Eosinophils and Bacteria, the Beginning of a Story
Abstract
1. Eosinophils in Context
2. Eosinophils and Innate Immunity
2.1. Eosinophils during Helminth Infections
2.2. Eosinophils during Viral Infections
2.3. Eosinophils during Bacterial Infections
3. Eosinophils and T-Cell Immunity
3.1. Eosinophils and TH2 Immunity
3.2. Eosinophils and TH1/TH17
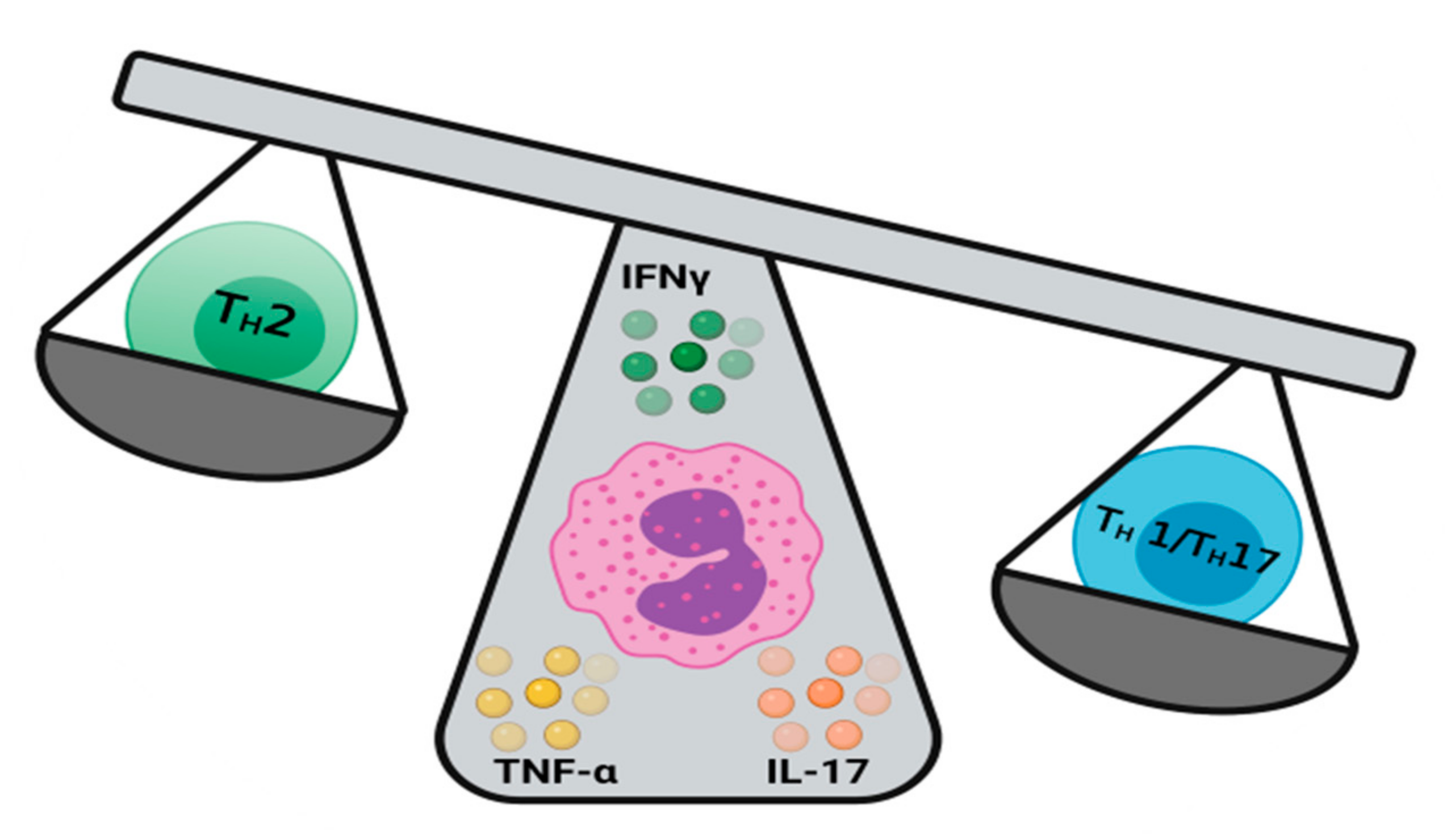
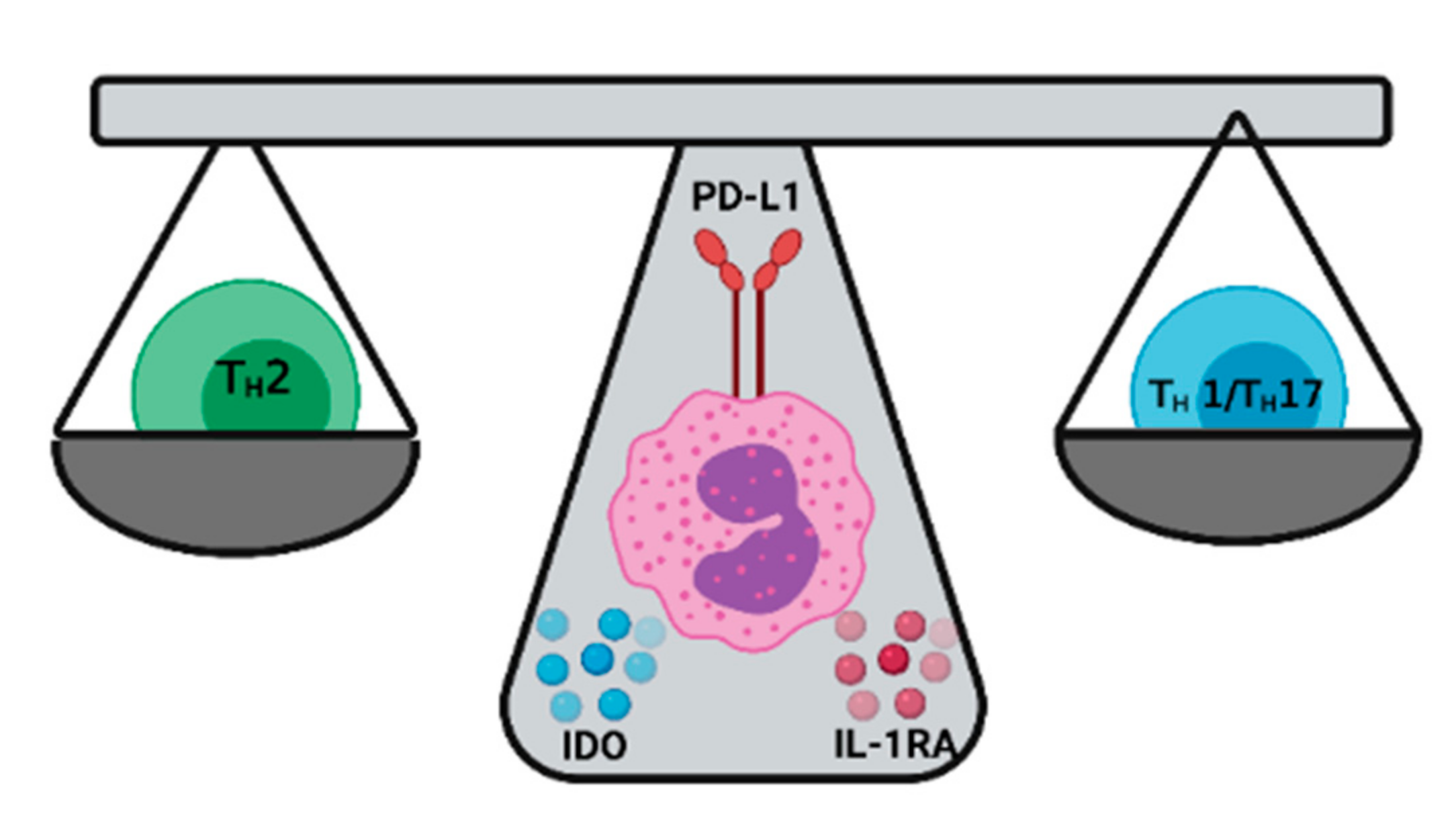
4. Eosinophils as Keepers of the TH1/TH2 Balance
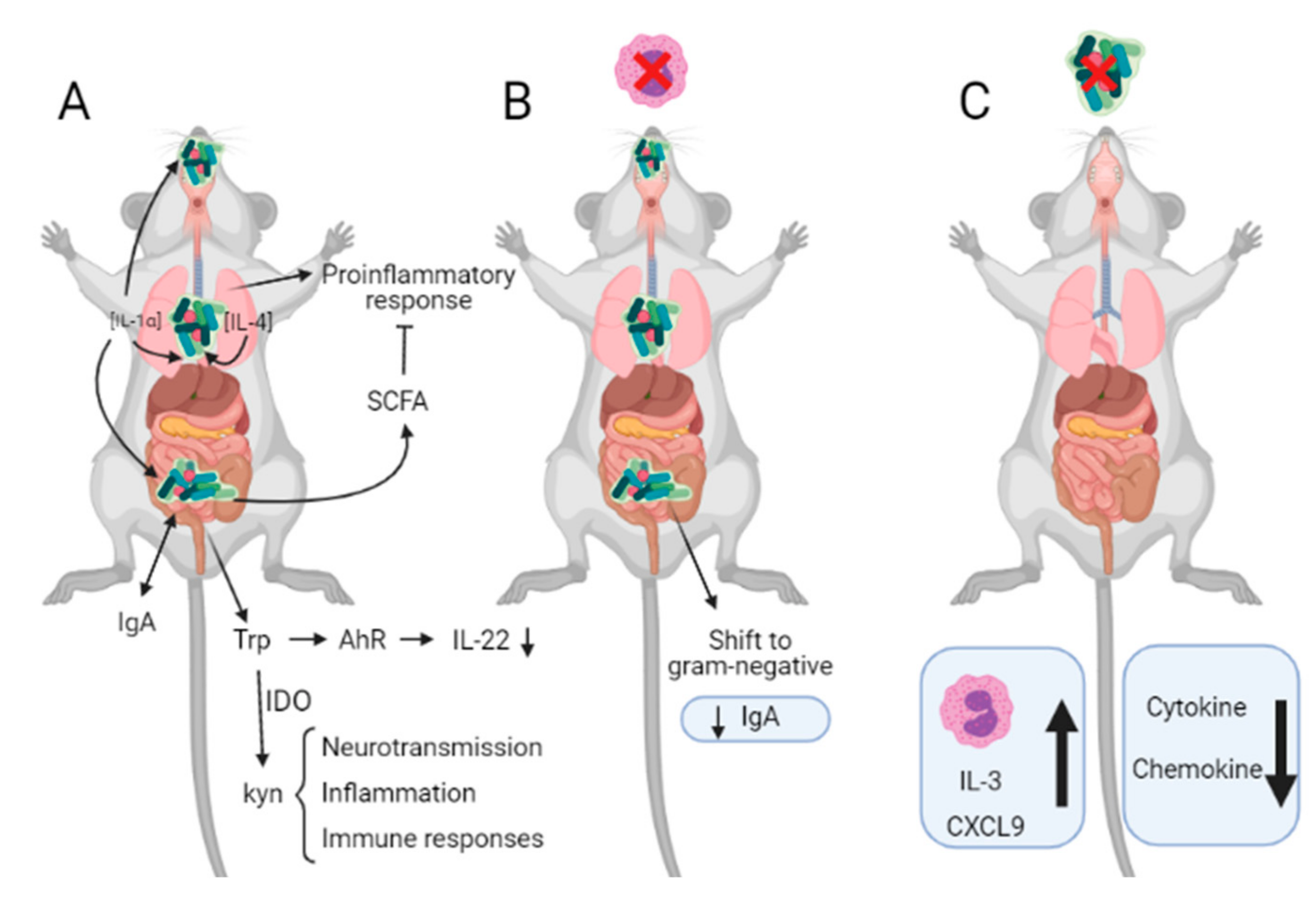
4.1. Eosinophils and Microbiota
4.1.1. Eosinophils and Gut Microbiota
4.1.2. Eosinophils and Lung Microbiota
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MBP | Major basic protein |
| EPX | Eosinophil peroxidase |
| ECP | Eosinophil cationic protein |
| EDN | Eosinophil derived neurotoxin |
| TLR | Toll-like receptor |
| CLC-P | Charcot-Leyden crystal protein |
| ILCs | Innate lymphoid cells |
| IDO | Indoleamine 2, 3-Dioxygenase |
| IFNγ | Interferon γ |
| PD-L1 | Programmed death-ligand 1 |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| IL-1Ra | IL-1 receptor antagonist |
| GF | Germ free |
| SCFA | Short-chain fatty acids |
| Kyn | Kynurenine |
| AhR | Aryl hydrocarbon receptor |
References
- Varga, S.M.; Sant, A.J. Editorial: Orchestration of an Immune Response to Respiratory Pathogens. Front. Immunol. 2019, 10, 690. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.; Tirouvanziam, R.; Laval, J.; Greene, C.M.; Habiel, D.; Sharma, L.; Yildirim, A.; Dela Cruz, C.S.; Hogaboam, C.M. Innate Immunity of the Lung: From Basic Mechanisms to Translational Medicine. J. Innate Immun. 2018, 10, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Montalban-Arques, A.; Chaparro, M.; Gisbert, J.P.; Bernardo, D. The Innate Immune System in the Gastrointestinal Tract: Role of Intraepithelial Lymphocytes and Lamina Propria Innate Lymphoid Cells in Intestinal Inflammation. Inflamm. Bowel Dis. 2018, 24, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Santaolalla, R.; Abreu, M.T. Innate immunity in the small intestine. Curr. Opin. Gastroenterol. 2012, 28, 124–129. [Google Scholar] [CrossRef]
- Shamri, R.; Xenakis, J.J.; Spencer, L.A. Eosinophils in innate immunity: An evolving story. Cell Tissue Res. 2011, 343, 57–83. [Google Scholar] [CrossRef]
- Jacobsen, E.A.; Jackson, D.J.; Heffler, E.; Mathur, S.K.; Bredenoord, A.J.; Pavord, I.D.; Akuthota, P.; Roufosse, F.; Rothenberg, M.E. Eosinophil Knockout Humans: Uncovering the Role of Eosinophils Through Eosinophil-Directed Biological Therapies. Annu. Rev. Immunol. 2021, 39, 719–757. [Google Scholar] [CrossRef]
- Williams, T.L.; Rada, B.; Tandon, E.; Gestal, M.C. NETs and EETs, a Whole Web of Mess. Microorganisms 2020, 8, 1925. [Google Scholar] [CrossRef]
- Jacobsen, E.A.; Helmers, R.A.; Lee, J.J.; Lee, N.A. The expanding role(s) of eosinophils in health and disease. Blood 2012, 120, 3882–3890. [Google Scholar] [CrossRef]
- Samarasinghe, A.E.; Melo, R.C.; Duan, S.; Le Messurier, K.S.; Liedmann, S.; Surman, S.L.; Lee, J.J.; Hurwitz, J.L.; Thomas, P.G.; McCullers, J.A. Eosinophils Promote Antiviral Immunity in Mice Infected with Influenza A Virus. J. Immunol. 2017, 198, 3214–3226. [Google Scholar] [CrossRef]
- Padigel, U.M.; Lee, J.J.; Nolan, T.J.; Schad, G.A.; Abraham, D. Eosinophils can function as antigen-presenting cells to induce primary and secondary immune responses to Strongyloides stercoralis. Infect. Immun. 2006, 74, 3232–3238. [Google Scholar] [CrossRef] [PubMed]
- Klion, A.D.; Nutman, T.B. The role of eosinophils in host defense against helminth parasites. J. Allergy Clin. Immunol. 2004, 113, 30–37. [Google Scholar] [CrossRef]
- Butterworth, A.E.; Wassom, D.L.; Gleich, G.J.; Loegering, D.A.; David, J.R. Damage to schistosomula of Schistosoma mansoni induced directly by eosinophil major basic protein. J. Immunol. 1979, 122, 221–229. [Google Scholar]
- Hamann, K.J.; Gleich, G.J.; Checkel, J.L.; Loegering, D.A.; McCall, J.W.; Barker, R.L. In vitro killing of microfilariae of Brugia pahangi and Brugia malayi by eosinophil granule proteins. J. Immunol. 1990, 144, 3166–3173. [Google Scholar]
- Martin, L.B.; Kita, H.; Leiferman, K.M.; Gleich, G.J. Eosinophils in allergy: Role in disease, degranulation, and cytokines. Int. Arch. Allergy Immunol. 1996, 109, 207–215. [Google Scholar] [CrossRef]
- Busse, W.W.; Sedgwick, J.B. Eosinophils in asthma. Ann. Allergy 1992, 68, 286–290. [Google Scholar]
- Nagata, M.; Nakagome, K.; Soma, T. Mechanisms of eosinophilic inflammation. Asia Pac. Allergy 2020, 10, e14. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.G.; Seoh, J.Y.; Jang, M.H. Regulatory Eosinophils in Inflammation and Metabolic Disorders. Immune Netw. 2017, 17, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Gleich, G.J. Mechanisms of eosinophil-associated inflammation. J. Allergy Clin. Immunol. 2000, 105, 651–663. [Google Scholar] [CrossRef]
