Effect of Erythropoietin on the Expression of Murine Transferrin Receptor 2
Abstract
:1. Introduction
2. Results
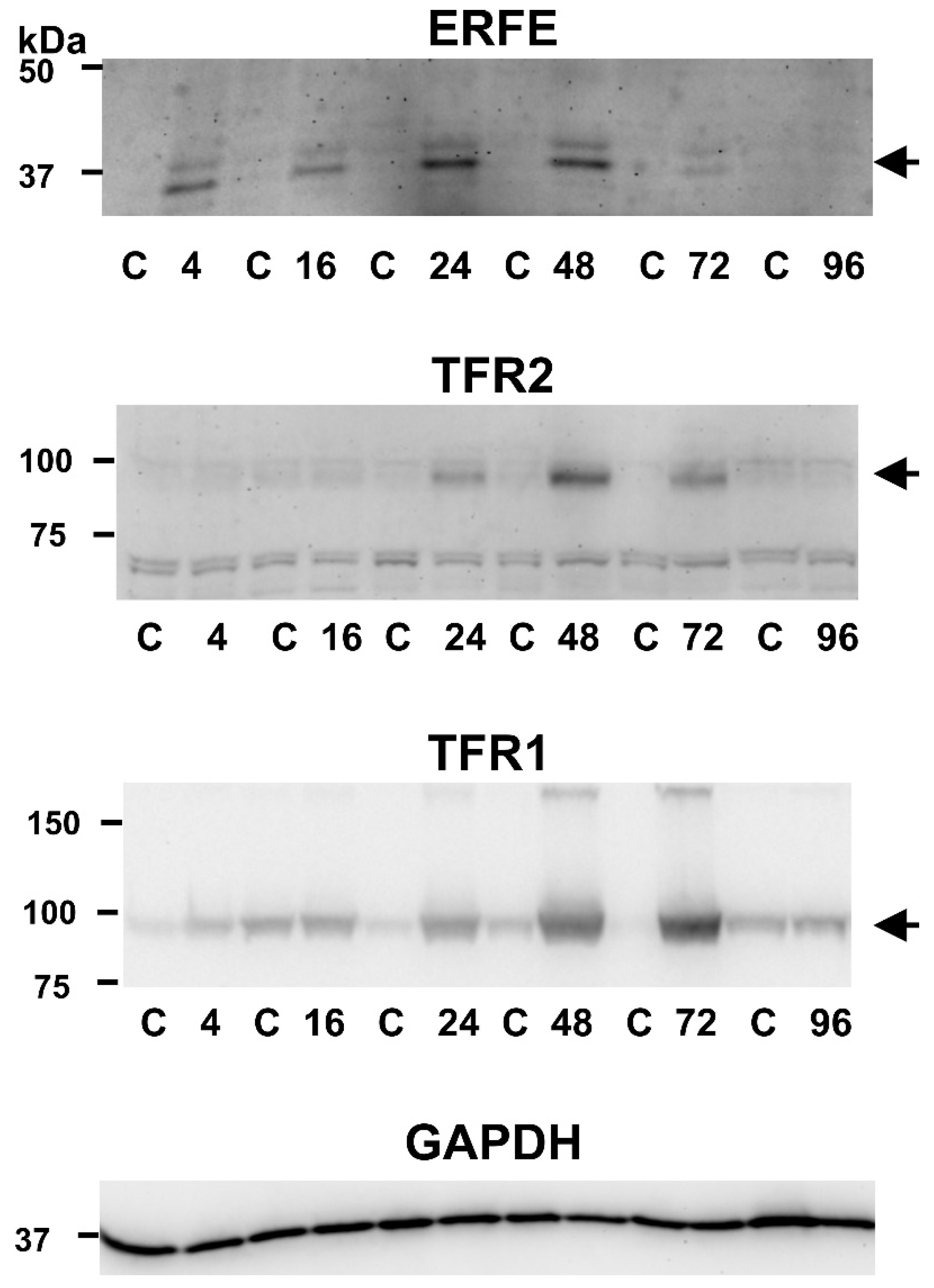
2.1. EPO Administration Induces Splenic TFR2 Protein Content at 48 and 72 h Post Injection
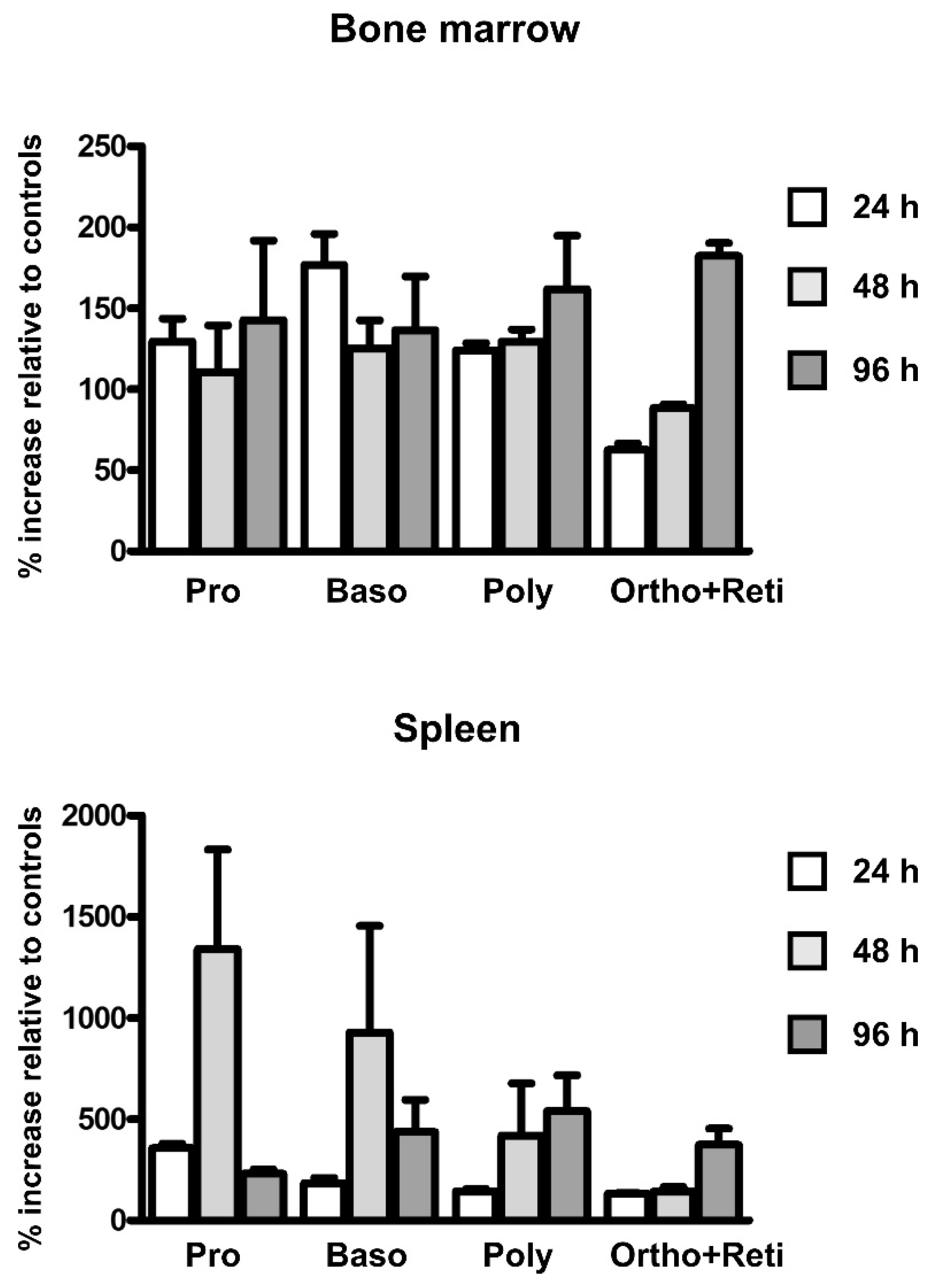
2.2. EPO Administration Increases the Number of Erythroid Precursors in the Spleen
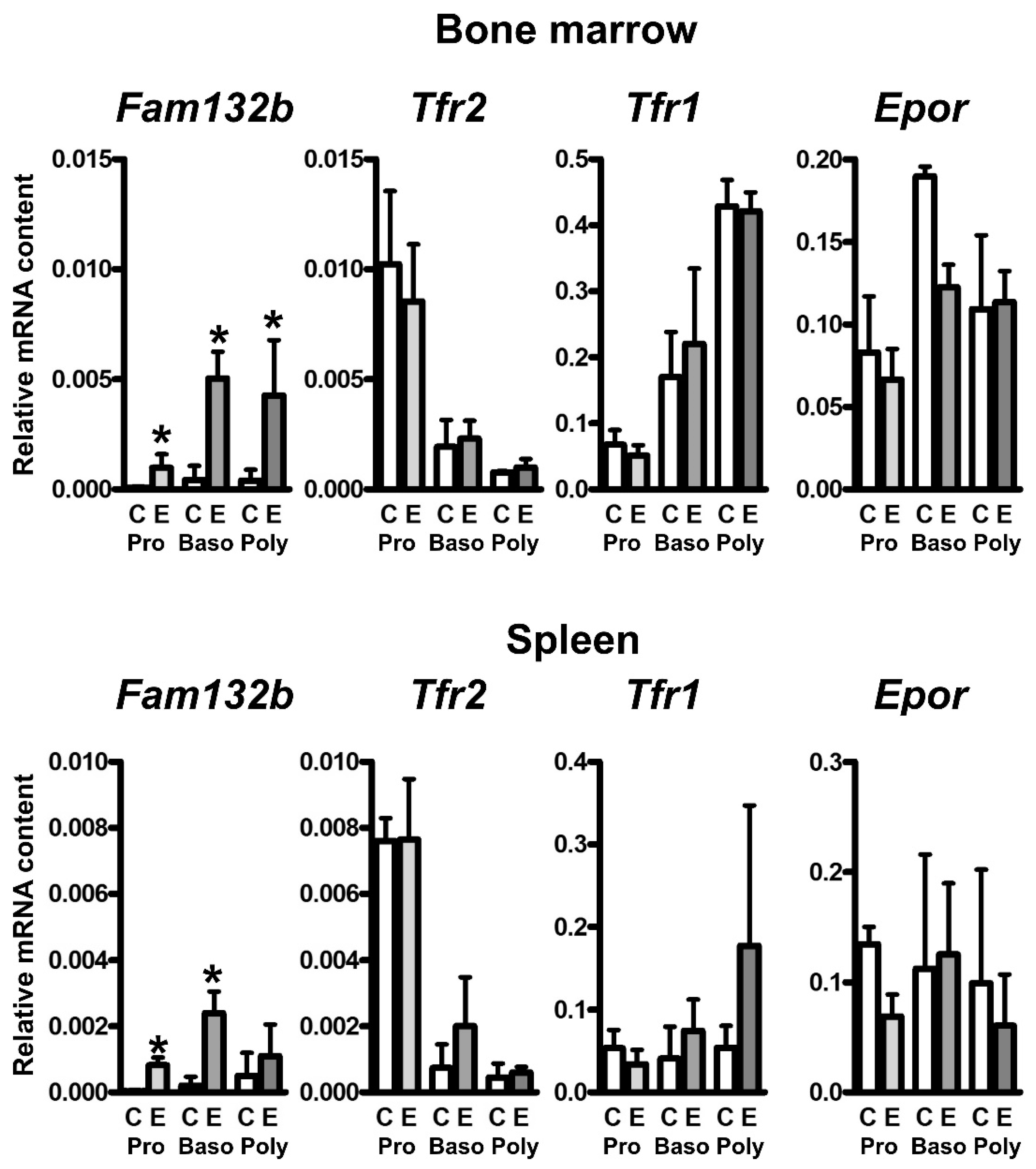
2.3. EPO Administration Increases the Content of Fam132b mRNA, but Not Tfr2 or Tfr1 mRNA, in Erythroid Precursors
2.4. TFR2 Is Not Detected in Mouse Plasma Exosomes after EPO Administration
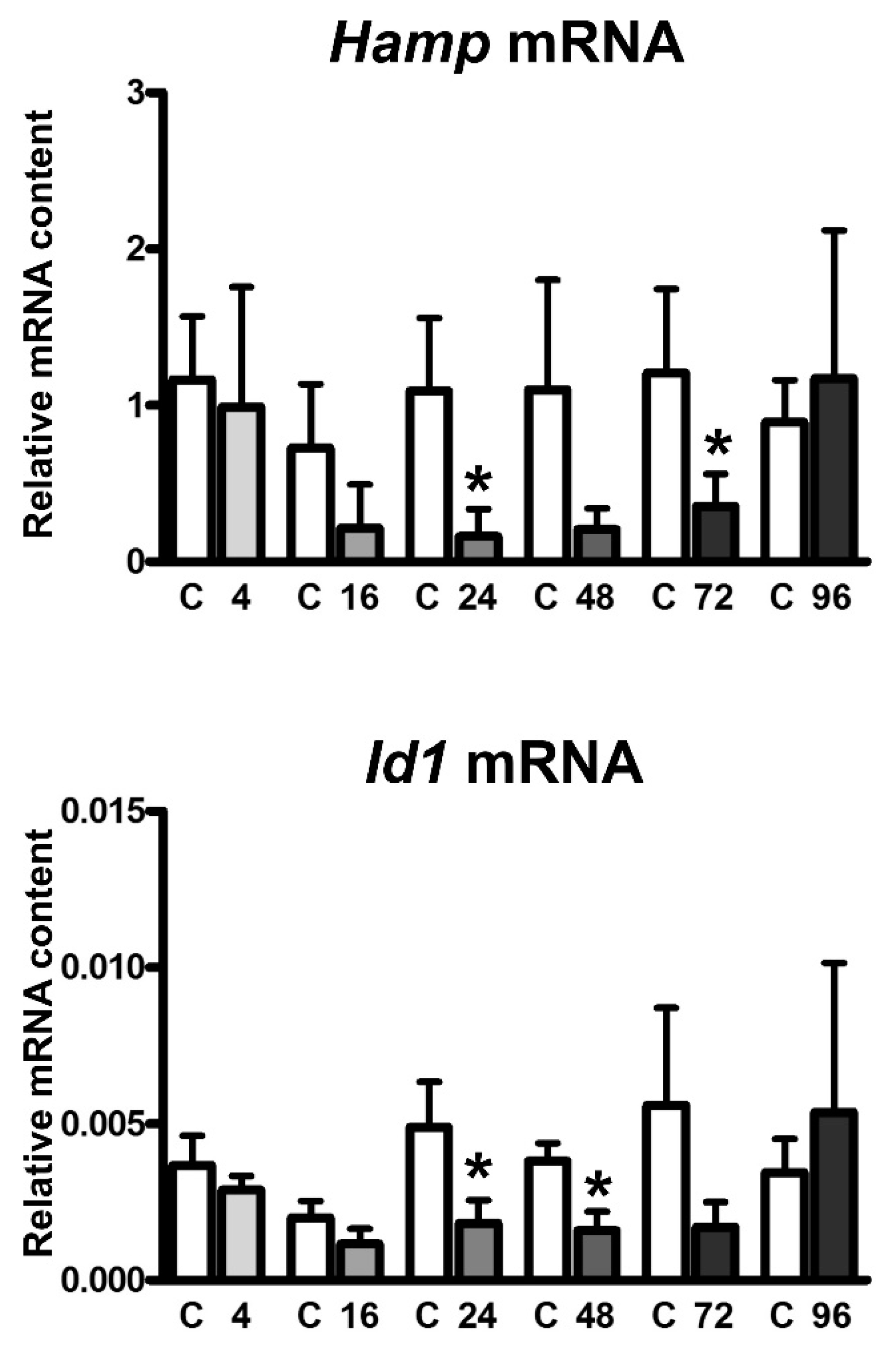
3. Discussion
4. Materials and Methods
4.1. Animals and Treatment
4.2. RNA Analyses
4.3. Immunoblotting
4.4. Flow Cytometry and Cell Sorting
4.5. Exosome Preparation
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Camaschella, C. Iron deficiency. Blood 2019, 133, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Sheftel, A.D.; Mason, A.B.; Ponka, P. The long history of iron in the Universe and in health and disease. Biochim. Biophys. Acta 2012, 1820, 161–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawabata, H.; Nakamaki, T.; Ikonomi, P.; Smith, R.D.; Germain, R.S.; Koeffler, H.P. Expression of transferrin receptor 2 in normal and neoplastic hematopoietic cells. Blood 2001, 98, 2714–2719. [Google Scholar] [CrossRef]
- Levy, J.E.; Jin, O.; Fujiwara, Y.; Kuo, F.; Andrews, N.C. Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nat. Genet. 1999, 21, 396–399. [Google Scholar] [CrossRef]
