Synthesis and Characterization of Polyvinylpyrrolidone-Modified ZnO Quantum Dots and Their In Vitro Photodynamic Tumor Suppressive Action
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of ZnO/PVP QDs
2.2. Characterization of ZnO/PVP QDs
2.3. Quantum Yield of the ZnO/PVP QDs
2.4. In Vitro Stability of ZnO/PVP QDs
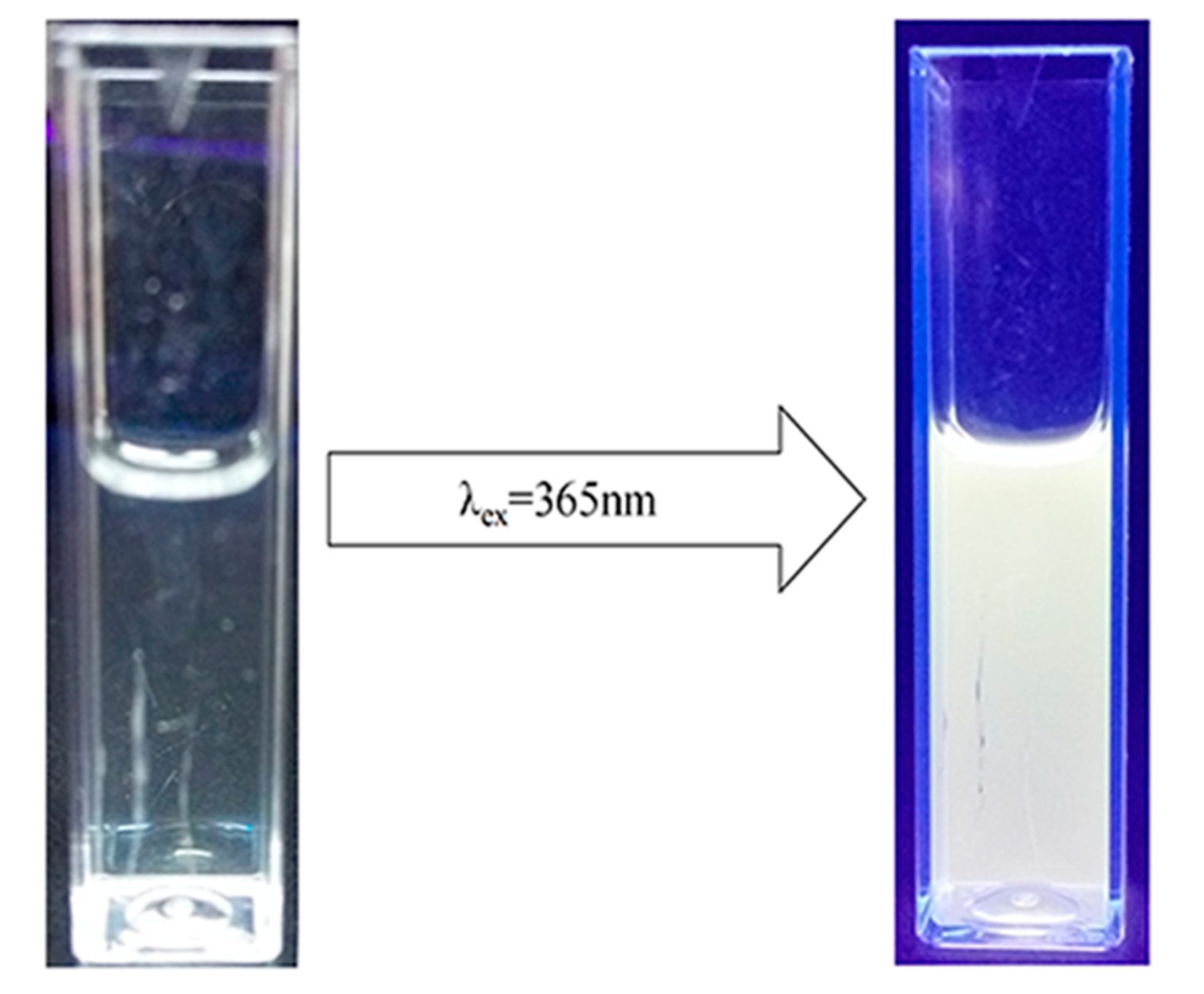
2.5. Photoluminescent Properties of the ZnO/PVP QDs
2.6. In Vitro ROS Production
2.7. Cytotoxicity of ZnO/PVP QDs
2.8. Photodynamic Experiment of the ZnO/PVP QDs In Vitro
2.9. Photodynamic Experiment of ZnO/PVP QDs In Vivo
3. Materials and Methods
3.1. Reagents and Cell Lines
3.2. Synthesis of the ZnO/PVP QDs
3.3. Characterizations of the ZnO/PVP QDs
3.4. Quantum Yield of ZnO/PVP QDs
3.5. In Vitro Stability Study of the ZnO/PVP QDs
3.6. Fluorescence Intensities of the ZnO/PVP QDs
3.7. Measurement of ROS Levels
3.8. Cytotoxicity Analysis
3.9. In Vitro Photodynamic Study
3.10. Experimental Animals
3.11. In Vivo Photodynamic Study
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhary, S.; Nouri, K.; Elsaie, M.L. Photodynamic therapy in dermatology: A review. Lasers Med. Sci. 2009, 24, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Moor, A.C.E. Signaling pathways in cell death and survival after photodynamic therapy. J. Photochem. Photobiol. B Biol. 2000, 57, 1–13. [Google Scholar] [CrossRef]
- Barra, F.; Roscetto, E.; Soriano, A.; Vollaro, A.; Postiglione, I.; Pierantoni, G.; Palumbo, G.; Catania, M. Photodynamic and antibiotic therapy in combination to fight biofilms and resistant surface bacterial infections. Int. J. Mol. Sci. 2015, 16, 20417–20430. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.A.; Evans, D.H.; Abrahamse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B Biol. 2009, 96, 1–8. [Google Scholar] [CrossRef]
- Kim, M.M.; Darafsheh, A. Light sources and dosimetry techniques for photodynamic therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef] [Green Version]
- Rong, P.; Yang, K.; Srivastan, A.; Kiesewetter, D.O.; Yue, X.; Wang, F.; Nie, L.; Bhirde, A.; Wang, Z.; Liu, Z.; et al. Photosensitizer loaded nano-graphene for multimodality imaging guided tumor photodynamic therapy. Theranostics 2014, 4, 229–239. [Google Scholar] [CrossRef] [Green Version]
- Allison, R.R.; Sibata, C.H.; Downie, G.H.; Cuenca, R.E. A clinical review of PDT for cutaneous malignancies. Photodiagn. Photodyn. Ther. 2006, 3, 214–226. [Google Scholar] [CrossRef]
