Micronutrient Food Supplements in Patients with Gastro-Intestinal and Hepatic Cancers
Abstract
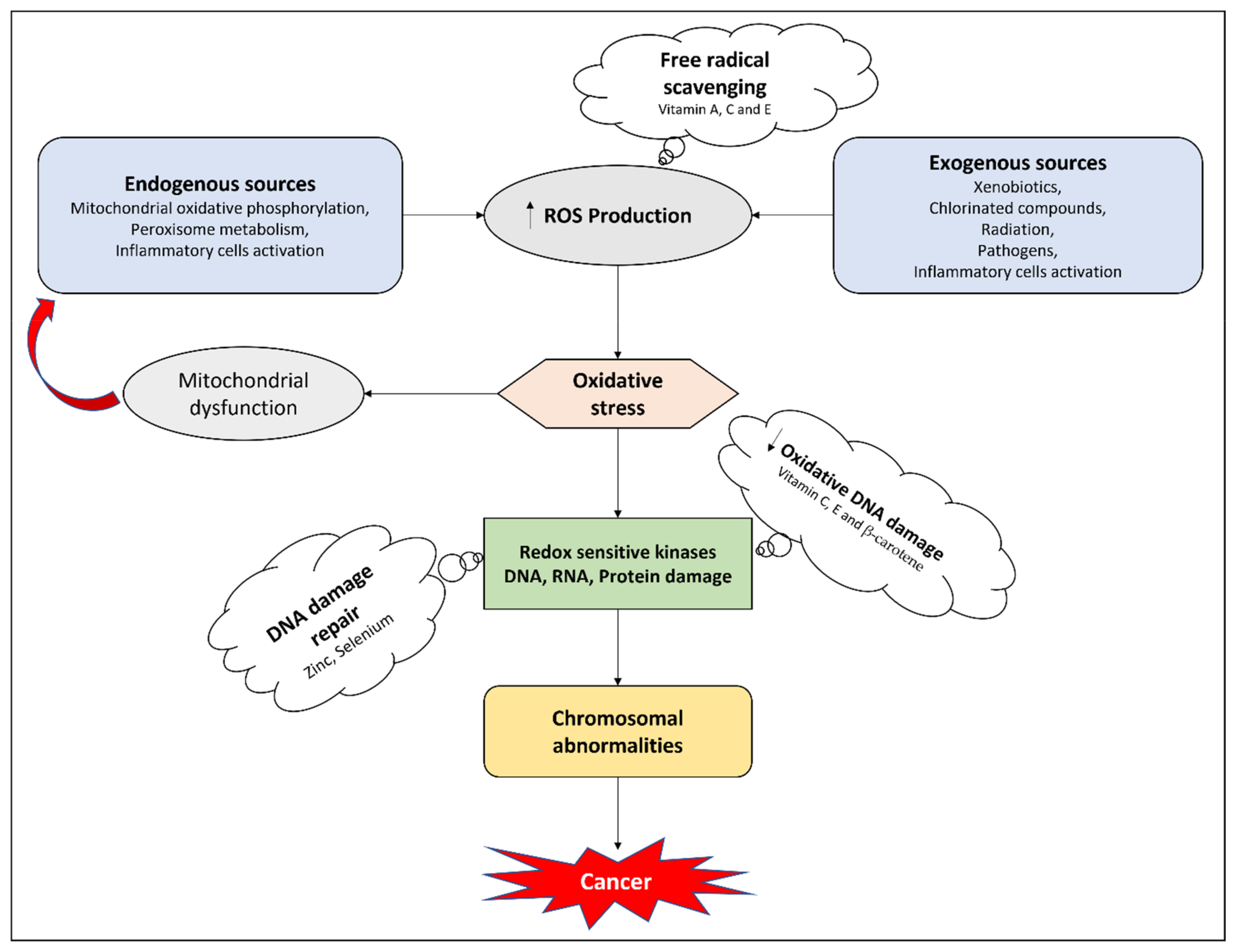
:1. Introduction
2. Micronutrients and Cancer: What We Need to Know?
3. A Possible Link of Micronutrients with GI and Hepatic Cancers
3.1. Vitamin D
3.2. Antioxidant Vitamins
3.2.1. Vitamin A
3.2.2. Vitamin E
3.2.3. Vitamin C
3.3. Zinc
3.4. Selenium
4. Molecular Mechanisms of Micronutrients
4.1. Antioxidant Effects
4.2. Apoptosis Targeting
4.3. Anti-Proliferative Mechanisms
4.4. Anti-Angiogenic Effects
5. Micronutrients and Cancer Therapy Related Side Effects
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 12 February 2021).
- Wild, C.; Weiderpass, E.; Stewart, B. World Cancer Report: Cancer Research for Cancer Prevention; IARC: Lyon, France, 2020; pp. 23–33. [Google Scholar]
- O’Connor, A.; McNamara, D.; O’Moráin, C.A. Surveillance of gastric intestinal metaplasia for the prevention of gastric cancer. Cochrane Database Syst. Rev. 2013, 9, CD009322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacFarlane, A.J.; Stover, P.J. Convergence of genetic, nutritional and inflammatory factors in gastrointestinal cancers. Nutr. Rev. 2007, 65, S157–S166. [Google Scholar] [CrossRef]
- Jayasekara, H.; English, D.R.; Haydon, A.; Hodge, A.M.; Lynch, B.M.; Rosty, C.; Williamson, E.J.; Clendenning, M.; Southey, M.C.; Jenkins, M.A. Associations of alcohol intake, smoking, physical activity and obesity with survival following colorectal cancer diagnosis by stage, anatomic site and tumor molecular subtype. Int. J. Cancer 2018, 142, 238–250. [Google Scholar] [CrossRef]
- Tuan, J.; Chen, Y.-X. Dietary and lifestyle factors associated with colorectal cancer risk and interactions with microbiota: Fiber, red or processed meat and alcoholic drinks. Gastrointest. Tumors 2016, 3, 17–24. [Google Scholar] [CrossRef]
- Landy, J.; Ronde, E.; English, N.; Clark, S.K.; Hart, A.L.; Knight, S.C.; Ciclitira, P.J.; Al-Hassi, H.O. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J. Gastroenterol. 2016, 22, 3117. [Google Scholar] [CrossRef]
- Alter, M.J. Epidemiology of hepatitis C virus infection. World J. Gastroenterol. 2007, 13, 2436. [Google Scholar] [CrossRef] [Green Version]
- Marengo, A.; Rosso, C.; Bugianesi, E. Liver cancer: Connections with obesity, fatty liver, and cirrhosis. Annu. Rev. Med. 2016, 67, 103–117. [Google Scholar] [CrossRef]
- White, D.L.; Kanwal, F.; El–Serag, H.B. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin. Gastroenterol. Hepatol. 2012, 10, 1342–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El–Serag, H.B.; Hampel, H.; Javadi, F. The association between diabetes and hepatocellular carcinoma: A systematic review of epidemiologic evidence. Clin. Gastroenterol. Hepatol. 2006, 4, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.E.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids nanoparticles in cancer: Treatment, prevention and clinical prospects. Semin. Cancer Biol. 2021, 69, 200–211. [Google Scholar] [CrossRef]
- Khan, H.; Reale, M.; Ullah, H.; Sureda, A.; Tejada, S.; Wang, Y.; Zhang, Z.-J.; Xiao, J. Anti-cancer effects of polyphenols via targeting p53 signaling pathway: Updates and future directions. Biotechnol. Adv. 2020, 38, 107385. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, V.; Perry, M.C. Classical chemotherapy: Mechanisms, toxicities and the therapeutic window. Cancer Biol. Ther. 2003, 2, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Gibson, R.J.; Keefe, D.M. Cancer chemotherapy-induced diarrhoea and constipation: Mechanisms of damage and prevention strategies. Support Care Cancer 2006, 14, 890–900. [Google Scholar] [CrossRef]
- Davis, M.P.; Hallerberg, G. A systematic review of the treatment of nausea and/or vomiting in cancer unrelated to chemotherapy or radiation. J. Pain Symptom Manag. 2010, 39, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Can, G.; Demir, M.; Erol, O.; Aydiner, A. A comparison of men and women’s experiences of chemotherapy-induced alopecia. Eur. J. Oncol. Nurs. 2013, 17, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Morrison, V.A. Immunosuppression associated with novel chemotherapy agents and monoclonal antibodies. Clin. Infect. Dis. 2014, 59, S360–S364. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Chen, B.; Hong, S.; Guo, Y. Acupuncture therapy for the treatment of myelosuppression after chemotherapy: A literature review over the past 10 years. J. Acupunct. Meridian Stud. 2015, 8, 122–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodgers, G.M.; Becker, P.S.; Blinder, M.; Cella, D.; Chanan-Khan, A.; Cleeland, C.; Coccia, P.F.; Djulbegovic, B.; Gilreath, J.A.; Kraut, E.H. Cancer-and chemotherapy-induced anemia. J. Natl. Compr. Cancer Netw. 2012, 10, 628–653. [Google Scholar] [CrossRef] [Green Version]
- Nesher, L.; Rolston, K.V. Neutropenic enterocolitis, a growing concern in the era of widespread use of aggressive chemotherapy. Clin. Infect. Dis. 2013, 56, 711–717. [Google Scholar] [CrossRef] [Green Version]
- Lorenzi, E.; Simonelli, M.; Santoro, A. Infertility risk and teratogenicity of molecularly targeted anticancer therapy: A challenging issue. Crit. Rev. Oncol. Hematol. 2016, 107, 1–13. [Google Scholar] [CrossRef]
- Martin, A.; Schneiderman, J.; Helenowski, I.B.; Morgan, E.; Dilley, K.; Danner-Koptik, K.; Hatahet, M.; Shimada, H.; Cohn, S.L.; Kletzel, M. Secondary malignant neoplasms after high-dose chemotherapy and autologous stem cell rescue for high-risk neuroblastoma. Pediatr. Blood Cancer 2014, 61, 1350–1356. [Google Scholar] [CrossRef]
- Carrier, X.; Gaur, S.; Philipovskiy, A. Tumor lysis syndrome after a single dose of atezolizumab with Nab-Paclitaxel: A case report and review of literature. Am. J. Med. Case Rep. 2020, 21, e925248-1. [Google Scholar]
- Liu, Z.; Huang, P.; Law, S.; Tian, H.; Leung, W.; Xu, C. Preventive effect of curcumin against chemotherapy-induced side-effects. Front. Pharmacol. 2018, 9, 1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, Y.; Wang, K.; Sheng, S.; Cui, J. Polydatin ameliorates chemotherapy-induced cognitive impairment (chemobrain) by inhibiting oxidative stress, inflammatory response, and apoptosis in rats. Biosci. Biotechnol. Biochem. 2020, 84, 1201–1210. [Google Scholar] [CrossRef]
- Waghray, D.; Zhang, Q. Inhibit or evade multidrug resistance p-glycoprotein in cancer treatment: Miniperspective. J. Med. Chem. 2017, 61, 5108–5121. [Google Scholar] [CrossRef]
- Luqmani, Y. Mechanisms of drug resistance in cancer chemotherapy. Med. Princ. Pract. 2005, 14, 35–48. [Google Scholar] [CrossRef]
- Saam, J.; Critchfield, G.C.; Hamilton, S.A.; Roa, B.B.; Wenstrup, R.J.; Kaldate, R.R. Body surface area–based dosing of 5-fluoruracil results in extensive interindividual variability in 5-fluorouracil exposure in colorectal cancer patients on FOLFOX regimens. Clin. Colorectal Cancer 2011, 10, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Gamelin, E.; Delva, R.; Jacob, J.; Merrouche, Y.; Raoul, J.L.; Pezet, D.; Dorval, E.; Piot, G.; Morel, A.; BoisdronCelle, M. Individual fluorouracil dose adjustment based on pharmacokinetic follow-up compared with conventional dosage: Results of a multicenter randomized trial of patients with metastatic colorectal cancer. J. Clin. Oncol. 2013, 31, 3612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoeft, B.; Weber, P.; Eggersdorfer, M. Micronutrients—A global perspective on intake, health benefits and economics. Int. J. Vitam. Nutr. Res. 2012, 82, 316–320. [Google Scholar] [CrossRef] [Green Version]
