Brillouin Spectroscopy: From Biomedical Research to New Generation Pathology Diagnosis
Abstract
:1. Introduction
2. Brillouin Light Scattering
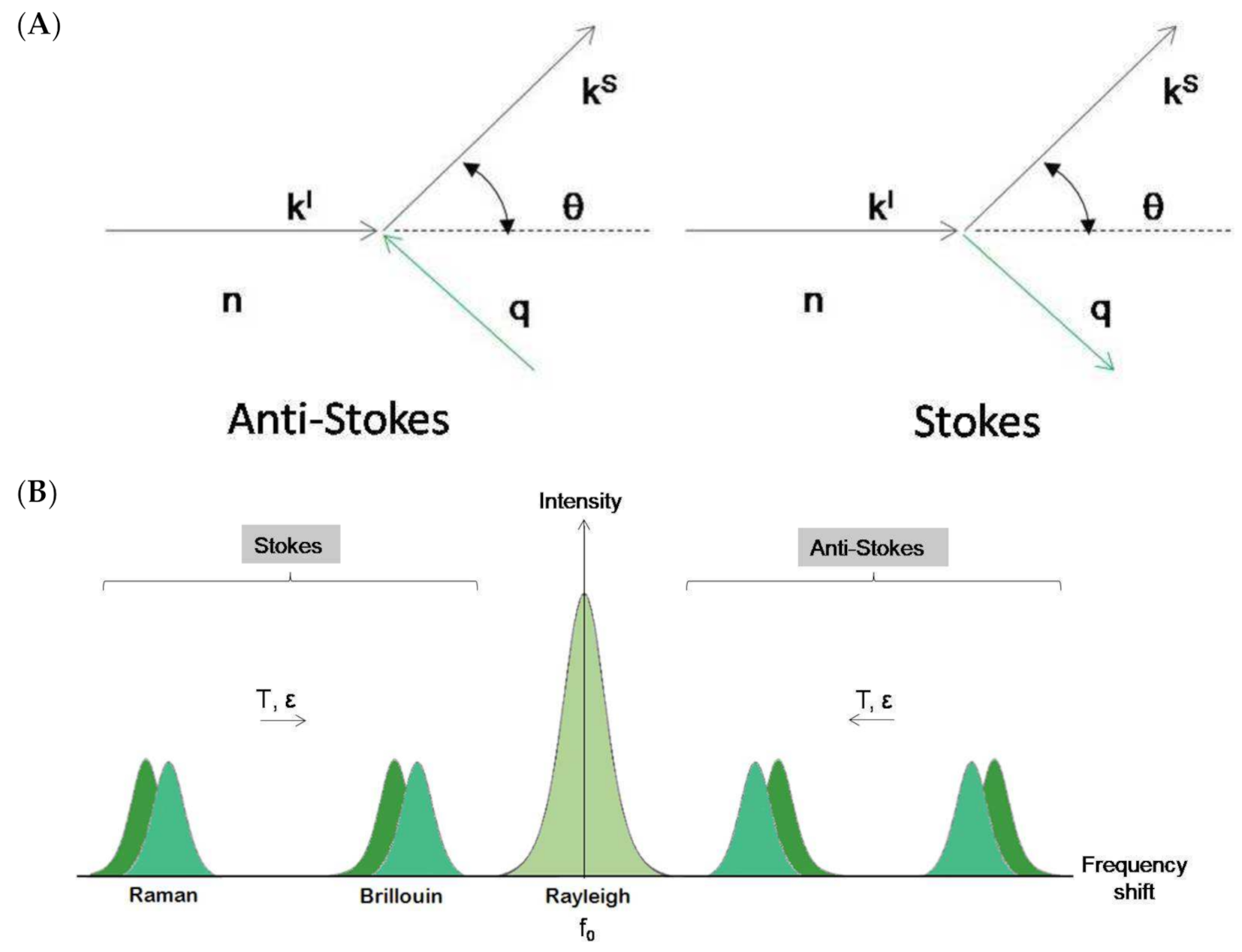
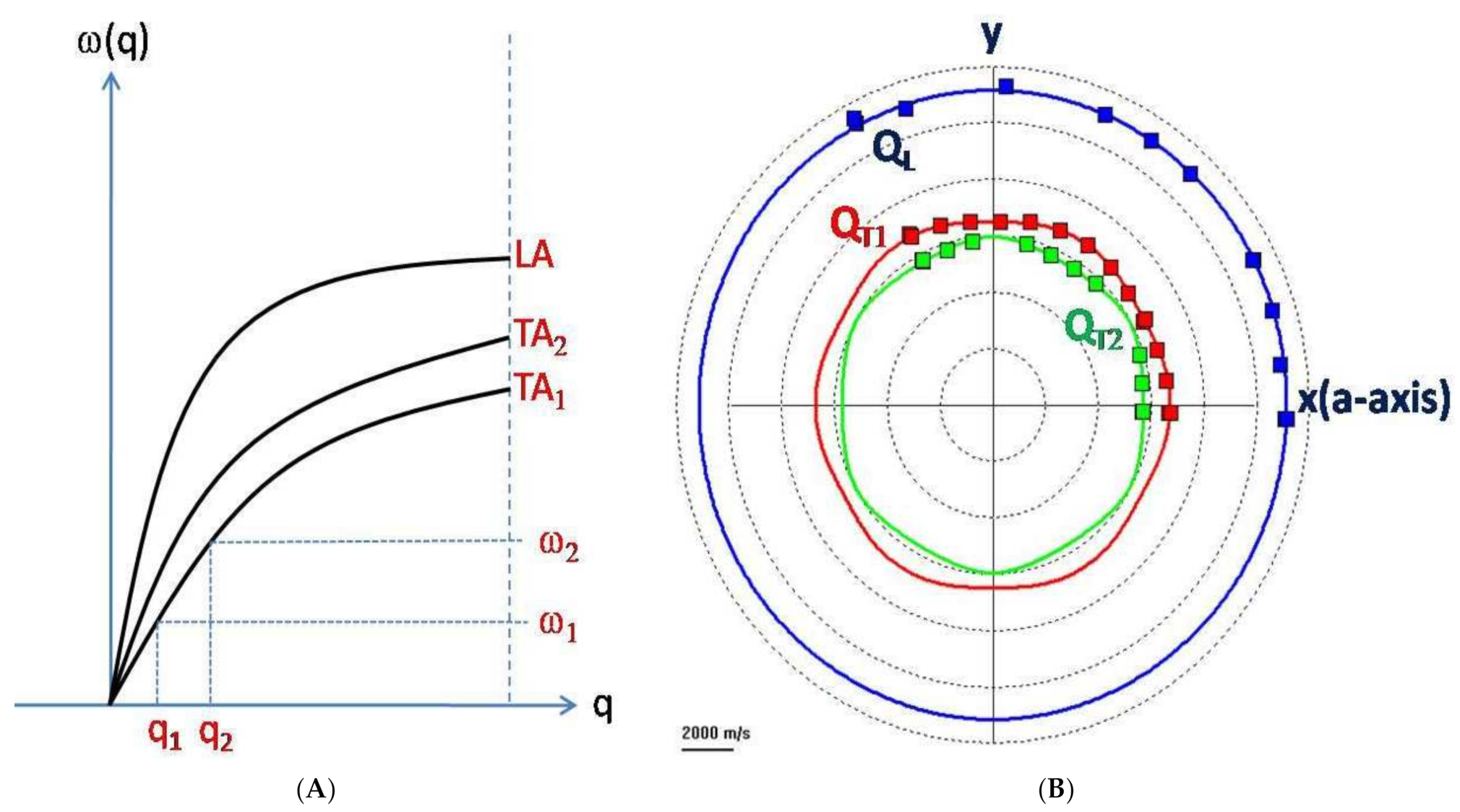
2.1. Physical Basis
ωs − ωi = ±ω(q) (energy conservation)
2.2. Experimental Setup: Scattering Geometries
2.3. Mechano–Elastic Information of the Brillouin Signal
- c12 = c11 − 2c44
- Shear modulus: μ, G = c44
- Young’s modulus: Y, E = c44 (3c11 − 4c44)/(c11 − c44)
- Poisson’s number: ν = (c11 − 2c44)/(2c11 − 2c44)
- Adiabatic Bulk Modulus: .
2.4. Instrumentation
Influence of the Testing Frequency
2.5. Brillouin Scattering and Optical Tissue Clearing
- Several clearing methods have high osmotic power and change the volume of the sample [52]. According to Scarcelli et al., sucrose-induced hyperosmotic shock shrinkage causes a linear increase in the Brillouin shift in NHC 3T3 cells [54]. This is a critical issue to be considered for any clearing method used in a Brillouin spectroscopic system. For instance, organic solvent-based methods such as 3DISCO or BABB or simple immersion RI matching agents such as TDE cause significant sample shrinkage during the dehydration step that may substantially alter the Brillouin frequency shift [55]. Although not reported for cellular expansion, hyperhydration clearing methods may cause similar artifacts as well.
- The basic premise when using optical clearing to improve the Brillouin signal is that the frequency shift must not be altered. Whether this is true is not yet clear. Some experiments revealed significant differences in Brillouin shifts after decalcification with EDTA [46,47]. Additionally, it is expected that delipidating clearing agents affect the elasticity of the sample due to lipid removal [32]. Further studies of different clearing protocols are recommended.
- Most clearing methods require 4% PFA fixation to prevent tissue degradation during optical clearing and to allow long-term preservation. PFA acts by linking lysine amino acids of contiguous protein networks and has been reported to increase the Brillouin shift of HeLa cells [49,52]. Therefore, prior fixation of optical tissue clearing methods likely hinders Brillouin signals of tissue samples.