- Nseyo, U.O.; De Haven, J.; Dougherty, T.J.; Potter, W.R.; Merrill, D.L.; Lundahl, S.L.; Lamm, D.L. Photodynamic therapy (PDT) in the treatment of patients with resistant superficial bladder cancer: A long term experience. J. Clin. Laser Med. Surg. 1998, 16, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Moghissi, K.; Dixon, K.; Thorpe, J.A.C.; Stringer, M.; Oxtoby, C. Photodynamic therapy (PDT) in early central lung cancer: A treatment option for patients ineligible for surgical resection. Thorax 2007, 62, 391–395. [Google Scholar] [CrossRef] [Green Version]
- Muroya, T.; Suehiro, Y.; Umayahara, K.; Akiya, T.; Iwabuchi, H.; Sakunaga, H.; Sakamoto, M.; Sugishita, T.; Tenjin, Y. Photodynamic therapy (PDT) for early cervical cancer. Gan Kagaku Ryoho 1996, 23, 47–56. [Google Scholar]
- Biel, M.A. Photodynamic therapy of head and neck cancers. In Methods in Molecular Biology; Humana Press: Tortowa, NJ, USA, 2010; pp. 281–293. [Google Scholar]
- Biel, M. Advances in photodynamic therapy for the treatment of head and neck cancers. Lasers Surg. Med. 2006, 38, 349–355. [Google Scholar] [CrossRef]
- Han, Z.; Wang, X.; Heng, C.; Han, Q.; Cai, S.; Li, J.; Qi, C.; Liang, W.; Yang, R.; Wang, C. Synergistically enhanced photocatalytic and chemotherapeutic effects of aptamer-functionalized ZnO nanoparticles towards cancer cells. Phys. Chem. Chem. Phys. 2015, 17, 21576–21582. [Google Scholar] [CrossRef]
- Xiong, H.-M.; Liu, D.-P.; Xia, Y.-Y.; Chen, J.-S. Polyether-grafted ZnO nanoparticles with tunable and stable photoluminescence at room temperature. Chem. Mater. 2005, 17, 3062–3064. [Google Scholar] [CrossRef]
- Jamieson, T.; Bakhshi, R.; Petrova, D.; Pocock, R.; Imani, M.; Seifalian, A.M. Biological applications of quantum dots. Biomaterials 2007, 28, 4717–4732. [Google Scholar] [CrossRef]
- Wu, W.; Shen, J.; Banerjee, P.; Zhou, S. A Multifuntional nanoplatform based on responsive fluorescent plasmonic ZnO-Au@PEG hybrid nanogels. Adv. Funct. Mater. 2011, 21, 2830–2839. [Google Scholar] [CrossRef]
- Pan, Z.-Y.; Liang, J.; Zheng, Z.-Z.; Wang, H.-H.; Xiong, H.-M. The application of ZnO luminescent nanoparticles in labeling mice. Contrast Media Mol. Imaging 2011, 6, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.-M. ZnO Nanoparticles applied to bioimaging and drug delivery. Adv. Mater. 2013, 25, 5329–5335. [Google Scholar] [CrossRef] [PubMed]
- Urban, B.E.; Neogi, P.; Senthilkumar, K.; Rajpurohit, S.K.; Jagadeeshwaran, P.; Kim, S.; Fujita, Y.; Neogi, A. Bioimaging using the optimized nonlinear optical properties of ZnO nanoparticles. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1451–1456. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Xiong, H.-M. Biological applications of ZnO nanoparticles. Curr. Mol. Imaging 2013, 2, 177–192. [Google Scholar] [CrossRef] [Green Version]