- Godswill, A.G.; Somtochukwu, I.V.; Ikechukwu, A.O.; Kate, E.C. Health benefits of micronutrients (vitamins and minerals) and their Associated Deficiency Diseases: A systematic review. Int. J. Food Sci. 2020, 3, 1–32. [Google Scholar]
- Gröber, U.; Holzhauer, P.; Kisters, K.; Holick, M.F.; Adamietz, I.A. Micronutrients in oncological intervention. Nutrients 2016, 8, 163. [Google Scholar] [CrossRef] [Green Version]
- Micke, O.; Bruns, F.; Glatzel, M.; Schönekaes, K.; Micke, P.; Mücke, R.; Büntzel, J. Predictive factors for the use of complementary and alternative medicine (CAM) in radiation oncology. Eur. J. Integr. Med. 2009, 1, 19–25. [Google Scholar] [CrossRef]
- Zirpoli, G.R.; Brennan, P.M.; Hong, C.-C.; McCann, S.E.; Ciupak, G.; Davis, W.; Unger, J.M.; Budd, G.T.; Hershman, D.L.; Moore, H.C. Supplement use during an intergroup clinical trial for breast cancer (S0221). Breast Cancer Res. Treat. 2013, 137, 903–913. [Google Scholar] [CrossRef] [Green Version]
- D’Andrea, G.M. Use of antioxidants during chemotherapy and radiotherapy should be avoided. Cancer J. Clin. 2005, 55, 319–321. [Google Scholar] [CrossRef]
- Lawenda, B.D.; Kelly, K.M.; Ladas, E.J.; Sagar, S.M.; Vickers, A.; Blumberg, J.B. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J. Natl. Cancer Inst. 2008, 100, 773–783. [Google Scholar] [CrossRef] [Green Version]
- Yasueda, A.; Urushima, H.; Ito, T. Efficacy and interaction of antioxidant supplements as adjuvant therapy in cancer treatment: A systematic review. Integr. Cancer Ther. 2016, 15, 17–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Block, K.I.; Koch, A.C.; Mead, M.N.; Tothy, P.K.; Newman, R.A.; Gyllenhaal, C. Impact of antioxidant supplementation on chemotherapeutic toxicity: A systematic review of the evidence from randomized controlled trials. Int. J. cancer 2008, 123, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Nechuta, S.; Lu, W.; Chen, Z.; Zheng, Y.; Gu, K.; Cai, H.; Zheng, W.; Shu, X.O. Vitamin supplement use during breast cancer treatment and survival: A prospective cohort study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 262–271. [Google Scholar] [CrossRef] [Green Version]
- Gröber, U.; Kisters, K.; Adamietz, I.A. Vitamin D in oncology: Update 2015. Med. Monatsschr. Pharm. 2015, 38, 512–516. [Google Scholar] [PubMed]
- Russell, S.T.; Tisdale, M.J. The role of glucocorticoids in the induction of zinc-α 2-glycoprotein expression in adipose tissue in cancer cachexia. Br. J. cancer 2005, 92, 876–881. [Google Scholar] [CrossRef]
- Büntzel, J.; Bruns, F.; Glatzel, M.; Garayev, A.; Mücke, R.; Kisters, K.; Schäfer, U.; Schönekaes, K.; Micke, O. Zinc concentrations in serum during head and neck cancer progression. Anticancer Res. 2007, 27, 1941–1943. [Google Scholar] [PubMed]
- Churilla, T.M.; Brereton, H.D.; Klem, M.; Peters, C.A. Vitamin D deficiency is widespread in cancer patients and correlates with advanced stage disease: A community oncology experience. Nutr. Cancer 2012, 64, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Cruciani, R.; Dvorkin, E.; Homel, P.; Culliney, B.; Malamud, S.; Shaiova, L.; Fleishman, S.; Lapin, J.; Klein, E.; Lesage, P. L-carnitine supplementation for the treatment of fatigue and depressed mood in cancer patients with carnitine deficiency: A preliminary analysis. Ann. N. Y. Acad. Sci. 2004, 1033, 168–176. [Google Scholar] [CrossRef]
- Mayland, C.R.; Bennett, M.I.; Allan, K. Vitamin C deficiency in cancer patients. Palliat. Med. 2005, 19, 17–20. [Google Scholar] [CrossRef]
- Babaknejad, N.; Sayehmiri, F.; Sayehmiri, K.; Rahimifar, P.; Bahrami, S.; Delpesheh, A.; Hemati, F.; Alizadeh, S. The relationship between selenium levels and breast cancer: A systematic review and meta-analysis. Biol. Trace Elem. Res. 2014, 159, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, S.S.; Ghone, R.A.; Suryakar, A.N.; Hundekar, P.S. Lipid peroxidation and antioxidant vitamin status in colorectal cancer patients. Indian J. Physiol. Pharmacol. 2011, 55, 72–76. [Google Scholar]
- Lin, C.-C.; Yin, M.-C. B vitamins deficiency and decreased anti-oxidative state in patients with liver cancer. Eur. J. Nutr. 2007, 46, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, M. Cutaneous bleeding related to zinc deficiency in two cases of advanced cancer. Cancer 1999, 86, 866–870. [Google Scholar] [CrossRef]
- Jatoi, A.; Williams, B.; Nichols, F.; Marks, R.; Aubry, M.-C.; Wampfler, J.; Finke, E.E.; Yang, P. Is voluntary vitamin and mineral supplementation associated with better outcome in non-small cell lung cancer patients?: Results from the Mayo Clinic lung cancer cohort. Lung Cancer 2005, 49, 77–84. [Google Scholar] [CrossRef]
- Sieja, K.; Talerczyk, M. Selenium as an element in the treatment of ovarian cancer in women receiving chemotherapy. Gynecol. Oncol. 2004, 93, 320–327. [Google Scholar] [CrossRef]
- Pathak, A.K.; Bhutani, M.; Guleria, R.; Bal, S.; Mohan, A.; Mohanti, B.K.; Sharma, A.; Pathak, R.; Bhardwaj, N.K.; Prasad, K.N. Chemotherapy alone vs. chemotherapy plus high dose multiple antioxidants in patients with advanced non small cell lung cancer. J. Am. Coll. Nutr. 2005, 24, 16–21. [Google Scholar] [CrossRef]
- Prasad, K.N. Multiple dietary antioxidants enhance the efficacy of standard and experimental cancer therapies and decrease their toxicity. Integr. Cancer Ther. 2004, 3, 310–322. [Google Scholar] [CrossRef] [Green Version]
- Ströhle, A.; Zänker, K.; Hahn, A. Nutrition in oncology: The case of micronutrients. Oncol. Rep. 2010, 24, 815–828. [Google Scholar] [CrossRef] [Green Version]
- Norman, H.A.; Butrum, R.R.; Feldman, E.; Heber, D.; Nixon, D.; Picciano, M.F.; Rivlin, R.; Simopoulos, A.; Wargovich, M.J.; Weisburger, E.K. The role of dietary supplements during cancer therapy. J. Nutr. 2003, 133, 3794S–3799S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M. Nutrition and physical activity guidelines for cancer survivors. Cancer J. Clin. 2012, 62, 242–274. [Google Scholar] [CrossRef] [Green Version]
- Marmot, M.; Atinmo, T.; Byers, T.; Chen, J.; Hirohata, T.; Jackson, A.; James, W.; Kolonel, L.; Kumanyika, S.; Leitzmann, C. Food, Nutrition, Physical activity, and the Prevention of Cancer: A Global Perspective; World Cancer Research Fund/American Institute for Cancer Research: Washington, DC, USA, 2007; pp. 4–29. [Google Scholar]
- Ja Kim, H.; Lee, S.S.; Choi, B.Y.; Kim, M.K. Nitrate intake relative to antioxidant vitamin intake affects gastric cancer risk: A case-control study in Korea. Nutr. Cancer 2007, 59, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Donma, O.; Donma, M.M.; Sonmez, S. Metal speciation, phytochemicals and Helicobacter pylori infection. Med. Hypotheses 2006, 67, 545–549. [Google Scholar] [CrossRef]
