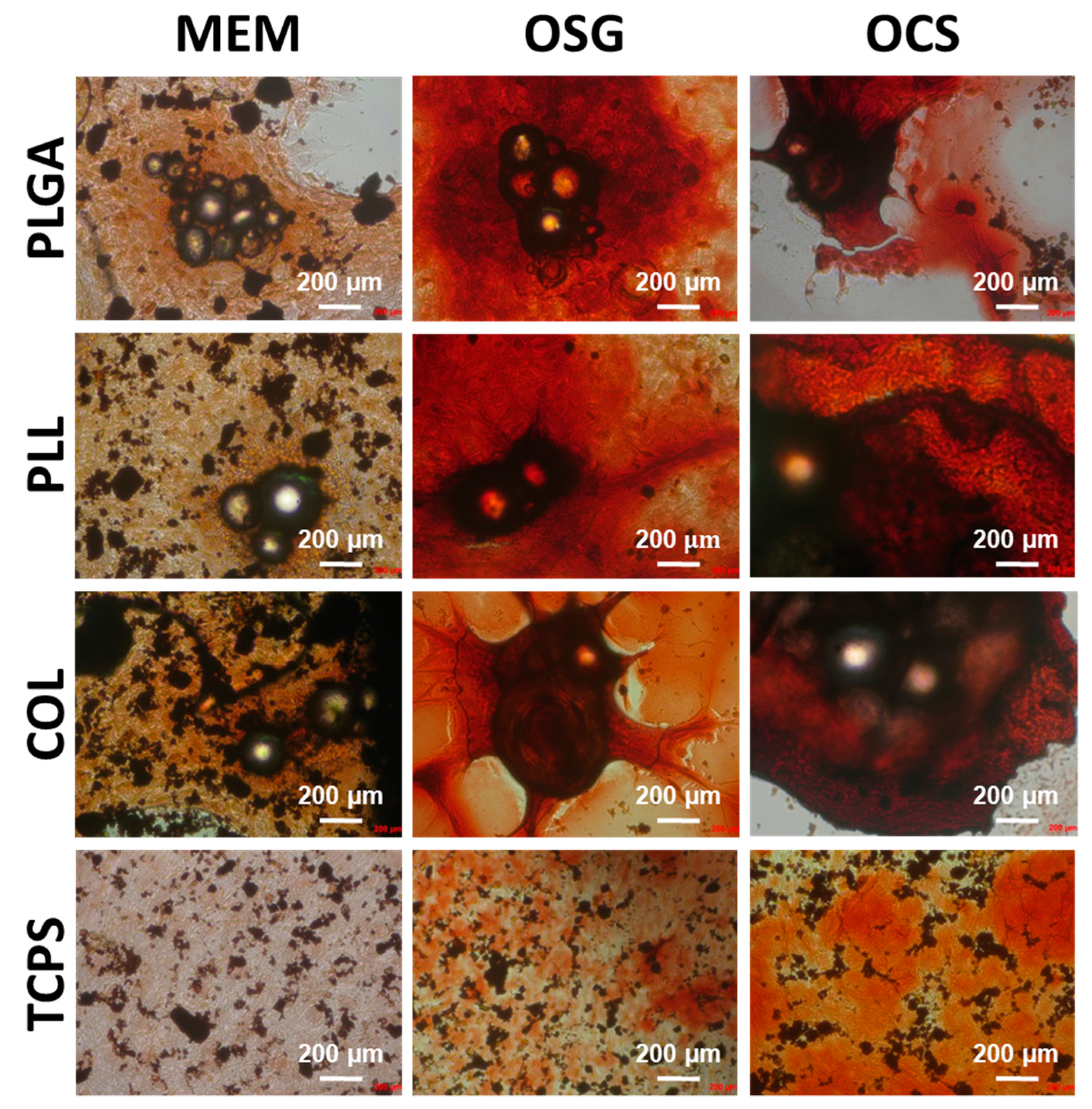
2.2. In Vitro Cell Culture on MS
3. Materials and Methods
3.1. MS Manufacturing and Modification
3.2. Optical and Scanning Electron Microscopy Characterization of MS
3.3. X-ray Photoelectron Spectroscopy Analysis of MS
3.4. In Vitro Cell Culture on MS
3.5. Cell Viability
3.6. Alkaline Phosphatase Assay
3.7. Mineralization Assay
3.8. Cell Morphology Assay—Confocal, Optical, and Scanning Electron Microscopy
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, M.; Jang, H.B.; Lee, S.; Park, J.; Hwang, Y. In Vitro Micro-Mineralized Tissue Formation by the Combinatory Condition of Adipose-Derived Stem Cells, Macroporous PLGA Microspheres and a Bioreactor. Macromol. Res. 2014, 22, 47–57. [Google Scholar] [CrossRef]
- Schon, B.; Hooper, G.J.; Woodfield, T.B.F. Modular Tissue Assembly Strategies for Biofabrication of Engineered Cartilage. Ann. Biomed. Eng. 2017, 45, 100–114. [Google Scholar] [CrossRef]
- Ciucurel, E.C.; Chamberlain, M.D.; Sefton, M.V. The Modular Approach, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Chapter 7. [Google Scholar]
- Clara-Trujillo, S.; Marín-Payá, J.C.; Cordón, L.; Sempere, A.; Gallego Ferrer, G.; Gómez Ribelles, J.L. Biomimetic Microspheres for 3D Mesenchymal Stem Cell Culture and Characterization. Colloids Surf. B Biointerfaces 2019, 177, 68–76. [Google Scholar] [CrossRef]
- Nichol, J.W.; Khademhosseini, A. Modular Tissue Engineering: Engineering Biological Tissues from the Bottom Up. Soft Matter 2010, 5, 1312–1319. [Google Scholar] [CrossRef] [Green Version]
- Yong, J.; Eun, S.; Yun, Y.; Choi, S.; Jeon, D.I.; Kim, H.; Park, K.; Song, H. Osteogenesis and New Bone Formation of Alendronate-Immobilized Porous PLGA Microspheres in a Rat Calvarial Defect Model. J. Ind. Eng. Chem. 2017, 52, 277–286. [Google Scholar] [CrossRef]
- Kang, S.; Bae, Y.H. Biomaterials Cryopreservable and Tumorigenic Three-Dimensional Tumor Culture in Porous Poly (Lactic-Co-Glycolic Acid) Microsphere. Biomaterials 2009, 30, 4227–4232. [Google Scholar] [CrossRef] [Green Version]