- Rothenberg, M.E. Eosinophilic gastrointestinal disorders (EGID). J. Allergy Clin. Immunol. 2004, 113, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Spencer, L.A.; Szela, C.T.; Perez, S.A.; Kirchhoffer, C.L.; Neves, J.S.; Radke, A.L.; Weller, P.F. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J. Leukoc. Biol. 2009, 85, 117–123. [Google Scholar] [CrossRef]
- Flores-Torres, A.S.; Salinas-Carmona, M.C.; Salinas, E.; Rosas-Taraco, A.G. Eosinophils and Respiratory Viruses. Viral Immunol. 2019, 32, 198–207. [Google Scholar] [CrossRef]
- Arnold, I.C.; Artola-Borán, M.; de Lara, P.T.; Kyburz, A.; Taube, C.; Ottemann, K.; van den Broek, M.; Yousefi, S.; Simon, H.U.; Müller, A. Eosinophils suppress Th1 responses and restrict bacterially induced gastrointestinal inflammation. J. Exp. Med. 2018, 215, 2055–2072. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, A.; Gonzalez-Perez, I.; Arnold, I.C. Intestinal eosinophils, homeostasis and response to bacterial intrusion. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–12. [Google Scholar]
- Zhang, X.; Arnold, I.C.; Müller, A. Mechanisms of persistence, innate immune activation and immunomodulation by the gastric pathogen Helicobacter pylori. Curr. Opin. Microbiol. 2020, 54, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Anthony, R.M.; Rutitzky, L.I.; Urban, J.F.; Stadecker, M.J.; Gause, W.C. Protective immune mechanisms in helminth infection. Nat. Rev. Immunol. 2007, 7, 975–987. [Google Scholar] [CrossRef]
- Turner, J.D.; Faulkner, H.; Kamgno, J.; Cormont, F.; Van Snick, J.; Else, K.J.; Grencis, R.K.; Behnke, J.M.; Boussinesq, M.; Bradley, J.E. Th2 cytokines are associated with reduced worm burdens in a human intestinal helminth infection. J. Infect. Dis. 2003, 188, 1768–1775. [Google Scholar] [CrossRef]
- McKean, J.R.; Anwar, A.R.; Kay, A.B. Schistosoma mansoni: Complement and antibody damage, mediated by human eosinophils and neutrophils, in killing schistosomula in vitro. Exp. Parasitol. 1981, 51, 307–317. [Google Scholar] [CrossRef]
- Capron, M.; Torpier, G.; Capron, A. In vitro killing of S. mansoni schistosomula by eosinophils from infected rats: Role of cytophilic antibodies. J. Immunol. 1979, 123, 2220–2230. [Google Scholar]
- Maizels, R.M.; Holland, M.J. Parasite immunity: Pathways for expelling intestinal helminths. Curr. Biol. 1998, 8, R711–R714. [Google Scholar] [CrossRef]
- Bacharier, L.B.; Geha, R.S. Molecular mechanisms of IgE regulation. J. Allergy Clin. Immunol. 2000, 105 Pt 2, S547–S558. [Google Scholar] [CrossRef]
- Brunet, L.R.; Kopf, M.A.; Pearce, E.J. Schistosoma mansoni: IL-4 is necessary for concomitant immunity in mice. J. Parasitol. 1999, 85, 734–736. [Google Scholar] [CrossRef]
- Strandmark, J.; Steinfelder, S.; Berek, C.; Kühl, A.; Rausch, S.; Hartmann, S. Eosinophils are required to suppress Th2 responses in Peyer’s patches during intestinal infection by nematodes. Mucosal Immunol. 2017, 10, 661–672. [Google Scholar] [CrossRef]
- Meeusen, E.N.; Balic, A. Do eosinophils have a role in the killing of helminth parasites? Parasitol. Today 2000, 16, 95–101. [Google Scholar] [CrossRef]
- Gebreselassie, N.G.; Moorhead, A.R.; Fabre, V.; Gagliardo, L.F.; Lee, N.A.; Lee, J.J.; Appleton, J.A. Eosinophils preserve parasitic nematode larvae by regulating local immunity. J. Immunol. 2012, 188, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Fabre, V.; Beiting, D.P.; Bliss, S.K.; Gebreselassie, N.G.; Gagliardo, L.F.; Lee, N.A.; Lee, J.J.; Appleton, J.A. Eosinophil deficiency compromises parasite survival in chronic nematode infection. J. Immunol. 2009, 182, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Behm, C.; Ovington, K. The role of eosinophils in parasitic helminth infections: Insights from genetically modified mice. Parasitol. Today 2000, 16, 202–209. [Google Scholar] [CrossRef]
- Yasuda, K.; Kuroda, E. Role of eosinophils in protective immunity against secondary nematode infections. Immunol. Med. 2019, 42, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.H.; Lee, Y.A.; Min, D.Y. Eosinophil-mediated tissue inflammatory responses in helminth infection. Korean J. Parasitol. 2009, 47, S125–S131. [Google Scholar] [CrossRef]