- Fleming, R.E.; Ahmann, J.R.; Migas, M.C.; Waheed, A.; Koeffler, H.P.; Kawabata, H.; Britton, R.S.; Bacon, B.R.; Sly, W.S. Targeted mutagenesis of the murine transferrin receptor-2 gene produces hemochromatosis. Proc. Natl. Acad. Sci. USA 2002, 99, 10653–10658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camaschella, C.; Roetto, A.; Calì, A.; De Gobbi, M.; Garozzo, G.; Carella, M.; Majorano, N.; Totaro, A.; Gasparini, P. The gene TFR2 is mutated in a new type of haemochromatosis mapping to 7q22. Nat. Genet. 2000, 25, 14–15. [Google Scholar] [CrossRef] [PubMed]
- Forejtníková, H.; Vieillevoye, M.; Zermati, Y.; Lambert, M.; Pellegrino, R.M.; Guihard, S.; Gaudry, M.; Camaschella, C.; Lacombe, C.; Roetto, A.; et al. Transferrin receptor 2 is a component of the erythropoietin receptor complex and is required for efficient erythropoiesis. Blood 2010, 116, 5357–5367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roetto, A.; Di Cunto, F.; Pellegrino, R.M.; Hirsch, E.; Azzolino, O.; Bondi, A.; Defilippi, I.; Carturan, S.; Miniscalco, B.; Riondato, F.; et al. Comparison of 3 Tfr2-deficient murine models suggests distinct functions for Tfr2-alpha and Tfr2-beta isoforms in different tissues. Blood 2010, 115, 3382–3389. [Google Scholar] [CrossRef] [PubMed]
- Nai, A.; Lidonnici, M.R.; Rausa, M.; Mandelli, G.; Pagani, A.; Silvestri, L.; Ferrari, G.; Camaschella, C. The second transferrin receptor regulates red blood cell production in mice. Blood 2015, 125, 1170–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rishi, G.; Secondes, E.S.; Wallace, D.F.; Subramaniam, V.N. Hematopoietic deletion of transferrin receptor 2 in mice leads to a block in erythroid differentiation during iron-deficient anemia. Am. J. Hematol. 2016, 91, 812–818. [Google Scholar] [CrossRef] [Green Version]
