Reduced Endothelin-2 and Hypoxic Signaling Pathways in Granulosa-Lutein Cells of PCOS Women
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics of PCOS and Control Patients
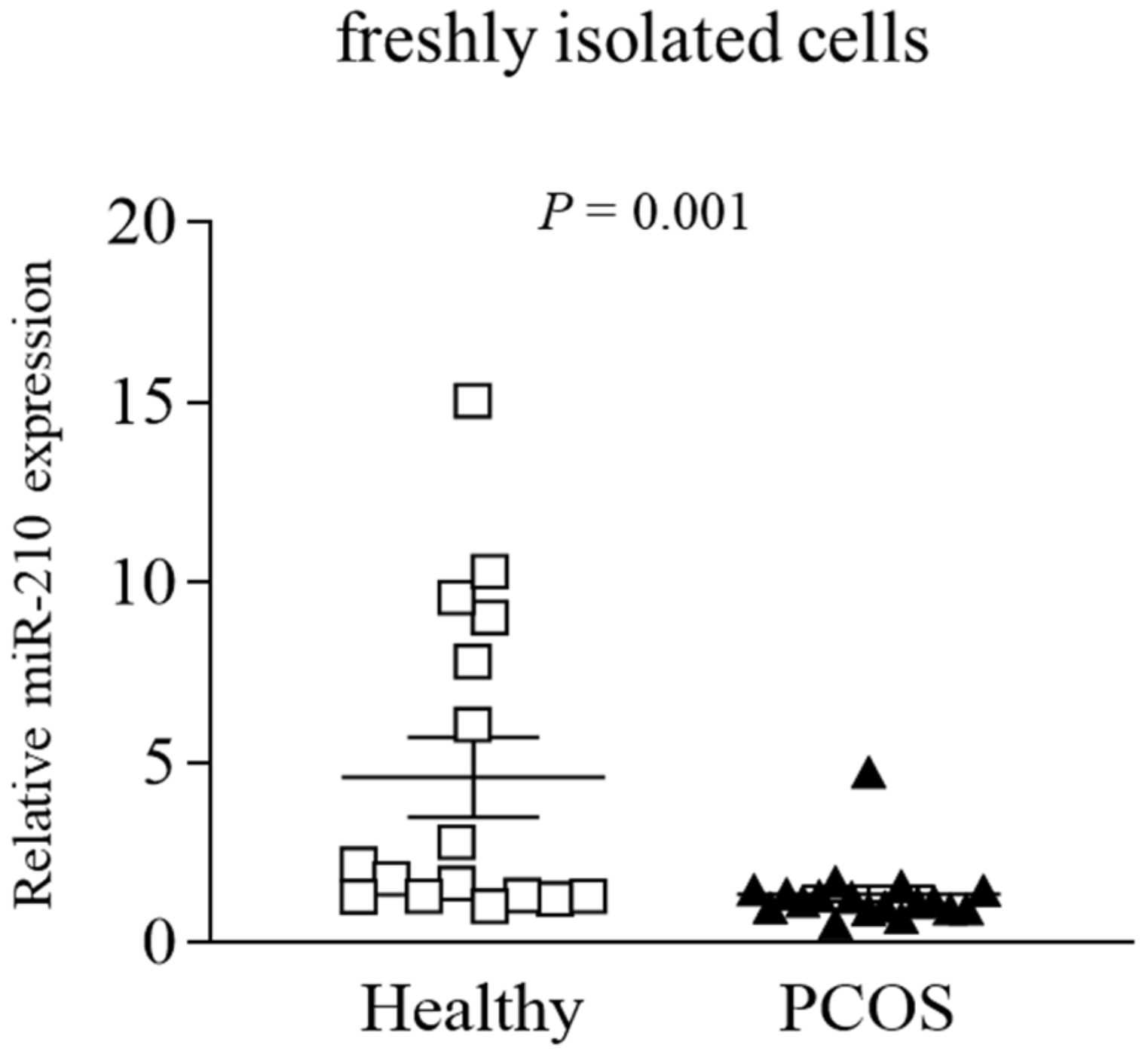
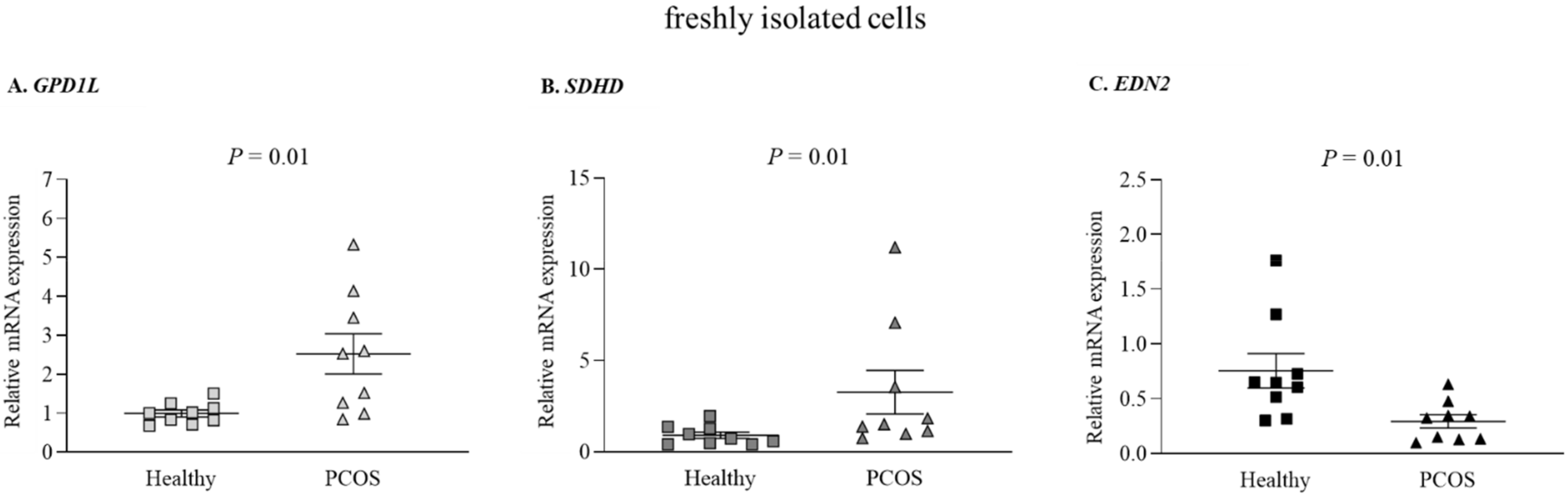
2.2. Altered miRs and Gene Expression Levels in Freshly Isolated GLCs from PCOS and Healthy Women
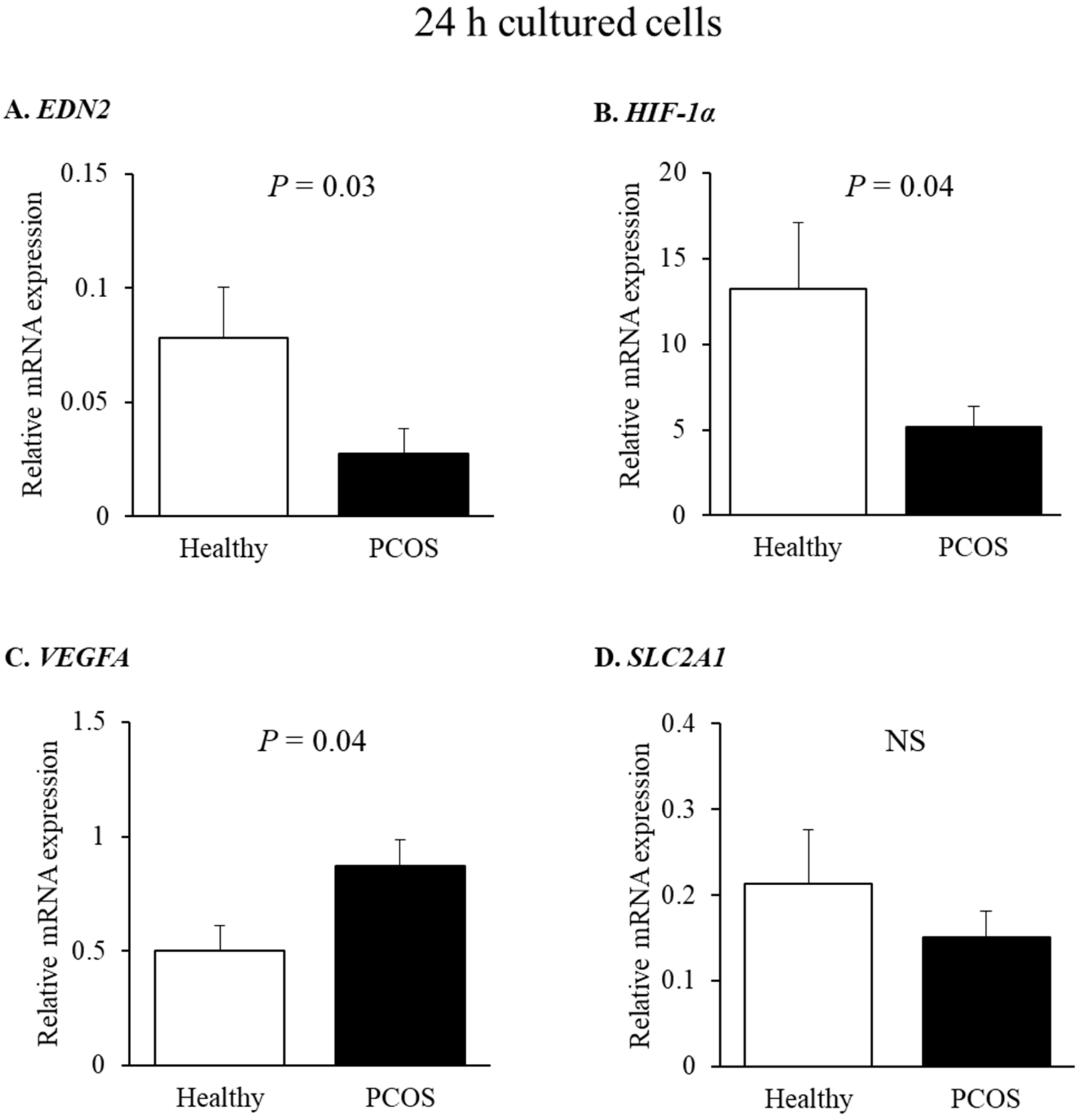
2.3. Effect of miR-210 on HIF-1α-Responsive Genes: EDN2 and VEGFA in Human GLCs
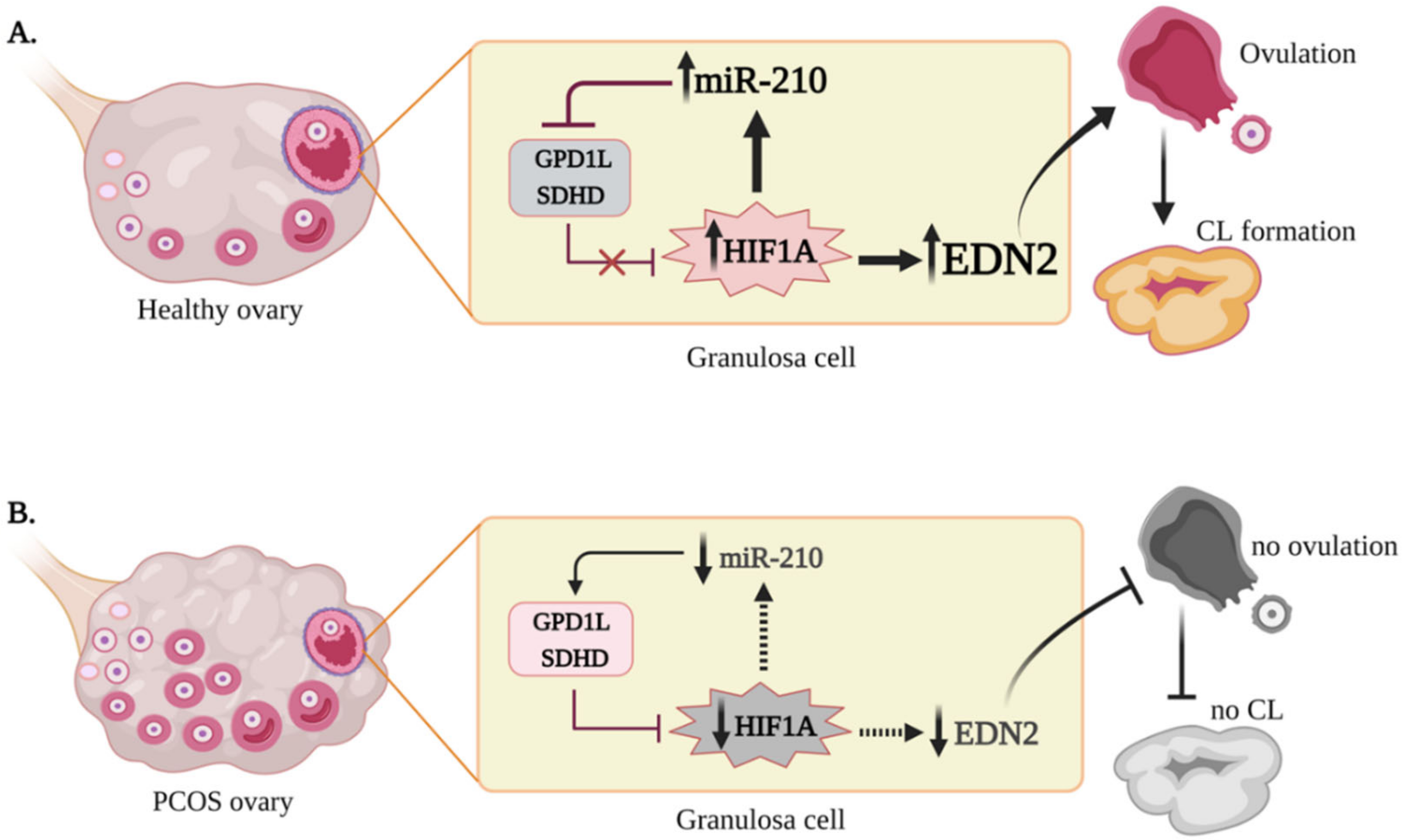
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Experiment Protocol
4.3. Cell Transfection
4.4. Quantitative PCR Analysis of mRNA and miRNA
4.5. MiRNA Expression Profiling
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azziz, R. Polycystic Ovary Syndrome. Obstet. Gynecol. 2018, 132, 321–336. [Google Scholar] [CrossRef]
- Diao, F.-Y.; Xu, M.; Hu, Y.; Li, J.; Xu, Z.; Lin, M.; Wang, L.; Zhou, Y.; Zhou, Z.; Liu, J.; et al. The molecular characteristics of polycystic ovary syndrome (PCOS) ovary defined by human ovary cDNA microarray. J. Mol. Endocrinol. 2004, 33, 59–72. [Google Scholar] [CrossRef] [PubMed]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef]
- Barber, T.M.; Franks, S. Obesity and polycystic ovary syndrome. Clin. Endocrinol. 2021. [Google Scholar] [CrossRef]
- Stubbs, S.A.; Stark, J.; Dilworth, S.M.; Franks, S.; Hardy, K. Abnormal Preantral Folliculogenesis in Polycystic Ovaries Is Associated with Increased Granulosa Cell Division. J. Clin. Endocrinol. Metab. 2007, 92, 4418–4426. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Djahanbakhch, O.; Hacihanefioglu, B.; Saridogan, E.; Ikram, M.; Ghali, L.; Raveendran, M.; Storey, A. Granulosa cell survival and proliferation are altered in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 881–887. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.-D.; Zhou, X.-Y.; Chen, S.-L.; Chen, X.; Zhe, J.; Zhang, J.; Zhang, Q.-Y.; Chen, Y.-X. MiR-29a regulates the proliferation, aromatase expression, and estradiol biosynthesis of human granulosa cells in polycystic ovary syndrome. Mol. Cell. Endocrinol. 2019, 498, 110540. [Google Scholar] [CrossRef]
- Imbar, T.; Klipper, E.; Greenfield, C.; Hurwitz, A.; Haimov-Kochman, R.; Meidan, R. Altered endothelin expression in granulosa-lutein cells of women with polycystic ovary syndrome. Life Sci. 2012, 91, 703–709. [Google Scholar] [CrossRef]
