Atrazine Inhalation Causes Neuroinflammation, Apoptosis and Accelerating Brain Aging
Abstract
:1. Introduction
2. Results
2.1. ATR Inhalation Induces Anxiety and Depression
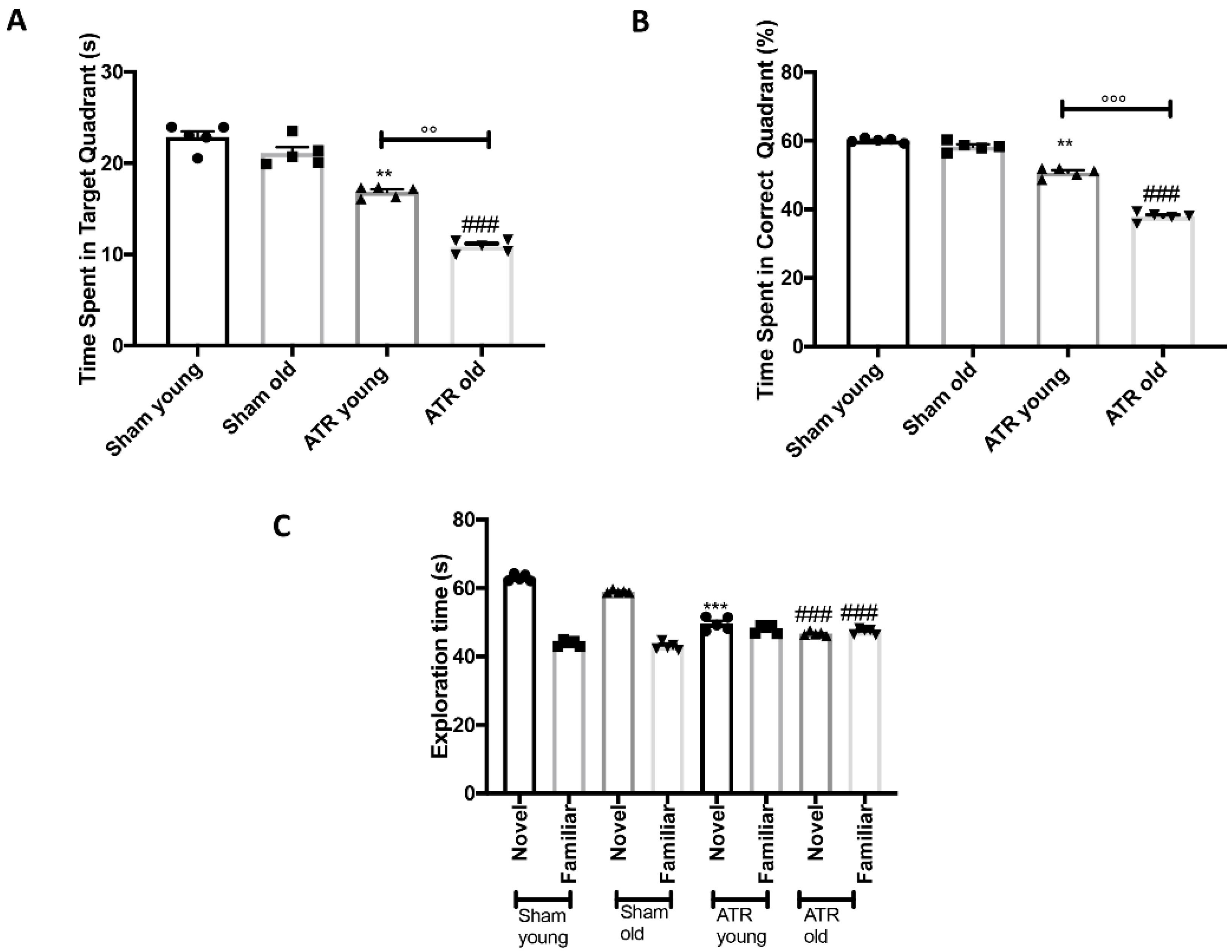
2.2. Effects of ATR Inhalation on Spatial Learning and Memory Function
2.3. Changes in Motor Activity after ATR Inhalation