3. Biological Applications of Brillouin
3.1. Cell Biology Research
3.1.1. Subcellular Analysis
3.1.2. Microbial Biofilms
3.2. Animal Tissue Biology: Preclinical Studies
3.2.1. Vascular Applications
3.2.2. Nervous System Applications
CSF Screening
Amyloid Plaques in Alzheimer’s Disease
3.2.3. Ophthalmological Applications
Cornea
Lens
3.2.4. The ECM and Its Fibrous Proteins
3.2.5. Other Applications in Animal Models
Melanoma
Muscle
Adipose Tissue
Bone and Articular Cartilage
3.3. Brillouin in Development Biology
3.3.1. Zebrafish Spinal Cord
3.3.2. Mouse Embryogenesis
3.3.3. Pharynx of Caenorhabditis elegans
3.4. Applications of Brillouin to Diagnosis
3.4.1. Ophthalmology
3.4.2. Bone
4. Brillouin as a Pathology Diagnosis Tool
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Moon, S.; Chen, J.J.; Zhu, Z.; Chen, Z. Ultrahigh-sensitive optical coherence elastography. Light Sci. Appl. 2020, 9, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brillouin, L. Diffusion de la lumière et des rayons X par un corps transparent homogène. Ann. Phys. 1922, 9, 88–122. [Google Scholar] [CrossRef]
- Brillouin, L. Influence de l’agitationthermique sur la viscosité des liquides. Propagationd’ondesélastiquesdans un milieu en mouvement. J. Phys. Radium 1922, 3, 362383. [Google Scholar] [CrossRef]
- Carlotti, G. Elastic Characterization of Transparent and Opaque Films, Multilayers and Acoustic Resonators by Surface Brillouin Scattering: A Review. Appl. Sci. 2018, 8, 124. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, P. Light Scattering in Inhomogeneous Media; McGraw-Hill Education: New York, NY, USA, 2010. [Google Scholar]
- Mandelstam, L.I. Light scattering by inhomogeneus media. Zh. Russ. Fiz-Khim. Ova. 1926, 58, 381. [Google Scholar]
- Pechenkin, A. Leonid Isaakovich Mandelstam; Springer: Cham, Switzerland, 2014; ISBN 9783319005713. [Google Scholar]
- Gross, E. überÄnderung der WellenlÄngebeiLichtzerstreuung in Kristallen. Z. Fur Phys. 1930, 63, 685. [Google Scholar] [CrossRef]
- Rank, D.H. Brillouin Effect in Liquids in the Prelaser Era. J. Acoust. Soc. Am. 1971, 49, 937–940. [Google Scholar] [CrossRef]
- Harley, R.; James, D.F.V.; Miller, A.J.; White, J.W. Phonons and the elastic moduli of collagen and muscle. Nature 1977, 267, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, J.M.; Randall, J.T. Brillouin scattering, density and elastic properties of the lens and cornea of the eye. Nature 1980, 284, 489–491. [Google Scholar] [CrossRef]
- Shirasaki, M. Large angular dispersion by a virtually imaged phased array and its application to a wavelength demultiplexer. Opt. Lett. 1996, 21, 366–368. [Google Scholar] [CrossRef]
- Koski, K.J.; Yarger, J.L. Brillouin imaging. Appl. Phys. Lett. 2005, 87, 061903. [Google Scholar] [CrossRef]
- Coker, Z.; Troyanova-Wood, M.; Traverso, A.J.; Yakupov, T.; Utegulov, Z.N.; Yakovlev, V.V. Assessing performance of modern Brillouin spectrometers. Opt. Express 2018, 26, 2400–2409. [Google Scholar] [CrossRef] [PubMed]
- Antonacci, G.; Beck, T.; Bilenca, A.; Czarske, J.; Elsayad, K.; Guck, J.; Kim, K.; Krug, B.; Palombo, F.; Prevedel, R.; et al. Recent progress and current opinions in Brillouin microscopy for life science applications. Biophys. Rev. 2020, 12, 615–624. [Google Scholar] [CrossRef]
- Kragh, H. The Lorenz-Lorentz formula: Origin and early history. Substantia 2018, 2, 7–18. [Google Scholar]
- Carpenter, D.K. Dynamic Light Scattering with Applications to Chemistry, Biology, and Physics (Berne, Bruce J.; Pecora, Robert). J. Chem. Educ. 1977, 54, A430. [Google Scholar] [CrossRef] [Green Version]
- Berne, B.J.; Pecora, R. Dynamic Light Scattering: With Applications to Chemistry, Biology, and Physics; Dover Publications: Mineola, NY, USA, 1976. [Google Scholar]
- Garmire, E. Stimulated Brillouin Review: Invented 50 Years Ago and Applied Today. Int. J. Opt. 2018, 2018, 2459501. [Google Scholar] [CrossRef]
- Remer, I.; Shaashoua, R.; Shemesh, N.; Ben-Zvi, A.; Bilenca, A. High-sensitivity and high-specificity biomechanical imaging by stimulated Brillouin scattering microscopy. Nat. Methods 2020, 17, 913–916. [Google Scholar] [CrossRef]
- Freudiger, C.W.; Min, W.; Saar, B.G.; Lu, S.; Holtom, G.R.; He, C.; Tsai, J.C.; Kang, J.X.; Xie, X.S. Label-Free Biomedical Imaging with High Sensitivity by Stimulated Raman Scattering Microscopy. Science 2008, 322, 1857–1861. [Google Scholar] [CrossRef] [Green Version]
- Scarponi, F.; Mattana, S.; Corezzi, S.; Caponi, S.; Comez, L.; Sassi, P.; Morresi, A.; Paolantoni, M.; Urbanelli, L.; Emiliani, C.; et al. High-Performance Versatile Setup for Simultaneous Brillouin-Raman Microspectroscopy. Phys. Rev. X 2017, 7, 031015. [Google Scholar] [CrossRef] [Green Version]
- Krüger, J.K. Optical Techniques to Characterize Polymer Systems; Bassler, H., Ed.; Elsevier: New York, NY, USA, 1989; 610p. [Google Scholar]