- Erickson, G.F.; Magoffin, D.A.; Garzo, V.; Cheung, A.P.; Chang, R. Granulosa cells of polycystic ovaries: Are they normal or abnormal? Hum. Reprod. 1992, 7, 293–299. [Google Scholar] [CrossRef]
- Haouzi, D.; Assou, S.; Monzo, C.; Vincens, C.; Dechaud, H.; Hamamah, S. Altered gene expression profile in cumulus cells of mature mii oocytes from patients with polycystic ovary syndrome. Hum. Reprod. 2012, 27, 3523–3530. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Y.-W.; Tong, X.-H.; Liu, Y.-S. Characterization of microRNA profile in human cumulus granulosa cells: Identification of microRNAs that regulate Notch signaling and are associated with PCOS. Mol. Cell. Endocrinol. 2015, 404, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.R.; Ho, C.; Nelson-Degrave, V.L.; McAllister, J.M.; Strauss, J.F. The molecular signature of polycystic ovary syndrome (PCOS) theca cells defined by gene expression profiling. J. Reprod. Immunol. 2004, 63, 51–60. [Google Scholar] [CrossRef]
- Ko, C.; Gieske, M.C.; Al-Alem, L.; Hahn, Y.; Su, W.; Gong, M.C.; Iglarz, M.; Koo, Y. Endothelin-2 in Ovarian Follicle Rupture. Endocrinology 2006, 147, 1770–1779. [Google Scholar] [CrossRef] [PubMed]
- Klipper, E.; Levit, A.; Mastich, Y.; Berisha, B.; Schams, D.; Meidan, R. Induction of Endothelin-2 Expression by Luteinizing Hormone and Hypoxia: Possible Role in Bovine Corpus Luteum Formation. Endocrinology 2010, 151, 1914–1922. [Google Scholar] [CrossRef]
- Cacioppo, J.; Oh, S.W.; Kim, H.-Y.; Cho, J.; Lin, P.-C.P.; Yanagisawa, M.; Ko, C. Loss of Function of Endothelin-2 Leads to Reduced Ovulation and CL Formation. PLoS ONE 2014, 9, e96115. [Google Scholar] [CrossRef]
- Palanisamy, G.S.; Cheon, Y.-P.; Kim, J.; Kannan, A.; Li, Q.; Sato, M.; Mantena, S.R.; Sitruk-Ware, R.L.; Bagchi, M.K.; Bagchi, I.C. A Novel Pathway Involving Progesterone Receptor, Endothelin-2, and Endothelin Receptor B Controls Ovulation in Mice. Mol. Endocrinol. 2006, 20, 2784–2795. [Google Scholar] [CrossRef]
- Kim, J.; Bagchi, I.C.; Bagchi, M.K. Signaling by Hypoxia-Inducible Factors Is Critical for Ovulation in Mice. Endocrinology 2009, 150, 3392–3400. [Google Scholar] [CrossRef]
- Na, G.; Bridges, P.J.; Koo, Y.; Ko, C. Role of hypoxia in the regulation of periovulatory EDN2 expression in the mouse. Can. J. Physiol. Pharmacol. 2008, 86, 310–319. [Google Scholar] [CrossRef]
