Effects of Visfatin on Intracellular Mechanics and Catabolism in Human Primary Chondrocytes through Glycogen Synthase Kinase 3β Inactivation
Abstract
:1. Introduction
2. Results
2.1. Visfatin Inactivates GSK3β to Increase Catabolic COX2 Expression in Human Primary Chondrocytes
2.2. P38 Signaling Regulates the Effect of Visfatin on GSK3β Inactivation and Subsequent COX2 Expression in Human Primary Chondrocytes
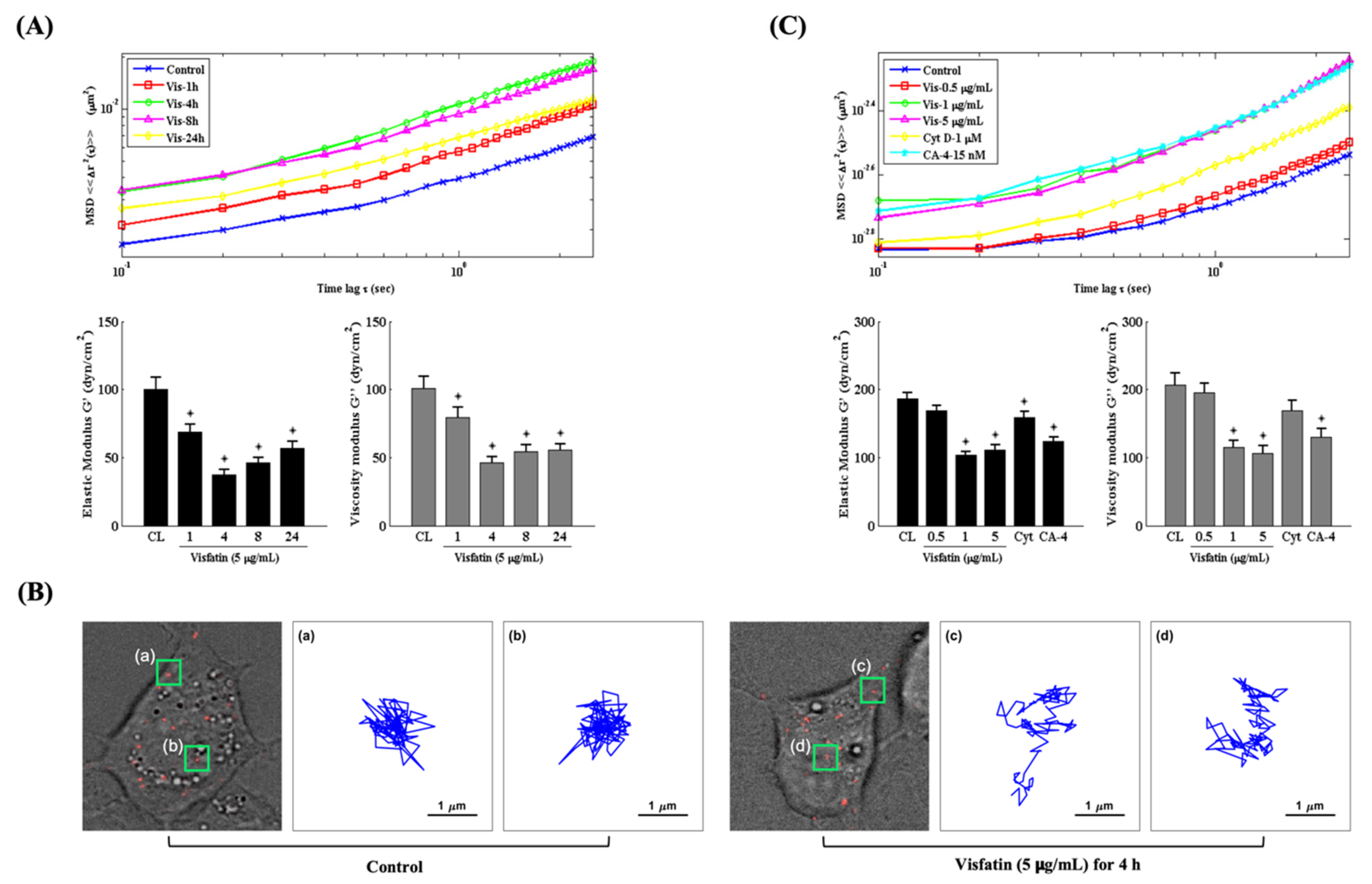
2.3. Visfatin Changes the Intracellular Mechanics of Human Primary Chondrocytes
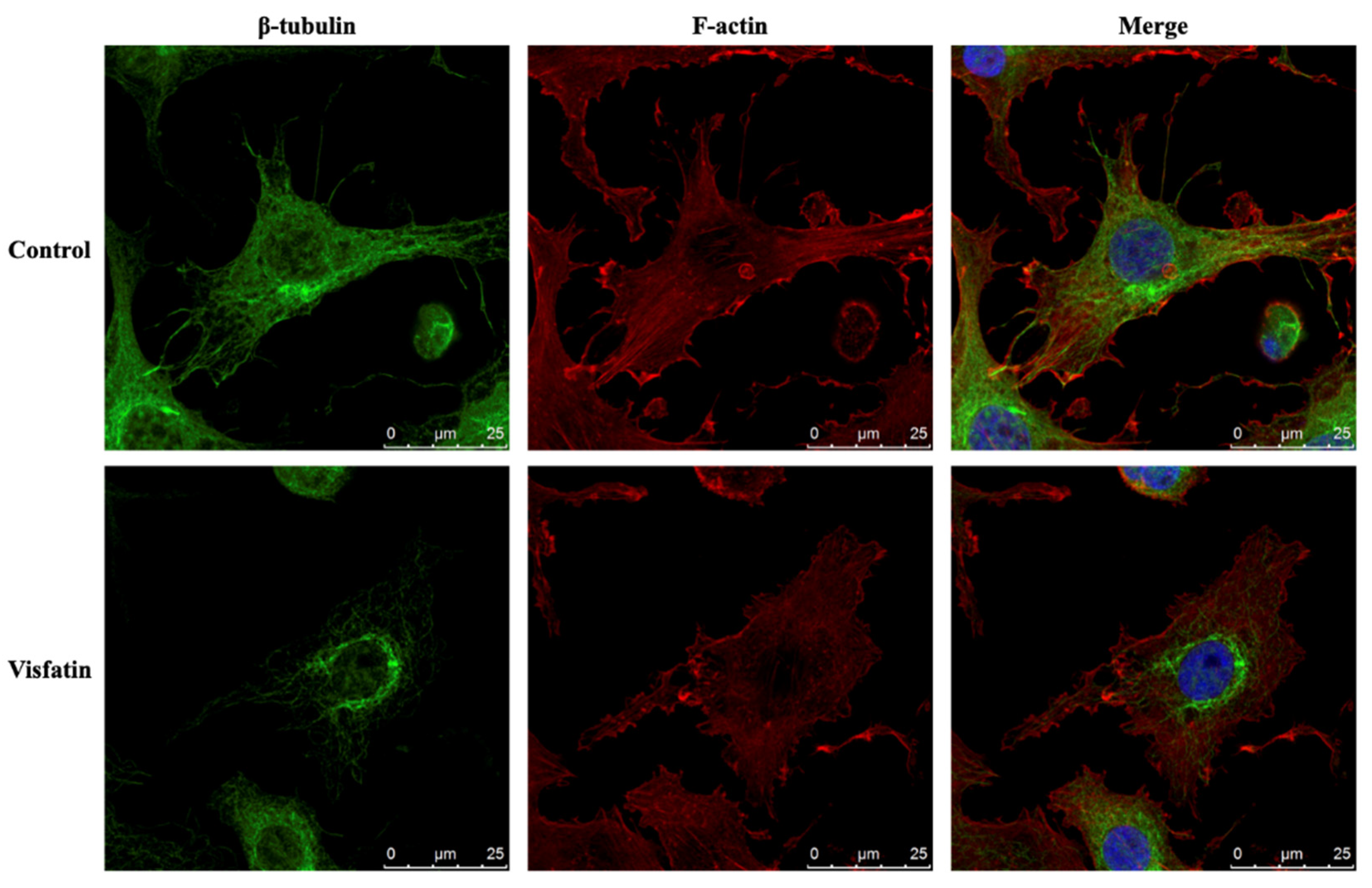
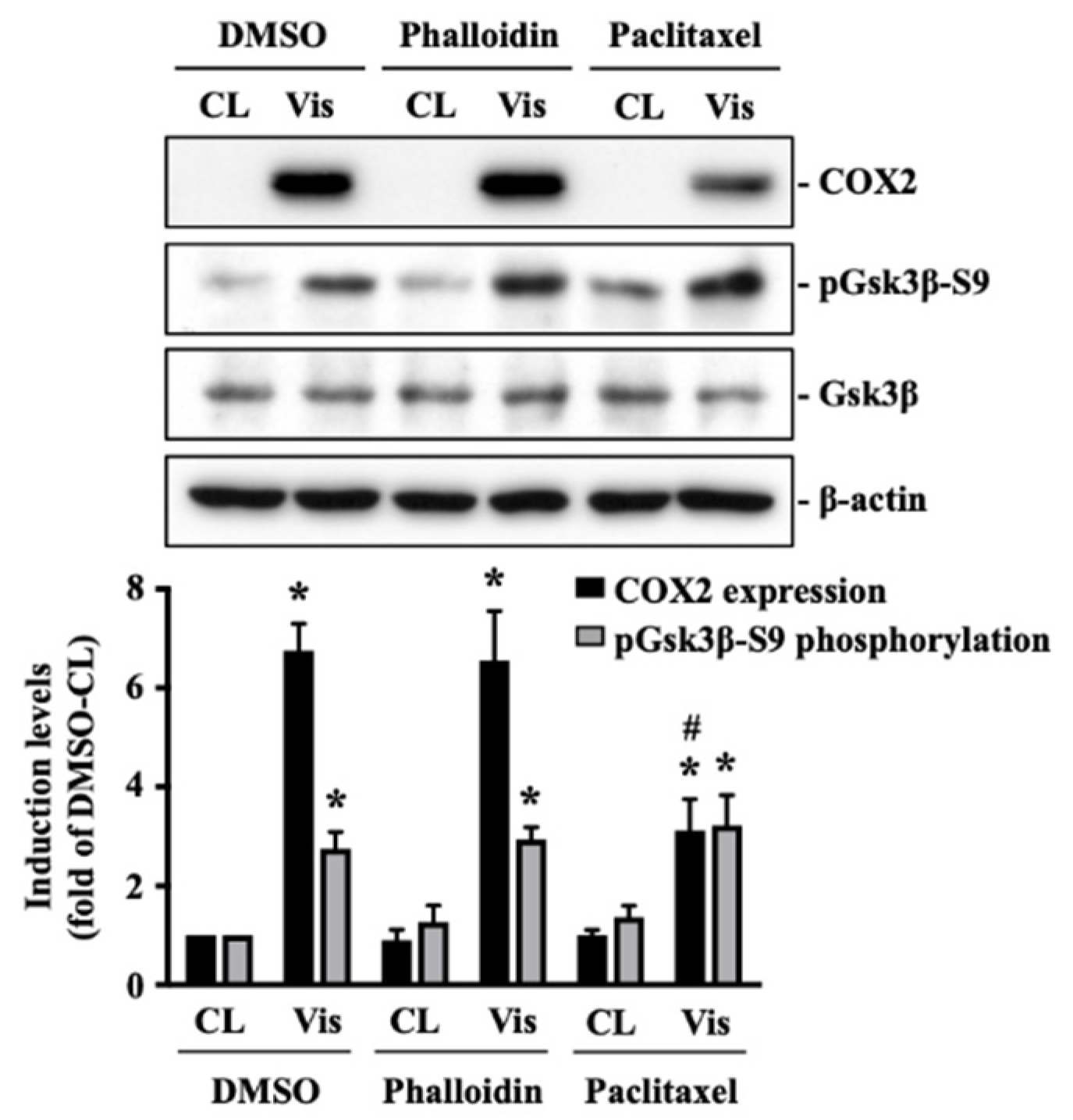
2.4. Visfatin Destabilizes the Microtubule Network to Influence Its Catabolic Effect on COX2 Expression in Human Primary Chondrocytes
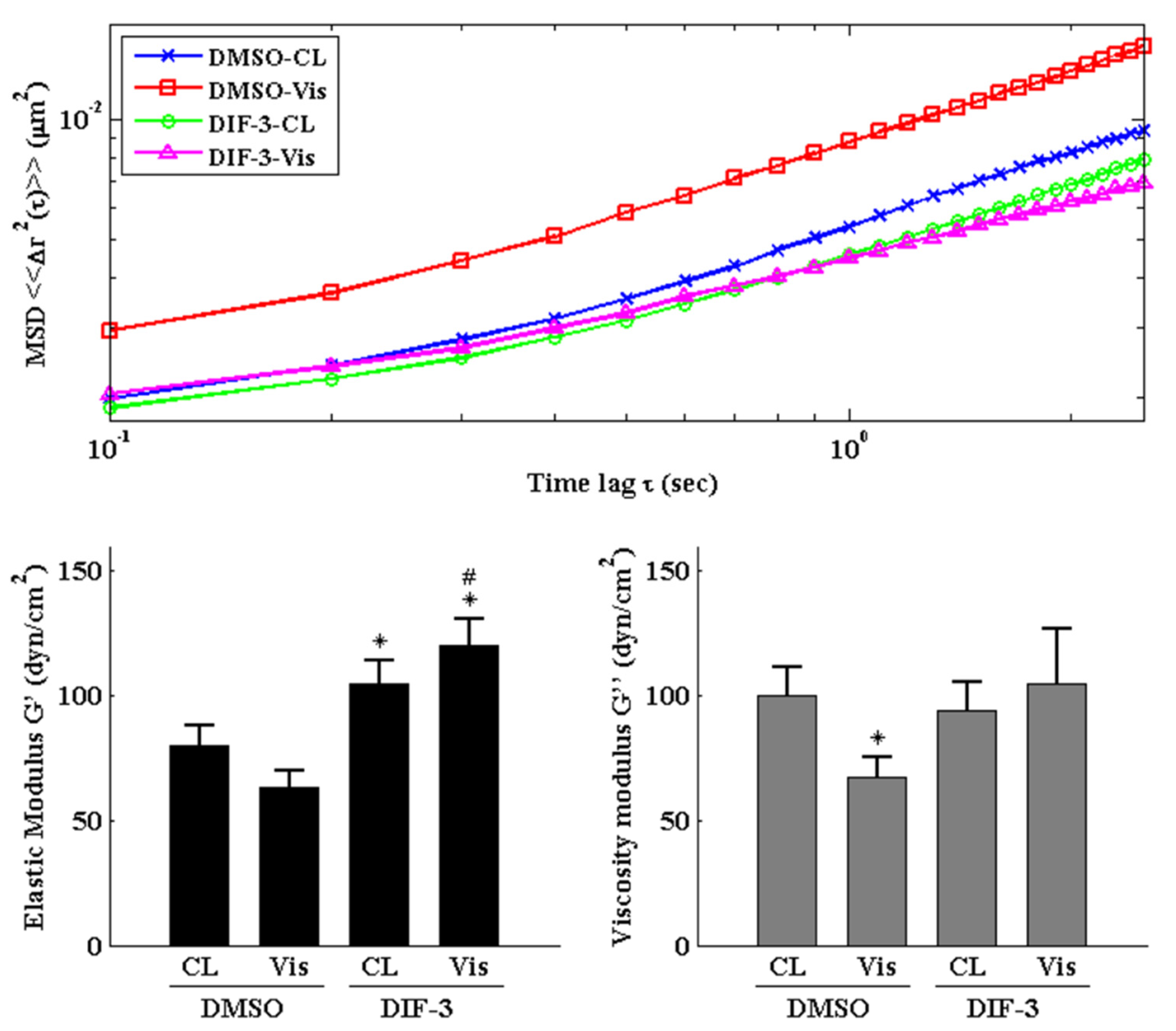
2.5. GSK3β Inactivation Regulates the Visfatin-Induced Changes in Intracellular Mechanics and Cytoskeletal Architecture in Human Primary Chondrocytes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Western Blot
4.4. Injection of Fluorescent Beads
4.5. Tracking of Fluorescent Beads
4.6. Immunofluorescent Staining
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jang, S.; Lee, K.; Ju, J. Recent Updates of Diagnosis, Pathophysiology, and Treatment on Osteoarthritis of the Knee. Int. J. Mol. Sci. 2021, 22, 2619. [Google Scholar] [CrossRef] [PubMed]
- Varela-Eirín, M.; Varela-Vázquez, A.; Guitián-Caamaño, A.; Paíno, C.L.; Mato, V.; Largo, R.; Aasen, T.; Tabernero, A.; Fonseca, E.; Kandouz, M.; et al. Targeting of chondrocyte plasticity via connexin43 modulation attenuates cellular senescence and fosters a pro-regenerative environment in osteoarthritis. Cell Death Dis. 2018, 9, 1166. [Google Scholar] [CrossRef] [Green Version]