- de Krömer, M.T.K.; la Garza, C.E.M.-D.; Brattig, N.W. Differences in eosinophil and neutrophil chemotactic responses in sowda and generalized form of onchocerciasis. Acta Trop. 1995, 60, 21–33. [Google Scholar] [CrossRef]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.N. Schistosomiasis. Nat. Rev. Dis. Primers 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Schaller, M.; Hogaboam, C.M.; Standiford, T.J.; Chensue, S.W.; Kunkel, S.L. TLR9 activation is a key event for the maintenance of a mycobacterial antigen-elicited pulmonary granulomatous response. Eur. J. Immunol. 2007, 37, 2847–2855. [Google Scholar] [CrossRef] [PubMed]
- Schramm, G.; Suwandi, A.; Galeev, A.; Sharma, S.; Braun, J.; Claes, A.K.; Braubach, P.; Grassl, G.A. Schistosome Eggs Impair Protective Th1/Th17 Immune Responses Against. Front. Immunol. 2018, 9, 2614. [Google Scholar] [CrossRef] [PubMed]
- Adamko, D.J.; Yost, B.L.; Gleich, G.J.; Fryer, A.D.; Jacoby, D.B. Ovalbumin sensitization changes the inflammatory response to subsequent parainfluenza infection. Eosinophils mediate airway hyperresponsiveness, m(2) muscarinic receptor dysfunction, and antiviral effects. J. Exp. Med. 1999, 190, 1465–1478. [Google Scholar] [CrossRef]
- Phipps, S.; Lam, C.E.; Mahalingam, S.; Newhouse, M.; Ramirez, R.; Rosenberg, H.F.; Foster, P.S.; Matthaei, K.I. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood 2007, 110, 1578–1586. [Google Scholar] [CrossRef]
- Mathur, S.K.; Fichtinger, P.S.; Kelly, J.T.; Lee, W.M.; Gern, J.E.; Jarjour, N.N. Interaction between allergy and innate immunity: Model for eosinophil regulation of epithelial cell interferon expression. Ann. Allergy Asthma Immunol. 2013, 111, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo-Muñoz, J.M.; Sastre, B.; Cañas, J.A.; Gil-Martínez, M.; Redondo, N.; Del Pozo, V. Eosinophil Response Against Classical and Emerging Respiratory Viruses: COVID-19. J. Investig. Allergol. Clin. Immunol. 2021, 31, 94–107. [Google Scholar] [CrossRef]
- Chonmaitree, T.; Patel, J.A.; Lett-Brown, M.A.; Uchida, T.; Garofalo, R.; Owen, M.J.; Howie, V.M. Virus and bacteria enhance histamine production in middle ear fluids of children with acute otitis media. J. Infect. Dis. 1994, 169, 1265–1270. [Google Scholar] [CrossRef]
- Graham, A.C.; Hilmer, K.M.; Zickovich, J.M.; Obar, J.J. Inflammatory response of mast cells during influenza A virus infection is mediated by active infection and RIG-I signaling. J. Immunol. 2013, 190, 4676–4684. [Google Scholar] [CrossRef] [PubMed]
- Piliponsky, A.M.; Pickholtz, D.; Gleich, G.J.; Levi-Schaffer, F. Human eosinophils induce histamine release from antigen-activated rat peritoneal mast cells: A possible role for mast cells in late-phase allergic reactions. J. Allergy Clin. Immunol. 2001, 107, 993–1000. [Google Scholar] [CrossRef]
- Lindsley, A.W.; Schwartz, J.T.; Rothenberg, M.E. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J. Allergy Clin. Immunol. 2020, 146, 1–7. [Google Scholar] [CrossRef]
- Ferastraoaru, D.; Hudes, G.; Jerschow, E.; Jariwala, S.; Karagic, M.; de Vos, G.; Rosenstreich, D.; Ramesh, M. Eosinophilia in Asthma Patients Is Protective Against Severe COVID-19 Illness. J. Allergy Clin. Immunol. Pract. 2021, 9, 1152–1162.e3. [Google Scholar] [CrossRef]
- Azkur, A.K.; Akdis, M.; Azkur, D.; Sokolowska, M.; van de Veen, W.; Brüggen, M.C.; O’Mahony, L.; Gao, Y.; Nadeau, K.; Akdis, C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020, 75, 1564–1581. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.; Pekkarinen, P.T.; Lakshmikanth, T.; Tan, Z.; Consiglio, C.R.; Pou, C.; Chen, Y.; Mugabo, C.H.; Nguyen, N.A.; Nowlan, K.; et al. Systems-Level Immunomonitoring from Acute to Recovery Phase of Severe COVID-19. Cell Rep. Med. 2020, 1, 100078. [Google Scholar] [CrossRef]
- Rosenberg, H.F.; Foster, P.S. Eosinophils and COVID-19: Diagnosis, prognosis, and vaccination strategies. In Seminars in immunopathology; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–10. [Google Scholar]
- Xie, G.; Ding, F.; Han, L.; Yin, D.; Lu, H.; Zhang, M. The role of peripheral blood eosinophil counts in COVID-19 patients. Allergy 2021, 76, 471–482. [Google Scholar] [CrossRef]
- Archer, G.T.; Hirsch, J.G. Isolation of Granules from Eosinophil Leucocytes and Study of Their Enzyme Content. J. Exp. Med. 1963, 118, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Harris, H. Role of chemotaxis in inflammation. Physiol. Rev. 1954, 34, 529–562. [Google Scholar] [CrossRef] [PubMed]
- Cline, M.J.; Hanifin, J.; Lehrer, R.I. Phagocytosis by human eosinophils. Blood 1968, 32, 922–934. [Google Scholar] [CrossRef]
- Persson, T.; Andersson, P.; Bodelsson, M.; Laurell, M.; Malm, J.; Egesten, A. Bactericidal activity of human eosinophilic granulocytes against Escherichia coli. Infect. Immun. 2001, 69, 3591–3596. [Google Scholar] [CrossRef]
- Lind, A. An eosinophilic response to an isolated staphylococcus aureus infection. J. Allergy 1961, 32, 283–287. [Google Scholar] [CrossRef]