- Shrestha, K.; Onasanya, A.E.; Eisenberg, I.; Wigoda, N.; Yagel, S.; Yalu, R.; Meidan, R.; Imbar, T. miR-210 and GPD1L regulate EDN2 in primary and immortalized human granulosa-lutein cells. Reproduction 2018, 155, 197–205. [Google Scholar] [CrossRef]
- Tam, K.K.; Russell, D.L.; Peet, D.J.; Bracken, C.P.; Rodgers, R.J.; Thompson, J.G.; Kind, K.L. Hormonally regulated follicle differentiation and luteinization in the mouse is associated with hypoxia inducible factor activity. Mol. Cell. Endocrinol. 2010, 327, 47–55. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.; Wu, Y.; Chen, L.; Luo, Q.; Chen, J.; Huang, X.; Cheng, Y.; Wang, Z. Regulatory Effect of Hypoxia-Inducible Factor-1α on hCG-Stimulated Endothelin-2 Expression in Granulosa Cells from the PMSG-Treated Rat Ovary. J. Reprod. Dev. 2012, 58, 678–684. [Google Scholar] [CrossRef][Green Version]
- Yalu, R.; Oyesiji, A.E.; Eisenberg, I.; Imbar, T.; Meidan, R. HIF1A-dependent increase in endothelin 2 levels in granulosa cells: Role of hypoxia, LH/cAMP, and reactive oxygen species. Reproduction 2015, 149, 11–20. [Google Scholar] [CrossRef]
- Devlin, C.; Greco, S.; Martelli, F.; Ivan, M. miR-210: More than a silent player in hypoxia. IUBMB Life 2011, 63, 94–100. [Google Scholar] [CrossRef]
- Chan, Y.C.; Banerjee, J.; Choi, S.Y.; Sen, C.K. miR-210: The Master Hypoxamir. Microcirculation 2012, 19, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.J.; Souza, A.L.; Clish, C.B.; Puigserver, P. A Hypoxia-Induced Positive Feedback Loop Promotes Hypoxia-Inducible Factor 1α Stability through miR-210 Suppression of Glycerol-3-Phosphate Dehydrogenase 1-Like. Mol. Cell. Biol. 2011, 31, 2696–2706. [Google Scholar] [CrossRef]
- Puissegur, M.-P.; Mazure, N.M.; Bertero, T.; Pradelli, L.; Grosso, S.; Robbe-Sermesant, K.; Maurin, T.; Lebrigand, K.; Cardinaud, B.; Hofman, V.; et al. miR-210 is overexpressed in late stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ. 2011, 18, 465–478. [Google Scholar] [CrossRef]
- Merlo, A.; de Quiros, S.B.; Secades, P.; Zambrano, I.; Balbín, M.; Astudillo, A.; Scola, B.; Arístegui, M.; Suarez, C.; Chiara, M.-D. Identification of a Signaling Axis HIF-1α/MicroRNA-210/ISCU Independent of SDH Mutation That Defines a Subgroup of Head and Neck Paragangliomas. J. Clin. Endocrinol. Metab. 2012, 97, E2194–E2200. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-Inducible Factor 1 (HIF-1) Pathway. Sci. STKE 2007, 2007, cm8. [Google Scholar] [CrossRef]