- Tu, M.; Yang, M.; Yu, N.; Zhen, G.; Wan, M.; Liu, W.; Ji, B.; Ma, H.; Guo, Q.; Tong, P.; et al. Inhibition of cyclooxygenase-2 activity in subchondrodral bone modifies a subtype of osteoarthritis. Bone Res. 2019, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Mᵃᶜdonald, I.J.; Liu, S.-C.; Huang, C.-C.; Kuo, S.-J.; Tsai, C.-H.; Tang, C.-H. Associations between Adipokines in Arthritic Disease and Implications for Obesity. Int. J. Mol. Sci. 2019, 20, 1505. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Chen, Q. Adipokines: New Therapeutic Target for Osteoarthritis? Curr. Rheumatol Rep. 2019, 21, 71. [Google Scholar] [CrossRef]
- Cheleschi, S.; Tenti, S.; Barbarino, M.; Giannotti, S.; Bellisai, F.; Frati, E.; Fioravanti, A. Exploring the Crosstalk between Hydrostatic Pressure and Adipokines: An In Vitro Study on Human Osteoarthritic Chondrocytes. Int. J. Mol. Sci. 2021, 22, 2745. [Google Scholar] [CrossRef]
- Tu, C.; He, J.; Wu, B.; Wang, W.; Li, Z. An extensive review regarding the adipokines in the pathogenesis and pro-gression of osteoarthritis. Cytokine 2019, 113, 1–12. [Google Scholar] [CrossRef]
- Fioravanti, A.; Cheleschi, S.; De Palma, A.; Addimanda, O.; Mancarella, L.; Pignotti, E.; Pulsatelli, L.; Galeazzi, M.; Meliconi, R. Can adipokines serum levels be used as biomarkers of hand osteoarthritis? Biomarkers 2017, 23, 265–270. [Google Scholar] [CrossRef]
- Philp, A.M.; Butterworth, S.; Davis, E.T.; Jones, S.W. eNAMPT is localised to areas of cartilage damage in patients with hip osteoarthritis and promotes cartilage catabolism and inflammation. Int. J. Mol. Sci. 2021, 22, 6719. [Google Scholar] [CrossRef] [PubMed]