- Barrias, C.C.; Ribeiro, C.C.; Lamghari, M.; Sa, C.; Gene, I. De Proliferation, Activity, and Osteogenic Differentiation of Bone Marrow Stromal Cells Cultured on Calcium Titanium Phosphate Microspheres. J. Biomed. Mater. Res. A 2005, 72, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Leong, W.; Wang, D. Cell-Laden Polymeric Microspheres for Biomedical Applications. Trends Biotechnol. 2015, 33, 653–666. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, Y.; Yan, S.; Liu, Z.; He, S.; Cui, L.; Yin, J. Poly (L -Glutamic Acid)/Chitosan Polyelectrolyte Complex Porous Microspheres as Cell Microcarriers for Cartilage Regeneration. Acta Biomater. 2014, 10, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Shi, X.; Gan, Z.; Wang, F. Modification of Porous PLGA Microspheres by Poly- l -Lysine for Use as Tissue Engineering Scaffolds. Colloids Surf. B Biointerfaces 2018, 161, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Eun, S.; Yun, Y.; Shim, K.; Park, K.; Choi, S.; Hun, D. Effect of Lactoferrin-Impregnated Porous Poly (Lactide-Co-Glycolide) (PLGA) Microspheres on Osteogenic Differentiation of Rabbit Adipose-Derived Stem Cells (RADSCs). Colloids Surf. B Biointerfaces 2014, 122, 457–464. [Google Scholar] [CrossRef]
- Chun, K.W.; Yoo, H.S.; Yoon, J.J.; Park, T.G. Biodegradable PLGA Microcarriers for Injectable Delivery of Chondrocytes: Effect of Surface Modification on Cell Attachment and Function. Biotechnol. Prog. 2004, 20, 1797–1801. [Google Scholar] [CrossRef]
- Lao, L.; Tan, H.; Wang, Y.; Gao, C. Chitosan Modified Poly (L-Lactide) Microspheres as Cell Microcarriers for Cartilage Tissue Engineering. Colloids Surf. B Biointerfaces 2008, 66, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Gabler, F.; Frauenschuh, S.; Ringe, J.; Brochhausen, C. Emulsion-Based Synthesis of PLGA-Microspheres for the in Vitro Expansion of Porcine Chondrocytes. Biomol. Eng. 2007, 24, 515–520. [Google Scholar] [CrossRef]
- Jain, R.A. The Manufacturing Techniques of Various Drug Loaded Biodegradable Poly (Lactide- Co -Glycolide) (PLGA) Devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef]
- Wanh, S.; Shi, X.; Gan, Z.; Wang, F. Preparation of PLGA Microspheres with Different Porous Morphologies. Chin. J. Polym. Sci. 2015, 33, 128–136. [Google Scholar] [CrossRef]
- Fan, Q.; Qi, F.; Miao, C.; Yue, H.; Gong, F.; Wu, J. Physicochemical and Engineering Aspects Direct and Controllable Preparation of Uniform PLGA Particles with Various Shapes and Surface Morphologies. Colloids Surf. A Physicochem. Eng. Asp. 2016, 500, 177–185. [Google Scholar] [CrossRef]