- Abreu, M.T.; Peek, R.M., Jr. Gastrointestinal malignancy and the microbiome. Gastroenterology 2014, 146, 1534–1546.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noto, J.M.; Peek, R.M., Jr. Micronutrients: A double-edged sword in microbe-induced gastric carcinogenesis. Trends Cancer 2015, 1, 136–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenab, M.; Riboli, E.; Ferrari, P.; Sabate, J.; Slimani, N.; Norat, T.; Friesen, M.; Tjønneland, A.; Olsen, A.; Overvad, K. Plasma and dietary vitamin C levels and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST). Carcinogenesis 2006, 27, 2250–2257. [Google Scholar] [CrossRef] [Green Version]
- Jenab, M.; Riboli, E.; Ferrari, P.; Friesen, M.; Sabate, J.; Norat, T.; Slimani, N.; Tjønneland, A.; Olsen, A.; Overvad, K. Plasma and dietary carotenoid, retinol and tocopherol levels and the risk of gastric adenocarcinomas in the European prospective investigation into cancer and nutrition. Br. J. Cancer 2006, 95, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Bergkvist, L.; Näslund, I.; Rutegård, J.; Wolk, A. Vitamin A, retinol, and carotenoids and the risk of gastric cancer: A prospective cohort study. Am. J. Clin. Nutr. 2007, 85, 497–503. [Google Scholar] [CrossRef]
- Pelucchi, C.; Tramacere, I.; Bertuccio, P.; Tavani, A.; Negri, E.; La Vecchia, C. Dietary intake of selected micronutrients and gastric cancer risk: An Italian case-control study. Ann. Oncol. 2009, 20, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Lazarević, K.; Nagorni, A.; Bogdanović, D.; Rančić, N.; Stošić, L.; Milutinović, S. Dietary micronutrients and gastric cancer: Hospital based study. Cent. Eur. J. Med. 2011, 6, 783–787. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Zhu, Y.; Wang, P.P.; Roebothan, B.; Zhao, J.; Zhao, J.; Dicks, E.; Cotterchio, M.; Buehler, S.; Campbell, P.T. Reported intake of selected micronutrients and risk of colorectal cancer: Results from a large population-based case–control study in Newfoundland, Labrador and Ontario, Canada. Anticancer Res. 2012, 32, 687–696. [Google Scholar] [PubMed]
- Da Costa, P.M.; Martins, I.; Neves, J.; Cortez-Pinto, H.; Velosa, J. Serum vitamin D levels correlate with the presence and histological grading of colorectal adenomas in peri and postmenopausal women. Clin. Nutr. 2019, 38, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Yaprak, G.; Gemici, C.; Temizkan, S.; Ozdemir, S.; Dogan, B.C.; Seseogullari, O.O. Osteoporosis development and vertebral fractures after abdominal irradiation in patients with gastric cancer. BMC Cancer 2018, 18, 1–6. [Google Scholar] [CrossRef]
- McCullough, M.L.; Zoltick, E.S.; Weinstein, S.J.; Fedirko, V.; Wang, M.; Cook, N.R.; Eliassen, A.H.; Zeleniuch-Jacquotte, A.; Agnoli, C.; Albanes, D. Circulating vitamin D and colorectal cancer risk: An international pooling project of 17 cohorts. J. Natl. Cancer Inst. 2019, 111, 158–169. [Google Scholar] [CrossRef]
- Sacco, R.; Conte, C.; Marceglia, S.; Mismas, V.; Bresci, G.; Romano, A.; Eggenhoffner, R.; Giacomelli, L. Beneficial and detrimental effects of natural dietary products on the risk of hepatocellular carcinoma, and their roles in its management. Hepatoma Res. 2016, 2, 53–61. [Google Scholar] [CrossRef]
- Kozeniecki, M.; Ludke, R.; Kerner, J.; Patterson, B. Micronutrients in liver disease: Roles, risk factors for deficiency, and recommendations for supplementation. Nutr. Clin. Pract. 2020, 35, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Schütte, K.; Schulz, C.; Malfertheiner, P. Nutrition and hepatocellular cancer. Gastrointest. Tumors 2015, 2, 188–194. [Google Scholar] [CrossRef]
- Fedirko, V.; Duarte-Salles, T.; Bamia, C.; Trichopoulou, A.; Aleksandrova, K.; Trichopoulos, D.; Trepo, E.; Tjønneland, A.; Olsen, A.; Overvad, K. Prediagnostic circulating vitamin D levels and risk of hepatocellular carcinoma in European populations: A nested case-control study. Hepatology 2014, 60, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Grüngreiff, K.; Reinhold, D.; Wedemeyer, H. The role of zinc in liver cirrhosis. Ann. Hepatol. 2016, 15, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Ebara, M.; Fukuda, H.; Hatano, R.; Saisho, H.; Nagato, Y.; Suzuki, K.; Nakajima, K.; Yukawa, M.; Kondo, F.; Nakayama, A. Relationship between copper, zinc and metallothionein in hepatocellular carcinoma and its surrounding liver parenchyma. J. Hepatol. 2000, 33, 415–422. [Google Scholar] [CrossRef]
- Alberino, F.; Gatta, A.; Amodio, P.; Merkel, C.; Di Pascoli, L.; Boffo, G.; Caregaro, L. Nutrition and survival in patients with liver cirrhosis. Nutrition 2001, 17, 445–450. [Google Scholar] [CrossRef]
- Maqbool, M.A.; Aslam, M.; Akbar, W.; Iqbal, Z. Biological importance of vitamins for human health: A review. J. Agric. Basic Sci. 2017, 2, 50–58. [Google Scholar]
- Chiang, K.C.; Yeh, C.N.; Chen, M.F.; Chen, T.C. Hepatocellular carcinoma and vitamin D: A review. J. Gastroenterol. Hepatol. 2011, 26, 1597–1603. [Google Scholar] [CrossRef]
- Zuo, S.; Wu, L.; Wang, Y.; Yuan, X. Long non-coding RNA MEG3 activated by vitamin d suppresses glycolysis in colorectal cancer via promoting c-myc degradation. Front. Oncol. 2020, 10, 274. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Shu, X.-O.; Li, H.; Yang, G.; Cai, H.; Ji, B.-T.; Gao, J.; Gao, Y.-T.; Zheng, W.; Xiang, Y.-B. Vitamin intake and liver cancer risk: A report from two cohort studies in China. J. Natl. Cancer Inst. 2012, 104, 1174–1182. [Google Scholar] [CrossRef] [Green Version]
- Taylor, P.R.; Qiao, Y.-L.; Abnet, C.C.; Dawsey, S.M.; Yang, C.S.; Gunter, E.W.; Wang, W.; Blot, W.J.; Dong, Z.-W.; Mark, S.D. Prospective study of serum vitamin E levels and esophageal and gastric cancers. J. Natl. Cancer Inst. 2003, 95, 1414–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnett, K.T.; Fokum, F.D.; Malafa, M.P. Vitamin E succinate inhibits colon cancer liver metastases. J. Surg. Res. 2002, 106, 292–298. [Google Scholar] [CrossRef]
- Correa, P.; Fontham, E.T.; Bravo, J.C.; Bravo, L.E.; Ruiz, B.; Zarama, G.; Realpe, J.L.; Malcom, G.T.; Li, D.; Johnson, W.D. Chemoprevention of gastric dysplasia: Randomized trial of antioxidant supplements and anti-Helicobacter pylori therapy. J. Natl. Cancer Inst. 2000, 92, 1881–1888. [Google Scholar] [CrossRef] [Green Version]
- Lv, H.; Wang, C.; Fang, T.; Li, T.; Lv, G.; Han, Q.; Yang, W.; Wang, H. Vitamin C preferentially kills cancer stem cells in hepatocellular carcinoma via SVCT-2. NPJ Precis. Oncol. 2018, 2, 1–13. [Google Scholar] [CrossRef] [Green Version]
- García-Closas, R.; Berenguer, A.; Tormo, M.J.; Sánchez, M.J.; Quiros, J.R.; Navarro, C.; Arnaud, R.; Dorronsoro, M.; Chirlaque, M.D.; Barricarte, A. Dietary sources of vitamin C, vitamin E and specific carotenoids in Spain. Br. J. Nutr. 2004, 91, 1005–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dani, V.; Goel, A.; Vaiphei, K.; Dhawan, D. Chemopreventive potential of zinc in experimentally induced colon carcinogenesis. Toxicol. Lett. 2007, 171, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Fong, L.Y.; Nguyen, V.T.; Farber, J.L. Esophageal cancer prevention in zinc-deficient rats: Rapid induction of apoptosis by replenishing zinc. J. Natl. Cancer Inst. 2001, 93, 1525–1533. [Google Scholar] [CrossRef]
- Ji, J.H.; Shin, D.G.; Kwon, Y.; Cho, D.H.; Lee, K.B.; Park, S.S.; Yoon, J. Clinical correlation between gastric cancer type and serum selenium and zinc levels. J. Gastric Cancer 2012, 12, 217. [Google Scholar] [CrossRef] [Green Version]