- Patil, K.; Hinduja, I.; Mukherjee, S. Alteration in angiogenic potential of granulosa-lutein cells and follicular fluid contributes to luteal defects in polycystic ovary syndrome. Hum. Reprod. 2021, 36, 1052–1064. [Google Scholar] [CrossRef]
- Gecaj, R.; Schanzenbach, C.I.; Kirchner, B.; Pfaffl, M.; Riedmaier, I.; Tweedie-Cullen, R.Y.; Berisha, B. The Dynamics of microRNA Transcriptome in Bovine Corpus Luteum during Its Formation, Function, and Regression. Front. Genet. 2017, 8, 213. [Google Scholar] [CrossRef]
- Van den Driesche, S.; Myers, M.; Gay, E.; Thong, K.J.; Duncan, W.C. HCG up-regulates hypoxia inducible factor-1 alpha in luteinized granulosa cells: Implications for the hormonal regulation of vascular endothelial growth factor A in the human corpus luteum. Mol. Hum. Reprod. 2008, 14, 455–464. [Google Scholar] [CrossRef]
- Basini, G.; Bianco, F.; Grasselli, F.; Tirelli, M.; Bussolati, S.; Tamanini, C. The effects of reduced oxygen tension on swine granulosa cell. Regul. Pept. 2004, 120, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Bridges, P.J.; Jo, M.; Al Alem, L.; Na, G.; Su, W.; Gong, M.C.; Jeoung, M.; Ko, C. Production and binding of endothelin-2 (EDN2) in the rat ovary: Endothelin receptor subtype A (EDNRA)-mediated contraction. Reprod. Fertil. Dev. 2010, 22, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Shozu, M.; Minami, N.; Yokoyama, H.; Inoue, M.; Kurihara, H.; Matsushima, K.; Kuno, K. ADAMTS-1 is involved in normal follicular development, ovulatory process and organization of the medullary vascular network in the ovary. J. Mol. Endocrinol. 2005, 35, 343–355. [Google Scholar] [CrossRef]
- Shrestha, K.; Rodler, D.; Sinowatz, F.; Meidan, R. Corpus Luteum Formation. In The Ovary, 3rd ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 255–267. [Google Scholar]
- Ferrara, N.; Frantz, G.; LeCouter, J.; Dillard-Telm, L.; Pham, T.; Draksharapu, A.; Giordano, T.; Peale, F. Differential Expression of the Angiogenic Factor Genes Vascular Endothelial Growth Factor (VEGF) and Endocrine Gland-Derived VEGF in Normal and Polycystic Human Ovaries. Am. J. Pathol. 2003, 162, 1881–1893. [Google Scholar] [CrossRef]
- Kamat, B.R.; Brown, L.F.; Manseau, E.J.; Senger, D.R.; Dvorak, H.F. Expression of vascular permeability factor/vascular endothelial growth factor by human granulosa and theca lutein cells. Role in corpus luteum development. Am. J. Pathol. 1995, 146, 157–165. [Google Scholar]