- Khalil, S.; Holy, M.; Grado, S.; Fleming, R.; Kurita, R.; Nakamura, Y.; Goldfarb, A. A specialized pathway for erythroid iron delivery through lysosomal trafficking of transferrin receptor 2. Blood Adv. 2017, 1, 1181–1194. [Google Scholar] [CrossRef] [Green Version]
- Artuso, I.; Lidonnici, M.R.; Altamura, S.; Mandelli, G.; Pettinato, M.; Muckenthaler, M.U.; Silvestri, L.; Ferrari, G.; Camaschella, C.; Nai, A. Transferrin receptor 2 is a potential novel therapeutic target for β-thalassemia: Evidence from a murine model. Blood 2018, 132, 2286–2297. [Google Scholar] [CrossRef] [Green Version]
- Wortham, A.M.; Goldman, D.C.; Chen, J.; Fleming, W.H.; Zhang, A.S.; Enns, C.A. Extrahepatic deficiency of transferrin receptor 2 is associated with increased erythropoiesis independent of iron overload. J. Biol. Chem. 2020, 295, 3906–3917. [Google Scholar] [CrossRef]
- Richard, C.; Viret, S.; Cantero Aguilar, L.; Lefevre, C.; Leduc, M.; Faouzi, E.H.; Azar, N.; Lavazec, C.; Mayeux, P.; Verdier, F. Myotonic dystrophy kinase-related CDC42-binding kinase α, a new transferrin receptor type 2-binding partner, is a regulator of erythropoiesis. Am. J. Hematol. 2021, 96, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Trenor, C.C.; Campagna, D.R.; Sellers, V.M.; Andrews, N.C.; Fleming, M.D. The molecular defect in hypotransferrinemic mice. Blood 2000, 96, 1113–1118. [Google Scholar] [CrossRef]