- Franco-Trepat, E.; Guillán-Fresco, M.; Alonso-Pérez, A.; Jorge-Mora, A.; Francisco, V.; Gualillo, O.; Gómez, R. Visfatin Connection: Present and Future in Osteoarthritis and Osteoporosis. J. Clin. Med. 2019, 8, 1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yavuz, G.Y.; Şimşek Kaya, G.; Kızıltunç, A. Analysis of synovial fluid visfatin level in temporomandibular joint disorders. CRANIO® 2018, 37, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Han, D.-F.; Li, Y.; Xu, H.-Y.; Li, R.-H.; Zhao, D. An Update on the Emerging Role of Visfatin in the Pathogenesis of Osteoarthritis and Pharmacological Intervention. Evid.-Based Complement. Altern. Med. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Wilson, J.A.; Kobsar, D. Osteoarthritis year in review 2020: Mechanics. Osteoarthr. Cartil. 2021, 29, 161–169. [Google Scholar] [CrossRef]
- O’Conor, C.J.; Case, N.; Guilak, F. Mechanical regulation of chondrogenesis. Stem Cell Res. Ther. 2013, 4, 61. [Google Scholar] [CrossRef] [Green Version]
- Guilak, F. The deformation behavior and viscoelastic properties of chondrocytes in articular cartilage. Biorheology 2000, 37, 27–44. [Google Scholar] [PubMed]
- Trickey, W.R.; Lee, G.M.; Guilak, F. Viscoelastic properties of chondrocytes from normal and osteoarthritic human cartilage. J. Orthop. Res. 2000, 18, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Trickey, W.R.; Vail, T.P.; Guilak, F. The role of the cytoskeleton in the viscoelastic properties of human articular chon-drocytes. J. Orthop. Res. 2004, 22, 131–139. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, K.S. Qualitative analysis of contribution of intracellular skeletal changes to cellular elasticity. Cell. Mol. Life Sci. 2019, 77, 1345–1355. [Google Scholar] [CrossRef]