- Staedler, D.; Magouroux, T.; Rachid, H.; Joulaud, C.; Extermann, J.; Schwung, S.; Passemard, S.; Kasparian, C.; Clarke, G.; Gerrmann, M.; et al. Harmonic nanocrystals for biolabeling: A survey of optical properties and biocompatibility. ACS Nano 2012, 6, 2542–2549. [Google Scholar] [CrossRef]
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 184–192. [Google Scholar] [CrossRef]
- Wang, A.; Qi, W.; Wang, N.; Zhao, J.; Muhammad, F.; Cai, K.; Ren, H.; Sun, F.; Chen, L.; Guo, Y.; et al. A smart nanoporous theranostic platform for simultaneous enhanced MRI and drug delivery. Microporous Mesoporous Mater. 2013, 180, 1–7. [Google Scholar] [CrossRef]
- Martinez-Carmona, M.; Gun’ko, Y.; Vallet-Regi, M. ZnO nanostructures for drug delivery and theranostic applications. Nanomaterials 2018, 8, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, X.; Jiang, L.; Gong, Y.; Li, J.; Liu, L.; Cao, Y. The presence of oleate stabilized ZnO nanoparticles (NPs) and reduced the toxicity of aged NPs to Caco-2 and HepG2 cells. Chem. Biol. Interact. 2017, 278, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Aswathanarayan, J.B.; Vittal, R.R.; Muddegowda, U. Anticancer activity of metal nanoparticles and their peptide conjugates against human colon adenorectal carcinoma cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Tang, M.; Zhang, T. Review of toxicological effect of quantum dots on the liver. J. Appl. Toxicol. 2019, 39, 72–86. [Google Scholar] [CrossRef] [Green Version]
- Marfavi, Z.H.; Farhadi, M.; Jameie, S.B.; Zahmatkeshan, M.; Pirhajati, V.; Jameie, M. Glioblastoma U-87MG tumour cells suppressed by ZnO folic acid-conjugated nanoparticles: An in vitro study. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2783–2790. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Shen, C.-L.; Lou, Q.; Zhao, W.B.; Wei, J.Y.; Liu, K.K.; Zang, J.H.; Dong, L.; Shan, C.-X. Efficient chemiluminescent ZnO nanoparticles for cellular imaging. J. Lumin. 2020, 221, 117111. [Google Scholar] [CrossRef]
- Wang, Y.; He, L.; Yu, B.; Chen, Y.; Shen, Y.; Cong, H. ZnO quantum dots modified by pH-activated charge-reversal polymer for tumor targeted drug delivery. Polymers 2018, 10, 1272. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, C.; Liu, L.; Gong, Y.; Xie, Y.; Cao, Y. The effects of baicalein or baicalin on the colloidal stability of ZnO nanoparticles (NPs) and toxicity of NPs to Caco-2 cells. Toxicol. Mech. Methods 2018, 28, 167–176. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef]