- Nicolas, G.; Viatte, L.; Bennoun, M.; Beaumont, C.; Kahn, A.; Vaulont, S. Hepcidin, a new iron regulatory peptide. Blood Cells Mol. Dis. 2002, 29, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Vokurka, M.; Krijt, J.; Šulc, K.; Nečas, E. Hepcidin mRNA levels in mouse liver respond to inhibition of erythropoiesis. Physiol. Res. 2006, 55, 667–674. [Google Scholar]
- Pak, M.; Lopez, M.A.; Gabayan, V.; Ganz, T.; Rivera, S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood 2006, 108, 3730–3735. [Google Scholar] [CrossRef]
- Kautz, L.; Jung, G.; Valore, E.V.; Rivella, S.; Nemeth, E.; Ganz, T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat. Genet. 2014, 46, 678–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arezes, J.; Foy, N.; McHugh, K.; Sawant, A.; Quinkert, D.; Terraube, V.; Brinth, A.; Tam, M.; LaVallie, E.R.; Taylor, S.; et al. Erythroferrone inhibits the induction of hepcidin by BMP6. Blood 2018, 132, 1473–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arezes, J.; Foy, N.; McHugh, K.; Quinkert, D.; Benard, S.; Sawant, A.; Frost, J.N.; Armitage, A.E.; Pasricha, S.R.; Lim, P.J.; et al. Antibodies against the erythroferrone N-terminal domain prevent hepcidin suppression and ameliorate murine thalassemia. Blood 2020, 135, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Xu, Y.; Traeger, L.; Dogan, D.Y.; Xiao, X.; Steinbicker, A.U.; Babitt, J.L. Erythroferrone lowers hepcidin by sequestering BMP2/6 heterodimer from binding to the BMP type I receptor ALK3. Blood 2020, 135, 453–456. [Google Scholar] [CrossRef]