- Lauer, J.C.; Selig, M.; Hart, M.L.; Kurz, B.; Rolauffs, B. Articular Chondrocyte Phenotype Regulation through the Cy-toskeleton and the Signaling Processes That Originate from or Converge on the Cytoskeleton: Towards a Novel Under-standing of the Intersection between Actin Dynamics and Chondrogenic Function. Int. J. Mol. Sci. 2021, 22, 3279. [Google Scholar] [CrossRef] [PubMed]
- Pegoraro, A.F.; Janmey, P.; Weitz, D.A. Mechanical Properties of the Cytoskeleton and Cells. Cold Spring Harb. Perspect. Biol. 2017, 9, a022038. [Google Scholar] [CrossRef]
- Blain, E.J. Involvement of the cytoskeletal elements in articular cartilage homeostasis and pathology. Int. J. Exp. Pathol. 2009, 90, 1–15. [Google Scholar] [CrossRef]
- Chen, C.; Xie, J.; Rajappa, R.; Deng, L.; Fredberg, J.; Yang, L. Interleukin-1β and tumor necrosis factor-α increase stiff-ness and impair contractile function of articular chondrocytes. Acta. Biochim. Biophys. Sin. (Shanghai) 2015, 47, 121–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haudenschild, D.R.; Chen, J.; Steklov, N.; Lotz, M.K.; D’Lima, D.D. Characterization of the chondrocyte actin cyto-skeleton in living three-dimensional culture: Response to anabolic and catabolic stimuli. Mol. Cell Biomech. 2009, 6, 135–144. [Google Scholar]
- Hsieh, C.; Lin, Y.-H.; Lin, S.; Tsai-Wu, J.-J.; Wu, C.H.; Jiang, C.-C. Surface ultrastructure and mechanical property of human chondrocyte revealed by atomic force microscopy. Osteoarthr. Cartil. 2008, 16, 480–488. [Google Scholar] [CrossRef] [Green Version]