- Agrawal, R.; Jacobs, H.; Payne, N.; Conway, G. Concentration of vascular endothelial growth factor released by cultured human luteinized granulosa cells is higher in women with polycystic ovaries than in women with normal ovaries. Fertil. Steril. 2002, 78, 1164–1169. [Google Scholar] [CrossRef]
- Agrawal, R.; Sladkevicius, P.; Engmann, L.; Conway, G.S.; Payne, N.N.; Bekis, J.; Tan, S.L.; Campbell, S.; Jacobs, H.S. Serum vascular endothelial growth factor concentrations and ovarian stromal blood flow are increased in women with polycystic ovaries. Hum. Reprod. 1998, 13, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Abd El Aal, D.E.; Mohamed, S.A.; Amine, A.F.; Meki, A.-R.M. Vascular endothelial growth factor and insulin-like growth factor-1 in polycystic ovary syndrome and their relation to ovarian blood flow. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 118, 219–224. [Google Scholar] [CrossRef]
- Artini, P.G.; Monti, M.; Matteucci, C.; Valentino, V.; Cristello, F.; Genazzani, A.R. Vascular endothelial growth factor and basic fibroblast growth factor in polycystic ovary syndrome during controlled ovarian hyperstimulation. Gynecol. Endocrinol. 2006, 22, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Artini, P.G.; Ruggiero, M.; Toldin, M.R.P.; Monteleone, P.; Monti, M.; Cela, V.; Genazzani, A.R. Vascular endothelial growth factor and its soluble receptor in patients with polycystic ovary syndrome undergoing IVF. Hum. Fertil. 2009, 12, 40–44. [Google Scholar] [CrossRef]
- Adams, J.; Liu, Z.; Ren, Y.A.; Wun, W.-S.; Zhou, W.; Kenigsberg, S.; Librach, C.; Valdes, C.; Gibbons, W.; Richards, J. Enhanced Inflammatory Transcriptome in the Granulosa Cells of Women with Polycystic Ovarian Syndrome. J. Clin. Endocrinol. Metab. 2016, 101, 3459–3468. [Google Scholar] [CrossRef] [PubMed]
- Abramovich, D.; Irusta, G.; Bas, D.; Cataldi, N.I.; Parborell, F.; Tesone, M. Angiopoietins/TIE2 System and VEGF Are Involved in Ovarian Function in a DHEA Rat Model of Polycystic Ovary Syndrome. Endocrinology 2012, 153, 3446–3456. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.-H.; Kim, E.K.; Kim, K.-H.; Lee, K.-A.; Kang, D.-W.; Kim, H.Y.; Bridges, P.; Ko, C. Expression pattern of endothelin system components and localization of smooth muscle cells in the human pre-ovulatory follicle. Hum. Reprod. 2011, 26, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Fraser, H.M.; Bell, J.; Wilson, H.; Taylor, P.D.; Morgan, K.; Anderson, R.A.; Duncan, W.C. Localization and Quantification of Cyclic Changes in the Expression of Endocrine Gland Vascular Endothelial Growth Factor in the Human Corpus Luteum. J. Clin. Endocrinol. Metab. 2005, 90, 427–434. [Google Scholar] [CrossRef][Green Version]