- Babitt, J.L.; Huang, F.W.; Wrighting, D.M.; Xia, Y.; Sidis, Y.; Samad, T.A.; Campagna, J.A.; Chung, R.T.; Schneyer, A.L.; Woolf, C.J.; et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat. Genet. 2006, 38, 531–539. [Google Scholar] [CrossRef]
- Meynard, D.; Kautz, L.; Darnaud, V.; Canonne-Hergaux, F.; Coppin, H.; Roth, M.P. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat. Genet. 2009, 41, 478–481. [Google Scholar] [CrossRef]
- Canali, S.; Wang, C.Y.; Zumbrennen-Bullough, K.B.; Bayer, A.; Babitt, J.L. Bone morphogenetic protein 2 controls iron homeostasis in mice independent of Bmp6. Am. J. Hematol. 2017, 92, 1204–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauner, M.; Baschant, U.; Roetto, A.; Pellegrino, R.M.; Rother, S.; Salbach-Hirsch, J.; Weidner, H.; Hintze, V.; Campbell, G.; Petzold, A.; et al. Transferrin receptor 2 controls bone mass and pathological bone formation via BMP and Wnt signaling. Nat. Metab. 2019, 1, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Frýdlová, J.; Rogalsky, D.W.; Truksa, J.; Nečas, E.; Vokurka, M.; Krijt, J. Effect of stimulated erythropoiesis on liver SMAD signaling pathway in iron-overloaded and iron-deficient mice. PLoS ONE 2019, 14, e0215028. [Google Scholar] [CrossRef]
- Chen, K.; Liu, J.; Heck, S.; Chasis, J.A.; An, X.; Mohandas, N. Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis. Proc. Natl. Acad. Sci. USA 2009, 106, 17413–17418. [Google Scholar] [CrossRef] [Green Version]
- Kohgo, Y.; Niitsu, Y.; Kondo, H.; Kato, J.; Tsushima, N.; Sasaki, K.; Hirayama, M.; Numata, T.; Nishisato, T.; Urushizaki, I. Serum transferrin receptor as a new index of erythropoiesis. Blood 1987, 70, 1955–1958. [Google Scholar] [CrossRef] [Green Version]