2.4. Changes in Grip Strength and Sociability after ATR Exposure
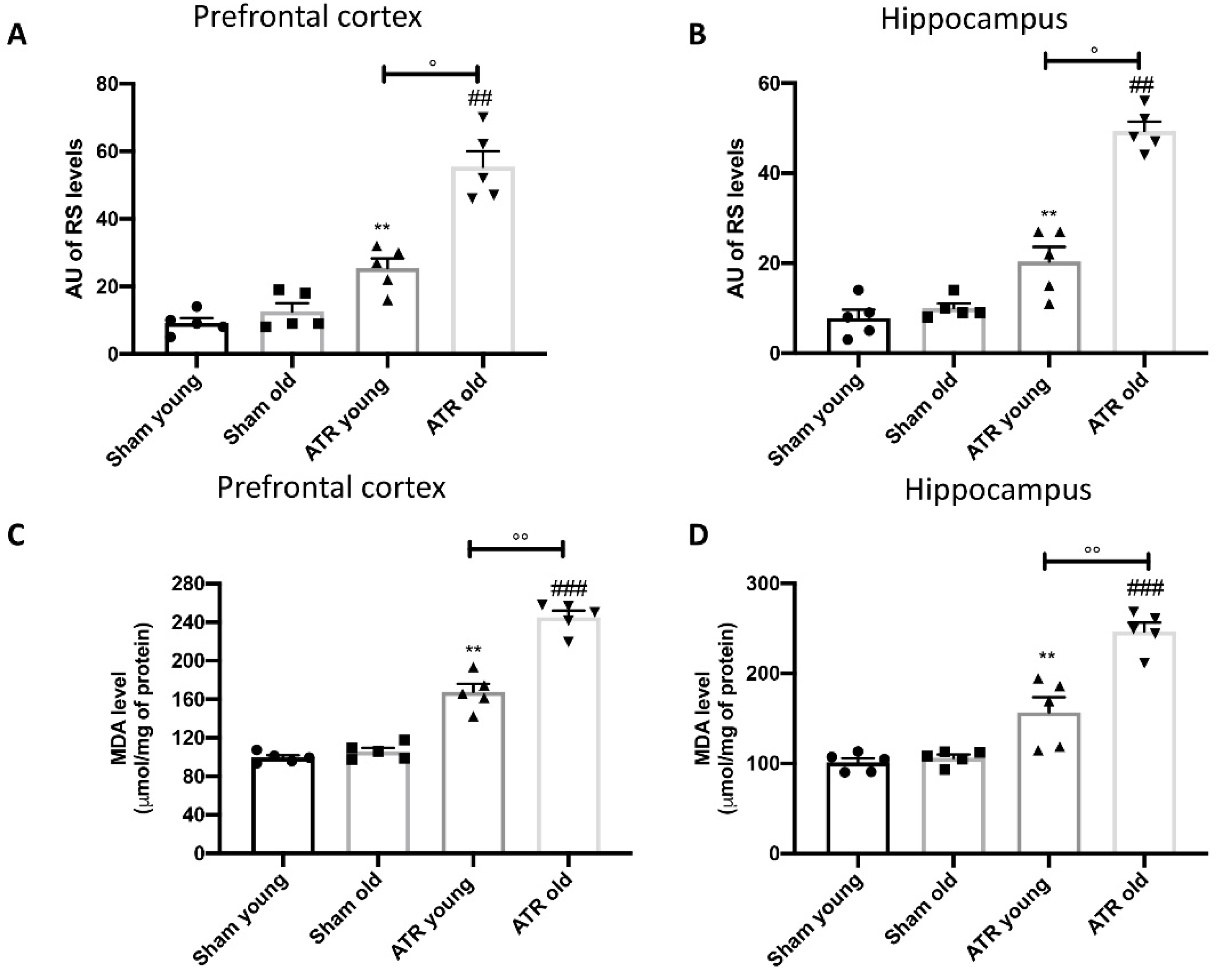
2.5. ATR Exposure Increases Oxidative Stress and Lipid Peroxidation
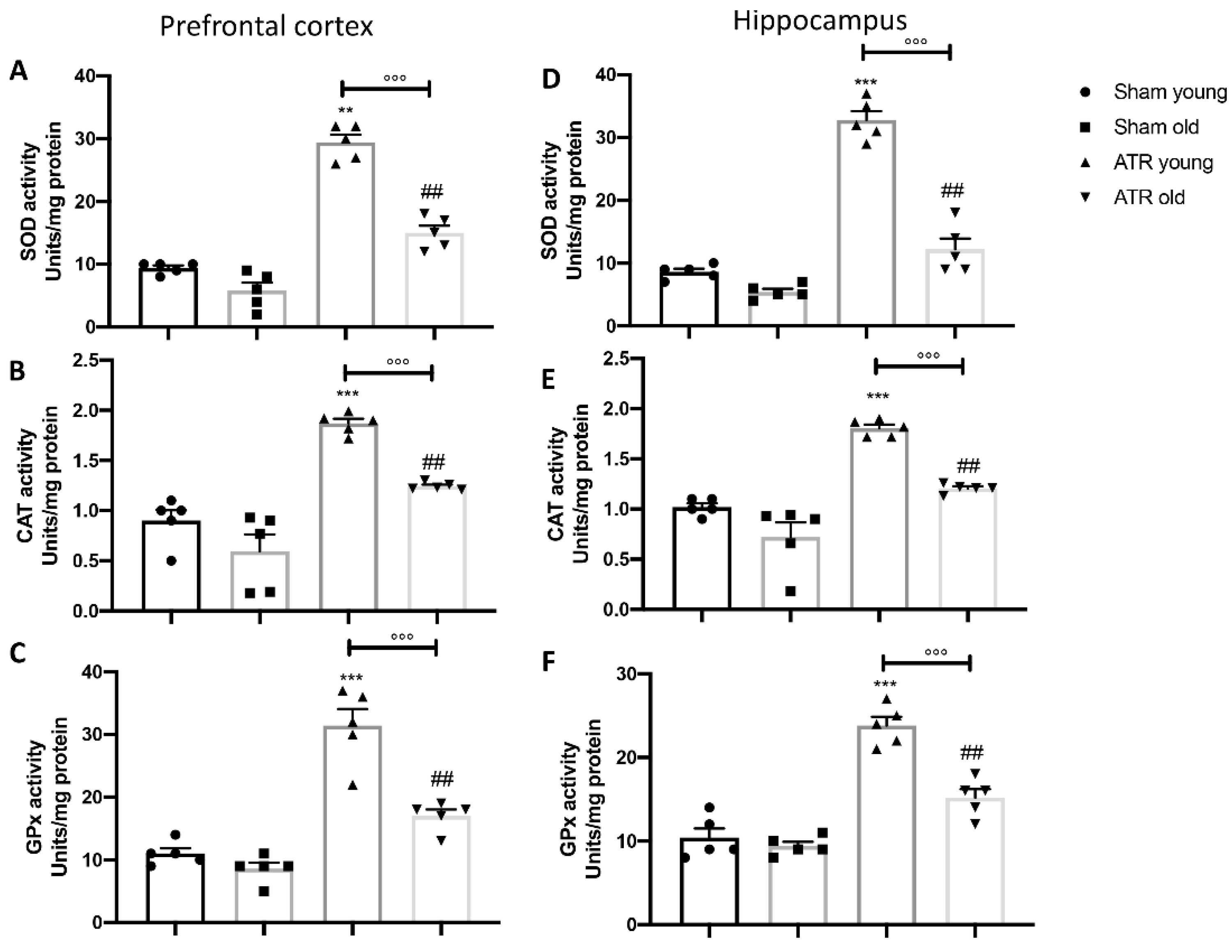
2.6. ATR Inhalation Causes an Imbalance in Physiological Antioxidant Response
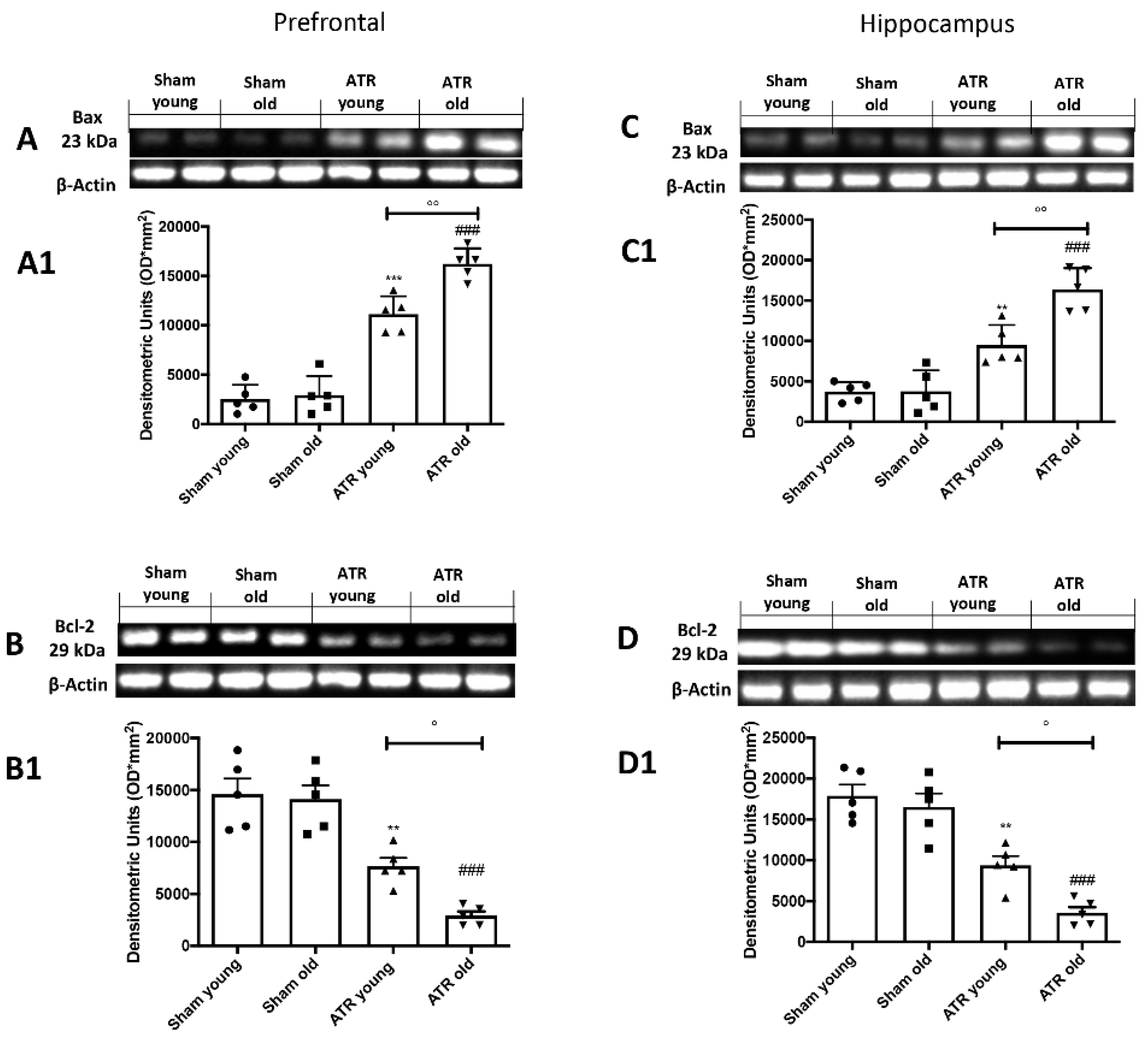
2.7. ATR Inhalation Induces Brain Apoptosis