- Costello, L.C.; Franklin, R.B. The status of zinc in the development of hepatocellular cancer: An important, but neglected, clinically established relationship. Cancer Biol. Ther. 2014, 15, 353–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rollison, D.E.; Cole, A.L.; Tung, K.-H.; Slattery, M.L.; Baumgartner, K.B.; Byers, T.; Wolff, R.K.; Giuliano, A.R. Vitamin D intake, vitamin D receptor polymorphisms, and breast cancer risk among women living in the southwestern US. Breast Cancer Res. Treat. 2012, 132, 683–691. [Google Scholar] [CrossRef] [Green Version]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef]
- Bishop, L.E.; Ismailova, A.; Dimeloe, S.; Hewison, M.; White, J.H. Vitamin D and immune regulation: Antibacterial, antiviral, anti-inflammatory. JBMR Plus 2021, 5, e10405. [Google Scholar] [CrossRef]
- Garland, C.F.; Garland, F.C. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int. J. Epidemiol. 1980, 9, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Meeker, S.; Seamons, A.; Paik, J.; Treuting, P.M.; Brabb, T.; Grady, W.M.; Maggio-Price, L. Increased dietary vitamin D suppresses MAPK signaling, colitis, and colon cancer. Cancer Res. 2014, 74, 4398–4408. [Google Scholar] [CrossRef] [Green Version]
- Pavlova, N.N.; Thompson, C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, D.M.; Thomas, S.D.; Islam, A.; Muench, D.; Sedoris, K. c-Myc and cancer metabolism. Clin. Cancer Res. 2012, 18, 5546–5553. [Google Scholar] [CrossRef] [Green Version]
- Osthus, R.C.; Shim, H.; Kim, S.; Li, Q.; Reddy, R.; Mukherjee, M.; Xu, Y.; Wonsey, D.; Lee, L.A.; Dang, C.V. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J. Biol. Chem. 2000, 275, 21797–21800. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-W.; Gao, P.; Liu, Y.-C.; Semenza, G.L.; Dang, C.V. Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol. Cell. Biol. 2007, 27, 7381–7393. [Google Scholar] [CrossRef] [Green Version]
- Johnson, C.H.; Dejea, C.M.; Edler, D.; Hoang, L.T.; Santidrian, A.F.; Felding, B.H.; Ivanisevic, J.; Cho, K.; Wick, E.C.; Hechenbleikner, E.M. Metabolism links bacterial biofilms and colon carcinogenesis. Cell Metab. 2015, 21, 891–897. [Google Scholar] [CrossRef] [Green Version]
- Mima, K.; Sukawa, Y.; Nishihara, R.; Qian, Z.R.; Yamauchi, M.; Inamura, K.; Kim, S.A.; Masuda, A.; Nowak, J.A.; Nosho, K. Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol. 2015, 1, 653–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, A.; Mach, N. Role of vitamin D in the hygiene hypothesis: The interplay between vitamin D, vitamin D receptors, gut microbiota, and immune response. Front. Immunol. 2016, 7, 627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ooi, J.H.; Li, Y.; Rogers, C.J.; Cantorna, M.T. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate–induced colitis. J. Nutr. 2013, 143, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, P.; Wang, F.; Yang, J.; Liu, Z.; Qin, H. Association between vitamin D and risk of colorectal cancer: A systematic review of prospective studies. J. Clin. Oncol. 2011, 29, 3775–3782. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; McCullough, M.L.; Gapstur, S.M.; Jacobs, E.J.; Bostick, R.M.; Fedirko, V.; Flanders, W.D.; Campbell, P.T. Calcium, vitamin D, dairy products, and mortality among colorectal cancer survivors: The Cancer Prevention Study-II Nutrition Cohort. J. Clin. Oncol. 2014, 32, 2335–2343. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Lamprecht, S.A.; Shinozaki, H.; Fan, K.; Yang, W.; Newmark, H.L.; Kopelovich, L.; Edelmann, W.; Jin, B.; Gravaghi, C. Dietary calcium and cholecalciferol modulate cyclin D1 expression, apoptosis, and tumorigenesis in intestine of adenomatous polyposis coli1638N/+ mice. J. Nutr. 2008, 138, 1658–1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahendra, A.; Karishma, B.K.C.; Sharma, T.; Bansal, N.; Bansal, R.; Gupta, S. Vitamin D and gastrointestinal cancer. J. Lab. Physicians 2018, 10, 1. [Google Scholar] [CrossRef]
- Bae, W.K.; Lee, J.H.; Park, M.S.; Ahn, J.S.; Hwang, J.E.; Hyun Jeong Shim, S.H.C.; Chung, I.-J. 19-nor-1Ձ-25-Dihydroxyvitamin D 2 (Paricalcitol) induces apoptosis in gastric cancer cells. Cancer Prev. Res. 2009, 14, 329–334. [Google Scholar]
- Bigelsen, S. Evidence-based complementary treatment of pancreatic cancer: A review of adjunct therapies including paricalcitol, hydroxychloroquine, intravenous vitamin C, statins, metformin, curcumin, and aspirin. Cancer Manag. Res. 2018, 10, 2003. [Google Scholar] [CrossRef] [Green Version]
- Park, M.R.; Lee, J.H.; Park, M.S.; Hwang, J.E.; Shim, H.J.; Cho, S.H.; Chung, I.-J.; Bae, W.K. Suppressive effect of 19-nor-1α-25-dihydroxyvitamin D2 on gastric cancer cells and peritoneal metastasis model. J. Korean Med. Sci. 2012, 27, 1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain-Hakimjee, E.A.; Peng, X.; Mehta, R.R.; Mehta, R.G. Growth inhibition of carcinogen-transformed MCF-12F breast epithelial cells and hormone-sensitive BT-474 breast cancer cells by 1α-hydroxyvitamin D5. Carcinogenesis 2006, 27, 551–559. [Google Scholar] [CrossRef] [Green Version]
- Hargrove, L.; Taylor Francis, H.F. Vitamin D and GI cancers: Shedding some light on dark diseases. Ann. Transl. Med. 2014, 2, 9. [Google Scholar]
- Duarte-Salles, T.; Fedirko, V.; Stepien, M.; Trichopoulou, A.; Bamia, C.; Lagiou, P.; Lukanova, A.; Trepo, E.; Overvad, K.; Tjønneland, A. Dairy products and risk of hepatocellular carcinoma: The European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2014, 135, 1662–1672. [Google Scholar] [CrossRef]
- Ventura, E.R.; Konigorski, S.; Rohrmann, S.; Schneider, H.; Stalla, G.K.; Pischon, T.; Linseisen, J.; Nimptsch, K. Association of dietary intake of milk and dairy products with blood concentrations of insulin-like growth factor 1 (IGF-1) in Bavarian adults. Eur. J. Nutr. 2020, 59, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Authority, E.F.S. Opinion of the Scientific Panel on contaminants in the food chain [CONTAM] related to Aflatoxin B1 as undesirable substance in animal feed. EFSA J. 2004, 2, 39. [Google Scholar]
- Food and Agricultural Organization; World Health Organization. Safety Evaluation of Certain Mycotoxins in Food; Food & Agriculture Org.: Rome, Italy, 2001. [Google Scholar]
- Finkelmeier, F.; Kronenberger, B.; Köberle, V.; Bojunga, J.; Zeuzem, S.; Trojan, J.; Piiper, A.; Waidmann, O. Severe 25-hydroxyvitamin D deficiency identifies a poor prognosis in patients with hepatocellular carcinoma–a prospective cohort study. Aliment. Pharmacol. Ther. 2014, 39, 1204–1212. [Google Scholar] [CrossRef] [Green Version]
- Pourgholami, M.; Akhter, J.; Lu, Y.; Morris, D. In vitro and in vivo inhibition of liver cancer cells by 1, 25-dihydroxyvitamin D3. Cancer Lett. 2000, 151, 97–102. [Google Scholar] [CrossRef]