- Wirtz, D. Particle-Tracking Microrheology of Living Cells: Principles and Applications. Annu. Rev. Biophys. 2009, 38, 301–326. [Google Scholar] [CrossRef] [Green Version]
- Guidotti, S.; Minguzzi, M.; Platano, D.; Cattini, L.; Trisolino, G.; Mariani, E.; Borzì, R.M. Lithium Chloride Dependent Glycogen Synthase Kinase 3 Inactivation Links Oxidative DNA Damage, Hypertrophy and Senescence in Human Articular Chondrocytes and Reproduces Chondrocyte Phenotype of Obese Osteoarthritis Patients. PLoS ONE 2015, 10, e0143865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guidotti, S.; Minguzzi, M.; Platano, D.; Santi, S.; Trisolino, G.; Filardo, G.; Mariani, E.; Borzì, R.M. Glycogen Synthase Kinase-3β Inhibition Links Mitochondrial Dysfunction, Extracellular Matrix Remodelling and Terminal Differentiation in Chondrocytes. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Jones, W.R.; Ting-Beall, H.P.; Lee, G.M.; Kelley, S.S.; Hochmuth, R.M.; Guilak, F. Alterations in the Young’s modulus and volumetric properties of chondrocytes isolated from normal and osteoarthritic human cartilage. J. Biomech. 1999, 32, 119–127. [Google Scholar] [CrossRef]
- Gardel, M.L.; Kasza, K.; Brangwynne, C.P.; Liu, J.; Weitz, D.A. Chapter 19 Mechanical Response of Cytoskeletal Networks. Micropatterning Cell Biol. Part B 2008, 89, 487–519. [Google Scholar] [CrossRef] [Green Version]
- Wong, B.L.; Bae, W.C.; Chun, J.; Gratz, K.R.; Lotz, M.; Sah, R.L. Biomechanics of cartilage articulation: Effects of lubri-cation and degeneration on shear deformation. Arthritis Rheum. 2008, 58, 2065–2074. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.T.; Jacobsen, T.D.; Chahine, N.O. Effects of Inflammation on Multiscale Biomechanical Properties of Carti-laginous Cells and Tissues. ACS Biomater. Sci. Eng. 2017, 3, 2644–2656. [Google Scholar] [CrossRef]