- Hosoki, K.; Nakamura, A.; Nagao, M.; Hiraguchi, Y.; Tanida, H.; Tokuda, R.; Wada, H.; Nobori, T.; Suga, S.; Fujisawa, T. Staphylococcus aureus directly activates eosinophils via platelet-activating factor receptor. J. Leukoc. Biol. 2012, 92, 333–341. [Google Scholar] [CrossRef]
- De Chatelet, L.R.; Migler, R.A.; Shirley, P.S.; Muss, H.B.; Szejda, P.; Bass, D.A. Comparison of intracellular bactericidal activities of human neutrophils and eosinophils. Blood 1978, 52, 609–617. [Google Scholar] [CrossRef]
- Yazdanbakhsh, M.; Eckmann, C.M.; Bot, A.A.; Roos, D. Bactericidal action of eosinophils from normal human blood. Infect. Immun. 1986, 53, 192–198. [Google Scholar] [CrossRef]
- Mukherjee, M.; Lacy, P.; Ueki, S. Eosinophil extracellular traps and inflammatory pathologies—Untangling the web! Front. Immunol. 2018, 9, 2763. [Google Scholar] [CrossRef]
- Fukuchi, M.; Miyabe, Y.; Furutani, C.; Saga, T.; Moritoki, Y.; Yamada, T.; Weller, P.F.; Ueki, S. How to detect eosinophil ETosis (EETosis) and extracellular traps. Allergol. Int. 2021, 70, 19–29. [Google Scholar] [CrossRef]
- Yousefi, S.; Simon, D.; Simon, H.U. Eosinophil extracellular DNA traps: Molecular mechanisms and potential roles in disease. Curr. Opin. Immunol. 2012, 24, 736–739. [Google Scholar] [CrossRef]
- Yousefi, S.; Gold, J.A.; Andina, N.; Lee, J.J.; Kelly, A.M.; Kozlowski, E.; Schmid, I.; Straumann, A.; Reichenbach, J.; Gleich, G.J.; et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 2008, 14, 949–953. [Google Scholar] [CrossRef]
- Ueki, S.; Tokunaga, T.; Melo, R.C.N.; Saito, H.; Honda, K.; Fukuchi, M.; Konno, Y.; Takeda, M.; Yamamoto, Y.; Hirokawa, M.; et al. Charcot-Leyden crystal formation is closely associated with eosinophil extracellular trap cell death. Blood 2018, 132, 2183–2187. [Google Scholar] [CrossRef]
- Ueki, S.; Miyabe, Y.; Yamamoto, Y.; Fukuchi, M.; Hirokawa, M.; Spencer, L.A.; Weller, P.F. Charcot-Leyden Crystals in Eosinophilic Inflammation: Active Cytolysis Leads to Crystal Formation. Curr. Allergy Asthma Rep. 2019, 19, 1–9. [Google Scholar] [CrossRef]
- Raqib, R.; Moly, P.K.; Sarker, P.; Qadri, F.; Alam, N.H.; Mathan, M.; Andersson, J. Persistence of mucosal mast cells and eosinophils in Shigella-infected children. Infect. Immun. 2003, 71, 2684–2692. [Google Scholar] [CrossRef] [PubMed]
- Bass, D.A. Behavior of eosinophil leukocytes in acute inflammation. II. Eosinophil dynamics during acute inflammation. J. Clin. Investig. 1975, 56, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Abidi, K.; Khoudri, I.; Belayachi, J.; Madani, N.; Zekraoui, A.; Zeggwagh, A.A.; Abouqal, R. Eosinopenia is a reliable marker of sepsis on admission to medical intensive care units. Crit. Care 2008, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Soni, M. Evaluation of eosinopenia as a diagnostic and prognostic indicator in COVID-19 infection. Int. J. Lab. Hematol. 2020. [Google Scholar] [CrossRef]
- Xia, Z. Eosinopenia as an early diagnostic marker of COVID-19 at the time of the epidemic. EClinicalMedicine 2020, 23, 100398. [Google Scholar] [CrossRef] [PubMed]
- Davido, B.; Makhloufi, S.; Matt, M.; Calin, R.; Senard, O.; Perronne, C.; Dinh, A.; Salomon, J. Changes in eosinophil count during bacterial infection: Revisiting an old marker to assess the efficacy of antimicrobial therapy. Int. J. Infect. Dis. 2017, 61, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.P.; Waddell, A.; Fulkerson, P.C. Eosinophils in infection and intestinal immunity. Curr. Opin. Gastroenterol. 2013, 29, 7–14. [Google Scholar] [CrossRef]
- Krishack, P.A.; Louviere, T.J.; Decker, T.S.; Kuzel, T.G.; Greenberg, J.A.; Camacho, D.F.; Hrusch, C.L.; Sperling, A.I.; Verhoef, P.A. Protection against Staphylococcus aureus bacteremia–induced mortality depends on ILC2s and eosinophils. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Poznanski, S.M.; Mukherjee, M.; Zhao, N.; Huang, C.; Radford, K.; Ashkar, A.A.; Nair, P. Asthma exacerbations on benralizumab are largely non-eosinophilic. Allergy 2021, 76, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Ghassemian, A.; Park, J.J.; Tsoulis, M.W.; Kim, H. Targeting the IL-5 pathway in eosinophilic asthma: A comparison of mepolizumab to benralizumab in the reduction of peripheral eosinophil counts. Allergy Asthma Clin. Immunol. 2021, 17, 1–7. [Google Scholar] [CrossRef]
- Shah, S.C.; Tepler, A.; Peek, R.M., Jr.; Colombel, J.-F.; Hirano, I.; Narula, N. Association between Helicobacter pylori exposure and decreased odds of eosinophilic esophagitis—A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2019, 17, 2185–2198.e3. [Google Scholar] [CrossRef]