- Mcbane, J.E.; Sharifpoor, S.; Cai, K.; Labow, R.S.; Santerre, J.P. Biomaterials Biodegradation and in Vivo Biocompatibility of a Degradable, Polar/Hydrophobic/Ionic Polyurethane for Tissue Engineering Applications. Biomaterials 2011, 32, 6034–6044. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Mujeeb, A.; Jin, Z.; Ge, Z. Optimization and Characterization of Chemically Modified Polymer Microspheres and Their Effect on Cell Behavior. Mater. Lett. 2015, 154, 68–72. [Google Scholar] [CrossRef]
- Capan, Y.; Woo, B.H.; Gebrekidan, S.; Ahmed, S. Influence of formulation parameters on the characteristics of poly (D, L-lactide-co-glycolide) microspheres containing poly (L-lysine) complexed plasmid DNA. J. Control. Release 1999, 60, 279–286. [Google Scholar] [CrossRef]
- Chen, R.; Curran, S.J.; Curran, J.M.; Hunt, J.A. The Use of Poly(l-Lactide) and RGD Modified Microspheres as Cell Carriers in a Flow Intermittency Bioreactor for Tissue Engineering Cartilage. Biomaterials 2006, 27, 4453–4460. [Google Scholar] [CrossRef] [PubMed]
- Gröhn, P.; Klöck, G.; Zimmermann, U. Collagen-Coated Ba2+-Alginate Microcarriers for the Culture of Anchorage-Dependent Mammalian Cells. Biotechniques 1997, 22, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Grässel, S.; Bauer, R.J. Collagen XVI in Health and Disease. Matrix Biol. 2013, 32, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Ungai-salánki, R.; Peter, B.; Gerecsei, T.; Orgovan, N.; Horvath, R.; Szabó, B. A Practical Review on the Measurement Tools for Cellular Adhesion Force. Adv. Colloid Interface Sci. 2019, 269, 309–333. [Google Scholar] [CrossRef] [Green Version]
- Barrantes, A.; Santos, O.; Sotres, J.; Arnebrant, T. Influence of PH on the Build-up of Poly-L-Lysine/Heparin Multilayers. J. Colloid Interface Sci. 2012, 388, 191–200. [Google Scholar] [CrossRef]
- Heath, G.R.; Li, M.; Polignano, I.L.; Richens, J.L.; Catucci, G.; O’Shea, P.; Sadeghi, S.J.; Gilardi, G.; Butt, J.N.; Jeuken, L.J.C. Layer-by-Layer Assembly of Supported Lipid Bilayer Poly-L-Lysine Multilayers. Biomacromolecules 2016, 17, 324–335. [Google Scholar] [CrossRef] [Green Version]
- Wei, F.; Li, M.; Crawford, R.; Zhou, Y.; Xiao, Y. Exosome-Integrated Titanium Oxide Nanotubes for Targeted Bone Regeneration. Acta Biomater. 2019, 86, 480–492. [Google Scholar] [CrossRef]