- Yang, Y.; Song, Z.; Wu, W.; Xu, A.; Lv, S.; Ji, S. ZnO quantum dots induced oxidative stress and apoptosis in HeLa and HEK-293T cell lines. Front. Pharmacol. 2020, 11, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowik, J.; Miodyńska, M.; Bajorowicz, B.; Mikolajczyk, A.; Lisowski, W.; Klimczuk, T.; Kaczor, D.; Medynska, A.Z.; Malankowska, A. Optical and photocatalytic properties of rare earth metal-modified ZnO quantum dots. Appl. Surf. Sci. 2019, 464, 651–663. [Google Scholar] [CrossRef]
- Hong, H.; Wang, F.; Zhang, Y.; Graves, S.A.; Eddine, S.B.Z.; Yang, Y.; Theuer, C.P.; Nickles, R.J.; Wang, X.; Cai, W. Red fluorescent zinc oxide nanoparticle: A novel platform for cancer targeting. ACS Appl. Mater. Interfaces 2015, 7, 3373–3381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Ai, K.; Yuan, Q.; Lu, L. Fluorescence-enhanced gadolinium-doped zinc oxide quantum dots for magnetic resonance and fluorescence imaging. Biomaterials 2011, 32, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Choo, E.S.G.; Li, L.; Ding, J.; Xue, J. Synthesis of ZnO nanoparticles with tunable emission colors and their cell labeling applications. Chem. Mater. 2010, 22, 3383–3388. [Google Scholar] [CrossRef]
- Wang, S.-L.; Liu, K.-K.; Shan, C.-X.; Liu, E.-S.; Shen, D.-Z. Oleylamine-assisted and temperature-controlled synthesis of ZnO nanoparticles and their application in encryption. Nanotechnology 2019, 30, 015702. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, R.; Zhao, L.-H.; Sun, S.-Q. Synthesis of water-soluble gamma-aminopropyl triethoxysilane-capped ZnO:MgO nanocrystals with biocompatibility. CrystEngComm 2012, 14, 613–619. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, B.; Ding, N.; Ding, W.; Wang, L.; Yu, M.; Zhang, Q. Fast synthesize ZnO quantum dots via ultrasonic method. Ultrason. Sonochem. 2016, 30, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobsson, T.J.; Viarbitskaya, S.; Mukhtar, E.; Edvinsson, T. A size dependent discontinuous decay rate for the exciton emission in ZnO quantum dots. Phys. Chem. Chem. Phys. 2014, 16, 13849–13857. [Google Scholar] [CrossRef]
- Chen, B.W.; Bi, Y.; Luo, X.; Zhang, L. Photoluminescence of monolithic zinc oxide aerogel synthesised by dispersed inorganic sol–gel method. Mater. Technol. 2014, 30, 65–69. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Senthilkumar, O.; Yamauchi, K.; Sato, M.; Morito, S.; Ohba, T.; Nakamura, M.; Fujita, Y. Preparation of ZnO nanoparticles for bio-imaging applications. Phys. Status Solidi 2009, 246, 885–888. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H. Optical properties of ZnO nanostructures. Small 2006, 2, 944–961. [Google Scholar] [CrossRef]