- Krüger, J.K.; Embs, J.; Brierley, J.; Jiménez, R. A new Brillouin scattering technique for the investigation of acoustic and opto-acoustic properties: Application to polymers. J. Phys. D Appl. Phys. 1998, 31, 1913–1917. [Google Scholar] [CrossRef]
- Mutti, P.; Bottani, C.; Ghislotti, G.; Beghi, M.; Briggs, G.; Sandercock, J. Surface Brillouin Scattering—Extending Surface Wave Measurements to 20 GHz. In Advances in Acoustic Microscopy; Springer: Boston, MA, USA, 2011. [Google Scholar]
- Sebastian, T.; Schultheiss, K.; Obry, B.; Hillebrands, B.; Schultheiss, H. Micro-focused Brillouin light scattering: Imaging spin waves at the nanoscale. Front. Phys. 2015, 3. [Google Scholar] [CrossRef] [Green Version]
- Kittel, C. Introduction to Solid State Physics, 3rd ed.; Wiley: New York, NY, USA, 1967. [Google Scholar]
- Ashcroft, N.W.; Mermin, N.D. Solid State Physics; International Edition; Saunders College Publishing: Rochester, NY, USA, 1988. [Google Scholar]
- Fecht, H.J.; Wunderlich, R.; Battezzati, L.; Etay, J.; Ricci, E.; Seetharaman, S.; Egry, I. Thermophysical Properties of Materials. Phys. Space 2008, 39, 19–21. [Google Scholar] [CrossRef] [Green Version]
- Grimvall, G. Thermophysical Properties of Materials; Elsevier BV: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Meng, Z.; Traverso, A.J.; Ballmann, C.W.; Troyanova-Wood, M.A.; Yakovlev, V.V. Seeing cells in a new light: A renaissance of Brillouin spectroscopy. Adv. Opt. Photonics 2016, 8, 300–327. [Google Scholar] [CrossRef]
- Poon, C.; Chou, J.; Cortie, M.; Kabakoba, I. Brillouin imaging for studies of micromechanics in biology and biomedicine: From current state-of-the-art to future clinical translation. J. Phys. Photonics 2020, 3, 012002. [Google Scholar] [CrossRef]
- Wu, P.-J.; Masouleh, M.I.; Dini, D.; Paterson, C.; Török, P.; Overby, D.R.; Kabakova, I.V. Detection of proteoglycan loss from articular cartilage using Brillouin microscopy, with applications to osteoarthritis. Biomed. Opt. Express 2019, 10, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Sandercock, J.R. Light Scattering in Solids III; Cardona, M., Güntherodt, G., Eds.; Springer: Berlin/Heidelberg, Germany, 1982. [Google Scholar]
- Scarcelli, G.; Yun, S.H. Confocal Brillouin microscopy for three-dimensional mechanical imaging. Nat. Photonics 2007, 2, 39–43. [Google Scholar] [CrossRef]
- So, P. Brillouin bioimaging. Nat. Photonics 2008, 2, 13–14. [Google Scholar] [CrossRef]
- Traverso, A.J.; Thompson, J.V.; Steelman, Z.A.; Meng, Z.; Scully, M.O.; Yakovlev, V.V. Dual Raman-Brillouin Microscope for Chemical and Mechanical Characterization and Imaging. Anal. Chem. 2015, 87, 7519–7523. [Google Scholar] [CrossRef]
- Fiore, A.; Scarcelli, G. Single etalon design for two-stage cross-axis VIPA spectroscopy. Biomed. Opt. Express 2019, 10, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Kabakova, I.V.; Xiang, Y.; Paterson, C.; Török, P. Fiber-integrated Brillouin microspectroscopy: Towards Brillouin endoscopy. J. Innov. Opt. Health Sci. 2017, 10, 1742002. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Y.; Basirun, C.; Chou, J.; Warkiani, M.E.; Török, P.; Wang, Y.; Gao, S.; Kabakova, I.V. Background-free fibre optic Brillouin probe for remote mapping of micromechanics. Biomed. Opt. Express 2020, 11, 6687–6698. [Google Scholar] [CrossRef] [PubMed]
- Vacher, R.; Boyer, L. Brillouin Scattering: A Tool for the Measurement of Elastic and Photoelastic Constants. Phys. Rev. B 1972, 6, 639–673. [Google Scholar] [CrossRef]
- Zhang, H.; Ferrer, M.L.; Ruiz, M.J.R.; Jiménez-Riobóo, R.J.; Gutierrez, M.C.; Del Monte, F. Brillouin Spectroscopy as a Suitable Technique for the Determination of the Eutectic Composition in Mixtures of Choline Chloride and Water. J. Phys. Chem. B 2020, 124, 4002–4009. [Google Scholar] [CrossRef]
- Jiménez-Riobóo, R.J.; Artús, L.; Cuscó, R.; Taniguchi, T.; Cassabois, G.; Gil, B. In- and out-of-plane longitudinal acoustic-wave velocities and elastic moduli in h-BN from Brillouin scattering measurements. Appl. Phys. Lett. 2018, 112, 051905. [Google Scholar] [CrossRef]
- Scarcelli, G.; Yun, S.H. In vivo Brillouin optical microscopy of the human eye. Opt. Express 2012, 20, 9197–9202. [Google Scholar] [CrossRef] [Green Version]
- Riobóo, R.J.J.; Desco, M.; Gómez-Gaviro, M.V. Impact of optical tissue clearing on the Brillouin signal from biological tissue samples. Biomed. Opt. Express 2019, 10, 2674–2683. [Google Scholar] [CrossRef]
- Fukui, K.; Takayanagi, S.; Suga, D.; Matsukawa, M. Measurement of Wave Velocity in Cortical Bone by Micro-Brillouin Scattering Technique: Effect of Bone Tissue Properties. Jpn. J. Appl. Phys. 2012, 51, 07GF20. [Google Scholar] [CrossRef]
- Matsukawa, M.; Tsubota, R.; Kawabe, M.; Fukui, K. Application of a micro-Brillouin scattering technique to characterize bone in the GHz range. Ultrasonics 2014, 54, 1155–1161. [Google Scholar] [CrossRef]