- Zuo, Z.T.; Ma, Y.; Sun, Y.; Bai, C.Q.; Ling, C.H.; Yuan, F.L. The Protective Effects of Helicobacter pylori Infection on Allergic Asthma. Int. Arch. Allergy Immunol. 2021, 182, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Darveaux, J.I.; Lemanske, R.F. Infection-related asthma. J. Allergy Clin. Immunol. Pract. 2014, 2, 658–663. [Google Scholar] [CrossRef]
- Busse, W.W.; Lemanske, R.F.; Gern, J.E. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet 2010, 376, 826–834. [Google Scholar] [CrossRef]
- Vogt, H.; Bråbäck, L.; Kling, A.M.; Grünewald, M.; Nilsson, L. Pertussis immunization in infancy and adolescent asthma medication. Pediatrics 2014, 134, 721–728. [Google Scholar] [CrossRef]
- Rubin, K.; Glazer, S. The pertussis hypothesis: Bordetella pertussis colonization in the etiology of asthma and diseases of allergic sensitization. Med. Hypotheses 2018, 120, 101–115. [Google Scholar] [CrossRef]
- Nilsson, L.; Kjellman, N.I.; Bjorksten, B. Allergic disease at the age of 7 years after pertussis vaccination in infancy: Results from the follow-up of a randomized controlled trial of 3 vaccines. Arch. Pediatr. Adolesc. Med. 2003, 157, 1184–1189. [Google Scholar] [CrossRef][Green Version]
- Gestal, M.C.; Blas-Machado, U.; Johnson, H.M.; Rubin, L.N.; Dewan, K.K.; Bryant, C.; Tiemeyer, M.; Harvill, E.T. Disrupting Bordetella Immunosuppression Reveals a Role for Eosinophils in Coordinating the Adaptive Immune Response in the Respiratory Tract. Microorganisms 2020, 8, 1808. [Google Scholar] [CrossRef]
- Berger, A. Th1 and Th2 responses: What are they? BMJ 2000, 321, 424. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K.; Tsai, M.; Starkl, P.; Marichal, T.; Galli, S.J. IgE and mast cells in host defense against parasites and venoms. Semin. Immunopathol. 2016, 38, 581–603. [Google Scholar] [CrossRef] [PubMed]
- Stone, K.D.; Prussin, C.; Metcalfe, D.D. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 2010, 125 (Suppl. 2), S73–S80. [Google Scholar] [CrossRef] [PubMed]
- Khader, S.A.; Gaffen, S.L.; Kolls, J.K. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal. Immunol. 2009, 2, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Li, Z.; Yang, X.O.; Chang, S.H.; Nurieva, R.; Wang, Y.H.; Wang, Y.; Hood, L.; Zhu, Z.; Tian, Q.; et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005, 6, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Miossec, P. IL-17 and Th17 cells in human inflammatory diseases. Microbes Infect. 2009, 11, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Blosse, A.; Peru, S.; Levy, M.; Marteyn, B.; Floch, P.; Sifré, E.; Giese, A.; Prochazkova-Carlotti, M.; Martin, L.A.; Dubus, P. APRIL-producing eosinophils are involved in gastric MALT lymphomagenesis induced by Helicobacter sp infection. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Davoine, F.; Lacy, P. Eosinophil cytokines, chemokines, and growth factors: Emerging roles in immunity. Front. Immunol. 2014, 5, 570. [Google Scholar] [CrossRef]
- Lacy, P.; Moqbel, R. Eosinophil cytokines. Chem. Immunol. 2000, 76, 134–155. [Google Scholar]
- Zheng, W.; Flavell, R.A. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 1997, 89, 587–596. [Google Scholar] [CrossRef]
- Zhu, J.; Guo, L.; Watson, C.J.; Hu-Li, J.; Paul, W.E. Stat6 is necessary and sufficient for IL-4′s role in Th2 differentiation and cell expansion. J. Immunol. 2001, 166, 7276–7281. [Google Scholar] [CrossRef] [PubMed]
- Gounni, A.S.; Lamkhioued, B.; Ochiai, K.; Tanaka, Y.; Delaporte, E.; Capron, A.; Kinet, J.P.; Capron, M. High-affinity IgE receptor on eosinophils is involved in defence against parasites. Nature 1994, 367, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Rajakulasingam, K.; Till, S.; Ying, S.; Humbert, M.; Barkans, J.; Sullivan, M.; Meng, Q.; Corrigan, C.J.; Bungre, J.; Grant, J.A.; et al. Increased expression of high affinity IgE (FcepsilonRI) receptor-alpha chain mRNA and protein-bearing eosinophils in human allergen-induced atopic asthma. Am. J. Respir. Crit. Care Med. 1998, 158, 233–240. [Google Scholar] [CrossRef]
- Carr, T.F.; Berdnikovs, S.; Simon, H.U.; Bochner, B.S.; Rosenwasser, L.J. Eosinophilic bioactivities in severe asthma. World Allergy Organ. J. 2016, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.Z. Eosinophils function as antigen—Presenting cells. J. Leukoc. Biol. 2004, 76, 520–527. [Google Scholar] [CrossRef]
- Shi, H.Z.; Humbles, A.; Gerard, C.; Jin, Z.; Weller, P.F. Lymph node trafficking and antigen presentation by endobronchial eosinophils. J. Clin. Investig. 2000, 105, 945–953. [Google Scholar] [CrossRef]
- Spencer, L.A.; Weller, P.F. Eosinophils and Th2 immunity: Contemporary insights. Immunol. Cell Biol. 2010, 88, 250–256. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef]
