Effect of Different Bone Grafting Materials and Mesenchymal Stem Cells on Bone Regeneration: A Micro-Computed Tomography and Histomorphometric Study in a Rabbit Calvarial Defect Model
Abstract
:1. Introduction
2. Results
2.1. Characterization of MBCP and Bio-Oss
2.2. Characterization of DPSCs and BMSCs
2.3. Micro-CT Measurements
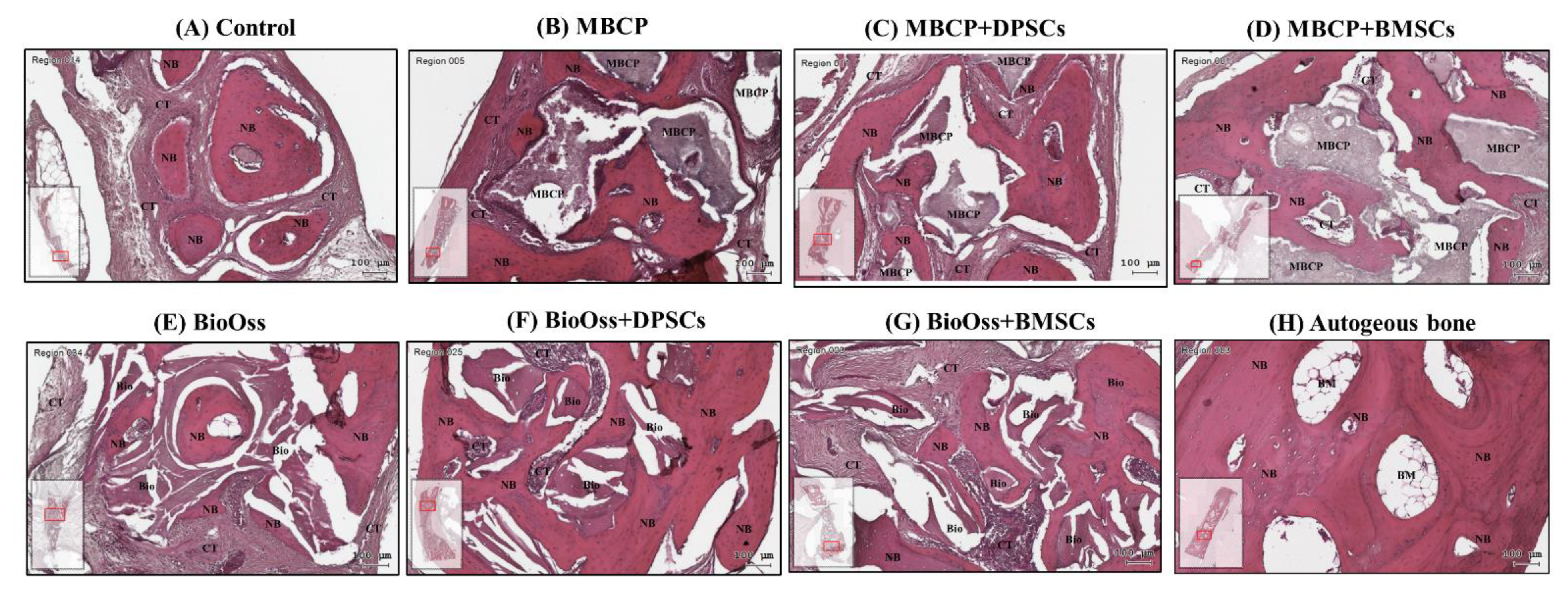
2.4. Histological Observations
2.5. Histomorphometric Results
3. Discussion
4. Materials and Methods
4.1. Characterization of MBCP and Bio-Oss Bone Grafting Materials
4.2. Isolation and Culture of DPSCs and BMSCs
4.3. Animals and Ethics
4.4. Animal Experiments and Surgical Procedures
4.5. Micro-Computed Tomography Measurements
4.6. Histology and Histomorphometric Analyses
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duan, R.; Barbieri, D.; Luo, X.; Weng, J.; Bao, C.; de Bruijn, J.D.; Yuan, H. Variation of the bone forming ability with the physicochemical properties of calcium phosphate bone substitutes. Biomater. Sci. 2017, 6, 136–145. [Google Scholar] [CrossRef]
- Halim, A.; Ariyanti, A.D.; Luo, Q.; Song, G. Recent Progress in Engineering Mesenchymal Stem Cell Differentiation. Stem Cell Rev. Rep. 2020, 16, 661–674. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Fonticoli, L.; Trubiani, O.; Rajan, T.; Marconi, G.; Bramanti, P.; Mazzon, E.; Pizzicannella, J.; Diomede, F. Oral Bone Tissue Regeneration: Mesenchymal Stem Cells, Secretome, and Biomaterials. Int. J. Mol. Sci. 2021, 22, 5236. [Google Scholar] [CrossRef]
- Diomede, F.; D’Aurora, M.; Gugliandolo, A.; Merciaro, I.; Orsini, T.; Gatta, V.; Piattelli, A.; Trubiani, O.; Mazzon, E. Biofunctionalized Scaffold in Bone Tissue Repair. Int. J. Mol. Sci. 2018, 19, 1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roato, I.; Belisario, D.C.; Compagno, M.; Verderio, L.; Sighinolfi, A.; Mussano, F.; Genova, T.; Veneziano, F.; Pertici, G.; Perale, G.; et al. Adipose-Derived Stromal Vascular Fraction/Xenohybrid Bone Scaffold: An Alternative Source for Bone Regeneration. Stem Cells Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Roato, I.; Perale, G.; Rossi, F.; Genova, T.; Mussano, F.; Ferracini, R.; Sorlini, M.; Torre, M.; Perteghella, S. Biohybrid Bovine Bone Matrix for Controlled Release of Mesenchymal Stem/Stromal Cell Lyosecretome: A Device for Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 4064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, S.; Xu, L.; Zhang, M.; Wang, G.; Jin, Y.; Zhang, X.; Jiang, X. Comparison of the Use of Adipose Tissue–Derived and Bone Marrow–Derived Stem Cells for Rapid Bone Regeneration. J. Dent. Res. 2013, 92, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-S.; Yang, S.-S.; Lee, J. Precoating of biphasic calcium phosphate bone substitute with atelocollagen enhances bone regeneration through stimulation of osteoclast activation and angiogenesis. J. Biomed. Mater. Res. Part. A 2017, 105, 1446–1456. [Google Scholar] [CrossRef]