- Lepert, G.; Gouveia, R.; Connon, C.; Paterson, C. Assessing corneal biomechanics with Brillouin spectro-microscopy. Faraday Discuss. 2016, 187, 415–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonacci, G.; De Turris, V.; Rosa, A.; Ruocco, G. Background-deflection Brillouin microscopy reveals altered biomechanics of intracellular stress granules by ALS protein FUS. Commun. Biol. 2018, 1, 139. [Google Scholar] [CrossRef]
- Mattana, S.; Mattarelli, M.; Urbanelli, L.; Sagini, K.; Emiliani, C.; Serra, M.D.; Fioretto, D.; Caponi, S. Non-contact mechanical and chemical analysis of single living cells by microspectroscopic techniques. Light Sci. Appl. 2018, 7, 17139. [Google Scholar] [CrossRef] [Green Version]
- Prevedel, R.; Diz-Muñoz, A.; Ruocco, G.; Antonacci, G. 2019. Brillouin microscopy—A revolutionary tool for mechanobiology. Nat. Methods 2019, 16, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Tainaka, K.; Kuno, A.; Kubota, S.I.; Murakami, T.; Ueda, H.R. Chemical Principles in Tissue Clearing and Staining Protocols for Whole-Body Cell Profiling. Annu. Rev. Cell Dev. Biol. 2016, 32, 713–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, S.T.; Twa, M.D.; Gump, J.C.; Venkiteshwar, M.; Bullimore, M.A.; Sooryakumar, R. Light-Scattering Study of the Normal Human Eye Lens: Elastic Properties and Age Dependence. IEEE Trans. Biomed. Eng. 2010, 57, 2910–2917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarcelli, G.; Polacheck, W.J.; Nia, H.; Patel, K.; Grodzinsky, A.J.; Kamm, R.D.; Yun, S.H. Noncontact three-dimensional mapping of intracellular hydromechanical properties by Brillouin microscopy. Nat. Methods 2015, 12, 1132–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, D.S.; Lichtman, J.W. Clarifying Tissue Clearing. Cell 2015, 162, 246257. [Google Scholar] [CrossRef] [Green Version]
- Antonacci, G.; Braakman, S. Biomechanics of subcellular structures by non-invasive Brillouin microscopy. Sci. Rep. 2016, 6, 37217. [Google Scholar] [CrossRef] [Green Version]
- Rossignol, C.; Chigarev, N.; Ducousso Audoin, B.; Forget, G.; Guillemot, F.; Durrieu, M.C. In vitro picosecond ultrasonics in a single cell. Appl. Phys. Lett. 2008, 93, 123901. [Google Scholar] [CrossRef]
- Doster, W.; Simon, B.; Schmidt, G.; Mayr, W. Compressibility of lysozyme in solution from time-resolved brillouin difference spectroscopy. Biopolymers 1985, 24, 15438. [Google Scholar] [CrossRef]
- Tao, N.J.; Lindsay, S.M.; Rupprecht, A. The dynamics of the DNA hydration shell at gigahertz frequencies. Biopolymers 1987, 26, 171–188. [Google Scholar] [CrossRef]
- Lee, S.A.; Lindsay, S.M.; Powell, J.W.; Weidlich, T.; Tao, N.J.; Lewen, G.D.; Rupprecht, A. A Brillouin scattering study of the hydration of Li- and Na-DNA films. Biopolymers 1987, 26, 1637–1665. [Google Scholar] [CrossRef]
- Tao, N.J.; Lindsay, S.M.; Rupprecht, A. Dynamic coupling between DNA and its primary hydration shell studied by brillouin scattering. Biopolymers 1988, 27, 1655–1671. [Google Scholar] [CrossRef]
- Hartschuh, R.D.; Wargacki, S.P.; Xiong, H.; Neiswinger, J.; Kisliuk, A.; Sihn, S.; Ward, V.; Vaia, R.A.; Sokolov, A.P. How rigid are viruses. Phys. Rev. E 2008, 78, 021907. [Google Scholar] [CrossRef]
- Zhang, J.; Nou, X.A.; Kim, H.; Scarcelli, G. Brillouin flow cytometry for label-free mechanical phenotyping of the nucleus. Lab Chip 2017, 17, 663–670. [Google Scholar] [CrossRef]
- Francesconi, R.; Ottani, S. Correlation of density and refraction index for liquid binary mixtures containing polyglycols. Use of the group contributions in the Lorentz–Lorenz, Gladstone–Dale and Vogel equations to evaluate the density of mixtures. J. Mol. Liq. 2007, 133, 125–133. [Google Scholar] [CrossRef]
- Hoenders, B.J. The Painful Derivation of the Refractive Index from Microscopical Considerations. In Lecture Notes in Electrical Engineering; Tata, D.B., Waynant, R.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Badur, T.; Dams, C.; Hampp, N. High Refractive Index Polymers by Design. Macromolecules 2018, 51, 4220–4228. [Google Scholar] [CrossRef]
- Matthews, J.N.A.; Yim, P.B.; Jacobs, D.T.; Forbes, J.G.; Peters, N.D.; Greer, S.C. The polymerization of actin: Extent of polymerization under pressure, volume change of polymerization, and relaxation after temperature jumps. J. Chem. Phys. 2005, 123, 074904. [Google Scholar] [CrossRef]
- Meng, Z.; Lopez, S.C.B.; Meissner, K.E.; Yakovlev, V.V. Subcellular measurements of mechanical and chemical properties using dual RamanBrillouin microspectroscopy. J. Biophotonics 2016, 9, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Alisafaei, F.; Nikolić, M.; Nou, X.A.; Kim, H.; Shenoy, V.B.; Scarcelli, G. Nuclear Mechanics within Intact Cells Is Regulated by Cytoskeletal Network and Internal Nanostructures. Small 2020, 16, e1907688. [Google Scholar] [CrossRef]