- Cook, J.D.; Skikne, B.S.; Baynes, R.D. Serum transferrin receptor. Annu. Rev. Med. 1993, 44, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Pagani, A.; Vieillevoye, M.; Nai, A.; Rausa, M.; Ladli, M.; Lacombe, C.; Mayeux, P.; Verdier, F.; Camaschella, C.; Silvestri, L. Regulation of cell surface transferrin receptor-2 by iron-dependent cleavage and release of a soluble form. Haematologica 2015, 100, 458–465. [Google Scholar] [CrossRef] [Green Version]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Johnstone, R.M. Cleavage of the transferrin receptor by human granulocytes: Preferential proteolysis of the exosome-bound TFR. J. Cell Physiol. 1996, 168, 333–345. [Google Scholar] [CrossRef]
- Paulson, R.F.; Shi, L.; Wu, D.C. Stress erythropoiesis: New signals and new stress progenitor cells. Curr. Opin. Hematol. 2011, 18, 139–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirciov, C.S.G.; Wilkins, S.J.; Hung, G.C.C.; Helman, S.L.; Anderson, G.J.; Frazer, D.M. Circulating iron levels influence the regulation of hepcidin following stimulated erythropoiesis. Haematologica 2018, 103, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Krijt, J.; Jonášová, A.; Neuwirtová, R.; Nečas, E. Effect of erythropoietin on hepcidin expression in hemojuvelin-mutant mice. Blood Cells Mol. Dis. 2010, 44, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Coffey, R.; Sardo, U.; Kautz, L.; Gabayan, V.; Nemeth, E.; Ganz, T. Erythroferrone is not required for the glucoregulatory and hematologic effects of chronic erythropoietin treatment in mice. Physiol. Rep. 2018, 6, e13890. [Google Scholar] [CrossRef]