- Park, J.C.; Oh, S.Y.; Lee, J.S.; Park, S.Y.; Choi, E.Y.; Cho, K.S.; Kim, C.S. In vivo bone formation by human alveolar-bone-derived mesenchymal stem cells obtained during implant osteotomy using biphasic calcium phosphate ceramics or Bio-Oss as carriers. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Van Dijk, L.A.; Barbieri, D.; De Groot, F.; Yuan, H.; De Bruijn, J.D. Accelerated bone formation by biphasic calcium phosphate with a novel sub-micron surface topography. Eur. Cells Mater. 2019, 37, 60–73. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, R.; Cooper, P.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef]
- Seo, S.-J.; Kim, Y.-G. Improved bone regeneration using collagen-coated biphasic calcium phosphate with high porosity in a rabbit calvarial model. Biomed. Mater. 2020, 16, 015012. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.; McKee, C.; Bakshi, S.; Walker, K.; Hakman, E.; Halassy, S.; Svinarich, D.; Dodds, R.; Govind, C.K.; Chaudhry, G.R. Mesenchymal stem cells: Cell therapy and regeneration potential. J. Tissue Eng. Regen. Med. 2019, 13, 1738–1755. [Google Scholar] [CrossRef]
- Shang, F.; Yu, Y.; Liu, S.; Ming, L.; Zhang, Y.; Zhou, Z.; Zhao, J.; Jin, Y. Advancing application of mesenchymal stem cell-based bone tissue regeneration. Bioact. Mater. 2021, 6, 666–683. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells—Current trends and future prospective. Biosci. Rep. 2015, 35. [Google Scholar] [CrossRef]
- Mohamed-Ahmed, S.; Fristad, I.; Lie, S.A.; Suliman, S.; Mustafa, K.; Vindenes, H.; Idris, S.B. Adipose-derived and bone marrow mesenchymal stem cells: A donor-matched comparison. Stem Cell Res. Ther. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Oliver, J.D.; Madhoun, W.; Graham, E.M.; Hendrycks, R.; Renouard, M.; Hu, M.S. Stem Cells Regenerating the Craniofacial Skeleton: Current State-Of-The-Art and Future Directions. J. Clin. Med. 2020, 9, 3307. [Google Scholar] [CrossRef]
- Futrega, K.; Mosaad, E.; Chambers, K.; Lott, W.B.; Clements, J.; Doran, M.R. Bone marrow-derived stem/stromal cells (BMSC) 3D microtissues cultured in BMP-2 supplemented osteogenic induction medium are prone to adipogenesis. Cell Tissue Res. 2018, 374, 541–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, A.G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef] [Green Version]
- La Noce, M.; Paino, F.; Spina, A.; Naddeo, P.; Montella, R.; Desiderio, V.; De Rosa, A.; Papaccio, G.; Tirino, V.; Laino, L. Dental pulp stem cells: State of the art and suggestions for a true translation of research into therapy. J. Dent. 2014, 42, 761–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lew, W.-Z.; Feng, S.-W.; Lin, C.-T.; Huang, H.-M. Use of 0.4-Tesla static magnetic field to promote reparative dentine formation of dental pulp stem cells through activation of p38 MAPK signalling pathway. Int. Endod. J. 2018, 52, 28–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishizaka, R.; Hayashi, Y.; Iohara, K.; Sugiyama, M.; Murakami, M.; Yamamoto, T.; Fukuta, O.; Nakashima, M. Stimulation of angiogenesis, neurogenesis and regeneration by side population cells from dental pulp. Biomaterials 2013, 34, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Janebodin, K.; Zeng, Y.; Buranaphatthana, W.; Ieronimakis, N.; Reyes, M. VEGFR2-dependent Angiogenic Capacity of Pericyte-like Dental Pulp Stem Cells. J. Dent. Res. 2013, 92, 524–531. [Google Scholar] [CrossRef]