- Nikolić, M.; Scarcelli, G. Long-term Brillouin imaging of live cells with reduced absorption-mediated damage at 660nm wavelength. Biomed. Opt. Express 2019, 10, 1567–1580. [Google Scholar] [CrossRef]
- Zhang, J.; Nou, X.A.; Kim, H.; Scarcelli, G. Mechanical properties of cellular nucleus characterized by Brillouin flow cytometry. In Proceedings of the Frontiers in Optics 2017, Washington, DC, USA, 18–21 September 2017; Optical Society of America: Washington, DC, USA, 2017. [Google Scholar]
- Roscini, L.; Vassiliou, A.; Corte, L.; Pierantoni, D.C.; Robert, V.; Tascini, C.; Mattana, S.; Cardinali, M.A.; Orfanos, S.E.; Fioretto, D.; et al. Yeast Biofilm as a Bridge Between Medical and Environmental Microbiology Across Different Detection Techniques. Infect. Dis. Ther. 2018, 7, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Mattana, S.; Cardinali, M.A.; Caponi, S.; Pierantoni, D.C.; Corte, L.; Roscini, L.; Cardinali, G.; Fioretto, D. High-contrast Brillouin and Raman micro-spectroscopy for simultaneous mechanical and chemical investigation of microbial biofilms. Biophys. Chem. 2017, 229, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Karampatzakis, A.; Song, C.Z.; Allsopp, L.; Filloux, A.; Rice, S.A.; Cohen, Y.; Wohland, T.; Török, P. Probing the internal micromechanical properties of Pseudomonas aeruginosa biofilms by Brillouin imaging. Npj Biofilms Microbiomes 2017, 3, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonacci, G.; Pedrigi, R.M.; Kondiboyina, A.; Mehta, V.V.; Silva, R.D.; Paterson, C.; Krams, R.; Török, P. Quantification of plaque stiffness by Brillouin microscopy in experimental thin cap fibroatheroma. J. R. Soc. Interface 2015, 12, 20150843. [Google Scholar] [CrossRef]
- Steelman, Z.; Meng, Z.; Traverso, A.J.; Yakovlev, V.V. Brillouin spectroscopy as a new method of screening for increased CSF total protein during bacterial meningitis. J. Biophotonics 2014, 8, 408–414. [Google Scholar] [CrossRef]
- Mattana, S.; Caponi, S.; Tamagnini, F.; Fioretto, D.; Palombo, F. Viscoelasticity of amyloid plaques in transgenic mouse brain studied by Brillouin microspectroscopy and correlative Raman analysis. J. Innov. Opt. Health Sci. 2017, 10, 1742001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palombo, F.; Masia, F.; Mattana, S.; Tamagnini, F.; Borri, P.; Langbein, W.; Fioretto, D. Hyperspectral analysis applied to micro-Brillouin maps of amyloid-beta plaques in Alzheimer’s disease brains. Analyst 2018, 143, 6095–6102. [Google Scholar] [CrossRef] [Green Version]
- Randall, J.T.; Vaughan, J.M. The measurement and interpretation of Brillouin scattering in the lens of the eye. Proc. R. Soc. London. Ser. B Biol. Sci. 1982, 214, 449–470. [Google Scholar]
- Scarcelli, G.; Kling, S.; Quijano, E.; Pineda, R.; Marcos, S.; Yun, S.H. Brillouin Microscopy of Collagen Crosslinking: Noncontact Depth-Dependent Analysis of Corneal Elastic Modulus. Investig. Opthalmol. Vis. Sci. 2013, 54, 1418–1425. [Google Scholar] [CrossRef] [Green Version]
- Scarcelli, G.; Pineda, R.; Yun, S.H. Brillouin Optical Microscopy for Corneal Biomechanics. Investig. Opthalmol. Vis. Sci. 2012, 53, 185–190. [Google Scholar] [CrossRef]
- Kochevar, I.; Gisel, T.; Verter, E.; Scarcelli, G.; Yun, S.; Webb, R.; Redmond, R.; Melki, S. Crosslinking Corneal Collagen using Rose Bengal and Green Light. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5286. [Google Scholar]
- Reiss, S.; Stachs, O.; Guthoff, R.; Stolz, H. Non-Invasive, Spatially Resolved Determination of Tissue Properties of the Crystalline Lens with regard to Rheology, Refractive Index, Density and Protein Concentration by using Brillouin Spectroscopy. Klin. Mon. Für Augenheilkd. 2011, 228, 1079–1085. [Google Scholar]
- Reiss, S.; Sperlich, K.; Hovakimyan, M.; Martius, P.; Guthoff, R.F.; Stolz, H.; Stachs, O. Ex vivo measurement of postmortem tissue changes in the crystalline lens by Brillouin spectroscopy and confocal reflectance microscopy. IEEE Trans. Biomed. Eng. 2012, 59, 2348–2354. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [Green Version]
- Wells, R.G. The role of matrix stiffness in regulating cell behavior. Hepatology 2008, 47, 1394–1400. [Google Scholar] [CrossRef]
- Cusack, S.; Miller, A. Determination of the elastic constants of collagen by Brillouin light scattering. J. Mol. Biol. 1979, 135, 39–51. [Google Scholar] [CrossRef]
- Randall, J.; Vaughan, J.M.; Cusak, S. Brillouin Scattering in Systems of Biological Significance [and Discussion]. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Sci. 1979, 293, 341–348. [Google Scholar]
- Lees, S.; Tao, N.-J.; Lindsay, S.M. Studies of Compact Hard Tissues and Collagen by Means of Brillouin Light Scattering. Connect. Tissue Res. 1990, 24, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Palombo, F.; Winlove, C.P.; Edginton, R.; Green, E.; Stone, N.; Caponi, S.; Madami, M.; Fioretto, D. Biomechanics of fibrous proteins of the extracellular matrix studied by Brillouin scattering. J. R. Soc. Interface 2014, 11, 20140739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edginton, R.S.; Mattana, S.; Caponi, S.; Fioretto, D.; Green, E.; Winlove, C.P.; Palombo, F. Preparation of Extracellular Matrix Protein Fibers for Brillouin Spectroscopy. J. Vis. Exp. 2016, 15, 54648. [Google Scholar] [CrossRef] [Green Version]