- Deeb, K.K.; Trump, D.L.; Johnson, C.S. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat. Rev. Cancer 2007, 7, 684–700. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.; Wong, M.K.; Flynn, G.; Yu, W.-d.; Johnson, C.S.; Trump, D.L. Differential antiproliferative effects of calcitriol on tumor-derived and matrigel-derived endothelial cells. Cancer Res. 2006, 66, 8565–8573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iseki, K.; Tatsuta, M.; Uehara, H.; Iishi, H.; Yano, H.; Sakai, N.; Ishiguro, S. Inhibition of angiogenesis as a mechanism for inhibition by Lα-hydroxyvitamin D3 and 1, 25-dihydroxyvitamin D3 of colon carcinogenesis induced by azoxymethane in Wistar rats. Int. J. Cancer 1999, 81, 730–733. [Google Scholar] [CrossRef]
- Dankers, W.; Colin, E.M.; van Hamburg, J.P.; Lubberts, E. Vitamin D in autoimmunity: Molecular mechanisms and therapeutic potential. Front. Immunol. 2017, 7, 697. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, A.V.; Feldman, D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 311–336. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, M.H.; Owen, J.; Ahearn, T.; Fedirko, V.; Flanders, W.D.; Jones, D.P.; Bostick, R.M. Effects of supplemental vitamin D and calcium on biomarkers of inflammation in colorectal adenoma patients: A randomized, controlled clinical trial. Cancer Prev. Res. 2011, 4, 1645–1654. [Google Scholar] [CrossRef] [Green Version]
- Leyssens, C.; Verlinden, L.; De Hertogh, G.; Kato, S.; Gysemans, C.; Mathieu, C.; Carmeliet, G.; Verstuyf, A. Impact on experimental colitis of vitamin D receptor deletion in intestinal epithelial or myeloid cells. Endocrinology 2017, 158, 2354–2366. [Google Scholar] [CrossRef] [PubMed]
- Protiva, P.; Pendyala, S.; Nelson, C.; Augenlicht, L.H.; Lipkin, M.; Holt, P.R. Calcium and 1, 25-dihydroxyvitamin D3 modulate genes of immune and inflammatory pathways in the human colon: A human crossover trial. Am. J. Clin. Nutr. 2016, 103, 1224–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrer-Mayorga, G.; Larriba, M.J.; Crespo, P.; Munoz, A. Mechanisms of action of vitamin D in colon cancer. J. Steroid Biochem. Mol. Biol. 2019, 185, 1–6. [Google Scholar] [CrossRef]
- García-Álvarez, M.; Pineda-Tenor, D.; Jiménez-Sousa, M.A.; Fernández-Rodríguez, A.; Guzmán-Fulgencio, M.; Resino, S. Relationship of vitamin D status with advanced liver fibrosis and response to hepatitis C virus therapy: A meta-analysis. Hepatology 2014, 60, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, H.; Chahal, S.; Gunton, J.E. Vitamin D in liver disease: Current evidence and potential directions. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 907–916. [Google Scholar] [CrossRef]
- Chapman, R.; Fevery, J.; Kalloo, A.; Nagorney, D.M.; Boberg, K.M.; Shneider, B.; Gores, G.J. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010, 51, 660–678. [Google Scholar] [CrossRef]
- Loft, S.; Møller, P.; Cooke, M.S.; Rozalski, R.; Olinski, R. Antioxidant vitamins and cancer risk: Is oxidative damage to DNA a relevant biomarker? Eur. J. Nutr. 2008, 47, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Seifried, H.E.; McDonald, S.S.; Anderson, D.E.; Greenwald, P.; Milner, J.A. The antioxidant conundrum in cancer. Cancer Res. 2003, 63, 4295–4298. [Google Scholar]
- García, O.P. Effect of vitamin A deficiency on the immune response in obesity. Proc. Nutr. Soc. 2012, 71, 290–297. [Google Scholar] [CrossRef] [Green Version]
- Dong, P.; Tao, Y.; Yang, Y.; Wang, W. Expression of retinoic acid receptors in intestinal mucosa and the effect of vitamin A on mucosal immunity. Nutrition 2010, 26, 740–745. [Google Scholar] [CrossRef]
- Okayasu, I.; Hana, K.; Nemoto, N.; Yoshida, T.; Saegusa, M.; Yokota-Nakatsuma, A.; Song, S.-Y.; Iwata, M. Vitamin A inhibits development of dextran sulfate sodium-induced colitis and colon cancer in a mouse model. BioMed Res. Int. 2016, 2016, 4874809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.-M.; Gao, Y.-T.; Ong, C.-N.; Ross, R.K.; Yu, M.C. Prediagnostic level of serum retinol in relation to reduced risk of hepatocellular carcinoma. J. Natl. Cancer Inst. 2006, 98, 482–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemente, C.; Elba, S.; Buongiorno, G.; Berloco, P.; Guerra, V.; Di Leo, A. Serum retinol and risk of hepatocellular carcinoma in patients with child-Pugh class A cirrhosis. Cancer Lett. 2002, 178, 123–129. [Google Scholar] [CrossRef]
- Lai, G.; Weinstein, S.; Taylor, P.; McGlynn, K.; Virtamo, J.; Gail, M.; Albanes, D.; Freedman, N. Effects of α-tocopherol and β-carotene supplementation on liver cancer incidence and chronic liver disease mortality in the ATBC study. Br. J. Cancer 2014, 111, 2220–2223. [Google Scholar] [CrossRef] [Green Version]
- Lan, Q.-Y.; Zhang, Y.-J.; Liao, G.-C.; Zhou, R.-F.; Zhou, Z.-G.; Chen, Y.-M.; Zhu, H.-L. The association between dietary vitamin A and carotenes and the risk of primary liver cancer: A case–control study. Nutrients 2016, 8, 624. [Google Scholar] [CrossRef] [Green Version]
- Freemantle, S.J.; Spinella, M.J.; Dmitrovsky, E. Retinoids in cancer therapy and chemoprevention: Promise meets resistance. Oncogene 2003, 22, 7305–7315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niles, R.M. Signaling pathways in retinoid chemoprevention and treatment of cancer. Mutat. Res.-Fund. Mol. Mech. Mutagen. 2004, 555, 97–105. [Google Scholar] [CrossRef]
- Tang, X.-H.; Gudas, L.J. Retinoids, retinoic acid receptors, and cancer. Annu. Rev. Pathol. 2011, 6, 345–364. [Google Scholar] [CrossRef]
- Duong, V.; Rochette-Egly, C. The molecular physiology of nuclear retinoic acid receptors. From health to disease. Biochim. Biophys. Acta Mol. Basis Dis. 2011, 1812, 1023–1031. [Google Scholar] [CrossRef] [Green Version]
- Al Tanoury, Z.; Piskunov, A.; Rochette-Egly, C. Vitamin A and retinoid signaling: Genomic and nongenomic effects: Thematic review series: Fat-soluble vitamins: Vitamin A. J. Lipid Res. 2013, 54, 1761–1775. [Google Scholar] [CrossRef] [Green Version]
- Carman, S.; Kamangar, F.; Freedman, N.D.; Wright, M.E.; Dawsey, S.M.; Dixon, L.B.; Subar, A.; Schatzkin, A.; Abnet, C.C. Vitamin E intake and risk of esophageal and gastric cancers in the NIH-AARP Diet and Health Study. Int. J. Cancer 2009, 125, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Slattery, M.L.; Edwards, S.L.; Anderson, K.; Caan, B. Vitamin E and colon cancer: Is there an association? Nutr. Cancer 1998, 50, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.; Weinstein, S.; Albanes, D.; Taylor, P.; Virtamo, J.; McGlynn, K.; Freedman, N. Association of serum α-tocopherol, β-carotene, and retinol with liver cancer incidence and chronic liver disease mortality. Br. J. Cancer 2014, 111, 2163–2171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das Gupta, S.; Suh, N. Tocopherols in cancer: An update. Mol. Nutr. Food Res. 2016, 60, 1354–1363. [Google Scholar] [CrossRef] [Green Version]
- Ju, J.; Picinich, S.C.; Yang, Z.; Zhao, Y.; Suh, N.; Kong, A.-N.; Yang, C.S. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis 2010, 31, 533–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.S.; Luo, P.; Zeng, Z.; Wang, H.; Malafa, M.; Suh, N. Vitamin E and cancer prevention: Studies with different forms of tocopherols and tocotrienols. Mol. Carcinog. 2020, 59, 365–389. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.; Kaddoumi, A. Vitamin E transporters in cancer therapy. AAPS J. 2015, 17, 313–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Brandt, P.A.; Goldbohm, R.A. Nutrition in the prevention of gastrointestinal cancer. Best Pract. Res. Clin. Gastroenterol. 2006, 20, 589–603. [Google Scholar] [CrossRef] [Green Version]