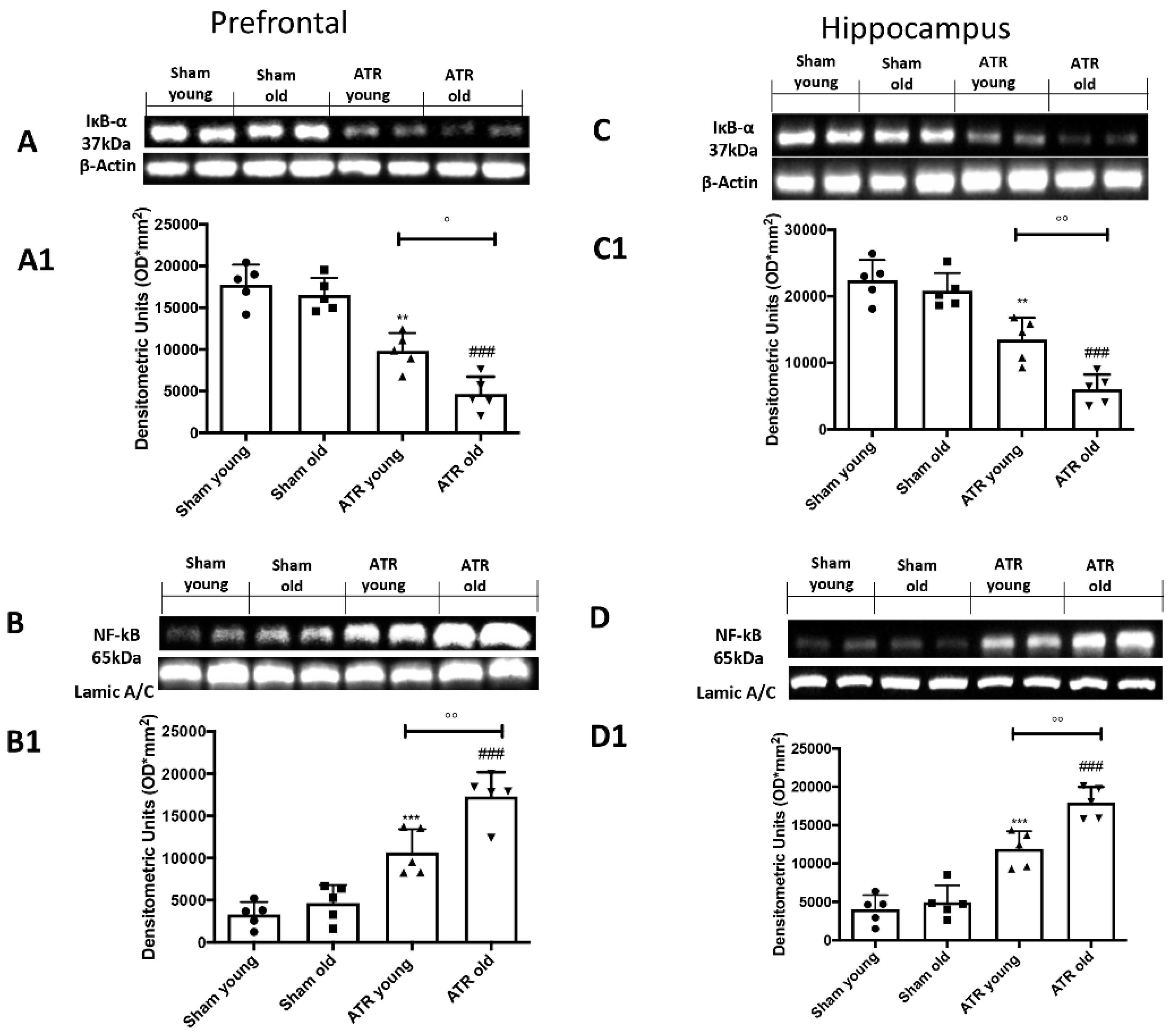
2.8. ATR Inhalation Induces Brain Inflammation
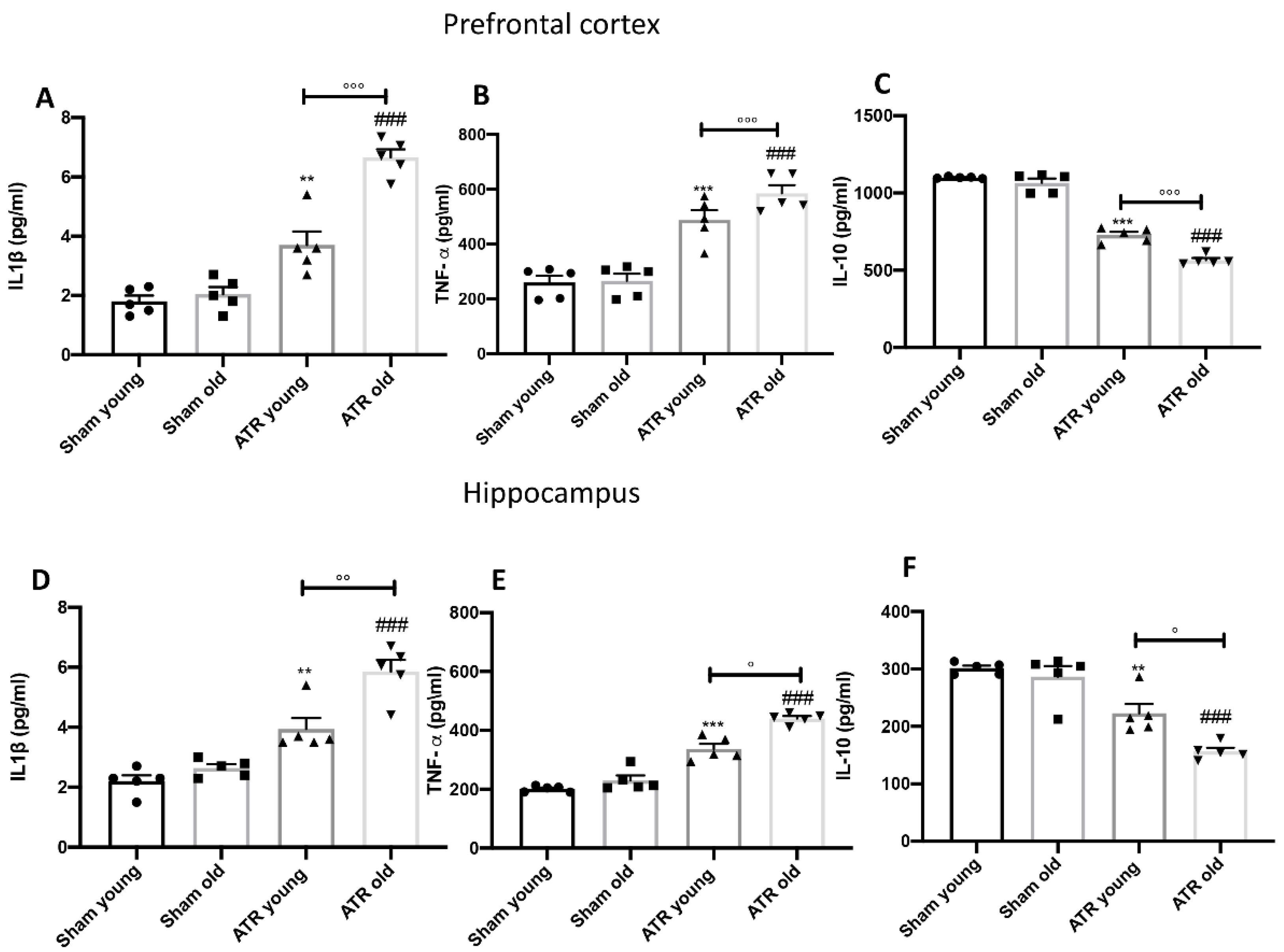
2.9. ATR Inhalation Induces Pro-Inflammatory Cytokines Release
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design and Groups
- (I)
- Sham group, i.e., animals that were exposed to the vehicle (saline with 10% of DMSO).