- Chien, S.; Sung, K. Effect of colchicine on viscoelastic properties of neutrophils. Biophys. J. 1984, 46, 383–386. [Google Scholar] [CrossRef] [Green Version]
- Sato, M.; Theret, D.P.; Wheeler, L.T.; Ohshima, N.; Nerem, R.M. Application of the Micropipette Technique to the Measurement of Cultured Porcine Aortic Endothelial Cell Viscoelastic Properties. J. Biomech. Eng. 1990, 112, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Z.; Zhang, G.; Long, M.; Wang, H.B.; Song, G.B.; Cai, S.X. Comparison of the viscoelastic properties of normal hepatocytes and hepatocellular carcinoma cells under cytoskeletal perturbation. Biorheology 2000, 37, 279–290. [Google Scholar]
- Jope, R.S.; Yuskaitis, C.J.; Beurel, E. Glycogen Synthase Kinase-3 (GSK3): Inflammation, Diseases, and Therapeutics. Neurochem. Res. 2006, 32, 577–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawasaki, Y.; Kugimiya, F.; Chikuda, H.; Kamekura, S.; Ikeda, T.; Kawamura, N.; Saito, T.; Shinoda, Y.; Higashikawa, A.; Yano, F.; et al. Phosphorylation of GSK-3beta by cGMP-dependent protein kinase II promotes hypertrophic differentiation of murine chondrocytes. J. Clin. Investig. 2008, 118, 2506–2515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, W.; Zhang, D.; Feng, X.; Zhou, Y. Low magnitude high frequency vibration promotes chondrogenic differentiation of bone marrow stem cells with involvement of β-catenin signaling pathway. Arch. Oral Biol. 2020, 118, 104860. [Google Scholar] [CrossRef] [PubMed]