- He, S.H.; Zhang, H.Y.; Zeng, X.N.; Chen, D.; Yang, P.C. Mast cells and basophils are essential for allergies: Mechanisms of allergic inflammation and a proposed procedure for diagnosis. Acta Pharmacol. Sin. 2013, 34, 1270–1283. [Google Scholar] [CrossRef]
- Chapman, D.G.; Irvin, C.G. Mechanisms of airway hyper—Responsiveness in asthma: The past, present and yet to come. Clin. Exp. Allergy 2015, 45, 706–719. [Google Scholar] [CrossRef]
- Mishra, A.; Rothenberg, M.E. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology 2003, 125, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Mould, A.W.; Matthaei, K.I.; Young, I.G.; Foster, P.S. Relationship between interleukin-5 and eotaxin in regulating blood and tissue eosinophilia in mice. J. Clin. Investig. 1997, 99, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- McBrien, C.N.; Menzies-Gow, A. The Biology of Eosinophils and Their Role in Asthma. Front. Med. 2017, 4, 93. [Google Scholar] [CrossRef] [PubMed]
- Erjefält, J.S.; Persson, C.G. New aspects of degranulation and fates of airway mucosal eosinophils. Am. J. Respir. Crit. Care Med. 2000, 161, 2074–2085. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.B.; Phipps, S.; Robinson, D.S. A role for eosinophils in airway remodelling in asthma. Trends Immunol. 2004, 25, 477–482. [Google Scholar] [CrossRef]
- Woerly, G.; Roger, N.; Loiseau, S.; Dombrowicz, D.; Capron, A.; Capron, M. Expression of Cd28 and Cd86 by human eosinophils and role in the secretion of type 1 cytokines (interleukin 2 and interferon γ) Inhibition by Immunoglobulin a Complexes. J. Exp. Med. 1999, 190, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Woerly, G.; Roger, N.; Loiseau, S.; Capron, M. Expression of Th1 and Th2 immunoregulatory cytokines by human eosinophils. Int. Arch. Allergy Immunol. 1999, 118, 95–97. [Google Scholar] [CrossRef]
- Nakajima, H.; Gleich, G.J.; Kita, H. Constitutive production of IL-4 and IL-10 and stimulated production of IL-8 by normal peripheral blood eosinophils. J. Immunol. 1996, 156, 4859–4866. [Google Scholar] [PubMed]
- Liu, L.Y.; Bates, M.E.; Jarjour, N.N.; Busse, W.W.; Bertics, P.J.; Kelly, E.A. Generation of Th1 and Th2 chemokines by human eosinophils: Evidence for a critical role of TNF-α. J. Immunol. 2007, 179, 4840–4848. [Google Scholar] [CrossRef] [PubMed]
- Handzel, Z.T.; Busse, W.W.; Sedgwick, J.B.; Vrtis, R.; Lee, W.M.; Kelly, E.A.; Gern, J.E. Eosinophils bind rhinovirus and activate virus-specific T cells. J. Immunol. 1998, 160, 1279–1284. [Google Scholar]
- Lee, J.J.; Jacobsen, E.A.; McGarry, M.P.; Schleimer, R.P.; Lee, N.A. Eosinophils in health and disease: The LIAR hypothesis. Clin. Exp. Allergy 2010, 40, 563–575. [Google Scholar] [CrossRef]
- Rothenberg, M.E.; Hogan, S.P. The eosinophil. Annu. Rev. Immunol. 2006, 24, 147–174. [Google Scholar] [CrossRef]
- Wu, D.; Molofsky, A.B.; Liang, H.E.; Ricardo-Gonzalez, R.R.; Jouihan, H.A.; Bando, J.K.; Chawla, A.; Locksley, R.M. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 2011, 332, 243–247. [Google Scholar] [CrossRef]
- Li, J.; Shi, W.; Sun, H.; Ji, Y.; Chen, Y.; Guo, X.; Sheng, H.; Shu, J.; Zhou, L.; Cai, T.; et al. Activation of DR3 signaling causes loss of ILC3s and exacerbates intestinal inflammation. Nat. Commun. 2019, 10, 1–5. [Google Scholar] [CrossRef]
- Odemuyiwa, S.O.; Ghahary, A.; Li, Y.; Puttagunta, L.; Lee, J.E.; Musat-Marcu, S.; Ghahary, A.; Moqbel, R. Cutting edge: Human eosinophils regulate T cell subset selection through indoleamine 2, 3-dioxygenase. J. Immunol. 2004, 173, 5909–5913. [Google Scholar] [CrossRef]
- Jürgens, B.; Hainz, U.; Fuchs, D.; Felzmann, T.; Heitger, A. Interferon-γ–triggered indoleamine 2, 3-dioxygenase competence in human monocyte-derived dendritic cells induces regulatory activity in allogeneic T cells. Blood J. Am. Soc. Hematol. 2009, 114, 3235–3243. [Google Scholar] [CrossRef]
- Taylor, M.W.; Feng, G. Relationship between interferon—γ, indoleamine 2, 3-dioxygenase, and tryptophan catabolism. FASEB J. 1991, 5, 2516–2522. [Google Scholar] [CrossRef] [PubMed]
- Akdis, M.; Trautmann, A.; Klunker, S.; Daigle, I.; Kucuksezer, U.; Deglmann, W.; Disch, R.; Blaser, K.; Akdis, C. T helper (Th) 2 predominance in atopy is due to preferential Th1 memory/effector cell apoptosis. J. Allergy Clin. Immunol. 2003, 111, S130. [Google Scholar] [CrossRef]
- Taher, Y.A.; Piavaux, B.J.; Gras, R.; van Esch, B.C.; Hofman, G.A.; Bloksma, N.; Henricks, P.A.; van Oosterhout, A.J. Indoleamine 2, 3-dioxygenase–dependent tryptophan metabolites contribute to tolerance induction during allergen immunotherapy in a mouse model. J. Allergy Clin. Immunol. 2008, 121, 983–991.e2. [Google Scholar] [CrossRef]
