Low-Molecular-Weight Fucoidan as Complementary Therapy of Fluoropyrimidine-Based Chemotherapy in Colorectal Cancer
Abstract
:1. Introduction
2. Results
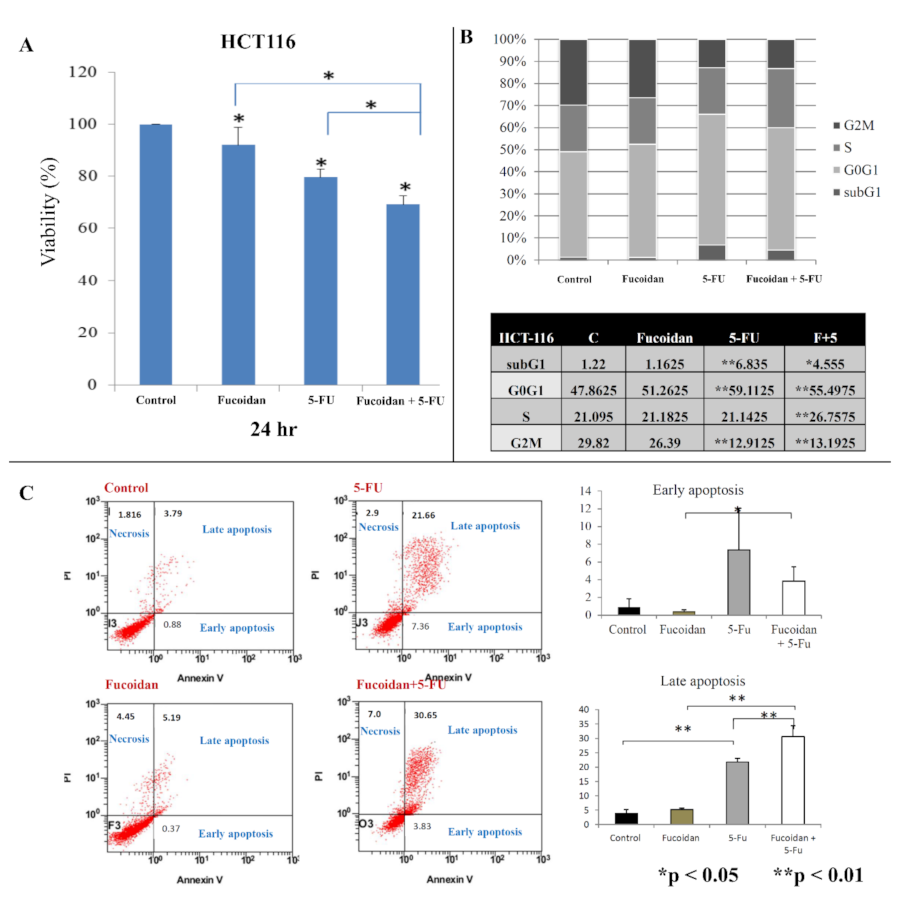
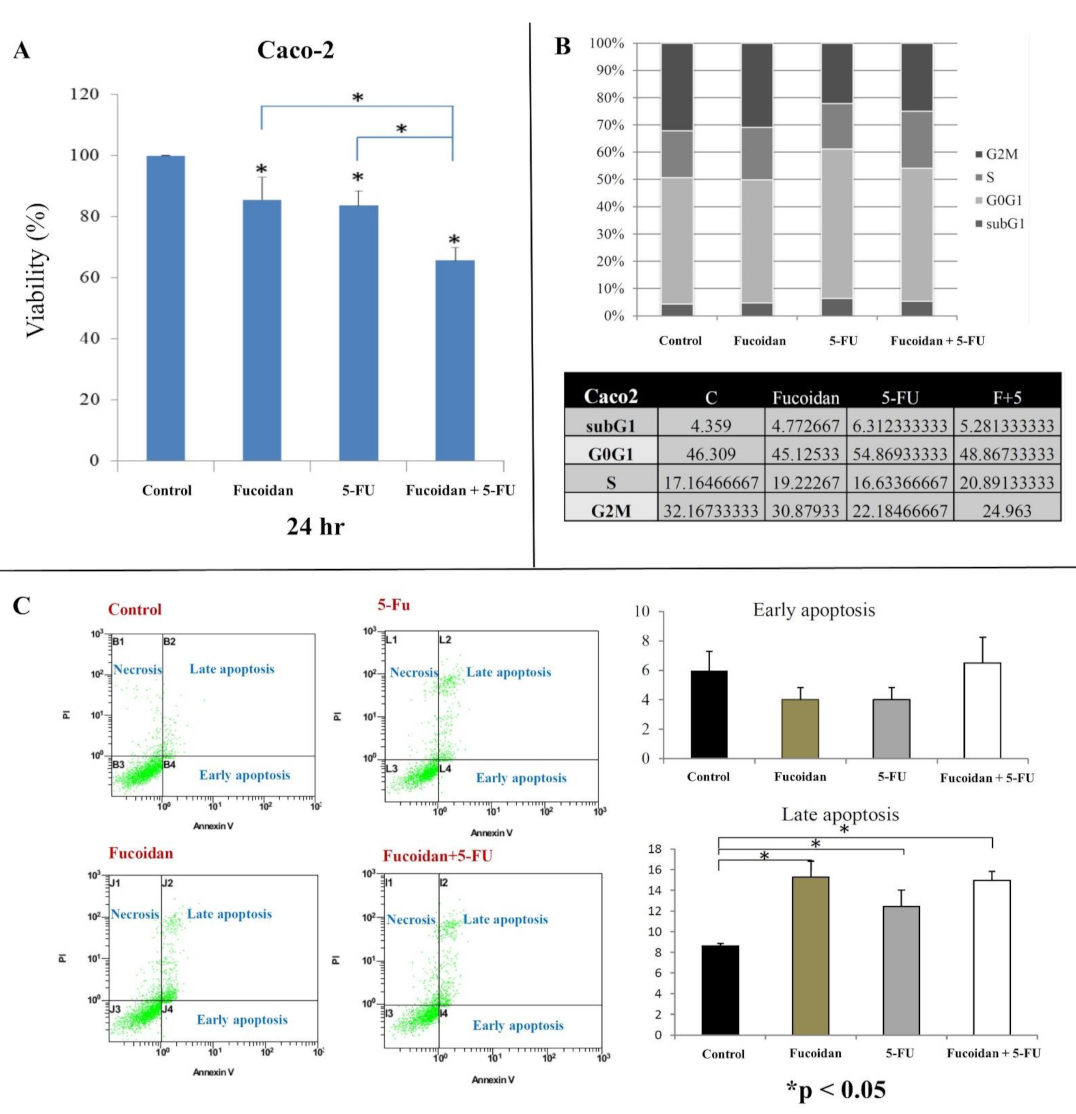