- Berisha, B.; Schams, D.; Kosmann, M.; Amselgruber, W.; Einspanier, R. Expression and Tissue Concentration of Vascular Endothelial Growth Factor, Its Receptors, and Localization in the Bovine Corpus Luteum During Estrous Cycle and Pregnancy. Biol. Reprod. 2000, 63, 1106–1114. [Google Scholar] [CrossRef]
- Lee, A.; Christenson, L.K.; Patton, P.E.; Burry, K.A.; Stouffer, R.L. Vascular endothelial growth factor production by human luteinized granulosa cells in vitro. Hum. Reprod. 1997, 12, 2756–2761. [Google Scholar] [CrossRef]
- Meidan, R.; Klipper, E.; Zalman, Y.; Yalu, R. The role of hypoxia-induced genes in ovarian angiogenesis. Reprod. Fertil. Dev. 2013, 25, 343–350. [Google Scholar] [CrossRef]
- Christenson, L.K.; Stouffer, R.L. Follicle-Stimulating Hormone and Luteinizing Hormone/Chorionic Gonadotropin Stimulation of Vascular Endothelial Growth Factor Production by Macaque Granulosa Cells from Pre- and Periovulatory Follicles. J. Clin. Endocrinol. Metab. 1997, 82, 2135–2142. [Google Scholar] [CrossRef]
- Beckmann, M.W.; Polacek, D.; Seung, L.; Schreiber, J.R. Human ovarian granulosa cell culture: Determination of blood cell contamination and evaluation of possible culture purification steps. Fertil. Steril. 1991, 56, 881–887. [Google Scholar] [CrossRef]
- Ferrero, H.; Delgado-Rosas, F.; Garcia-Pascual, C.M.; Monterde, M.; Zimmermann, R.C.; Simón, C.; Pellicer, A.; Gómez, R. Efficiency and purity provided by the existing methods for the isolation of luteinized granulosa cells: A comparative study. Hum. Reprod. 2012, 27, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Fedorcsák, P.; Ráki, M.; Storeng, R. Characterization and depletion of leukocytes from cells isolated from the pre-ovulatory ovarian follicle. Hum. Reprod. 2007, 22, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Flannery, G.; Randle, B.; Jenkins, J.; Holmes, C. Leukocyte origin and profile in follicular aspirates at oocyte retrieval. Hum. Reprod. 2005, 20, 3526–3531. [Google Scholar] [CrossRef]
- Sasson, R.; Rimon, E.; Dantes, A.; Cohen, T.; Shinder, V.; Land-Bracha, A.; Amsterdam, A. Gonadotrophin-induced gene regulation in human granulosa cells obtained from IVF patients. Modulation of steroidogenic genes, cytoskeletal genes and genes coding for apoptotic signalling and protein kinases. Mol. Hum. Reprod. 2004, 10, 299–311. [Google Scholar] [CrossRef]