- Pourakbari, R.; Khodadadi, M.; Aghebati-maleki, A.; Aghebati-maleki, L. The Potential of Exosomes in the Therapy of the Cartilage and Bone Complications; Emphasis on Osteoarthritis. Life Sci. 2019, 236, 116861. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.E.; Shao, J.; van den Beucken, J.J.J.P. Combinatorial Surface Roughness Effects on Osteoclastogenesis and Osteogenesis. Appl. Mater. Interfaces 2018, 10, 36652–36663. [Google Scholar] [CrossRef] [Green Version]
- Mielan, B.; Pamuła, E. Optimizing Manufacturing Conditions of Polymer Microspheres as Cell Carriers for Modular Tissue Engineering. Eng. Biomater. 2020, 156, 2–9. [Google Scholar] [CrossRef]
- Sousa, D.M.; Conceição, F.; Silva, D.I.; Leitão, L.; Neto, E.; Alves, C.J.; Alencastre, I.S.; Herzog, H.; Aguiar, P. Ablation of Y 1 Receptor Impairs Osteoclast Bone-Resorbing Activity. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| MS Sample | C (at. %) | O (at. %) | n (at. %) |
|---|---|---|---|
| PLGA pristine | 65.27 | 34.72 | bdl |
| PLGA after NaOH treatment | 58.18 | 41.81 | bdl |
| PLL | 59.7–64.4 | 35.2–39.8 | 0.3–0.5 |
| COL | 58.0–60.7 | 29.2–39.2 | 1.0–3.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mielan, B.; Sousa, D.M.; Krok-Borkowicz, M.; Eloy, P.; Dupont, C.; Lamghari, M.; Pamuła, E. Polymeric Microspheres/Cells/Extracellular Matrix Constructs Produced by Auto-Assembly for Bone Modular Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 7897. https://doi.org/10.3390/ijms22157897
Mielan B, Sousa DM, Krok-Borkowicz M, Eloy P, Dupont C, Lamghari M, Pamuła E. Polymeric Microspheres/Cells/Extracellular Matrix Constructs Produced by Auto-Assembly for Bone Modular Tissue Engineering. International Journal of Molecular Sciences. 2021; 22(15):7897. https://doi.org/10.3390/ijms22157897
Chicago/Turabian StyleMielan, Bartosz, Daniela M. Sousa, Małgorzata Krok-Borkowicz, Pierre Eloy, Christine Dupont, Meriem Lamghari, and Elżbieta Pamuła. 2021. "Polymeric Microspheres/Cells/Extracellular Matrix Constructs Produced by Auto-Assembly for Bone Modular Tissue Engineering" International Journal of Molecular Sciences 22, no. 15: 7897. https://doi.org/10.3390/ijms22157897
APA StyleMielan, B., Sousa, D. M., Krok-Borkowicz, M., Eloy, P., Dupont, C., Lamghari, M., & Pamuła, E. (2021). Polymeric Microspheres/Cells/Extracellular Matrix Constructs Produced by Auto-Assembly for Bone Modular Tissue Engineering. International Journal of Molecular Sciences, 22(15), 7897. https://doi.org/10.3390/ijms22157897