- (II)
- ATR group, i.e., animals that were exposed to 25 mg of ATR every day for 28 days.
4.3. Behavioral Testing
4.3.1. Pole Test (PT)
4.3.2. Rotarod Test (RT)
4.3.3. Catalepsy Test (CT)
4.3.4. Elevated Plus-Maze (EPM)
4.3.5. Open Field Test (OFT)
4.3.6. Morris Water Maze (MWM)
4.3.7. Grip Strength Test
4.3.8. Forced Swim Test (FST)
4.3.9. Novel Object Recognition (NOR) Test
4.3.10. Social Interaction Test
4.4. Western Blot Analysis of Cytosolic and Nuclear Extracts
4.5. Evaluation of Tissue Lipid Peroxidation
4.6. Cytokine Measurement
4.7. Reactive Species (RS) Detemination
4.8. SOD, CAT and GPx Evaluation
4.9. Materials
4.10. Statistical Evaluation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bondy, S.C. Anthropogenic pollutants may increase the incidence of neurodegenerative disease in an aging population. Toxicology 2016, 341–343, 41–46. [Google Scholar] [CrossRef]
- Sharman, E.H.; Bondy, S.C.; Sharman, K.G.; Lahiri, D.; Cotman, C.W.; Perreau, V.M. Effects of melatonin and age on gene expression in mouse CNS using microarray analysis. Neurochem. Int. 2007, 50, 336–344. [Google Scholar] [CrossRef] [Green Version]
- Bondy, S.C.; Sharman, E.H. Melatonin and the aging brain. Neurochem. Int. 2007, 50, 571–580. [Google Scholar] [CrossRef] [Green Version]
- Bondy, S.C.; Lahiri, D.K.; Perreau, V.M.; Sharman, K.Z.; Campbell, A.; Zhou, J.; Sharman, E.H. Retardation of brain aging by chronic treatment with melatonin. Ann. N. Y. Acad. Sci. 2004, 1035, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Sharman, E.H.; Vaziri, N.D.; Ni, Z.; Sharman, K.G.; Bondy, S.C. Reversal of biochemical and behavioral parameters of brain aging by melatonin and acetyl L-carnitine. Brain Res. 2002, 957, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.S.; Knapp, D.J.; Crews, F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Becaria, A.; Lahiri, D.K.; Bondy, S.C.; Chen, D.; Hamadeh, A.; Li, H.; Taylor, R.; Campbell, A. Aluminum and copper in drinking water enhance inflammatory or oxidative events specifically in the brain. J. Neuroimmunol. 2006, 176, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Cambier, S.; Gonzalez, P.; Mesmer-Dudons, N.; Brethes, D.; Fujimura, M.; Bourdineaud, J.P. Effects of dietary methylmercury on the zebrafish brain: Histological, mitochondrial, and gene transcription analyses. Biometals 2012, 25, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Sipos, E.; Chen, L.; Andras, I.E.; Wrobel, J.; Zhang, B.; Pu, H.; Park, M.; Eum, S.Y.; Toborek, M. Proinflammatory adhesion molecules facilitate polychlorinated biphenyl-mediated enhancement of brain metastasis formation. Toxicol. Sci. 2012, 126, 362–371. [Google Scholar] [CrossRef]
- Santos, D.; Batoreu, M.C.; Tavares de Almeida, I.; Davis Randall, L.; Mateus, M.L.; Andrade, V.; Ramos, R.; Torres, E.; Aschner, M.; Marreilha dos Santos, A.P. Evaluation of neurobehavioral and neuroinflammatory end-points in the post-exposure period in rats sub-acutely exposed to manganese. Toxicology 2013, 314, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Backman, L.; Nyberg, L.; Lindenberger, U.; Li, S.C.; Farde, L. The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neurosci. Biobehav. Rev. 2006, 30, 791–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalphin, J.C. [Respiratory pathology in the agricultural environment]. Rev. Prat. 1998, 48, 1313–1318. [Google Scholar] [PubMed]
- Hoppin, J.A.; Umbach, D.M.; London, S.J.; Alavanja, M.C.; Sandler, D.P. Animal production and wheeze in the Agricultural Health Study: Interactions with atopy, asthma, and smoking. Occup. Environ. Med. 2003, 60, e3. [Google Scholar] [CrossRef] [Green Version]
- Song, X.Y.; Li, J.N.; Wu, Y.P.; Zhang, B.; Li, B.X. Atrazine Causes Autophagy- and Apoptosis-Related Neurodegenerative Effects in Dopaminergic Neurons in the Rat Nigrostriatal Dopaminergic System. Int. J. Mol. Sci. 2015, 16, 13490–13506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzzella, L.; Pozzoni, F.; Giuliano, G. Herbicide contamination of surficial groundwater in Northern Italy. Environ. Pollut. 2006, 142, 344–353. [Google Scholar] [CrossRef]
- Abraxis, L.; Kit, A.E.; Kit, A.T. Immunoassay Test. Kits for Atrazine; Environmental Protection Agency: Washington, DC, USA, 2007.
- EPAUS. Interim Reregistration Eligibility Decision for Atrazine; Appendices C and K; US Environmental Protection Agency: Washington, DC, USA, 2003.