- Casey, J.L.; Hentze, M.W.; Koeller, D.M.; Caughman, S.W.; Rouault, T.A.; Klausner, R.D.; Harford, J.B. Iron-responsive elements: Regulatory RNA sequences that control mRNA levels and translation. Science 1988, 240, 924–928. [Google Scholar] [CrossRef]
- Lok, C.N.; Ponka, P. Identification of an erythroid active element in the transferrin receptor gene. J. Biol. Chem. 2000, 275, 24185–24190. [Google Scholar] [CrossRef] [Green Version]
- Vogt, T.M.; Blackwell, A.D.; Giannetti, A.M.; Bjorkman, P.J.; Enns, C.A. Heterotypic interactions between transferrin receptor and transferrin receptor 2. Blood 2003, 101, 2008–2014. [Google Scholar] [CrossRef] [Green Version]
- Guillemot, J.; Canuel, M.; Essalmani, R.; Prat, A.; Seidah, N.G. Implication of the proprotein convertases in iron homeostasis: Proprotein convertase 7 sheds human transferrin receptor 1 and furin activates hepcidin. Hepatology 2013, 57, 2514–2524. [Google Scholar] [CrossRef]
- Gautier, E.F.; Leduc, M.; Ladli, M.; Schulz, V.P.; Lefèvre, C.; Boussaid, I.; Fontenay, M.; Lacombe, C.; Verdier, F.; Guillonneau, F.; et al. Comprehensive proteomic analysis of murine terminal erythroid differentiation. Blood Adv. 2020, 4, 1464–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calzolari, A.; Raggi, C.; Deaglio, S.; Sposi, N.M.; Stafsnes, M.; Fecchi, K.; Parolini, I.; Malavasi, F.; Peschle, C.; Sargiacomo, M.; et al. TfR2 localizes in lipid raft domains and is released in exosomes to activate signal transduction along the MAPK pathway. J. Cell Sci. 2006, 119, 