- Plummer, M.; Vivas, J.; Lopez, G.; Bravo, J.C.; Peraza, S.; Carillo, E.; Cano, E.; Castro, D.; Andrade, O.; Sánchez, V. Chemoprevention of precancerous gastric lesions with antioxidant vitamin supplementation: A randomized trial in a high-risk population. J. Natl. Cancer Inst. 2007, 99, 137–146. [Google Scholar] [CrossRef]
- Jacobs, E.J.; Connell, C.J.; Patel, A.V.; Chao, A.; Rodriguez, C.; Seymour, J.; McCullough, M.L.; Calle, E.E.; Thun, M.J. Vitamin C and vitamin E supplement use and colorectal cancer mortality in a large American Cancer Society cohort. Cancer Epidemiol. Biomark. Prev. 2001, 10, 17–23. [Google Scholar]
- Su, M.; Chen, H.; Wei, C.; Chen, N.; Wu, W. Potential protection of vitamin C against liver-lesioned mice. Int. Immunopharmacol. 2014, 22, 492–497. [Google Scholar] [CrossRef]
- Dhawan, D.; Chadha, V.D. Zinc: A promising agent in dietary chemoprevention of cancer. Indian J. Med. Res. 2010, 132, 676. [Google Scholar]
- Prasad, A.S.; Kucuk, O. Zinc in cancer prevention. Cancer Metastasis Rev. 2002, 21, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of zinc in immune system and anti-cancer defense mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef] [Green Version]
- Chadha, V.D.; Vaiphei, K.; Dhawan, D. Zinc mediated normalization of histoarchitecture and antioxidant status offers protection against initiation of experimental carcinogenesis. Mol. Cell. Biochem. 2007, 304, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Chadha, V.D.; Dhawan, D. Membrane fluidity and surface changes during initiation of 1, 2 dimethylhydrazine-induced colon carcinogenesis: Protection by zinc. Oncol. Res. 2009, 18, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Fong, L.; Farber, J.L.; Magee, P.N. Zinc replenishment reduces esophageal cell proliferation and N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumor incidence in zinc-deficient rats. Carcinogenesis 1998, 19, 1591–1596. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, A.S.; Narayan, S. Zinc stabilizes adenomatous polyposis coli (APC) protein levels and induces cell cycle arrest in colon cancer cells. J. Cell. Biochem. 2004, 93, 345–357. [Google Scholar] [CrossRef] [PubMed]
- De Figueiredo Ribeiro, S.M.; Braga, C.B.M.; Peria, F.M.; Domenici, F.A.; Martinez, E.Z.; Feres, O.; Da Rocha, J.J.R.; Da Cunha, S.F.D.C. Effect of zinc supplementation on antioxidant defenses and oxidative stress markers in patients undergoing chemotherapy for colorectal cancer: A placebo-controlled, prospective randomized trial. Biol. Trace Elem. Res. 2016, 169, 8–16. [Google Scholar] [CrossRef]
- Reding, P.; Duchateau, J.; Bataille, C. Oral zinc supplementation improves hepatic encephalopathy: Results of a randomised controlled trial. Lancet 1984, 324, 493–495. [Google Scholar] [CrossRef]
- Stepien, M.; Hughes, D.J.; Hybsier, S.; Bamia, C.; Tjønneland, A.; Overvad, K.; Affret, A.; His, M.; Boutron-Ruault, M.-C.; Katzke, V. Circulating copper and zinc levels and risk of hepatobiliary cancers in Europeans. Br. J. Cancer 2017, 116, 688–696. [Google Scholar] [CrossRef]
- Tamai, Y.; Iwasa, M.; Eguchi, A.; Shigefuku, R.; Sugimoto, K.; Hasegawa, H.; Takei, Y. Serum copper, zinc and metallothionein serve as potential biomarkers for hepatocellular carcinoma. PLoS ONE 2020, 15, e0237370. [Google Scholar] [CrossRef] [PubMed]
- Fang, A.P.; Chen, P.Y.; Wang, X.Y.; Liu, Z.Y.; Zhang, D.M.; Luo, Y.; Liao, G.C.; Long, J.A.; Zhong, R.H.; Zhou, Z.G. Serum copper and zinc levels at diagnosis and hepatocellular carcinoma survival in the Guangdong Liver Cancer Cohort. Int. J. Cancer 2019, 144, 2823–2832. [Google Scholar] [CrossRef] [PubMed]
- Hosui, A.; Kimura, E.; Abe, S.; Tanimoto, T.; Onishi, K.; Kusumoto, Y.; Sueyoshi, Y.; Matsumoto, K.; Hirao, M.; Yamada, T. Long-term zinc supplementation improves liver function and decreases the risk of developing hepatocellular carcinoma. Nutrients 2018, 10, 1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, R.; Mei, X.; Ye, Y.; Xue, T.; Wang, J.; Sun, W.; Lin, C.; Xue, R.; Zhang, J.; Xu, D. Zn (II)-curcumin solid dispersion impairs hepatocellular carcinoma growth and enhances chemotherapy by modulating gut microbiota-mediated zinc homeostasis. Pharmacol. Res. 2019, 150, 104454. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Filippini, T.; Del Giovane, C.; Dennert, G.; Zwahlen, M.; Brinkman, M.; Zeegers, M.P.; Horneber, M.; D’Amico, R.; Crespi, C.M. Selenium for preventing cancer. Cochrane Database of Syst. Rev. 2018, 1, CD005195. [Google Scholar] [CrossRef]
- Fischer, J.L.; Mihelc, E.M.; Pollok, K.E.; Smith, M.L. Chemotherapeutic selectivity conferred by selenium: A role for p53-dependent DNA repair. Mol. Cancer Ther. 2007, 6, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Goel, A.; Fuerst, F.; Hotchkiss, E.; Boland, C.R. Selenomethionine induces p53 mediated cell cycle arrest and apoptosis in human colon cancer cells. Cancer Biol. Ther. 2006, 5, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Durrani, F.A.; Rustum, Y.M. Selective modulation of the therapeutic efficacy of anticancer drugs by selenium containing compounds against human tumor xenografts. Clin. Cancer Res. 2004, 10, 2561–2569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schomburg, L.; Hughes, D.J. The missing link? The potential role of selenium in the development of liver cancer and significance for the general population. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.-P.; Martitz, J.; Renko, K.; Stoedter, M.; Hybsier, S.; Cramer, T.; Schomburg, L. Hypoxia reduces and redirects selenoprotein biosynthesis. Metallomics 2014, 6, 1079–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohr-Udilova, N.; Sieghart, W.; Eferl, R.; Stoiber, D.; Björkhem-Bergman, L.; Eriksson, L.C.; Stolze, K.; Hayden, H.; Keppler, B.; Sagmeister, S. Antagonistic effects of selenium and lipid peroxides on growth control in early hepatocellular carcinoma. Hepatology 2012, 55, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.J.; Duarte-Salles, T.; Hybsier, S.; Trichopoulou, A.; Stepien, M.; Aleksandrova, K.; Overvad, K.; Tjønneland, A.; Olsen, A.; Affret, A. Prediagnostic selenium status and hepatobiliary cancer risk in the European Prospective Investigation into Cancer and Nutrition cohort. Am. J. Clin. Nutr. 2016, 104, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N. Micronutrients prevent cancer and delay aging. Toxicol. Lett. 1998, 102, 5–18. [Google Scholar] [CrossRef]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L., Jr.; Valanis, B.; Williams, J.H., Jr. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1150–1155. [Google Scholar] [CrossRef] [Green Version]
- Block, G. The data support a role for antioxidants in reducing cancer risk. Nutr. Rev. 1992, 50, 207–213. [Google Scholar] [CrossRef]
- Block, G.; Patterson, B.; Subar, A. Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutr. Cancer 1992, 18, 1–29. [Google Scholar] [CrossRef]
- Byers, T.; Guerrero, N. Epidemiologic evidence for vitamin C and vitamin E in cancer prevention. Am. J. Clin. Nutr. 1995, 62, 1385S–1392S. [Google Scholar] [CrossRef] [PubMed]