- Singh, M.; Kaur, P.; Sandhir, R.; Kiran, R. Protective effects of vitamin E against atrazine-induced genotoxicity in rats. Mutat Res. 2008, 654, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Simpkins, J.W.; Swenberg, J.A.; Weiss, N.; Brusick, D.; Eldridge, J.C.; Stevens, J.T.; Handa, R.J.; Hovey, R.C.; Plant, T.M.; Pastoor, T.P.; et al. Atrazine and Breast Cancer: A Framework Assessment of the Toxicological and Epidemiological Evidence. Toxicol. Sci. 2017, 159, 480. [Google Scholar] [CrossRef] [Green Version]
- Inoue-Choi, M.; Weyer, P.J.; Jones, R.R.; Booth, B.J.; Cantor, K.P.; Robien, K.; Ward, M.H. Atrazine in public water supplies and risk of ovarian cancer among postmenopausal women in the Iowa Women’s Health Study. Occup. Environ. Med. 2016, 73, 582–587. [Google Scholar] [CrossRef]
- Hu, K.; Tian, Y.; Du, Y.; Huang, L.; Chen, J.; Li, N.; Liu, W.; Liang, Z.; Zhao, L. Atrazine promotes RM1 prostate cancer cell proliferation by activating STAT3 signaling. Int. J. Oncol. 2016, 48, 2166–2174. [Google Scholar] [CrossRef]
- Albanito, L.; Lappano, R.; Madeo, A.; Chimento, A.; Prossnitz, E.R.; Cappello, A.R.; Dolce, V.; Abonante, S.; Pezzi, V.; Maggiolini, M. Effects of atrazine on estrogen receptor alpha-and G protein-coupled receptor 30-mediated signaling and proliferation in cancer cells and cancer-associated fibroblasts. Environ. Health Perspect. 2015, 123, 493–499. [Google Scholar] [CrossRef]
- Boffetta, P.; Adami, H.O.; Berry, S.C.; Mandel, J.S. Atrazine and cancer: A review of the epidemiologic evidence. Eur. J. Cancer Prev. 2013, 22, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.E.; Rusiecki, J.A.; Hoppin, J.A.; Lubin, J.H.; Koutros, S.; Andreotti, G.; Zahm, S.H.; Hines, C.J.; Coble, J.B.; Barone-Adesi, F.; et al. Atrazine and cancer incidence among pesticide applicators in the agricultural health study (1994-2007). Environ. Health Perspect. 2011, 119, 1253–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McElroy, J.A.; Gangnon, R.E.; Newcomb, P.A.; Kanarek, M.S.; Anderson, H.A.; Brook, J.V.; Trentham-Dietz, A.; Remington, P.L. Risk of breast cancer for women living in rural areas from adult exposure to atrazine from well water in Wisconsin. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusiecki, J.A.; De Roos, A.; Lee, W.J.; Dosemeci, M.; Lubin, J.H.; Hoppin, J.A.; Blair, A.; Alavanja, M.C. Cancer incidence among pesticide applicators exposed to atrazine in the Agricultural Health Study. J. Natl. Cancer Inst. 2004, 96, 1375–1382. [Google Scholar] [CrossRef]
- Hessel, P.A.; Kalmes, R.; Smith, T.J.; Lau, E.; Mink, P.J.; Mandel, J. A nested case-control study of prostate cancer and atrazine exposure. J. Occup. Environ. Med. 2004, 46, 379–385. [Google Scholar] [CrossRef]
- Van Leeuwen, J.A.; Waltner-Toews, D.; Abernathy, T.; Smit, B.; Shoukri, M. Associations between stomach cancer incidence and drinking water contamination with atrazine and nitrate in Ontario (Canada) agroecosystems, 1987–1991. Int. J. Epidemiol. 1999, 28, 836–840. [Google Scholar] [CrossRef] [Green Version]
- Xing, H.; Li, S.; Wang, Z.; Gao, X.; Xu, S.; Wang, X. Histopathological changes and antioxidant response in brain and kidney of common carp exposed to atrazine and chlorpyrifos. Chemosphere 2012, 88, 377–383. [Google Scholar] [CrossRef]
- Schmidel, A.J.; Assmann, K.L.; Werlang, C.C.; Bertoncello, K.T.; Francescon, F.; Rambo, C.L.; Beltrame, G.M.; Calegari, D.; Batista, C.B.; Blaser, R.E.; et al. Subchronic atrazine exposure changes defensive behaviour profile and disrupts brain acetylcholinesterase activity of zebrafish. Neurotoxicol. Teratol. 2014, 44, 62–69. [Google Scholar] [CrossRef]
- Horzmann, K.A.; Reidenbach, L.S.; Thanki, D.H.; Winchester, A.E.; Qualizza, B.A.; Ryan, G.A.; Egan, K.E.; Hedrick, V.E.; Sobreira, T.J.P.; Peterson, S.M.; et al. Embryonic atrazine exposure elicits proteomic, behavioral, and brain abnormalities with developmental time specific gene expression signatures. J. Proteom. 2018, 186, 71–82. [Google Scholar] [CrossRef]
- Chavez-Pichardo, M.E.; Reyes-Bravo, D.Y.; Mendoza-Trejo, M.S.; Marin-Lopez, A.G.; Giordano, M.; Hernandez-Chan, N.; Dominguez-Marchan, K.; Ortega-Rosales, L.C.; Rodriguez, V.M. Brain alterations in GABA, glutamate and glutamine markers after chronic atrazine exposure in the male albino rat. Arch. Toxicol. 2020, 94, 3217–3230. [Google Scholar] [CrossRef]
- Abarikwu, S.O. Protective effect of quercetin on atrazine-induced oxidative stress in the liver, kidney, brain, and heart of adult wistar rats. Toxicol. Int. 2014, 21, 148–155. [Google Scholar] [CrossRef]
- Mizota, K.; Ueda, H. Endocrine disrupting chemical atrazine causes degranulation through Gq/11 protein-coupled neurosteroid receptor in mast cells. Toxicol. Sci. 2006, 90, 362–368. [Google Scholar] [CrossRef] [Green Version]
- Jestadi, D.B.; Phaniendra, A.; Babji, U.; Srinu, T.; Shanmuganathan, B.; Periyasamy, L. Effects of short term exposure of atrazine on the liver and kidney of normal and diabetic rats. J. Toxicol. 2014, 2014, 536759. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Li, K.; Zhao, L.; Liu, J.; Suo, Q.; Zhao, J.; Wang, H.; Zhao, S. Effect of Nrf2 on rat ovarian tissues against atrazine-induced anti-oxidative response. Int. J. Clin. Exp. Pathol. 2014, 7, 2780–2789. [Google Scholar]
- Zhang, X.; Wang, M.; Gao, S.; Ren, R.; Zheng, J.; Zhang, Y. Atrazine-induced apoptosis of splenocytes in BALB/C mice. BMC Med. 2011, 9, 117. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Du, Y.; Liu, J.; Wang, H.; Sun, D.; Liang, D.; Zhao, L.; Shang, J. Effects of atrazine on the oxidative damage of kidney in Wister rats. Int. J. Clin. Exp. Med. 2014, 7, 3235–3243. [Google Scholar]
- Li, P.; Li, X.; Yao, L.; Wu, Y.; Li, B. Soybean isoflavones prevent atrazine-induced neurodegenerative damage by inducing autophagy. Ecotoxicol. Environ. Saf. 2020, 190, 110065. [Google Scholar] [CrossRef] [PubMed]
- Figueira, F.H.; de Quadros Oliveira, N.; de Aguiar, L.M.; Escarrone, A.L.; Primel, E.G.; Barros, D.M.; da Rosa, C.E. Exposure to atrazine alters behaviour and disrupts the dopaminergic system in Drosophila melanogaster. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2017, 202, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; He, X.; Ma, K.; Wu, Y.P.; Li, B.X. The Effect of Exposure to Atrazine on Dopaminergic Development in Pubertal Male SD Rats. Birth. Defects Res. Dev. Reprod. Toxicol. 2015, 104, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, Y.S.; Yang, J.W.; Yu, J.; Wu, Y.P.; Li, B.X. Exposure to atrazine during gestation and lactation periods: Toxicity effects on dopaminergic neurons in offspring by downregulation of Nurr1 and VMAT2. Int. J. Mol. Sci. 2014, 15, 2811–2825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.; Dodd, C.A.; Filipov, N.M. Differentiation state-dependent effects of in vitro exposure to atrazine or its metabolite diaminochlorotriazine in a dopaminergic cell line. Life Sci. 2013, 92, 81–90. [Google Scholar] [CrossRef]
- Bardullas, U.; Giordano, M.; Rodriguez, V.M. Chronic atrazine exposure causes disruption of the spontaneous locomotor activity and alters the striatal dopaminergic system of the male Sprague-Dawley rat. Neurotoxicol Teratol. 2011, 33, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Coban, A.; Filipov, N.M. Dopaminergic toxicity associated with oral exposure to the herbicide atrazine in juvenile male C57BL/6 mice. J. Neurochem. 2007, 100, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, N.; Hirose, Y.; Ohara, S.; Ono, T.; Watanabe, Y. A simple quantitative bradykinesia test in MPTP-treated mice. Res. Commun. Chem. Pathol. Pharm. 1985, 50, 435–441. [Google Scholar]
- Lucchini, R.; Zimmerman, N. Lifetime cumulative exposure as a threat for neurodegeneration: Need for prevention strategies on a global scale. Neurotoxicology 2009, 30, 1144–1148. [Google Scholar] [CrossRef] [PubMed]
- (EPA), U.S.E.P.A. Atrazine Background; US Environmental Protection Agency: Washington, DC, USA, 2008.