- Jin, Q.; Yuan, K.; Lin, W.; Niu, C.; Ma, R.; Huang, Z. Comparative characterization of mesenchymal stem cells from human dental pulp and adipose tissue for bone regeneration potential. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zou, X.-Y.; El-Ayachi, I.; Romero, L.; Yu, Z.; Iglesias-Linares, A.; Morales, J.C.; Huang, G.T.-J. Human Dental Pulp Stem Cells and Gingival Mesenchymal Stem Cells Display Action Potential Capacity In Vitro after Neuronogenic Differentiation. Stem Cell Rev. Rep. 2018, 15, 67–81. [Google Scholar] [CrossRef]
- Chan, Y.H.; Lee, Y.C.; Hung, C.Y.; Yang, P.J.; Lai, P.C.; Feng, S.W. Three-dimensional Spheroid Culture Enhances Multipotent Differentiation and Stemness Capacities of Human Dental Pulp-derived Mesenchymal Stem Cells by Modulating MAPK and NF-kB Signaling Pathways. Stem Cell Rev. Rep. 2021. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Kunimatsu, R.; Ando, K.; Ando, T.; Hayashi, Y.; Kihara, T.; Hiraki, T.; Tsuka, Y.; Abe, T.; Kaku, M.; et al. Comparison of the bone regeneration ability between stem cells from human exfoliated deciduous teeth, human dental pulp stem cells and human bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 497, 876–882. [Google Scholar] [CrossRef]
- Noda, S.; Kawashima, N.; Yamamoto, M.; Hashimoto, K.; Nara, K.; Sekiya, I.; Okiji, T. Effect of cell culture density on dental pulp-derived mesenchymal stem cells with reference to osteogenic differentiation. Sci. Rep. 2019, 9, 5430. [Google Scholar] [CrossRef]
- Novais, A.; Lesieur, J.; Sadoine, J.; Slimani, L.; Baroukh, B.; Saubaméa, B.; Schmitt, A.; Vital, S.; Poliard, A.; Helary, C.; et al. Priming Dental Pulp Stem Cells from Human Exfoliated Deciduous Teeth with Fibroblast Growth Factor-2 Enhances Mineralization Within Tissue-Engineered Constructs Implanted in Craniofacial Bone Defects. STEM CELLS Transl. Med. 2019, 8, 844–857. [Google Scholar] [CrossRef] [Green Version]
- Shiu, S.-T.; Lew, W.-Z.; Lee, S.-Y.; Feng, S.-W.; Huang, H.-M. Effects of Sapindus mukorossi Seed Oil on Proliferation, Osteogenetic/Odontogenetic Differentiation and Matrix Vesicle Secretion of Human Dental Pulp Mesenchymal Stem Cells. Materials 2020, 13, 4063. [Google Scholar] [CrossRef] [PubMed]
- Janko, M.; Sahm, J.; Schaible, A.; Brune, J.C.; Bellen, M.; Schröder, K.; Seebach, C.; Marzi, I.; Henrich, D. Comparison of three different types of scaffolds preseeded with human bone marrow mononuclear cells on the bone healing in a femoral critical size defect model of the athymic rat. J. Tissue Eng. Regen. Med. 2017, 12, 653–666. [Google Scholar] [CrossRef]
- Moya, A.; Larochette, N.; Bourguignon, M.; El-Hafci, H.; Potier, E.; Petite, H.; Logeart-Avramoglou, D. Osteogenic potential of adipogenic predifferentiated human bone marrow-derived multipotent stromal cells for bone tissue-engineering. J. Tissue Eng. Regen. Med. 2017, 12, e1511–e1524. [Google Scholar] [CrossRef]