2.1. Effect of LMWF and 5-FU on Cell Viability
2.2. Effects of LMWF and 5-FU on Cell Cycle
2.3. Effects of LMWF and 5-FU on Apoptosis
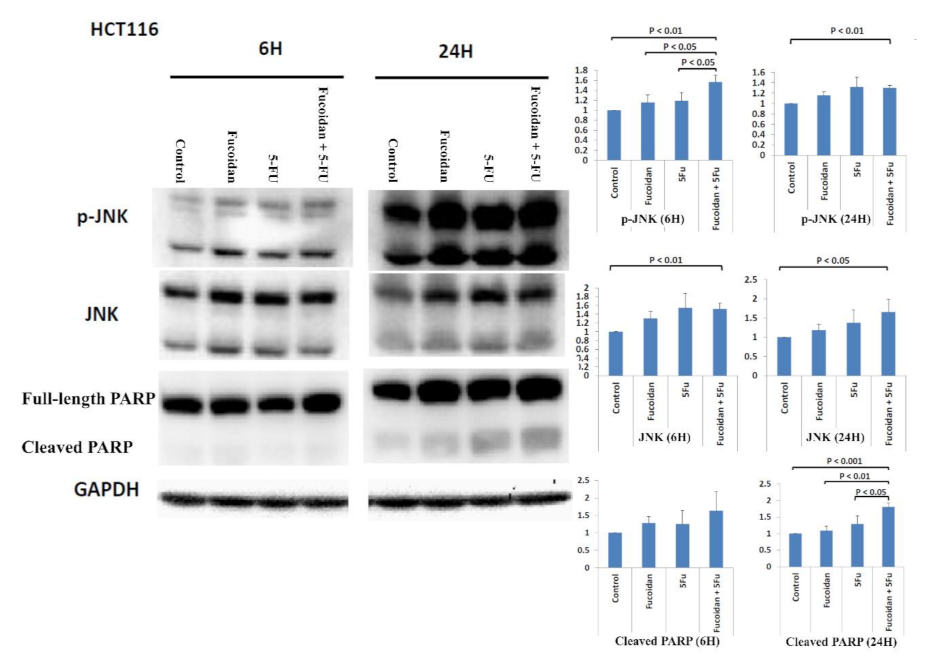
2.4. Signaling Pathways Responsible for LMWF and 5-FU Effects on Apoptosis in HCT116 Cells