- Ackerman, F. The economics of atrazine. Int. J. Occup. Environ. Health 2007, 13, 437–445. [Google Scholar] [CrossRef]
- Pohl, H.; Kolman, J. Interaction Profile for Atrazine, Deethylatrazine, Diazinon, Nitrate and Simazine; Department of Health and Human Services, Public Health; Agency for Toxic Substances and Disease Registry (ATSDR): Atlanta, GA, USA, 2006.
- Kavlock, R. Overview of endocrine disruptor research activity in the United States. Chemosphere 1999, 39, 1227–1236. [Google Scholar] [CrossRef]
- Munger, R.; Isacson, P.; Hu, S.; Burns, T.; Hanson, J.; Lynch, C.F.; Cherryholmes, K.; Van Dorpe, P.; Hausler, W.J., Jr. Intrauterine growth retardation in Iowa communities with herbicide-contaminated drinking water supplies. Environ. Health Perspect. 1997, 105, 308–314. [Google Scholar] [CrossRef]
- Arbuckle, T.E.; Lin, Z.; Mery, L.S. An exploratory analysis of the effect of pesticide exposure on the risk of spontaneous abortion in an Ontario farm population. Environ. Health Perspect. 2001, 109, 851–857. [Google Scholar] [CrossRef]
- Hayes, T.B.; Collins, A.; Lee, M.; Mendoza, M.; Noriega, N.; Stuart, A.A.; Vonk, A. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc. Natl. Acad. Sci. USA 2002, 99, 5476–5480. [Google Scholar] [CrossRef] [Green Version]
- Swan, S.H. Semen quality in relation to pesticide exposure in Missouri males. Mo Med. 2003, 100, 554. [Google Scholar] [PubMed]
- Swan, S.H.; Kruse, R.L.; Liu, F.; Barr, D.B.; Drobnis, E.Z.; Redmon, J.B.; Wang, C.; Brazil, C.; Overstreet, J.W.; Study for Future Families Research Group. Semen quality in relation to biomarkers of pesticide exposure. Environ. Health Perspect. 2003, 111, 1478–1484. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, V.M.; Limon-Pacheco, J.H.; Mendoza-Trejo, M.S.; Gonzalez-Gallardo, A.; Hernandez-Plata, I.; Giordano, M. Repeated exposure to the herbicide atrazine alters locomotor activity and the nigrostriatal dopaminergic system of the albino rat. Neurotoxicology 2013, 34, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Dodd, C.A.; Filipov, N.M. Short-term atrazine exposure causes behavioral deficits and disrupts monoaminergic systems in male C57BL/6 mice. Neurotoxicol. Teratol. 2013, 39, 26–35. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Yang, J.; Wu, Y.; Yu, J.; Li, B. The long-term effects of the herbicide atrazine on the dopaminergic system following exposure during pubertal development. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 763, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, Y.; Yang, J.; Wu, Y.; Yu, J.; Li, B. Age-dependent dopaminergic dysfunction following fetal exposure to atrazine in SD rats. Environ. Toxicol. Pharmacol. 2014, 37, 1275–1282. [Google Scholar] [CrossRef]
- Lin, Z.; Roede, J.R.; He, C.; Jones, D.P.; Filipov, N.M. Short-term oral atrazine exposure alters the plasma metabolome of male C57BL/6 mice and disrupts alpha-linolenate, tryptophan, tyrosine and other major metabolic pathways. Toxicology 2014, 326, 130–141. [Google Scholar] [CrossRef] [Green Version]
- Belloni, V.; Dessi-Fulgheri, F.; Zaccaroni, M.; Di Consiglio, E.; De Angelis, G.; Testai, E.; Santochirico, M.; Alleva, E.; Santucci, D. Early exposure to low doses of atrazine affects behavior in juvenile and adult CD1 mice. Toxicology 2011, 279, 19–26. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Cordaro, M.; D’Amico, R.; Morabito, R.; Fusco, R.; Siracusa, R.; Peritore, A.F.; Impellizzeri, D.; Genovese, T.; Crupi, R.; Gugliandolo, E.; et al. Physiological and Biochemical Changes in NRF2 Pathway in Aged Animals Subjected to Brain Injury. Cell. Physiol. Biochem. 2021, 55, 160–179. [Google Scholar] [CrossRef]
- Schramm, C.M.; Puddington, L.; Wu, C.; Guernsey, L.; Gharaee-Kermani, M.; Phan, S.H.; Thrall, R.S. Chronic inhaled ovalbumin exposure induces antigen-dependent but not antigen-specific inhalational tolerance in a murine model of allergic airway disease. Am. J. Pathol. 2004, 164, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Vaughan, R.P.; Szewczyk, M.T., Jr.; Lanosa, M.J.; Desesa, C.R.; Gianutsos, G.; Morris, J.B. Adenosine sensory transduction pathways contribute to activation of the sensory irritation response to inspired irritant vapors. Toxicol. Sci. 2006, 93, 411–421. [Google Scholar] [CrossRef] [Green Version]
- Ramona, D.; Monaco, F.; Fusco, R.; Peritore, A.F.; Genovese, T.; Impellizzeri, D.; Crupi, R.; Interdonato, L.; Sforza, A.M.; Gugliandolo, E.; et al. Exposure to Atrazine Induces Lung Inflammation through Nrf2-HO1 and Beclin 1/LC3 Pathways. Cell. Physiol. Biochem. 2021, 55, 413–427. [Google Scholar] [CrossRef]
- Lin, J.; Xia, J.; Zhao, H.S.; Hou, R.; Talukder, M.; Yu, L.; Guo, J.Y.; Li, J.L. Lycopene Triggers Nrf2-AMPK Cross Talk to Alleviate Atrazine-Induced Nephrotoxicity in Mice. J. Agric. Food Chem. 2018, 66, 12385–12394. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Bi, H.; Ma, K.; Li, B. Developmental Exposure to Atrazine Impairs Spatial Memory and Downregulates the Hippocampal D1 Dopamine Receptor and cAMP-Dependent Signaling Pathway in Rats. Int. J. Mol. Sci. 2018, 19, 2241. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Wang, Z.; Zhang, C.; Jia, L.; Zhang, Y. Oral Exposure to Atrazine Induces Oxidative Stress and Calcium Homeostasis Disruption in Spleen of Mice. Oxid. Med. Cell. Longev. 2016, 2016, 7978219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filipov, N.M.; Pinchuk, L.M.; Boyd, B.L.; Crittenden, P.L. Immunotoxic effects of short-term atrazine exposure in young male C57BL/6 mice. Toxicol. Sci. 2005, 86, 324–332. [Google Scholar] [CrossRef]
- Sedelis, M.; Schwarting, R.K.; Huston, J.P. Behavioral phenotyping of the MPTP mouse model of Parkinson’s disease. Behav. Brain Res. 2001, 125, 109–125. [Google Scholar] [CrossRef]
- Cordaro, M.; Siracusa, R.; Crupi, R.; Impellizzeri, D.; Peritore, A.F.; D’Amico, R.; Gugliandolo, E.; Di Paola, R.; Cuzzocrea, S. 2-Pentadecyl-2-Oxazoline Reduces Neuroinflammatory Environment in the MPTP Model of Parkinson Disease. Mol. Neurobiol. 2018, 55, 9251–9266. [Google Scholar] [CrossRef]
- Fleming, S.M.; Mulligan, C.K.; Richter, F.; Mortazavi, F.; Lemesre, V.; Frias, C.; Zhu, C.; Stewart, A.; Gozes, I.; Morimoto, B.; et al. A pilot trial of the microtubule-interacting peptide (NAP) in mice overexpressing alpha-synuclein shows improvement in motor function and reduction of alpha-synuclein inclusions. Mol. Cell. Neurosci. 2011, 46, 597–606. [Google Scholar] [CrossRef] [Green Version]