- Diplock, A.T. Will the ‘good fairies’ please prove to us that vitamin E lessens human degenerative disease? Free Radic. Res. 1997, 27, 511–532. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ames, B.N.; Gold, L.S. Environmental pollution, pesticides, and the prevention of cancer: Misconceptions 1. FASEB J. 1997, 11, 1041–1052. [Google Scholar] [CrossRef] [Green Version]
- Gerster, H. β-carotene, vitamin E and vitamin C in different stages of experimental carcinogenesis. Eur. J. Clin. Nutr. 1995, 49, 155–168. [Google Scholar] [PubMed]
- Hagen, T.M.; Yowe, D.L.; Bartholomew, J.C.; Wehr, C.M.; Do, K.L.; Park, J.-Y.; Ames, B.N. Mitochondrial decay in hepatocytes from old rats: Membrane potential declines, heterogeneity and oxidants increase. Proc. Natl. Acad. Sci. USA 1997, 94, 3064–3069. [Google Scholar] [CrossRef] [Green Version]
- Berlett, B.S.; Stadtman, E.R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997, 272, 20313–20316. [Google Scholar] [CrossRef] [Green Version]
- Ames, B.N.; Gold, L.S.; Willett, W.C. The causes and prevention of cancer. Proc. Natl. Acad. Sci. USA 1995, 92, 5258–5265. [Google Scholar] [CrossRef] [Green Version]
- Kukovetz, E.M.; Bratschitsch, G.; Hofer, H.P.; Egger, G.; Schaur, R.J. Influence of age on the release of reactive oxygen species by phagocytes as measured by a whole blood chemiluminescence assay. Free Radic. Biol. Med. 1997, 22, 433–438. [Google Scholar] [CrossRef]
- Steinmetz, K.A.; Potter, J.D. Vegetables, fruit, and cancer prevention: A review. J. Am. Diet. Assoc. 1996, 96, 1027–1039. [Google Scholar] [CrossRef]
- Blot, W.J. Vitamin/mineral supplementation and cancer risk: International chemoprevention trials. Proc. Soc. Exp. Biol. Med. 1997, 216, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Duthie, S.J.; Ma, A.; Ross, M.A.; Collins, A.R. Antioxidant supplementation decreases oxidative DNA damage in human lymphocytes. Cancer Res. 1996, 56, 1291–1295. [Google Scholar]
- Cooney, R.V.; Harwood, P.J.; Franke, A.A.; Narala, K.; Sundström, A.-K.; Berggren, P.-O.; Mordan, L.J. Products of γ-tocopherol reaction with NO2 and their formation in rat insulinoma (RINm5F) cells. Free Radic. Biol. Med. 1995, 19, 259–269. [Google Scholar] [CrossRef]
- Shigenaga, M.K.; Lee, H.H.; Blount, B.C.; Christen, S.; Shigeno, E.T.; Yip, H.; Ames, B.N. Inflammation and NOx-induced nitration: Assay for 3-nitrotyrosine by HPLC with electrochemical detection. Proc. Natl. Acad. Sci. USA 1997, 94, 3211–3216. [Google Scholar] [CrossRef] [Green Version]
- Fleet, J.C.; DeSmet, M.; Johnson, R.; Li, Y. Vitamin D and cancer: A review of molecular mechanisms. Biochem. J. 2012, 441, 61–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, R.A.; Sotoudeh, G. Vitamin C function and status in chronic disease. Nutr. Clin. Care 2002, 5, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, J.J.; Kuypers, F.A.; Roelofsen, B.; den Kamp, J.A.O. The cooperative action of vitamins E and C in the protection against peroxidation of parinaric acid in human erythrocyte membranes. Chem. Phys. Lipids 1990, 53, 309–320. [Google Scholar] [CrossRef]
- Du, J.; Martin, S.M.; Levine, M.; Wagner, B.A.; Buettner, G.R.; Wang, S.-H.; Taghiyev, A.F.; Du, C.; Knudson, C.M.; Cullen, J.J. Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer. Clin. Cancer Res. 2010, 16, 509–520. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shacter, E.; Levine, M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 13604–13609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, G.; Wronska, U.; Stone, L.; Foster, D.; Ingold, K. Biokinetics of dietary RRR-α-tocopherol in the male guinea pig at three dietary levels of vitamin C and two levels of vitamin E. Evidence that vitamin C does not “spare” vitamin E in vivo. Lipids 1990, 25, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Action of ascorbic acid as a scavenger of active and stable oxygen radicals. Am. J. Clin. Nutr. 1991, 54, 1119S–1124S. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.-j.; Duan, F.-d.; Zhang, H.-h.; Xiong, Y.; Wang, J. Sodium selenite-induced apoptosis mediated by ROS attack in human osteosarcoma U2OS cells. Biol. Trace Elem. Res. 2012, 145, 1–9. [Google Scholar] [CrossRef]
- Pang, K.-L.; Chin, K.-Y. Emerging anticancer potentials of selenium on osteosarcoma. Int. J. Mol. Sci. 2019, 20, 5318. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, J.; Hao, H.; Cai, M.; Wang, S.; Ma, J.; Li, Y.; Mao, C.; Zhang, S. In vitro and in vivo mechanism of bone tumor inhibition by selenium-doped bone mineral nanoparticles. ACS Nano 2016, 10, 9927–9937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Chen, Y.; Clair, D.K.S. ROS and p53: A versatile partnership. Free Radic. Biol. Med. 2008, 44, 1529–1535. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Meng, F.B.; Wang, Z.X.; Li, X.; Zhou, D.S. Selenocysteine inhibits human osteosarcoma cells growth through triggering mitochondrial dysfunction and ROS-mediated p53 phosphorylation. Cell Biol. Int. 2018, 42, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Du, W.; Heese, K.; Wu, M. The Bad guy cooperates with good cop p53: Bad is transcriptionally up-regulated by p53 and forms a Bad/p53 complex at the mitochondria to induce apoptosis. Mol. Cell. Biol. 2006, 26, 9071–9082. [Google Scholar] [CrossRef] [Green Version]
- Stambolic, V.; MacPherson, D.; Sas, D.; Lin, Y.; Snow, B.; Jang, Y.; Benchimol, S.; Mak, T. Regulation of PTEN transcription by p53. Mol. Cell 2001, 8, 317–325. [Google Scholar] [CrossRef]
- Boulares, A.H.; Yakovlev, A.G.; Ivanova, V.; Stoica, B.A.; Wang, G.; Iyer, S.; Smulson, M. Role of poly (ADP-ribose) polymerase (PARP) cleavage in apoptosis: Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J. Biol. Chem. 1999, 274, 22932–22940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaitanya, G.V.; Alexander, J.S.; Babu, P.P. PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration. Cell Commun. Signal. 2010, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Baudet, C.; Chevalier, G.; Chassevent, A.; Canova, C.; Filmon, R.; Larra, F.; Brachet, P.; Wion, D. 1,25-Dihydroxyvitamin D3 induces programmed cell death in a rat glioma cell line. J. Neurosci. Res. 1996, 46, 540–550. [Google Scholar] [CrossRef]
- Pitot, H.C.; Dragan, Y.P. The multistage nature of chemically induced hepatocarcinogenesis in the rat. Drug Metab. Rev. 1994, 26, 209–220. [Google Scholar] [CrossRef]
- Bodmer, W.; Tomlinson, I. Population Genetics of Tumours, Ciba Foundation Symposium, 1996; Wiley Online Library: Hoboken, NJ, USA, 1996; pp. 181–193. [Google Scholar]
- Moreno, J.; Krishnan, A.V.; Feldman, D. Molecular mechanisms mediating the anti-proliferative effects of Vitamin D in prostate cancer. J. Steroid Biochem. Mol. Biol. 2005, 97, 31–36. [Google Scholar] [CrossRef]
- Konety, B.R.; Getzenberg, R.H. Vitamin D and prostate cancer. Urol. Clin. 2002, 29, 95–106. [Google Scholar] [CrossRef]
- Wyllie, A. Apoptosis: Cell death in tissue regulation. J. Pathol. 1987, 153, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.S.; Madsen, M.W.; Lukas, J.; Binderup, L.; Bartek, J. Inhibitory effects of 1α, 25-dihydroxyvitamin D3 on the G1–S phase-controlling machinery. Mol. Endocrinol. 2001, 15, 1370–1380. [Google Scholar] [PubMed] [Green Version]