- Tan, S.-L.; Ahmad, T.S.; Selvaratnam, L.; Kamarul, T. Isolation, characterization and the multi-lineage differentiation potential of rabbit bone marrow-derived mesenchymal stem cells. J. Anat. 2013, 222, 437–450. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Krafts, K.P. Tissue repair: The hidden drama. Organogenesis 2010, 6, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, J.R.B.; Sartoretto, S.C.; Alves, A.T.N.N.; Mourão, C.F.A.B.; Martinez-Zelaya, V.R.; Uzeda, M.J.; Granjeiro, J.M.; Montemezzi, P.; Calasans-Maia, M.D.; Calasans-Maia, J.A. In Vitro and In Vivo Evaluation of Nanostructured Biphasic Calcium Phosphate in Granules and Putty Configurations. Int. J. Environ. Res. Public Health 2021, 18, 533. [Google Scholar] [CrossRef]
- De Grado, G.F.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yang, L.; Zheng, Z.; Li, Z.; Deng, T.; Ren, W.; Wu, C.; Guo, L. Bio-Oss modified by calcitonin gene-related peptide promotes osteogenesis in vitro. Exp. Ther. Med. 2017, 14, 4001–4008. [Google Scholar] [PubMed]
- Tapety, F.I.; Amizuka, N.; Uoshima, K.; Nomura, S.; Maeda, T. A histological evaluation of the involvement of Bio-OssR in osteoblastic differentiation and matrix synthesis. Clin. Oral Implant. Res. 2004, 15, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Saiki, C.E.T.; Silva, K.; Massuda, C.K.M.; Faloni, A.P.D.S.; Braz-Silva, P.H.; Pallos, D.; Sendyk, W.R. Bone Formation in Grafts with Bio-Oss and Autogenous Bone at Different Proportions in Rabbit Calvaria. Int. J. Dent. 2020, 2020, 1–6. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Aludden, H.; Mordenfeld, A.; Hallman, M.; Dahlin, C.; Jensen, T. Lateral ridge augmentation with Bio-Oss alone or Bio-Oss mixed with particulate autogenous bone graft: A systematic review. Int. J. Oral Maxillofac. Surg. 2017, 46, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Xing, Y.; Jia, L.; Ji, Y.; Zhao, B.; Wen, Y.; Xu, X. An In Vitro Comparative Study of Multisource Derived Human Mesenchymal Stem Cells for Bone Tissue Engineering. Stem Cells Dev. 2018, 27, 1634–1645. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.-C.; Lai, Y.-C.; Li, L.-H.; Liao, K.; Lai, H.-C.; Kao, S.-Y.; Wang, J.; Chuong, C.-M.; Hung, S.-C. Methylation and PTEN activation in dental pulp mesenchymal stem cells promotes osteogenesis and reduces oncogenesis. Nat. Commun. 2019, 10, 2226. [Google Scholar] [CrossRef]
- Jensen, J.; Tvedesøe, C.; Rölfing, J.H.D.; Foldager, C.B.; Lysdahl, H.; Kraft, D.C.E.; Chen, M.; Baas, J.; Le, D.Q.S.; Bünger, C.E. Dental pulp-derived stromal cells exhibit a higher osteogenic potency than bone marrow-derived stromal cells in vitro and in a porcine critical-size bone defect model. SICOT-J. 2016, 2, 16. [Google Scholar] [CrossRef]
- Lin, H.; Sohn, J.; Shen, H.; Langhans, M.; Tuan, R.S. Bone marrow mesenchymal stem cells: Aging and tissue engineering applications to enhance bone healing. Biomaterials 2019, 203, 96–110. [Google Scholar] [CrossRef]
- Nantavisai, S.; Pisitkun, T.; Osathanon, T.; Pavasant, P.; Kalpravidh, C.; Dhitavat, S.; Makjaroen, J.; Sawangmake, C. Systems biology analysis of osteogenic differentiation behavior by canine mesenchymal stem cells derived from bone marrow and dental pulp. Sci. Rep. 2020, 10, 1–18. [Google Scholar] [CrossRef]