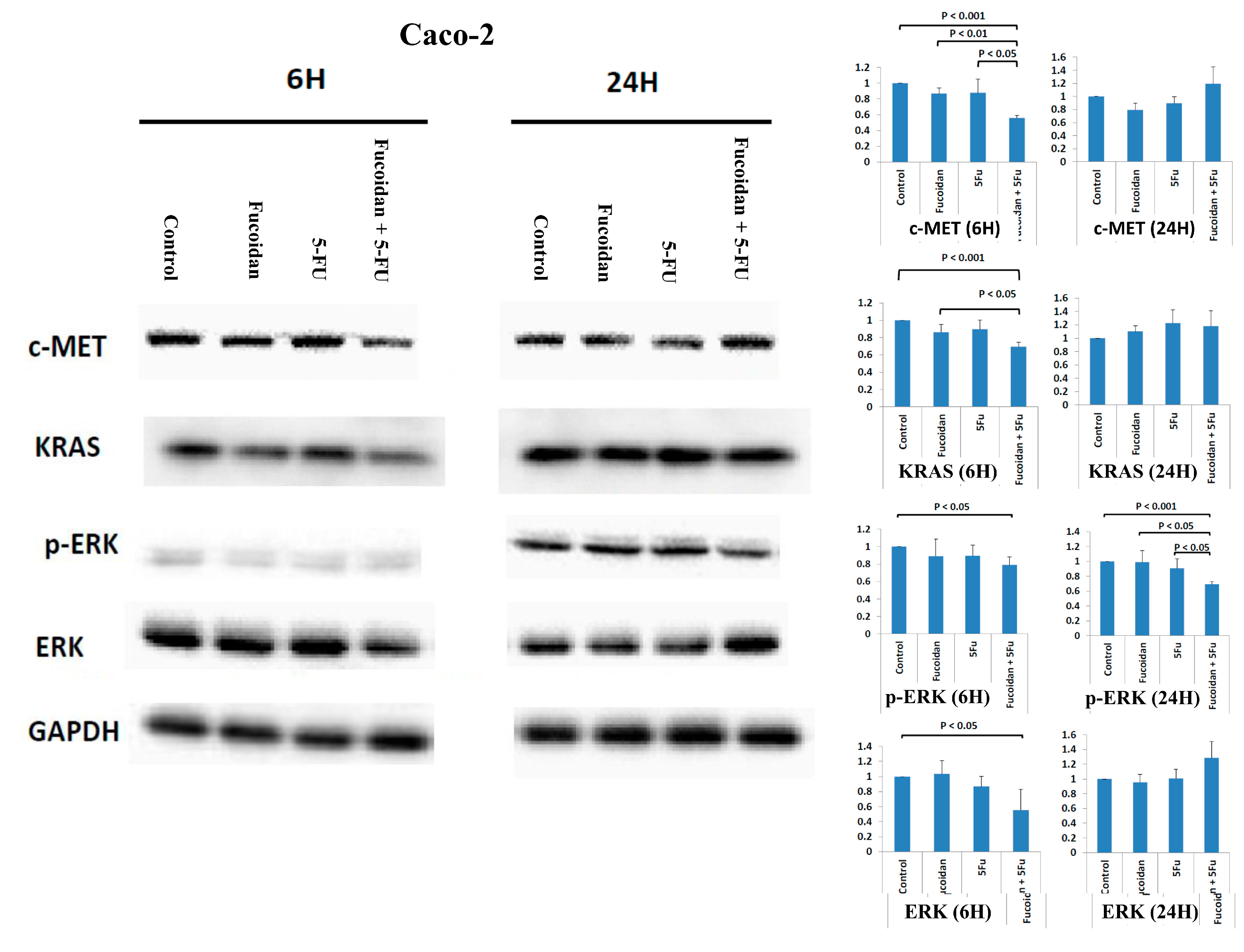
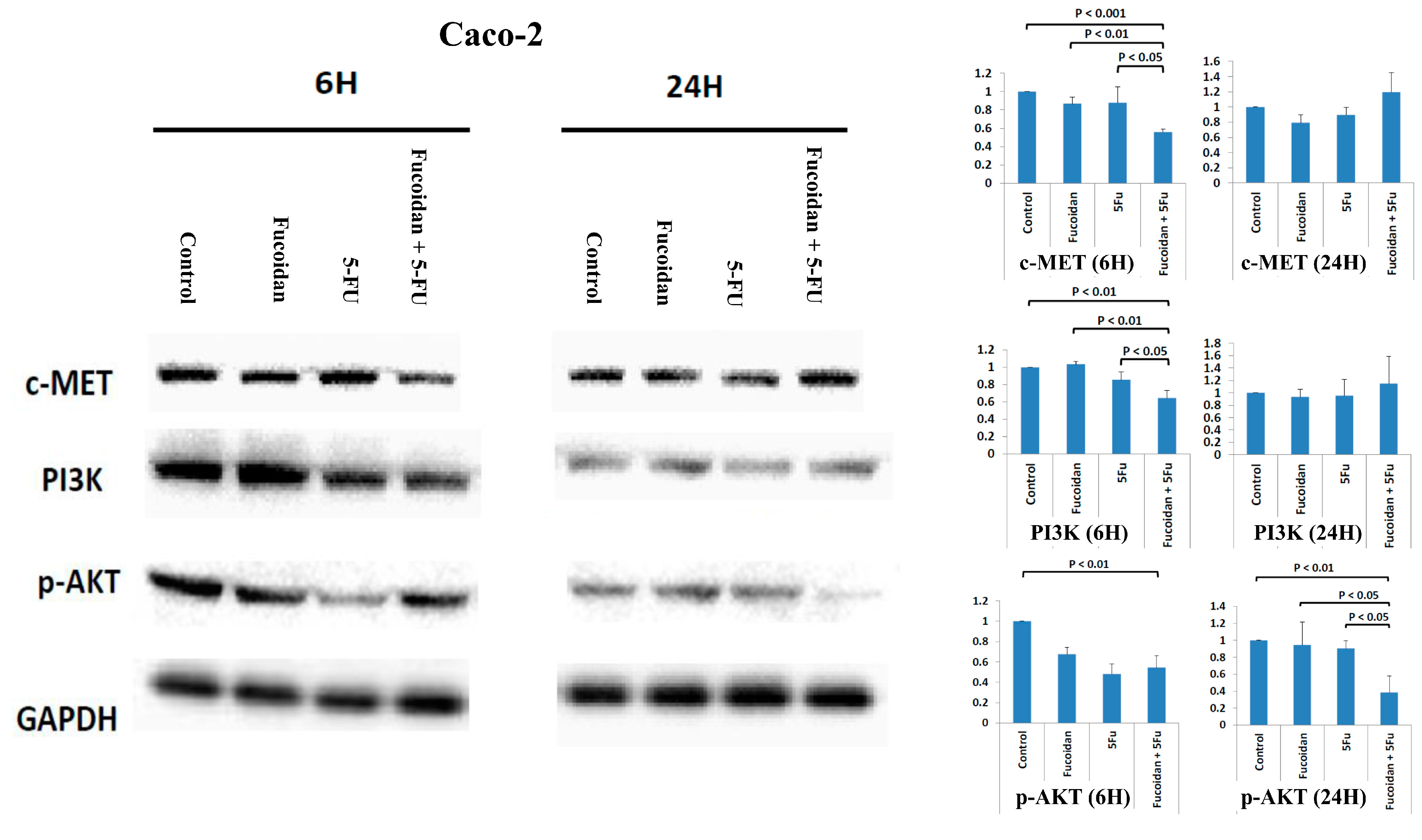
2.5. Effects of Signaling Pathways Responsible for LMWF and 5-FU on Decreased Viability of Caco-2 Cells
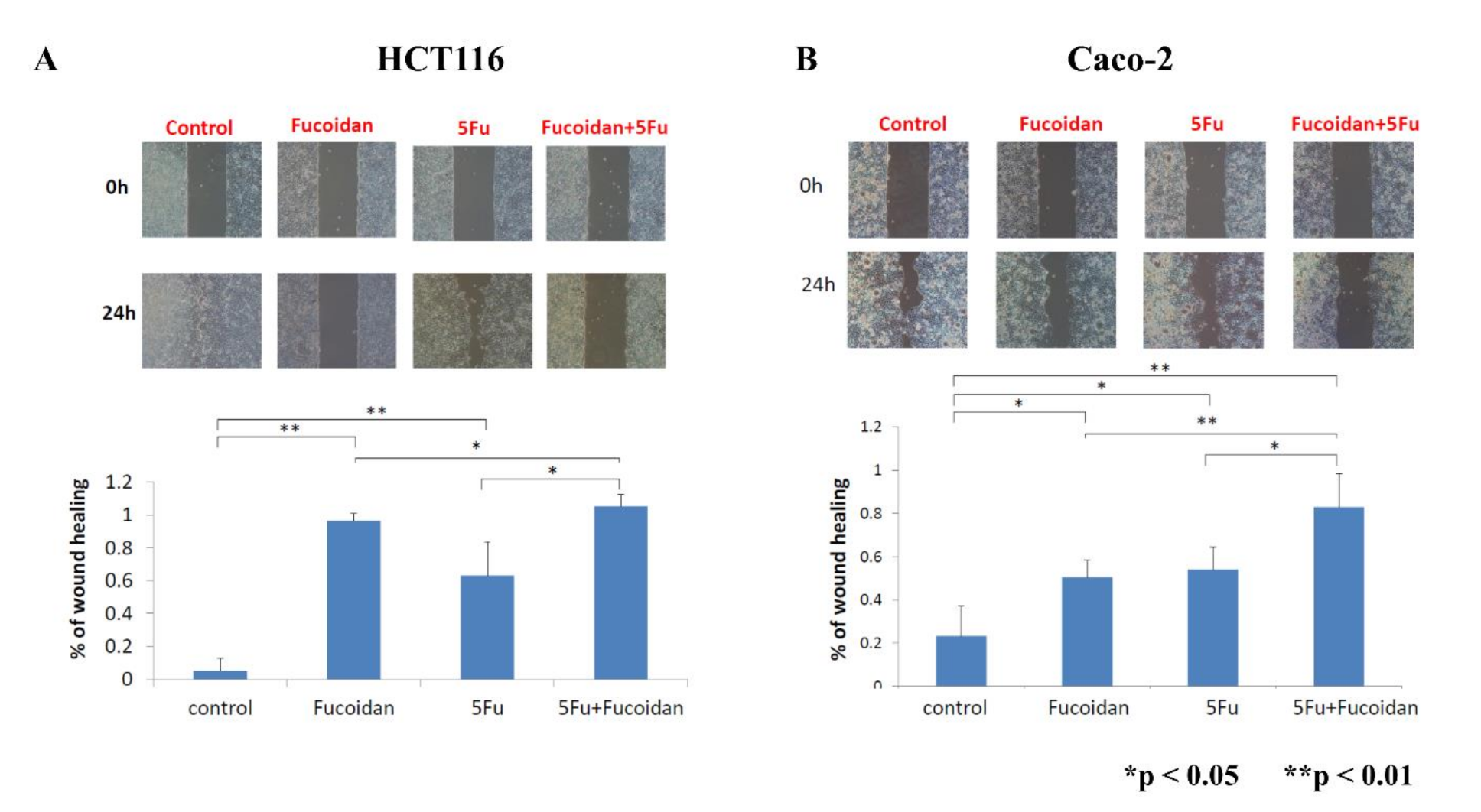
2.6. Effects of LMWF and 5-FU on Migration of Colon Cancer Cells