- Edginton, R.; Green, E.; Winlove, C.; Fioretto, D.; Palombo, F. Dual scale biomechanics of extracellular matrix proteins probed by Brillouin scattering and quasistatic tensile testing. In Proceedings of the SPIE BiOS 2018, San Francisco, CA, USA, 27 January–1 February 2018. [Google Scholar]
- Bevilacqua, C.; Sánchez-Iranzo, H.; Richter, D.; Diz-Munoz, A.; Prevedel, R. Imaging mechanical properties of sub-micron ECM in live zebrafish using Brillouin microscopy. Biomed. Opt. Express 2019, 10, 1420–1431. [Google Scholar] [CrossRef]
- Troyanova-Wood, M.; Meng, Z.; Yakovlev, V.V. Differentiating melanoma and healthy tissues based on elasticity-specific Brillouin microspectroscopy. Biomed. Opt. Express 2019, 10, 1774–1781. [Google Scholar] [CrossRef]
- Troyanova-Wood, M.; Meng, Z.; Yakovlev, V.V. Elasticity-based identification of tumor margins using Brillouin spectroscopy. In Proceedings of the SPIE BiOS 2016, San Francisco, CA, USA, 13–18 February 2016; p. 97190P. [Google Scholar]
- Berovic, N.; Thomas, N.; Thornhill, R.A.; Vaughan, J.M. Observation of Brillouin scattering from single muscle fibres. Eur. Biophys. J. 1989, 17, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Troyanova-Wood, M.; Gobbell, C.; Meng, Z.; Gashev, A.A.; Yakovlev, V.V. Optical assessment of changes in mechanical and chemical properties of adipose tissue in diet-induced obese rats. J. Biophotonics 2017, 10, 1694–1702. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Kawabe, M.; Matsukawa, M.; Koizumi, N.; Ohtori, N. Measurement of Wave Velocity in Bovine Bone Tissue by Micro-Brillouin Scattering. Jpn. J. Appl. Phys. 2008, 47, 4205–4208. [Google Scholar] [CrossRef]
- Mathieu, V.; Fukui, K.; Matsukawa, M.; Kawabe, M.; Vayron, R.; Soffer, E.; Anagnostou, F.; Haiat, G. Micro-Brillouin Scattering Measurements in Mature and Newly Formed Bone Tissue Surrounding an Implant. J. Biomech. Eng. 2011, 133, 021006. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, M.; Fukui, K.; Matsukawa, M.; Granke, M.; Saïed, A.; Grimal, Q.; Laugier, P. Comparative investigation of elastic properties in a trabecula using micro-Brillouin scattering and scanning acoustic microscopy. J. Acoust. Soc. Am. 2012, 132, EL54–EL60. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, R.; Fukui, K.; Matsukawa, M. Local ultrasonic wave velocities in trabeculae measured by micro-Brillouin scattering. J. Acoust. Soc. Am. 2014, 135, EL109–EL114. [Google Scholar] [CrossRef]
- Akilbekova, D.; Ogay, V.; Yakupov, T.; Sarsenova, M.; Umbayev, B.; Nurakhmetov, A.; Tazhin, K.; Yakovlev, V.V.; Utegulov, Z.N. Brillouin spectroscopy and radiography for assessment of viscoelastic and regenerative properties of mammalian bones. J. Biomed. Opt. 2018, 23, 097004. [Google Scholar] [CrossRef] [Green Version]
- Kawase, M.; Yasui, H.; Shibagaki, Y.; Kawabe, M.; Matsukawa, M. Wave velocities in articular cartilage measured by micro-Brillouin scattering technique. J. Acoust. Soc. Am. 2018, 144, EL492–EL496. [Google Scholar] [CrossRef]
- Schlüßler, R.; Möllmert, S.; Abuhattum, S.; Cojoc, G.; Müller, P.; Kim, K.; Möckel, C.; Zimmermann, C.; Czarske, J.; Guck, J. Mechanical Mapping of Spinal Cord Growth and Repair in Living Zebrafish Larvae by Brillouin Imaging. Biophys. J. 2018, 115, 911–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raghunathan, R.; Zhang, J.; Wu, C.; Rippy, J.; Singh, M.; Larin, K.V.; Scarcelli, G. Evaluating biomechanical properties of murine embryos using Brillouin microscopy and optical coherence tomography. J. Biomed. Opt. 2017, 22, 1–6. [Google Scholar] [CrossRef]
- Zhang, J.; Raghunathan, R.; Rippy, J.; Wu, C.; Finnell, R.H.; Larin, K.V.; Scarcelli, G. Tissue biomechanics during cranial neural tube closure measured by Brillouin microscopy and optical coherence tomography. Birth Defects Res. 2019, 111, 991–998. [Google Scholar] [CrossRef]
- Subbaram, M.; Gump, J.; Bullimore, M.; Sooryakumar, R. The Elasticity of the Human Lens. Investig. Ophthalmol. Vis. Sci. 2002, 43, 468. [Google Scholar]
- Besner, S.; Scarcelli, G.; Pineda, R.; Yun, S.-H. In Vivo Brillouin Analysis of the Aging Crystalline Lens. Investig. Opthalmol. Vis. Sci. 2016, 57, 5093–5100. [Google Scholar] [CrossRef] [Green Version]
- Seiler, T.G.; Shao, P.; Eltony, A.; Yun, S.-H. Brillouin Spectroscopy of Normal and Keratoconus Corneas. Am. J. Ophthalmol. 2019, 202, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Eltony, A.M.; Seiler, T.G.; Tavakol, B.; Pineda, R.; Koller, T.; Seiler, T.; Yun, S.-H. Spatially-resolved Brillouin spectroscopy reveals biomechanical abnormalities in mild to advanced keratoconus in vivo. Sci. Rep. 2019, 9, 7467. [Google Scholar] [CrossRef] [PubMed]