- Fleming, R.; Haxton, M.; Hamilton, M.; Conaghan, C.; Black, W.; Yates, R.; Coutts, J. Combined gonadotropin-releasing hormone analog and exogenous gonadotropins for ovulation induction in infertile women: Efficacy related to ovarian function assessment. Am. J. Obstet. Gynecol. 1988, 159, 376–381. [Google Scholar] [CrossRef]
- Szymanska, M.; Manthe, S.; Shrestha, K.; Girsh, E.; Harlev, A.; Kisliouk, T.; Meidan, R. Sirtuin-1 inhibits endothelin-2 expression in human granulosa-lutein cells via hypoxia inducible factor 1 alpha and epigenetic modifications. Biol. Reprod. 2021, 104, 387–398. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Geiss, G.K.; Bumgarner, R.; Birditt, B.; Dahl, T.; Dowidar, N.; Dunaway, D.L.; Fell, H.P.; Ferree, S.; George, R.D.; Grogan, T.; et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat. Biotechnol. 2008, 26, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Cortez, M.A.; Bueso-Ramos, C.; Ferdin, J.; Lopez-Berestein, G.; Sood, A.K.; Calin, G.A. MicroRNAs in body fluids—The mix of hormones and biomarkers. Nat. Rev. Clin. Oncol. 2011, 8, 467–477. [Google Scholar] [CrossRef]
- Eisenberg, I.; Kotaja, N.; Goldman-Wohl, D.; Imbar, T. microRNA in Human Reproduction. Adv. Exp. Med. Biol. 2015, 888, 353–387. [Google Scholar] [CrossRef] [PubMed]
- Sang, Q.; Yao, Z.; Wang, H.; Feng, R.; Wang, H.; Zhao, X.; Xing, Q.; Jin, L.; He, L.; Wu, L.; et al. Identification of MicroRNAs in Human Follicular Fluid: Characterization of MicroRNAs That Govern Steroidogenesis in Vitro and Are Associated with Polycystic Ovary Syndrome in Vivo. J. Clin. Endocrinol. Metab. 2013, 98, 3068–3079. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, S.S.; Nygaard, A.-B.; Christensen, T. miRNA expression profiles in cerebrospinal fluid and blood of patients with Alzheimer’s disease and other types of dementia—An exploratory study. Transl. Neurodegener. 2016, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]

| PCOS (n = 41) | Control (MFI; n = 40) | p-Value | |
|---|---|---|---|
| Age (years) | 27.8 ± 4.1 | 27.6 ± 4.8 | NS |
| BMI (kg/m2) | 27.0 ± 5.26 | 23 ± 5.4 | 0.001 |
| Serum early follicular FSH (IU/L) | 5.7 ± 1.86 | 6.2 ± 2.26 | NS |
| Serum early follicular LH (IU/L) | 7.5 ± 3.59 | 5.4 ± 2.77 | 0.009 |