- Jones, M.; Nedeljkovic, J.; Ellingson, R.J.; Nozik, A.J.; Rumbles, G. Photoenhancement of luminescence in colloidal CdSe quantum dot solutions. J. Phys. Chem. B 2003, 107, 11346–11352. [Google Scholar] [CrossRef]
- Moussodia, R.-O.; Balan, L.; Merlin, C.; Mustin, C.; Schneider, R. Biocompatible and stable ZnO quantum dots generated by functionalization with siloxane-core PAMAM dendrons. J. Mater. Chem. 2010, 20, 1147–1155. [Google Scholar] [CrossRef] [Green Version]
- Moussodia, R.-O.; Balan, L.; Schneider, R. Synthesis and characterization of water-soluble ZnO quantum dots prepared through PEG-siloxane coating. New J. Chem. 2008, 32, 1388. [Google Scholar] [CrossRef]
- Saliba, S.; Serrano, C.V.; Keilitz, J.; Kahn, M.L.; Mingotaud, C.; Haag, R.; Marty, J.-D. Hyperbranched polymers for the formation and stabilization of ZnO nanoparticles. Chem. Mater. 2010, 22, 6301–6309. [Google Scholar] [CrossRef]
- Hancock, J.M.; Rankin, W.M.; Hammad, T.M.; Salem, J.S.; Chesnel, K.; Harrison, R.G. Optical and magnetic properties of ZnO nanoparticles doped with Co, Ni and Mn and synthesized at low temperature. J. Nanosci. Nanotechnol. 2015, 15, 3809–3815. [Google Scholar] [CrossRef]
- Ostrovsky, S.; Kazimirsky, G.; Gedanken, A.; Brodie, C. Selective cytotoxic effect of ZnO nanoparticles on glioma cells. Nano Res. 2009, 2, 882–890. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Wu, C.; Jiang, H.; Li, Q.; Wang, X.; Chen, B. Synergistic cytotoxic effect of different sized ZnO nanoparticles and daunorubicin against leukemia cancer cells under UV irradiation. J. Photochem. Photobiol. B Biol. 2008, 93, 119–126. [Google Scholar] [CrossRef]
- Ng, S.M.; Wong, D.S.N.; Phung, J.H.C.; Chin, S.F.; Chua, H.S. Integrated miniature fluorescent probe to leverage the sensing potential of ZnO quantum dots for the detection of copper (II) ions. Talanta 2014, 119, 639. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, W.; Wang, S.; Liu, Y.; Pope, C. Phototoxicity of zinc oxide nanoparticle conjugates in human ovarian cancer NIH: OVCAR-3 cells. J. Biomed. Nanotechnol. 2008, 4, 432–438. [Google Scholar] [CrossRef]
- Depan, D.; Misra, R.D. Structural and physicochemical aspects of silica encapsulated ZnO quantum dots with high quantum yield and their natural uptake in HeLa cells. J. Biomed. Mater. Res. Part A 2014, 102, 2934–2941. [Google Scholar] [CrossRef]
- Sandmann, A.; Kompch, A.; Mackert, V.; Liebscher, C.H.; Winterer, M. Interaction of L-cysteine with ZnO: Structure, surface chemistry and optical properties. Langmuir 2015, 31, 5701–5711. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Darroudi, M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceram. Int. 2017, 43, 907–914. [Google Scholar] [CrossRef]