- Scarcelli, G.; Besner, S.; Pineda, R.; Yun, S.H. Biomechanical Characterization of Keratoconus Corneas Ex Vivo With Brillouin Microscopy. Investig. Opthalmol. Vis. Sci. 2014, 55, 4490–4495. [Google Scholar] [CrossRef] [Green Version]
- Scarcelli, G.; Besner, S.; Pineda, R.; Kalout, P.; Yun, S.H. In vivo biomechanical mapping of normal and keratoconus corneas. JAMA Ophthalmol. 2015, 133, 480–482. [Google Scholar] [CrossRef] [Green Version]
- Mercatelli, R.; Mattana, S.; Capozzoli, L.; Ratto, F.; Rossi, F.; Pini, R.; Fioretto, D.; Pavone, F.S.; Caponi, S.; Cicchi, R. Morpho-mechanics of human collagen superstructures revealed by all-optical correlative microspectroscopies. Commun. Biol. 2019, 2, 117. [Google Scholar] [CrossRef] [Green Version]
- Gouveia, R.M.; Lepert, G.; Gupta, S.; Mohan, R.R.; Paterson, C.; Connon, C.J. Assessment of corneal substrate biomechanics and its effect on epithelial stem cell maintenance and differentiation. Nat. Commun. 2019, 10, 1496. [Google Scholar] [CrossRef] [Green Version]
- Shao, P.; Seiler, T.G.; Eltony, A.M.; Ramier, A.; Kwok, S.J.J.; Scarcelli, G.; Ii, R.P.; Yun, S.-H.; Pineda, R. Effects of Corneal Hydration on Brillouin Microscopy In Vivo. Investig. Opthalmol. Vis. Sci. 2018, 59, 3020–3027. [Google Scholar] [CrossRef]
- Cardinali, M.A.; Dallari, D.; Govoni, M.; Stagni, C.; Marmi, F.; Tschon, M.; Brogini, S.; Fioretto, D.; Morresi, A. Brillouin micro-spectroscopy of subchondral, trabecular bone and articular cartilage of the human femoral head. Biomed. Opt. Express 2019, 10, 2606–2611. [Google Scholar] [CrossRef]
- Yun, S.H.; Chernyak, D. Brillouin microscopy: Assessing ocular tissue biomechanics. Curr. Opin. Ophthalmol. 2018, 29, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Girard, M.J.A.; Dupps, W.; Baskaran, M.; Scarcelli, G.; Yun, S.H.; Quigley, H.A.; Sigal, I.A.; Strouthidis, N.G. Translating Ocular Biomechanics into Clinical Practice: Current State and Future Prospects. Curr. Eye Res. 2015, 40, 1–18. [Google Scholar] [CrossRef]
- Eltony, A.M.; Shao, P.; Yun, S.H. Measuring mechanical anisotropy of the cornea with Brillouin microscopy. arXiv 2020, arXiv:2003.04344. [Google Scholar]
- Lainović, T.; Margueritat, J.; Martinet, Q.; Dagany, X.; Blažić, L.; Pantelić, D.; Rabasović, M.D.; Krmpot, A.J.; Dehoux, T. Micromechanical imaging of dentin with Brillouin microscopy. Acta Biomater. 2020, 105, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Palombo, F.; Madami, M.; Fioretto, D.; Nallala, J.; Barr, H.; David, A.; Stone, N. Chemico-mechanical imaging of Barrett’s oesophagus. J. Biophotonics 2016, 9, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Palombo, F.; Madami, M.; Stone, N.; Fioretto, D. Mechanical mapping with chemical specificity by confocal Brillouin and Raman microscopy. Analyst 2014, 139, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Day, J.C.C.; Bennett, R.; Smith, B.; Kendall, C.; Hutchings, J.; Meaden, G.M.; Born, C.; Yu, S.; Stone, N. A miniature confocal Raman probe for endoscopic use. Phys. Med. Biol. 2009, 54, 7077–7087. [Google Scholar] [CrossRef]
- Shu, C.; Zheng, W.; Lin, K.; Lim, C.; Huang, Z. Label-Free Follow-Up Surveying of Post-Treatment Efficacy and Recurrence in Nasopharyngeal Carcinoma Patients with Fiberoptic Raman Endoscopy. Anal. Chem. 2021, 93, 2053–2061. [Google Scholar] [CrossRef]
- Day, J.C.C.; Stone, N. A Subcutaneous Raman Needle Probe. Appl. Spectrosc. 2013, 67, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Yakovlev, V. Brillouin spectroscopy characterizes microscopic viscoelasticity associated with skin injury. Prog. Biomed. Opt. Imaging Proc. SPIE 2015, 9321, 93210C. [Google Scholar]
- Coker, Z.; Meng, Z.; Troyanova-Wood, M.; Traverso, A.; Ballmann, C.; Petrov, G.; Ibey, B.; Yakovlev, V. Investigation of burn effect on skin using simultaneous Raman-Brillouin spectroscopy, and fluorescence microspectroscopy. Proc. SPIE 2017, 10057. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rioboó, R.J.J.; Gontán, N.; Sanderson, D.; Desco, M.; Gómez-Gaviro, M.V. Brillouin Spectroscopy: From Biomedical Research to New Generation Pathology Diagnosis. Int. J. Mol. Sci. 2021, 22, 8055. https://doi.org/10.3390/ijms22158055
Rioboó RJJ, Gontán N, Sanderson D, Desco M, Gómez-Gaviro MV. Brillouin Spectroscopy: From Biomedical Research to New Generation Pathology Diagnosis. International Journal of Molecular Sciences. 2021; 22(15):8055. https://doi.org/10.3390/ijms22158055
Chicago/Turabian StyleRioboó, Rafael J. Jiménez, Nuria Gontán, Daniel Sanderson, Manuel Desco, and Maria Victoria Gómez-Gaviro. 2021. "Brillouin Spectroscopy: From Biomedical Research to New Generation Pathology Diagnosis" International Journal of Molecular Sciences 22, no. 15: 8055. https://doi.org/10.3390/ijms22158055