- Lories, R.J.; Corr, M.; Lane, N.E. To Wnt or not to Wnt: The bone and joint health dilemma. Nat. Rev. Rheumatol. 2013, 9, 328–339. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Tang, D.; Wu, Q.; Hao, S.; Chen, M.; Xie, C.; Rosier, R.N.; O’Keefe, R.J.; Zuscik, M.; Chen, D. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional ac-tivation mice. J. Bone Miner. Res. 2009, 24, 12–21. [Google Scholar] [CrossRef]
- Altman, R.; Asch, E.; Bloch, D.; Bole, G.; Borenstein, D.; Brandt, K.; Christy, W.; Cooke, T.D.; Greenwald, R.; Hochberg, M.; et al. Development of criteria for the classification and reporting of osteoarthritis: Classification of osteoarthritis of the knee. Arthritis Rheum. 1986, 29, 1039–1049. [Google Scholar] [CrossRef]
- Chang, S.F.; Huang, K.C.; Chang, H.I.; Lee, K.C.; Su, Y.P.; Chen, C.N. 2 dyn/cm(2) shear force upregulates kruppel-like factor 4 expression in human chondrocytes to inhibit the interleukin-1β-activated nuclear factor-κB. J. Cell. Physiol. 2018, 234, 958–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, R.N.; Webb, W.W. Automated detection and tracking of individual and clustered cell surface low density lipo-protein receptor molecules. Biophys. J. 1994, 66, 1301–1318. [Google Scholar] [CrossRef] [Green Version]
- Selvaggi, L.; Salemme, M.; Vaccaro, C.; Pesce, G.; Rusciano, G.; Sasso, A.; Campanella, C.; Carotenuto, R. Multi-ple-Particle-Tracking to investigate viscoelastic properties in living cells. Methods 2010, 51, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.; Kole, T.P.; Wirtz, D. Micromechanical Mapping of Live Cells by Multiple-Particle-Tracking Microrheology. Biophys. J. 2002, 83, 3162–3176. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.Q.; Kuo, C.Y.; Wei, M.T.; Wu, K.; Su, P.T.; Huang, C.S.; Chiou, A. Intracellular viscoelasticity of HeLa cells during cell division studied by video particle-tracking microrheology. J. Biomed. Opt. 2013, 19, 011008. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, S.-F.; Huang, K.-C.; Lee, K.-H.; Chiang, Y.-C.; Lee, W.-R.; Hsieh, R.-Z.; Su, Y.-P.; Wu, S.-C. Effects of Visfatin on Intracellular Mechanics and Catabolism in Human Primary Chondrocytes through Glycogen Synthase Kinase 3β Inactivation. Int. J. Mol. Sci. 2021, 22, 8107. https://doi.org/10.3390/ijms22158107
Chang S-F, Huang K-C, Lee K-H, Chiang Y-C, Lee W-R, Hsieh R-Z, Su Y-P, Wu S-C. Effects of Visfatin on Intracellular Mechanics and Catabolism in Human Primary Chondrocytes through Glycogen Synthase Kinase 3β Inactivation. International Journal of Molecular Sciences. 2021; 22(15):8107. https://doi.org/10.3390/ijms22158107
Chicago/Turabian StyleChang, Shun-Fu, Kuo-Chin Huang, Kuan-Han Lee, Yao-Chang Chiang, Wei-Ru Lee, Rong-Ze Hsieh, Yu-Ping Su, and Shun-Chi Wu. 2021. "Effects of Visfatin on Intracellular Mechanics and Catabolism in Human Primary Chondrocytes through Glycogen Synthase Kinase 3β Inactivation" International Journal of Molecular Sciences 22, no. 15: 8107. https://doi.org/10.3390/ijms22158107
APA StyleChang, S.-F., Huang, K.-C., Lee, K.-H., Chiang, Y.-C., Lee, W.-R., Hsieh, R.-Z., Su, Y.-P., & Wu, S.-C. (2021). Effects of Visfatin on Intracellular Mechanics and Catabolism in Human Primary Chondrocytes through Glycogen Synthase Kinase 3β Inactivation. International Journal of Molecular Sciences, 22(15), 8107. https://doi.org/10.3390/ijms22158107