- Meyer, M.B.; Goetsch, P.D.; Pike, J.W. VDR/RXR and TCF4/β-catenin cistromes in colonic cells of colorectal tumor origin: Impact on c-FOS and c-MYC gene expression. Mol. Endocrinol. 2012, 26, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Salehi-Tabar, R.; Nguyen-Yamamoto, L.; Tavera-Mendoza, L.E.; Quail, T.; Dimitrov, V.; An, B.-S.; Glass, L.; Goltzman, D.; White, J.H. Vitamin D receptor as a master regulator of the c-MYC/MXD1 network. Proc. Natl. Acad. Sci. USA 2012, 109, 18827–18832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Washington, M.N.; Kim, J.S.; Weigel, N.L. 1α,25-dihydroxyvitamin D3 inhibits C4-2 prostate cancer cell growth via a retinoblastoma protein (Rb)-independent G1 arrest. Prostate 2011, 71, 98–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Li, C.; Zhao, X.; Zhang, X.; Nicosia, S.V.; Bai, W. p27Kip1 stabilization and G1 arrest by 1,25-dihydroxyvitamin D3 in ovarian cancer cells mediated through down-regulation of cyclin E/cyclin-dependent kinase 2 and Skp1-Cullin-F-box protein/Skp2 ubiquitin ligase. J. Biol. Chem. 2004, 279, 25260–25267. [Google Scholar] [CrossRef] [Green Version]
- Bao, B.-Y.; Hu, Y.-C.; Ting, H.-J.; Lee, Y.-F. Androgen signaling is required for the vitamin D-mediated growth inhibition in human prostate cancer cells. Oncogene 2004, 23, 3350–3360. [Google Scholar] [CrossRef] [Green Version]
- Sunil Kumar, B.; Singh, S.; Verma, R. Anticancer potential of dietary vitamin D and ascorbic acid: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2623–2635. [Google Scholar] [CrossRef] [PubMed]
- Losso, J.N.; Bawadi, H.A. Hypoxia inducible factor pathways as targets for functional foods. J. Agric. Food Chem. 2005, 53, 3751–3768. [Google Scholar] [CrossRef]
- Ben-Shoshan, M.; Amir, S.; Dang, D.T.; Dang, L.H.; Weisman, Y.; Mabjeesh, N.J. 1α,25-dihydroxyvitamin D3 (Calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Mol. Cancer Ther. 2007, 6, 1433–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peyman, G.; Kivilcim, M.; Dellacroce, J.; Morales, A.M. Inhibition of corneal neovascularization by ascorbic acid in rat model. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1634. [Google Scholar]
- Mikirova, N.A.; Ichim, T.E.; Riordan, N.H. Anti-angiogenic effect of high doses of ascorbic acid. J. Transl. Med. 2008, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassett, M.J.; O’Malley, A.J.; Pakes, J.R.; Newhouse, J.P.; Earle, C.C. Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J. Natl. Cancer Inst. 2006, 98, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Berkey, F.J. Managing the adverse effects of radiation therapy. Am. Fam. Physician 2010, 82, 381–388. [Google Scholar] [PubMed]
- Goodman, M. Managing the Side Effects of Chemotherapy. Semin. Oncol. Nurs. 1989, 5, 29–52. [Google Scholar] [CrossRef]
- Donaldson, S.S.; Lenon, R.A. Alterations of nutritional status. Impact of chemotherapy and radiation therapy. Cancer 1979, 43, 2036–2052. [Google Scholar] [CrossRef]
- Conklin, K.A. Dietary antioxidants during cancer chemotherapy: Impact on chemotherapeutic effectiveness and development of side effects. Nutr. Cancer 2000, 37, 1–18. [Google Scholar] [CrossRef]
- Simone, C.B., II; Simone, N.L.; Simone, V.; Simone, C.B. Antioxidants and other nutrients do not interfere with chemotherapy or radiation therapy and can increase kill and increase survival, Part 2. Altern. Ther. Health Med. 2007, 13, 22–29. [Google Scholar]
- Weijl, N.; Elsendoorn, T.; Lentjes, E.; Hopman, G.; Wipkink-Bakker, A.; Zwinderman, A.; Cleton, F.; Osanto, S. Supplementation with antioxidant micronutrients and chemotherapy-induced toxicity in cancer patients treated with cisplatin-based chemotherapy: A randomised, double-blind, placebo-controlled study. Eur. J. Cancer 2004, 40, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.D.; Tucker, K.L.; Ladas, E.D.; Rheingold, S.R.; Blumberg, J.; Kelly, K.M. Low Antioxidant vitamin intakes are associated with increases in adverse effects of chemotherapy in children with acute lymphoblastic leukemia. Am. J. Clin. Nutr. 2004, 79, 1029–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolson, G.L. Lipid replacement/antioxidant therapy as an adjunct supplement to reduce the adverse effects of cancer therapy and restore mitochondrial function. Pathol. Oncol. Res. 2005, 11, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Dennert, G.; Horneber, M. Selenium for alleviating the side effects of chemotherapy, radiotherapy and surgery in cancer patients. Cochrane Database Syst. Rev. 2006, 19, CD005037. [Google Scholar] [CrossRef] [PubMed]

| Micronutrients | Underlined Cancer Types | RDA | Dietary Sources | References |
|---|---|---|---|---|
| Vitamin D | Colorectal cancer, HCC | 15 µg | Egg yolks, tuna, salmon, sardines, mushrooms, cow’s milk, soy milk, orange juice, and fortified foods. | [32,79,80,81] |
| Vitamin A | Gastric cancer, Colorectal cancer, HCC | 900 µg | Liver (animals and fishes) and egg yolk. Provitamin A carotenoids obtained from plant sources including deep green, yellow and orange fruits and vegetables such as carrots, spinach, broccoli, mangoes, turnips, and sweet potatoes. | [32,65,79,82,83] |
| Vitamin E | Upper GI cancers, Colon cancer, HCC | 15 mg | Vegetable oils (cotton seed oil, wheat germ oil, corn germ oil, and peanut oil). All green plants contain some concentration of tocopherol but some green leafy vegetables and rose hips contain more than wheat germ. | [32,79,82,83,84] |
| Vitamin C | Intestinal metaplasia, HCC | 90 mg | Fruits (especially citrus fruits) and vegetables (especially peppers andpotatoes). | [32,85,86,87] |
| Zinc | Esophageal tumors, Gastric cancers, Colon cancer, HCC | 11 mg | Oysters, red meat, nuts, whole grains, poultry, and dairy products. | [32,88,89,90,91] |
| Selenium | Colon cancer, HCC | 0.055 mg | Brazil nuts, seafoods, meats, grains, dairy products, eggs, and organ meats. | [32,92,93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, W.; Ullah, H.; Santarcangelo, C.; Di Minno, A.; Khan, H.; Daglia, M.; Arciola, C.R. Micronutrient Food Supplements in Patients with Gastro-Intestinal and Hepatic Cancers. Int. J. Mol. Sci. 2021, 22, 8014. https://doi.org/10.3390/ijms22158014
Alam W, Ullah H, Santarcangelo C, Di Minno A, Khan H, Daglia M, Arciola CR. Micronutrient Food Supplements in Patients with Gastro-Intestinal and Hepatic Cancers. International Journal of Molecular Sciences. 2021; 22(15):8014. https://doi.org/10.3390/ijms22158014
Chicago/Turabian StyleAlam, Waqas, Hammad Ullah, Cristina Santarcangelo, Alessandro Di Minno, Haroon Khan, Maria Daglia, and Carla Renata Arciola. 2021. "Micronutrient Food Supplements in Patients with Gastro-Intestinal and Hepatic Cancers" International Journal of Molecular Sciences 22, no. 15: 8014. https://doi.org/10.3390/ijms22158014
APA StyleAlam, W., Ullah, H., Santarcangelo, C., Di Minno, A., Khan, H., Daglia, M., & Arciola, C. R. (2021). Micronutrient Food Supplements in Patients with Gastro-Intestinal and Hepatic Cancers. International Journal of Molecular Sciences, 22(15), 8014. https://doi.org/10.3390/ijms22158014