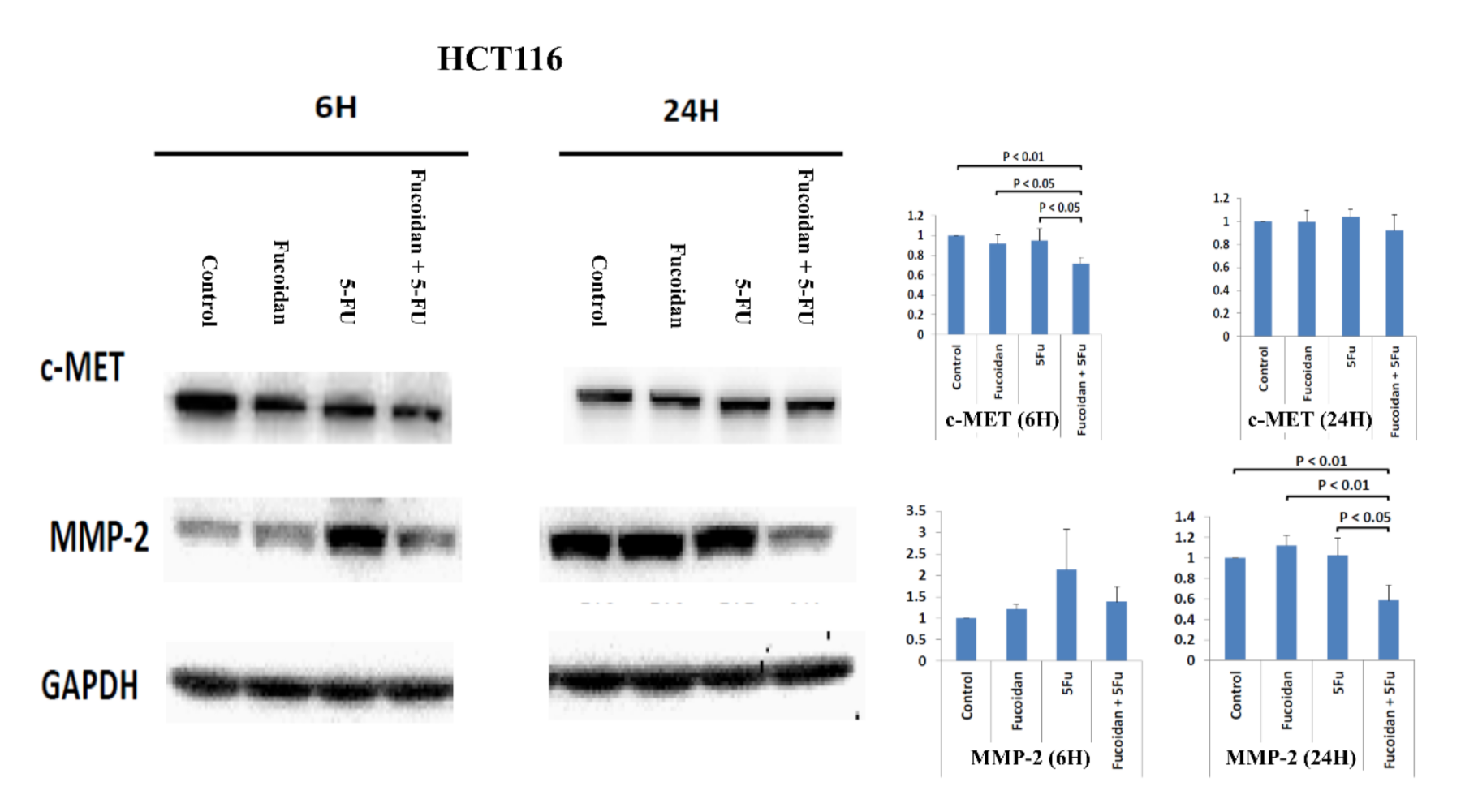
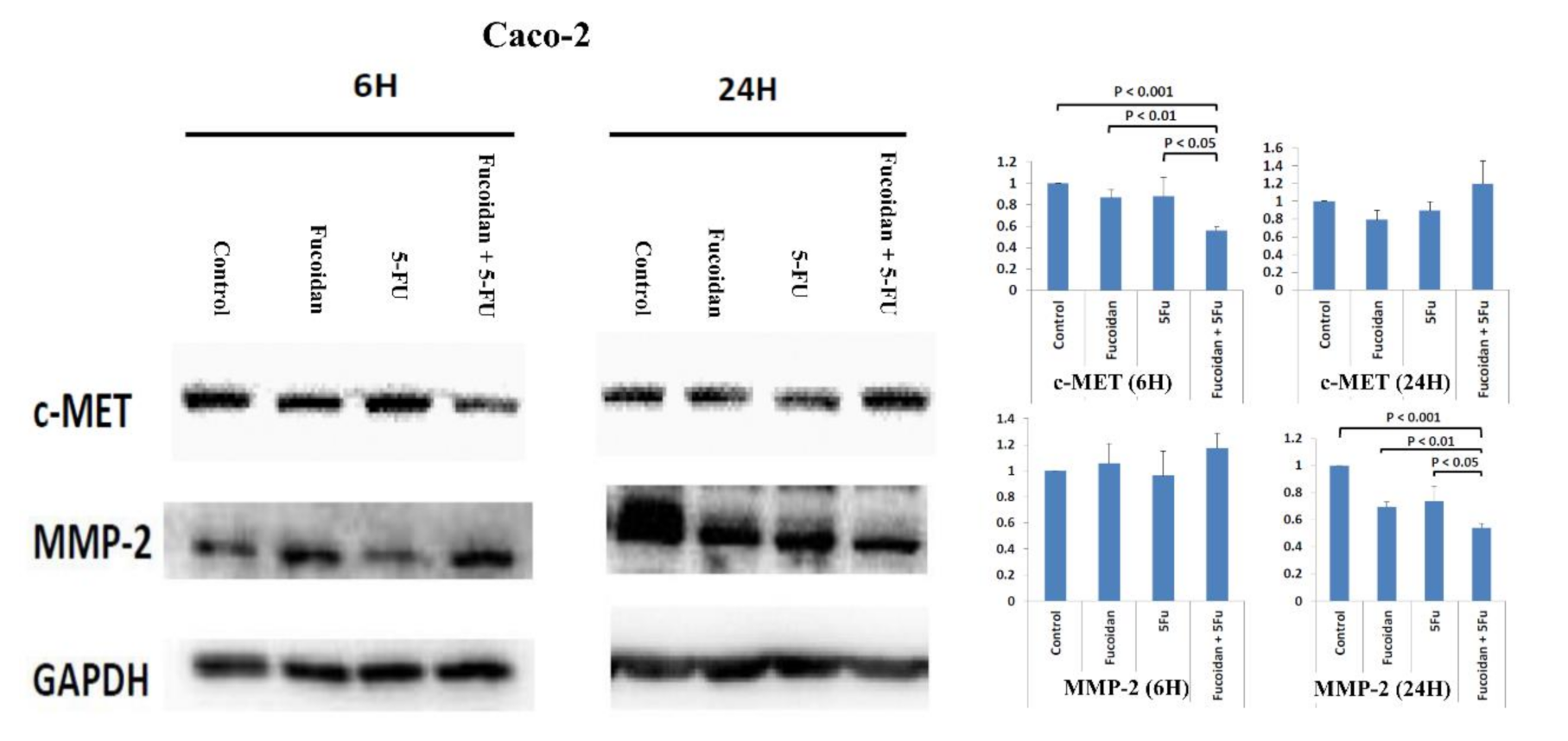
2.7. Effects of Signal Pathways Responsible for LMWF and 5-FU on Suppressive Effects of Migration in HCT 116 and Caco-2 Cells
3. Discussion
4. Materials and Methods
4.1. Preparation of LMF
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Wound-Healing Migration Assay
4.5. Cell Cycle Analysis
4.6. Analysis of Cell Apoptosis and Necrosis
4.7. Western Blot Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA 2019, 5, 1749–1768. [Google Scholar]
- Ministry of Health and Welfare, the Executive Yuan, Republic of China. Health and Vital Statistics. Available online: http://www.mohw.gov.tw/CHT/DOS/Statistic.aspx (accessed on 25 March 2021).