- Fan, X.-L.; Zhang, Y.; Li, X.; Fu, Q.-L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. 2020, 77, 2771–2794. [Google Scholar] [CrossRef] [Green Version]
- Johnson, Z.M.; Yuan, Y.; Li, X.; Jashashvili, T.; Jamieson, M.; Urata, M.; Chen, Y.; Chai, Y. Mesenchymal stem cells and three-dimensional-osteoconductive scaffold regenerate calvarial bone in critical size defects in swine. STEM CELLS Transl. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, H. MicroRNAs in the Migration of Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2018, 15, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Humbert, P.; Brennan, M.; Davison, N.; Rosset, P.; Trichet, V.; Blanchard, F.; Layrolle, P. Immune Modulation by Transplanted Calcium Phosphate Biomaterials and Human Mesenchymal Stromal Cells in Bone Regeneration. Front. Immunol. 2019, 10, 663. [Google Scholar] [CrossRef] [PubMed]
- Sagaradze, G.D.; Basalova, N.A.; Efimenko, A.Y.; Tkachuk, V.A. Mesenchymal Stromal Cells as Critical Contributors to Tissue Regeneration. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef]
- Liu, J.; Ding, Y.; Liu, Z.; Liang, X. Senescence in Mesenchymal Stem Cells: Functional Alterations, Molecular Mechanisms, and Rejuvenation Strategies. Front. Cell Dev. Biol. 2020, 8, 258. [Google Scholar] [CrossRef]
- Sui, B.; Hu, C.-H.; Liu, A.-Q.; Zheng, C.-X.; Xuan, K.; Jin, Y. Stem cell-based bone regeneration in diseased microenvironments: Challenges and solutions. Biomaterials 2019, 196, 18–30. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiu, S.-T.; Lee, W.-F.; Chen, S.-M.; Hao, L.-T.; Hung, Y.-T.; Lai, P.-C.; Feng, S.-W. Effect of Different Bone Grafting Materials and Mesenchymal Stem Cells on Bone Regeneration: A Micro-Computed Tomography and Histomorphometric Study in a Rabbit Calvarial Defect Model. Int. J. Mol. Sci. 2021, 22, 8101. https://doi.org/10.3390/ijms22158101
Shiu S-T, Lee W-F, Chen S-M, Hao L-T, Hung Y-T, Lai P-C, Feng S-W. Effect of Different Bone Grafting Materials and Mesenchymal Stem Cells on Bone Regeneration: A Micro-Computed Tomography and Histomorphometric Study in a Rabbit Calvarial Defect Model. International Journal of Molecular Sciences. 2021; 22(15):8101. https://doi.org/10.3390/ijms22158101
Chicago/Turabian StyleShiu, Shiau-Ting, Wei-Fang Lee, Sheng-Min Chen, Liu-Ting Hao, Yuan-Ting Hung, Pin-Chuang Lai, and Sheng-Wei Feng. 2021. "Effect of Different Bone Grafting Materials and Mesenchymal Stem Cells on Bone Regeneration: A Micro-Computed Tomography and Histomorphometric Study in a Rabbit Calvarial Defect Model" International Journal of Molecular Sciences 22, no. 15: 8101. https://doi.org/10.3390/ijms22158101
APA StyleShiu, S.-T., Lee, W.-F., Chen, S.-M., Hao, L.-T., Hung, Y.-T., Lai, P.-C., & Feng, S.-W. (2021). Effect of Different Bone Grafting Materials and Mesenchymal Stem Cells on Bone Regeneration: A Micro-Computed Tomography and Histomorphometric Study in a Rabbit Calvarial Defect Model. International Journal of Molecular Sciences, 22(15), 8101. https://doi.org/10.3390/ijms22158101