- Siracusa, R.; Paterniti, I.; Cordaro, M.; Crupi, R.; Bruschetta, G.; Campolo, M.; Cuzzocrea, S.; Esposito, E. Neuroprotective Effects of Temsirolimus in Animal Models of Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 2403–2419. [Google Scholar] [CrossRef]
- Araki, T.; Kumagai, T.; Tanaka, K.; Matsubara, M.; Kato, H.; Itoyama, Y.; Imai, Y. Neuroprotective effect of riluzole in MPTP-treated mice. Brain Res. 2001, 918, 176–181. [Google Scholar] [CrossRef]
- Paterniti, I.; Campolo, M.; Siracusa, R.; Cordaro, M.; Di Paola, R.; Calabrese, V.; Navarra, M.; Cuzzocrea, S.; Esposito, E. Liver X receptors activation, through TO901317 binding, reduces neuroinflammation in Parkinson’s disease. PLoS ONE 2017, 12, e0174470. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Siebold, L.; Krueger, A.C.; Abdala, J.A.; Figueroa, J.D.; Bartnik-Olson, B.; Holshouser, B.; Wilson, C.G.; Ashwal, S. Cosyntropin Attenuates Neuroinflammation in a Mouse Model of Traumatic Brain Injury. Front. Mol. Neurosci. 2020, 13, 109. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhou, R.; Zhu, X.Y.; Liu, G.; Zhao, Y.P.; Ma, P.S.; Wu, W.; Niu, Y.; Sun, T.; Li, Y.X.; et al. Neuroprotective Effects of Lycium barbarum Polysaccharide on Focal Cerebral Ischemic Injury in Mice. Neurochem. Res. 2017, 42, 2798–2813. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Bertin, A.; Blavet, N.; Deniel, M.; Jalfre, M. Immobility induced by forced swimming in rats: Effects of agents which modify central catecholamine and serotonin activity. Eur. J. Pharm. 1979, 57, 201–210. [Google Scholar] [CrossRef]
- Pan, Z.; Cui, M.; Dai, G.; Yuan, T.; Li, Y.; Ji, T.; Pan, Y. Protective Effect of Anthocyanin on Neurovascular Unit in Cerebral Ischemia/Reperfusion Injury in Rats. Front. Neurosci. 2018, 12, 947. [Google Scholar] [CrossRef]
- Siracusa, R.; Impellizzeri, D.; Cordaro, M.; Crupi, R.; Esposito, E.; Petrosino, S.; Cuzzocrea, S. Anti-Inflammatory and Neuroprotective Effects of Co-UltraPEALut in a Mouse Model of Vascular Dementia. Front. Neurol. 2017, 8, 233. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Cronin, C.G.; Scranton, V.L.; Jacobson, K.A.; Liang, B.T.; Verma, R. Neuroprotective and neuro-rehabilitative effects of acute purinergic receptor P2X4 (P2X4R) blockade after ischemic stroke. Exp. Neurol. 2020, 329, 113308. [Google Scholar] [CrossRef] [PubMed]
- Boccella, S.; Iannotta, M.; Cristiano, C.; Iannotti, F.A.; Bello, F.D.; Guida, F.; Belardo, C.; Infantino, R.; Ricciardi, F.; Giannella, M.; et al. Treatment With 2-Pentadecyl-2-Oxazoline Restores Mild Traumatic Brain Injury-Induced Sensorial and Neuropsychiatric Dysfunctions. Front. Pharm. 2020, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Paterniti, I.; Siracusa, R.; Impellizzeri, D.; Esposito, E.; Cuzzocrea, S. KU0063794, a Dual mTORC1 and mTORC2 Inhibitor, Reduces Neural Tissue Damage and Locomotor Impairment After Spinal Cord Injury in Mice. Mol. Neurobiol. 2017, 54, 2415–2427. [Google Scholar] [CrossRef] [PubMed]
- Campolo, M.; Esposito, E.; Ahmad, A.; Di Paola, R.; Paterniti, I.; Cordaro, M.; Bruschetta, G.; Wallace, J.L.; Cuzzocrea, S. Hydrogen sulfide-releasing cyclooxygenase inhibitor ATB-346 enhances motor function and reduces cortical lesion volume following traumatic brain injury in mice. J. Neuroinflammation 2014, 11, 196. [Google Scholar] [CrossRef] [Green Version]
- Paterniti, I.; Di Paola, R.; Campolo, M.; Siracusa, R.; Cordaro, M.; Bruschetta, G.; Tremolada, G.; Maestroni, A.; Bandello, F.; Esposito, E.; et al. Palmitoylethanolamide treatment reduces retinal inflammation in streptozotocin-induced diabetic rats. Eur J. Pharm. 2015, 769, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Impellizzeri, D.; Gugliandolo, E.; Siracusa, R.; Crupi, R.; Esposito, E.; Cuzzocrea, S. Adelmidrol, a Palmitoylethanolamide Analogue, as a New Pharmacological Treatment for the Management of Inflammatory Bowel Disease. Mol. Pharm. 2016, 90, 549–561. [Google Scholar] [CrossRef] [Green Version]
- Di Paola, R.; Cordaro, M.; Crupi, R.; Siracusa, R.; Campolo, M.; Bruschetta, G.; Fusco, R.; Pugliatti, P.; Esposito, E.; Cuzzocrea, S. Protective Effects of Ultramicronized Palmitoylethanolamide (PEA-um) in Myocardial Ischaemia and Reperfusion Injury in VIVO. Shock 2016, 46, 202–213. [Google Scholar] [CrossRef]
- Di Paola, R.; Impellizzeri, D.; Fusco, R.; Cordaro, M.; Siracusa, R.; Crupi, R.; Esposito, E.; Cuzzocrea, S. Ultramicronized palmitoylethanolamide (PEA-um((R))) in the treatment of idiopathic pulmonary fibrosis. Pharm. Res. 2016, 111, 405–412. [Google Scholar] [CrossRef]
- Esposito, E.; Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Siracusa, R.; Gugliandolo, E.; Crupi, R.; Cuzzocrea, S. A new co-micronized composite containing palmitoylethanolamide and polydatin shows superior oral efficacy compared to their association in a rat paw model of carrageenan-induced inflammation. Eur. J. Pharm. 2016, 782, 107–118. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Cordaro, M.; Bruschetta, G.; Crupi, R.; Pascali, J.; Alfonsi, D.; Marcolongo, G.; Cuzzocrea, S. 2-pentadecyl-2-oxazoline: Identification in coffee, synthesis and activity in a rat model of carrageenan-induced hindpaw inflammation. Pharm. Res. 2016, 108, 23–30. [Google Scholar] [CrossRef]
- Fusco, R.; D’Amico, R.; Cordaro, M.; Gugliandolo, E.; Siracusa, R.; Peritore, A.F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Absence of formyl peptide receptor 1 causes endometriotic lesion regression in a mouse model of surgically-induced endometriosis. Oncotarget 2018, 9, 31355–31366. [Google Scholar] [CrossRef] [Green Version]
- Gugliandolo, E.; D’Amico, R.; Cordaro, M.; Fusco, R.; Siracusa, R.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Effect of PEA-OXA on neuropathic pain and functional recovery after sciatic nerve crush. J. Neuroinflam. 2018, 15, 264. [Google Scholar] [CrossRef] [Green Version]
- Fusco, R.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; D’Amico, R.; Cordaro, M.; Crupi, R.; Mandalari, G.; Impellizzeri, D.; et al. The Role of Cashew (Anacardium occidentale L.) Nuts on an Experimental Model of Painful Degenerative Joint Disease. Antioxidants 2020, 9, 511. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Fusco, R.; Peritore, A.F.; Cordaro, M.; D’Amico, R.; Genovese, T.; Gugliandolo, E.; Crupi, R.; Smeriglio, A.; Mandalari, G.; et al. The Antioxidant and Anti-Inflammatory Properties of Anacardium occidentale L. Cashew Nuts in a Mouse Model of Colitis. Nutrients 2020, 12, 834. [Google Scholar] [CrossRef] [Green Version]