- Han, Y.S.; Lee, J.H.; Lee, S.H. Fucoidan inhibits the migration and proliferation of HT-29 human colon cancer cells via the phosphoinositide-3 kinase/Akt/mechanistic target of rapamycin pathways. Mol. Med. Rep. 2015, 12, 3446–3452. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.; Vinosha, M.; Marudhupandi, T.; Rajasekar, P.; Prabhu, N.M. Isolation of fucoidan from Sargassum polycystum brown algae: Structural characterization, in vitro antioxidant and anticancer activity. Int. J. Biol. Macromol. 2017, 102, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Van Weelden, G.; Bobiński, M.; Okła, K.; van Weelden, W.J.; Romano, A.; Pijnenborg, J.M.A. Fucoidan Structure and Activity in Relation to Anti-Cancer Mechanisms. Mar. Drugs 2019, 17, 32. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Qi, X.; Liu, H.; Xue, K.; Xu, S.; Tian, Z. The anti-cancer effects of fucoidan: A review of both in vivo and in vitro investigations. Cancer Cell Int. 2020, 20, 154. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Wu, H.; Liu, R. Overview on biological activities and molecular characteristics of sulfated polysaccharides from marine green algae in recent years. Mar. Drugs 2014, 12, 4984–5020. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.K. Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Sun, J.; Su, X.; Yu, Q.; Yu, Q.; Zhang, P. A review about the development of fucoidan in antitumor activity: Progress and challenges. Carbohydr. Polym. 2016, 154, 96–111. [Google Scholar] [CrossRef]
- Mabate, B.; Daub, C.D.; Malgas, S.; Edkins, A.L.; Pletschke, B.I. Fucoidan Structure and Its Impact on Glucose Metabolism: Implications for Diabetes and Cancer Therapy. Mar. Drugs. 2021, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Lee, J.S.; Kim, W.S.; Jeon, Y.J. The potential of brown-algae polysaccharides for the development of anticancer agents: An update on anticancer effects reported for fucoidan and laminaran. Carbohydr. Polym. 2017, 177, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.C.; Chang, T.K.; Su, W.C.; Tsai, H.L.; Wang, J.Y. Narrative review of the influence of diabetes mellitus and hyperglycemia on colorectal cancer risk and oncological outcomes. Transl. Oncol. 2021, 14, 101089. [Google Scholar] [CrossRef]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef]
- Tsai, H.L.; Tai, C.J.; Huang, C.W.; Chang, F.R.; Wang, J.Y. Efficacy of Low-Molecular-Weight Fucoidan as a Supplemental Therapy in Metastatic Colorectal Cancer Patients: A Double-Blind Randomized Controlled Trial. Mar. Drugs 2017, 15, 122. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.W.; Ma, C.J.; Su, W.C.; Chen, Y.T.; Tsai, H.L.; Yeh, Y.S.; Chang, T.K.; Hsu, W.H.; Yu, F.J.; Wang, J.Y. Prognostic Value of EGFR Expression for Patients with Stage III Colorectal Cancer Receiving Fluoropyrimidine Metronomic Maintenance Therapy after Radical Resection and Adjuvant Oxaliplatin-Based Chemotherapy. Oncol. Res. 2020. [Google Scholar] [CrossRef]
- Berg, K.C.G.; Eide, P.W.; Eilertsen, I.A.; Johannessen, B.; Bruun, J.; Danielsen, S.A.; Bjørnslett, M.; Meza-Zepeda, L.A.; Eknæs, M.; Lind, G.E.; et al. Multi-omics of 34 colorectal cancer cell lines—A resource for biomedical studies. Mol. Cancer 2017, 16, 116. [Google Scholar] [CrossRef]
- Dhanasekaran, D.N.; Reddy, E.P. JNK signaling in apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sui, X.; Kong, N.; Ye, L.; Han, W.; Zhou, J.; Zhang, Q.; He, C.; Pan, H. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014, 344, 174–179. [Google Scholar] [CrossRef]
- Dhanasekaran, D.N.; Reddy, E.P. JNK-signaling: A multiplexing hub in programmed cell death. Genes Cancer 2017, 8, 682–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Z.; El-Deiry, W.S. Overview of cell death signaling pathways. Cancer Biol. Ther. 2005, 4, 139–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Safaie Qamsari, E.; Safaei Ghaderi, S.; Zarei, B.; Dorostkar, R.; Bagheri, S.; Jadidi-Niaragh, F.; Somi, M.H.; Yousefi, M. The c-Met receptor: Implication for targeted therapies in colorectal cancer. Tumour Biol. 2017, 39, 1010428317699118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Feng, Q.; Chen, W.D.; Wang, Y.D. HGF/c-MET: A Promising Therapeutic Target in the Digestive System Cancers. Int. J. Mol. Sci. 