| LH:FSH ratio | 1.4 ± 0.87 | 0.9 ± 0.55 | 0.007 |
| Serum total testosterone (nmol/L) | 2.2 ± 1.18 | 1.5 ± 0.85 | 0.006 |
| Peak E2 (pmol/L) | 7124.0 ± 3511 | 6965 ± 3663 | NS |
| Oocytes retrieved | 12.9 ± 8.0 | 14.3 ± 5.6 | NS |
| miRNA | Molecule Count | Fold Change | p-Value | |
|---|---|---|---|---|
| PCOS | Control | |||
| hsa-miR-4284 | 62 | 262 | –4.2 | 0.009 |
| hsa-miR-132-3p | 2193 | 7273 | –3.3 | 0.018 |
| hsa-miR-210 | 408 | 1321 | –3.2 | 0.025 |
| hsa-miR-483-3p | 233 | 1022 | –3.1 | 0.032 |
| hsa-miR-1973 | 63 | 185 | –2.9 | 0.018 |
| Gene Name | Sequence (5’-3’) | Accession No. |
|---|---|---|
| GPD1L | F: GATGCAGACACTGTTGAACTC R: AGGTGGCTGTAGACACTTGG | NM_015141 [19] |
| SDHD | F: TCCTTGCTCTGCGATGGAC R: GCTTTGCAGATGCCCACAT | NM_003002 [19] |
| EDN2 | F: GCCAGCGTCCTCATCTAT R: GCCGTAAGGAGCTGTCTGTTC | NM_001956 [19] |
| VEGFA | F: ATCGAGACCCTGGTGGACA R: CCTCGGCTTGTCACATCTGC | NM_001025366 [22] |
| HIF-1α | F: ACTCATCCATGTGACCACG R: TAGTTCTCCCCCGGCTAG | NM_001530 [19] |
| SLC2A1 | F: CGCTTCCTGCTCATTAACCG R: CCTTCTTCTCCCGCATCAT | NM_006516 [22] |
| ACTB | F: CGGGACCTGACGGACTACCTC R: GCCATCTCCTGCTCGAAGTCC | NM_001100 [19] |
| RPS18 | F: CACCAAGAGGGCGGGAGA R: CTTCTTCAGTCGCTCCAGG | NM_022551 [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymanska, M.; Shrestha, K.; Girsh, E.; Harlev, A.; Eisenberg, I.; Imbar, T.; Meidan, R. Reduced Endothelin-2 and Hypoxic Signaling Pathways in Granulosa-Lutein Cells of PCOS Women. Int. J. Mol. Sci. 2021, 22, 8216. https://doi.org/10.3390/ijms22158216
Szymanska M, Shrestha K, Girsh E, Harlev A, Eisenberg I, Imbar T, Meidan R. Reduced Endothelin-2 and Hypoxic Signaling Pathways in Granulosa-Lutein Cells of PCOS Women. International Journal of Molecular Sciences. 2021; 22(15):8216. https://doi.org/10.3390/ijms22158216
Chicago/Turabian StyleSzymanska, Magdalena, Ketan Shrestha, Eliezer Girsh, Avi Harlev, Iris Eisenberg, Tal Imbar, and Rina Meidan. 2021. "Reduced Endothelin-2 and Hypoxic Signaling Pathways in Granulosa-Lutein Cells of PCOS Women" International Journal of Molecular Sciences 22, no. 15: 8216. https://doi.org/10.3390/ijms22158216
APA StyleSzymanska, M., Shrestha, K., Girsh, E., Harlev, A., Eisenberg, I., Imbar, T., & Meidan, R. (2021). Reduced Endothelin-2 and Hypoxic Signaling Pathways in Granulosa-Lutein Cells of PCOS Women. International Journal of Molecular Sciences, 22(15), 8216. https://doi.org/10.3390/ijms22158216