- Van Dijken, A.; Meulenkamp, E.A.; Vanmaekelbergh, D.; Meijerink, A. Identification of the transition responsible for the visible emission in ZnO using quantum size effects. J. Lumin. 2000, 90, 123–128. [Google Scholar] [CrossRef]
- Shi, H.Q.; Li, W.N.; Sun, L.W.; Liu, Y.; Xiao, H.M.; Fu, S.Y. Synthesis of silane surface modified ZnO quantum dots with ultrastable, strong and tunable luminescence. Chem. Commun. 2011, 47, 11921–11923. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Group | Number of Nude Mice | Tumor Volume of Nude Mice (mm3) | Tumor Inhibition Rate (%) | |||
|---|---|---|---|---|---|---|
| M1 | M2 | M3 | ± S | |||
| Control group | 3 | 5292 | 4200 | 6050 | 5180 ± 759 | – |
| UV group | 3 | 4400 | 5320 | 6348 | 5356 ± 795 | −3.3 |
| ZnO/PVP group | 3 | 5000 | 4630.5 | 4400 | 4543 ± 102 | 12.3 |
| ZnO/PVP+UV group | 3 | 2025 | 2048 | 1960 | 2011 ± 37 | 61.1 |
| Group | Number of Nude Mice | Tumor Volume of Nude Mice (mm3) | |||
|---|---|---|---|---|---|
| M1 | M2 | M3 | ± S | ||
| Control group | 3 | 2.27 | 2.16 | 2.08 | 2.17 ± 0.07 |
| UV group | 3 | 2.3 | 2.22 | 2.01 | 2.17 ± 0.12 |
| ZnO/PVP group | 3 | 2.14 | 2.13 | 2.24 | 2.17 ± 0.04 |
| ZnO/PVP+UV group | 3 | 1.5 | 1.43 | 1.52 | 1.48 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, T.; Qu, Y.; Ren, Z.; Yu, S.; Sun, M.; Yu, X.; Yu, X. Synthesis and Characterization of Polyvinylpyrrolidone-Modified ZnO Quantum Dots and Their In Vitro Photodynamic Tumor Suppressive Action. Int. J. Mol. Sci. 2021, 22, 8106. https://doi.org/10.3390/ijms22158106
Song T, Qu Y, Ren Z, Yu S, Sun M, Yu X, Yu X. Synthesis and Characterization of Polyvinylpyrrolidone-Modified ZnO Quantum Dots and Their In Vitro Photodynamic Tumor Suppressive Action. International Journal of Molecular Sciences. 2021; 22(15):8106. https://doi.org/10.3390/ijms22158106
Chicago/Turabian StyleSong, Tianming, Yawei Qu, Zhe Ren, Shuang Yu, Mingjian Sun, Xiaoyu Yu, and Xiaoyang Yu. 2021. "Synthesis and Characterization of Polyvinylpyrrolidone-Modified ZnO Quantum Dots and Their In Vitro Photodynamic Tumor Suppressive Action" International Journal of Molecular Sciences 22, no. 15: 8106. https://doi.org/10.3390/ijms22158106
APA StyleSong, T., Qu, Y., Ren, Z., Yu, S., Sun, M., Yu, X., & Yu, X. (2021). Synthesis and Characterization of Polyvinylpyrrolidone-Modified ZnO Quantum Dots and Their In Vitro Photodynamic Tumor Suppressive Action. International Journal of Molecular Sciences, 22(15), 8106. https://doi.org/10.3390/ijms22158106