2018, 19, 3295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunt, S.J.; Chaudary, N.; Hill, R.P. The tumor microenvironment and metastatic disease. Clin. Exp. Metastasis 2009, 26, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Hsu, W.L.; Hwang, P.A.; Chen, Y.L.; Chou, T.C. Combined administration of fucoidan ameliorates tumor and chemotherapy-induced skeletal muscle atrophy in bladder cancer-bearing mice. Oncotarget 2016, 7, 51608–51618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troiani, T.; Napolitano, S.; Della Corte, C.M.; Martini, G.; Martinelli, E.; Morgillo, F.; Ciardiello, F. Therapeutic value of EGFR inhibition in CRC and NSCLC: 15 years of clinical evidence. ESMO Open 2016, 1, e000088. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.H.; Kwon, M.J.; Nam, T.J. Differences in cell death and cell cycle following fucoidan treatment in high-density HT-29 colon cancer cells. Mol. Med. Rep. 2017, 15, 4116–4122. [Google Scholar] [CrossRef] [Green Version]
- Park, H.Y.; Park, S.H.; Jeong, J.W.; Yoon, D.; Han, M.H.; Lee, D.S.; Choi, G.; Yim, M.J.; Lee, J.M.; Kim, D.H.; et al. Induction of p53-Independent Apoptosis and G1 Cell Cycle Arrest by Fucoidan in HCT116 Human Colorectal Carcinoma Cells. Mar. Drugs 2017, 15, 154. [Google Scholar] [CrossRef] [Green Version]
- Narayani, S.S.; Saravanan, S.; Ravindran, J.; Ramasamy, M.S.; Chitra, J. In vitro anticancer activity of fucoidan extracted from Sargassum cinereum against Caco-2 cells. Int. J. Biol. Macromol. 2019, 138, 618–628. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Hwang, P.A. Clinical applications of fucoidan in translational medicine for adjuvant cancer therapy. Clin. Transl. Med. 2019, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. The effect of sulfated (1→3)-α-l-fucan from the brown alga Saccharina cichorioides Miyabe on resveratrol-induced apoptosis in colon carcinoma Cells. Mar. Drugs 2013, 11, 194–212. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.O.; Song, M.G.; Kim, Y.N.; Park, J.I.; Kwak, J.Y. The mechanism of fucoidan-induced apoptosis in leukemic cells: Involvement of ERK1/2, JNK, glutathione, and nitric oxide. Mol. Carcinog. 2010, 49, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, Y.; Zhang, Y.; Zhang, D. Fucoidan induces cancer cell apoptosis by modulating the endoplasmic reticulum stress cascades. PLoS ONE 2014, 9, e108157. [Google Scholar] [CrossRef]
- Kim, I.H.; Nam, T.J. Fucoidan downregulates insulin-like growth factor-I receptor levels in HT-29 human colon cancer cells. Oncol. Rep. 2018, 39, 1516–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, H.; Yang, Y.; Wei, H.; Liu, Z.; Liu, Z.; Ma, Y.; Gao, Z.; Hou, L.; Zou, X. Fucoidan Suppresses Hypoxia-Induced Lymphangiogenesis and Lymphatic Metastasis in Mouse Hepatocarcinoma. Mar. Drugs 2015, 13, 3514–3530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-W.; Chen, Y.-C.; Yin, T.-C.; Chen, P.-J.; Chang, T.-K.; Su, W.-C.; Ma, C.-J.; Li, C.-C.; Tsai, H.-L.; Wang, J.-Y. Low-Molecular-Weight Fucoidan as Complementary Therapy of Fluoropyrimidine-Based Chemotherapy in Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 8041. https://doi.org/10.3390/ijms22158041
Huang C-W, Chen Y-C, Yin T-C, Chen P-J, Chang T-K, Su W-C, Ma C-J, Li C-C, Tsai H-L, Wang J-Y. Low-Molecular-Weight Fucoidan as Complementary Therapy of Fluoropyrimidine-Based Chemotherapy in Colorectal Cancer. International Journal of Molecular Sciences. 2021; 22(15):8041. https://doi.org/10.3390/ijms22158041
Chicago/Turabian StyleHuang, Ching-Wen, Yen-Cheng Chen, Tzu-Chieh Yin, Po-Jung Chen, Tsung-Kun Chang, Wei-Chih Su, Cheng-Jen Ma, Ching-Chun Li, Hsiang-Lin Tsai, and Jaw-Yuan Wang. 2021. "Low-Molecular-Weight Fucoidan as Complementary Therapy of Fluoropyrimidine-Based Chemotherapy in Colorectal Cancer" International Journal of Molecular Sciences 22, no. 15: 8041. https://doi.org/10.3390/ijms22158041
APA StyleHuang, C.-W., Chen, Y.-C., Yin, T.-C., Chen, P.-J., Chang, T.-K., Su, W.-C., Ma, C.-J., Li, C.-C., Tsai, H.-L., & Wang, J.-Y. (2021). Low-Molecular-Weight Fucoidan as Complementary Therapy of Fluoropyrimidine-Based Chemotherapy in Colorectal Cancer. International Journal of Molecular Sciences, 22(15), 8041. https://doi.org/10.3390/ijms22158041