4486–4498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadvakassova, G.; Tiedemann, K.; Steer, K.J.D.; Mikolajewicz, N.; Stavnichuk, M.; In-Kyung Lee, I.; Sabirova, Z.; Schranzhofer, M.; Komarova, S.V. Active hematopoiesis triggers exosomal release of PRDX2 that promotes osteoclast formation. Physiol. Rep. 2021, 9, e14745. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berezovsky, B.; Báječný, M.; Frýdlová, J.; Gurieva, I.; Rogalsky, D.W.; Přikryl, P.; Pospíšil, V.; Nečas, E.; Vokurka, M.; Krijt, J. Effect of Erythropoietin on the Expression of Murine Transferrin Receptor 2. Int. J. Mol. Sci. 2021, 22, 8209. https://doi.org/10.3390/ijms22158209
Berezovsky B, Báječný M, Frýdlová J, Gurieva I, Rogalsky DW, Přikryl P, Pospíšil V, Nečas E, Vokurka M, Krijt J. Effect of Erythropoietin on the Expression of Murine Transferrin Receptor 2. International Journal of Molecular Sciences. 2021; 22(15):8209. https://doi.org/10.3390/ijms22158209
Chicago/Turabian StyleBerezovsky, Betty, Martin Báječný, Jana Frýdlová, Iuliia Gurieva, Daniel Wayne Rogalsky, Petr Přikryl, Vít Pospíšil, Emanuel Nečas, Martin Vokurka, and Jan Krijt. 2021. "Effect of Erythropoietin on the Expression of Murine Transferrin Receptor 2" International Journal of Molecular Sciences 22, no. 15: 8209. https://doi.org/10.3390/ijms22158209
APA StyleBerezovsky, B., Báječný, M., Frýdlová, J., Gurieva, I., Rogalsky, D. W., Přikryl, P., Pospíšil, V., Nečas, E., Vokurka, M., & Krijt, J. (2021). Effect of Erythropoietin on the Expression of Murine Transferrin Receptor 2. International Journal of Molecular Sciences, 22(15), 8209. https://doi.org/10.3390/ijms22158209