- Xu, H.; Oriss, T.B.; Fei, M.; Henry, A.C.; Melgert, B.N.; Chen, L.; Mellor, A.L.; Munn, D.H.; Irvin, C.G.; Ray, P. Indoleamine 2, 3-dioxygenase in lung dendritic cells promotes Th2 responses and allergic inflammation. Proc. Natl. Acad. Sci. USA 2008, 105, 6690–6695. [Google Scholar] [CrossRef] [PubMed]
- Astigiano, S.; Morandi, B.; Costa, R.; Mastracci, L.; D’Agostino, A.; Ratto, G.B.; Melioli, G.; Frumento, G. Eosinophil granulocytes account for indoleamine 2, 3-dioxygenase-mediated immune escape in human non small cell lung cancer. Neoplasia 2005, 7, 390–396. [Google Scholar] [CrossRef]
- Cravetchi, O.V. The Role of Eosinophils in the Neonatal Murine Thymus; Expression of Indoleamine 2, 3-Dioxygenase. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2009. [Google Scholar]
- Tulic, M.K.; Sly, P.D.; Andrews, D.; Crook, M.; Davoine, F.; Odemuyiwa, S.O.; Charles, A.; Hodder, M.L.; Prescott, S.L.; Holt, P.G. Thymic indoleamine 2, 3-dioxygenase-positive eosinophils in young children: Potential role in maturation of the naive immune system. Am. J. Pathol. 2009, 175, 2043–2052. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Bell, A.; Ladomersky, E.; Lauing, K.L.; Bollu, L.; Sosman, J.A.; Zhang, B.; Wu, J.D.; Miller, S.D.; Meeks, J.J.; et al. Immunosuppressive IDO in Cancer: Mechanisms of Action, Animal Models, and Targeting Strategies. Front. Immunol. 2020, 11, 1185. [Google Scholar] [CrossRef]
- Meireson, A.; Devos, M.; Brochez, L. IDO Expression in Cancer: Different Compartment, Different Functionality? Front. Immunol. 2020, 11, 531491. [Google Scholar] [CrossRef]
- Boomer, J.S.; To, K.; Chang, K.C.; Takasu, O.; Osborne, D.F.; Walton, A.H.; Bricker, T.L.; Jarman, S.D.; Kreisel, D.; Krupnick, A.S. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 2011, 306, 2594–2605. [Google Scholar] [CrossRef]
- Patil, N.K.; Luan, L.; Bohannon, J.K.; Hernandez, A.; Guo, Y.; Sherwood, E.R. Frontline Science: Anti-PD-L1 protects against infection with common bacterial pathogens after burn injury. J. Leukoc. Biol. 2018, 103, 23–33. [Google Scholar] [CrossRef]
- Dinarello, C.A. The IL-1 family of cytokines and receptors in rheumatic diseases. Nat. Rev. Rheumatol. 2019, 15, 612–632. [Google Scholar] [CrossRef]
- Sugawara, R.; Lee, E.J.; Jang, M.S.; Jeun, E.J.; Hong, C.P.; Kim, J.H.; Park, A.; Yun, C.H.; Hong, S.W.; Kim, Y.M.; et al. Small intestinal eosinophils regulate Th17 cells by producing IL-1 receptor antagonist. J. Exp. Med. 2016, 213, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Newcomb, D.C.; Peebles, R.S. Th17-mediated inflammation in asthma. Curr. Opin. Immunol. 2013, 25, 755–760. [Google Scholar] [CrossRef]
- Ikeda, S.; Saijo, S.; Murayama, M.A.; Shimizu, K.; Akitsu, A.; Iwakura, Y. Excess IL-1 Signaling Enhances the Development of Th17 Cells by Downregulating TGF-β–Induced Foxp3 Expression. J. Immunol. 2014, 192, 1449–1458. [Google Scholar] [CrossRef]
- Esnault, S.; Kelly, E.A.; Nettenstrom, L.M.; Cook, E.B.; Seroogy, C.M.; Jarjour, N.N. Human eosinophils release IL-1ß and increase expression of IL-17A in activated CD 4+ T lymphocytes. Clin. Exp. Allergy 2012, 42, 1756–1764. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.F.; Wong, C.K.; Lam, C.W. Molecular mechanisms of cytokine and chemokine release from eosinophils activated by IL-17A, IL-17F, and IL-23: Implication for Th17 lymphocytes-mediated allergic inflammation. J. Immunol. 2008, 180, 5625–5635. [Google Scholar] [CrossRef]
- Hynes, G.M.; Hinks, T.S.C. The role of interleukin-17 in asthma: A protective response? ERJ Open Res. 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Brembilla, N.C.; Senra, L.; Boehncke, W.H. The IL-17 Family of Cytokines in Psoriasis: IL-17A and Beyond. Front. Immunol. 2018, 9, 1682. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Roh, J.Y.; Jung, Y. Eosinophils Accelerate Pathogenesis of Psoriasis by Supporting an Inflammatory Milieu that Promotes Neutrophil Infiltration. J. Investig. Dermatol. 2018, 138, 2185–2194. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, D.B.; Fagarasan, S. Gut reactions: Eosinophils add another string to their bow. Immunity 2014, 40, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, H.F.; Masterson, J.C.; Furuta, G.T. Eosinophils, probiotics, and the microbiome. J. Leukoc. Biol. 2016, 100, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Al-Haddad, S.; Riddell, R.H. The role of eosinophils in inflammatory bowel disease. Gut 2005, 54, 1674–1675. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Hogan, S.P.; Lee, J.J.; Foster, P.S.; Rothenberg, M.E. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J. Clin. Investig. 1999, 103, 1719–1727. [Google Scholar] [CrossRef]