- Di Paola, R.; Fusco, R.; Impellizzeri, D.; Cordaro, M.; Britti, D.; Morittu, V.M.; Evangelista, M.; Cuzzocrea, S. Adelmidrol, in combination with hyaluronic acid, displays increased anti-inflammatory and analgesic effects against monosodium iodoacetate-induced osteoarthritis in rats. Arthritis Res. Ther. 2016, 18, 291. [Google Scholar] [CrossRef] [Green Version]
- Cuzzocrea, S.; Mazzon, E.; Esposito, E.; Muia, C.; Abdelrahman, M.; Di Paola, R.; Crisafulli, C.; Bramanti, P.; Thiemermann, C. Glycogen synthase kinase-3beta inhibition attenuates the development of ischaemia/reperfusion injury of the gut. Intensive Care Med. 2007, 33, 880–893. [Google Scholar] [CrossRef]
- Costantino, G.; Cuzzocrea, S.; Mazzon, E.; Caputi, A.P. Protective effects of melatonin in zymosan-activated plasma-induced paw inflammation. Eur J. Pharmacol. 1998, 363, 57–63. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Esposito, E.; Di Paola, R.; Ahmad, A.; Campolo, M.; Peli, A.; Morittu, V.M.; Britti, D.; Cuzzocrea, S. Palmitoylethanolamide and luteolin ameliorate development of arthritis caused by injection of collagen type II in mice. Arthritis Res. Ther. 2013, 15, R192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Paola, R.; Fusco, R.; Gugliandolo, E.; D’Amico, R.; Campolo, M.; Latteri, S.; Carughi, A.; Mandalari, G.; Cuzzocrea, S. The Antioxidant Activity of Pistachios Reduces Cardiac Tissue Injury of Acute Ischemia/Reperfusion (I/R) in Diabetic Streptozotocin (STZ)-Induced Hyperglycaemic Rats. Front. Pharmacol. 2018, 9, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordaro, M.; Siracusa, R.; Impellizzeri, D.; D’ Amico, R.; Peritore, A.F.; Crupi, R.; Gugliandolo, E.; Fusco, R.; Di Paola, R.; Schievano, C.; et al. Safety and efficacy of a new micronized formulation of the ALIAmide palmitoylglucosamine in preclinical models of inflammation and osteoarthritis pain. Arthritis Res. Ther. 2019, 21, 254. [Google Scholar] [CrossRef] [Green Version]
- Fusco, R.; Siracusa, R.; D’Amico, R.; Peritore, A.F.; Cordaro, M.; Gugliandolo, E.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Melatonin Plus Folic Acid Treatment Ameliorates Reserpine-Induced Fibromyalgia: An Evaluation of Pain, Oxidative Stress, and Inflammation. Antioxidants 2019, 8, 628. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Wu, S.; Xie, W.; He, H. Ketamine ameliorates oxidative stress-induced apoptosis in experimental traumatic brain injury via the Nrf2 pathway. Drug Des. Devel Ther. 2018, 12, 845–853. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Liu, C.; Li, Y.; Chen, L.; Xiang, J. Tenacissoside H Promotes Neurological Recovery of Cerebral Ischemia-reperfusion Injury in Mice by Modulating Inflammation and Oxidative stress via TrkB Pathway. Clin. Exp. Pharm. Physiol. 2020. [Google Scholar] [CrossRef]
- Shi, D.D.; Huang, Y.H.; Lai, C.S.W.; Dong, C.M.; Ho, L.C.; Wu, E.X.; Li, Q.; Wang, X.M.; Chung, S.K.; Sham, P.C.; et al. Chemotherapy-Induced Cognitive Impairment Is Associated with Cytokine Dysregulation and Disruptions in Neuroplasticity. Mol. Neurobiol. 2019, 56, 2234–2243. [Google Scholar] [CrossRef]
- Zhu, N.; Liang, X.; Zhang, M.; Yin, X.; Yang, H.; Zhi, Y.; Ying, G.; Zou, J.; Chen, L.; Yao, X.; et al. Astaxanthin protects cognitive function of vascular dementia. Behav. Brain Funct. 2020, 16, 10. [Google Scholar] [CrossRef]
- Peritore, A.F.; Siracusa, R.; Fusco, R.; Gugliandolo, E.; D’Amico, R.; Cordaro, M.; Crupi, R.; Genovese, T.; Impellizzeri, D.; Cuzzocrea, S.; et al. Ultramicronized Palmitoylethanolamide and Paracetamol, a New Association to Relieve Hyperalgesia and Pain in a Sciatic Nerve Injury Model in Rat. Int. J. Mol. Sci. 2020, 21, 3509. [Google Scholar] [CrossRef] [PubMed]
- Loetchutinat, C.; Kothan, S.; Dechsupa, S.; Meesungnoen, J.; Jay-Gerin, J.-P.; Mankhetkorn, S. Spectrofluorometric determination of intracellular levels of reactive oxygen species in drug-sensitive and drug-resistant cancer cells using the 2′, 7′-dichlorofluorescein diacetate assay. Radiat. Phys. Chem. 2005, 72, 323–331. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Souza, L.C.; Antunes, M.S.; Filho, C.B.; Del Fabbro, L.; de Gomes, M.G.; Goes, A.T.; Donato, F.; Prigol, M.; Boeira, S.P.; Jesse, C.R. Flavonoid Chrysin prevents age-related cognitive decline via attenuation of oxidative stress and modulation of BDNF levels in aged mouse brain. Pharm. Biochem. Behav. 2015, 134, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Wendel, A. Detoxi¢ cation and drug metabolism; conjugation and related systems. Methods Enzym. 1981, 77, 325–333. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genovese, T.; Siracusa, R.; Fusco, R.; D’Amico, R.; Impellizzeri, D.; Peritore, A.F.; Crupi, R.; Gugliandolo, E.; Morabito, R.; Cuzzocrea, S.; et al. Atrazine Inhalation Causes Neuroinflammation, Apoptosis and Accelerating Brain Aging. Int. J. Mol. Sci. 2021, 22, 7938. https://doi.org/10.3390/ijms22157938
Genovese T, Siracusa R, Fusco R, D’Amico R, Impellizzeri D, Peritore AF, Crupi R, Gugliandolo E, Morabito R, Cuzzocrea S, et al. Atrazine Inhalation Causes Neuroinflammation, Apoptosis and Accelerating Brain Aging. International Journal of Molecular Sciences. 2021; 22(15):7938. https://doi.org/10.3390/ijms22157938
Chicago/Turabian StyleGenovese, Tiziana, Rosalba Siracusa, Roberta Fusco, Ramona D’Amico, Daniela Impellizzeri, Alessio Filippo Peritore, Rosalia Crupi, Enrico Gugliandolo, Rossana Morabito, Salvatore Cuzzocrea, and et al. 2021. "Atrazine Inhalation Causes Neuroinflammation, Apoptosis and Accelerating Brain Aging" International Journal of Molecular Sciences 22, no. 15: 7938. https://doi.org/10.3390/ijms22157938
APA StyleGenovese, T., Siracusa, R., Fusco, R., D’Amico, R., Impellizzeri, D., Peritore, A. F., Crupi, R., Gugliandolo, E., Morabito, R., Cuzzocrea, S., Trovato Salinaro, A., Cordaro, M., & Di Paola, R. (2021). Atrazine Inhalation Causes Neuroinflammation, Apoptosis and Accelerating Brain Aging. International Journal of Molecular Sciences, 22(15), 7938. https://doi.org/10.3390/ijms22157938