- Jiménez-Saiz, R.; Anipindi, V.C.; Galipeau, H.; Ellenbogen, Y.; Chaudhary, R.; Koenig, J.F.; Gordon, M.E.; Walker, T.D.; Mandur, T.S.; Abed, S.; et al. Microbial Regulation of Enteric Eosinophils and Its Impact on Tissue Remodeling and Th2 Immunity. Front. Immunol. 2020, 11, 155. [Google Scholar] [CrossRef]
- Shah, K.; Ignacio, A.; Bernier-Latmani, J.; Koeller, Y.; Coakley, G.; Moyat, M.; Hamelin, R.; Armand, F.; Wong, N.C.; Remay, H. Small Intestinal Resident Eosinophils Maintain Gut Homeostasis Following Microbial Colonisation. bioRxiv 2021. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Chu, V.T.; Beller, A.; Rausch, S.; Strandmark, J.; Zänker, M.; Arbach, O.; Kruglov, A.; Berek, C. Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity 2014, 40, 582–593. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.U.; Yousefi, S.; Germic, N.; Arnold, I.C.; Haczku, A.; Karaulov, A.V.; Simon, D.; Rosenberg, H.F. The Cellular Functions of Eosinophils: Collegium Internationale Allergologicum (CIA) Update 2020. Int. Arch. Allergy Immunol. 2020, 181, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Wen, T.; Mingler, M.; Caldwell, J.; Wang, Y.; Chaplin, D.; Lee, E.; Jang, M.; Woo, S.; Seoh, J. IL-1β in eosinophil-mediated small intestinal homeostasis and IgA production. Mucosal Immunol. 2015, 8, 930–942. [Google Scholar] [CrossRef]
- Buonomo, E.L.; Cowardin, C.A.; Wilson, M.G.; Saleh, M.M.; Pramoonjago, P.; Petri, W.A. Microbiota-Regulated IL-25 Increases Eosinophil Number to Provide Protection during Clostridium difficile Infection. Cell Rep. 2016, 16, 432–443. [Google Scholar] [CrossRef]
- Singh, G.; Brass, A.; Knight, C.G.; Cruickshank, S.M. Gut eosinophils and their impact on the mucus—Resident microbiota. Immunology 2019, 158, 194–205. [Google Scholar] [CrossRef]
- Barcik, W.; Boutin, R.C.T.; Sokolowska, M.; Finlay, B.B. The Role of Lung and Gut Microbiota in the Pathology of Asthma. Immunity 2020, 52, 241–255. [Google Scholar] [CrossRef]
- Huffnagle, G.B.; Dickson, R.P.; Lukacs, N.W. The respiratory tract microbiome and lung inflammation: A two-way street. Mucosal Immunol. 2017, 10, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.P.; Erb-Downward, J.R.; Falkowski, N.R.; Hunter, E.M.; Ashley, S.L.; Huffnagle, G.B. The Lung Microbiota of Healthy Mice Are Highly Variable, Cluster by Environment, and Reflect Variation in Baseline Lung Innate Immunity. Am. J. Respir. Crit. Care Med. 2018, 198, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Mesnil, C.; Raulier, S.; Paulissen, G.; Xiao, X.; Birrell, M.A.; Pirottin, D.; Janss, T.; Starkl, P.; Ramery, E.; Henket, M.; et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J. Clin. Investig. 2016, 126, 3279–3295. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.I.; Vijverberg, S.J.H.; Neerincx, A.H.; Kraneveld, A.D.; Maitland-van der Zee, A.H. The crosstalk between microbiome and asthma: Exploring associations and challenges. Clin. Exp. Allergy 2019, 49, 1067–1086. [Google Scholar] [CrossRef]
- Rosenberg, H.F.; Dyer, K.D.; Foster, P.S. Eosinophils: Changing perspectives in health and disease. Nat. Rev. Immunol. 2013, 13, 9–22. [Google Scholar] [CrossRef]
- Lee, J.J.; Rosenberg, H.F. Eosinophils in Health and Disease; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Klion, A.D.; Ackerman, S.J.; Bochner, B.S. Contributions of Eosinophils to Human Health and Disease. Annu. Rev. Pathol. 2020, 15, 179–209. [Google Scholar] [CrossRef]
- Hogan, S.P.; Rosenberg, H.F.; Moqbel, R.; Phipps, S.; Foster, P.S.; Lacy, P.; Kay, A.B.; Rothenberg, M.E. Eosinophils: Biological properties and role in health and disease. Clin. Exp. Allergy 2008, 38, 709–750. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ondari, E.; Calvino-Sanles, E.; First, N.J.; Gestal, M.C. Eosinophils and Bacteria, the Beginning of a Story. Int. J. Mol. Sci. 2021, 22, 8004. https://doi.org/10.3390/ijms22158004
Ondari E, Calvino-Sanles E, First NJ, Gestal MC. Eosinophils and Bacteria, the Beginning of a Story. International Journal of Molecular Sciences. 2021; 22(15):8004. https://doi.org/10.3390/ijms22158004
Chicago/Turabian StyleOndari, Edna, Esther Calvino-Sanles, Nicholas J. First, and Monica C. Gestal. 2021. "Eosinophils and Bacteria, the Beginning of a Story" International Journal of Molecular Sciences 22, no. 15: 8004. https://doi.org/10.3390/ijms22158004
APA StyleOndari, E., Calvino-Sanles, E., First, N. J., & Gestal, M. C. (2021). Eosinophils and Bacteria, the Beginning of a Story. International Journal of Molecular Sciences, 22(15), 8004. https://doi.org/10.3390/ijms22158004






