Gamma-Aminobutyric Acid (GABA) Inhibits α-Melanocyte-Stimulating Hormone-Induced Melanogenesis through GABAA and GABAB Receptors
Abstract
:1. Introduction
2. Results
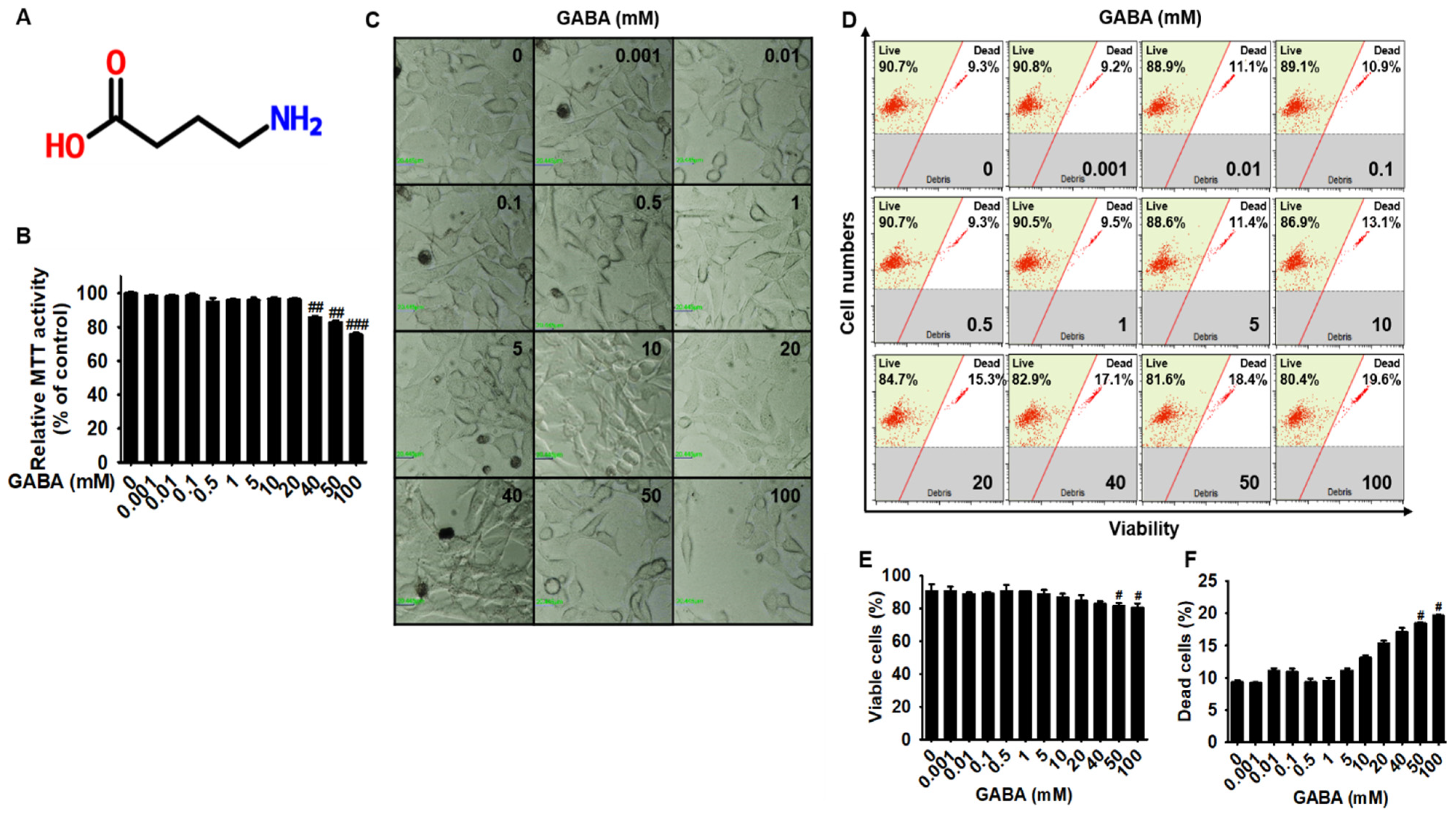
2.1. Low Concentrations of GABA Present No Cytotoxicity in B16F10 Melanoma Cells
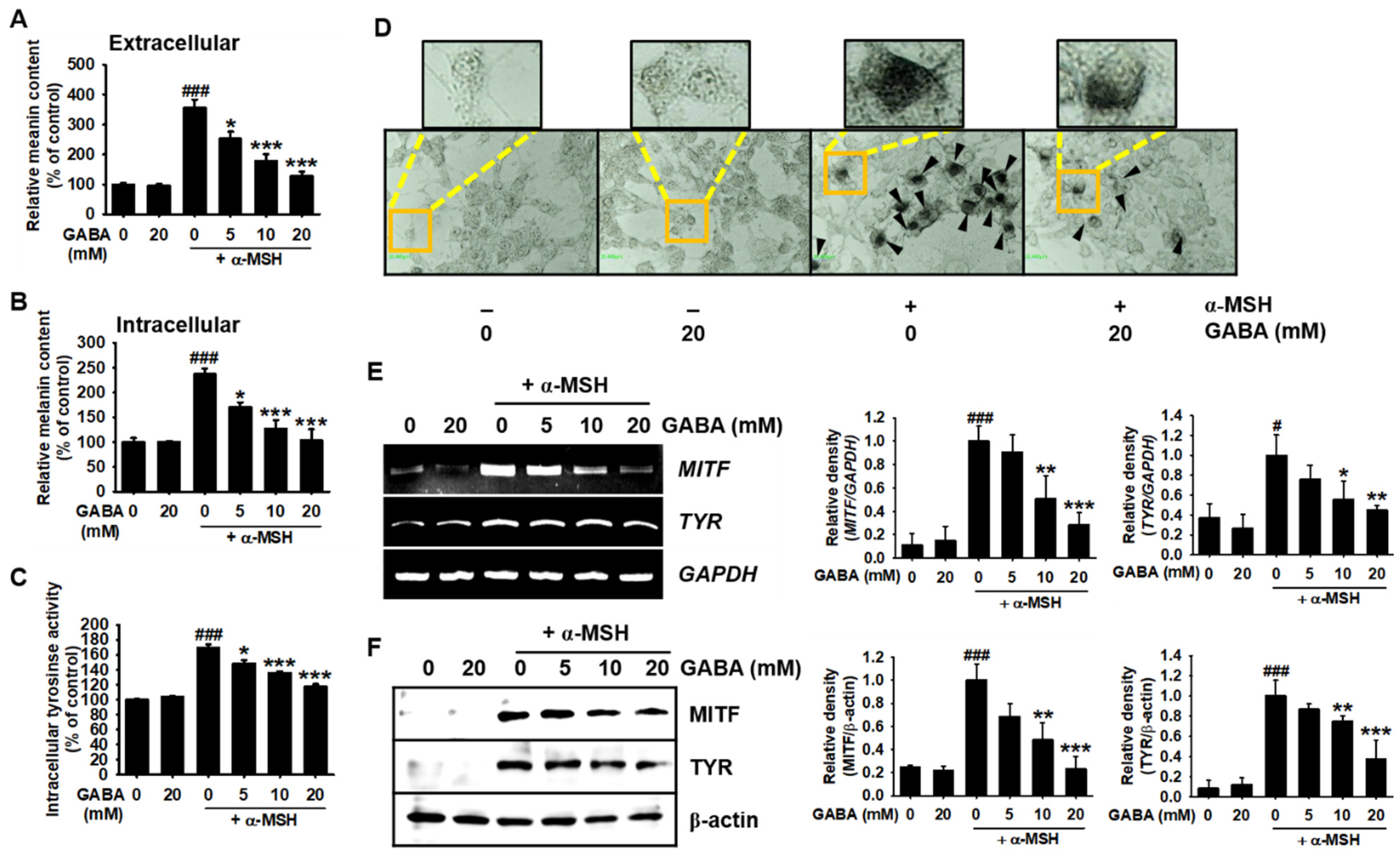
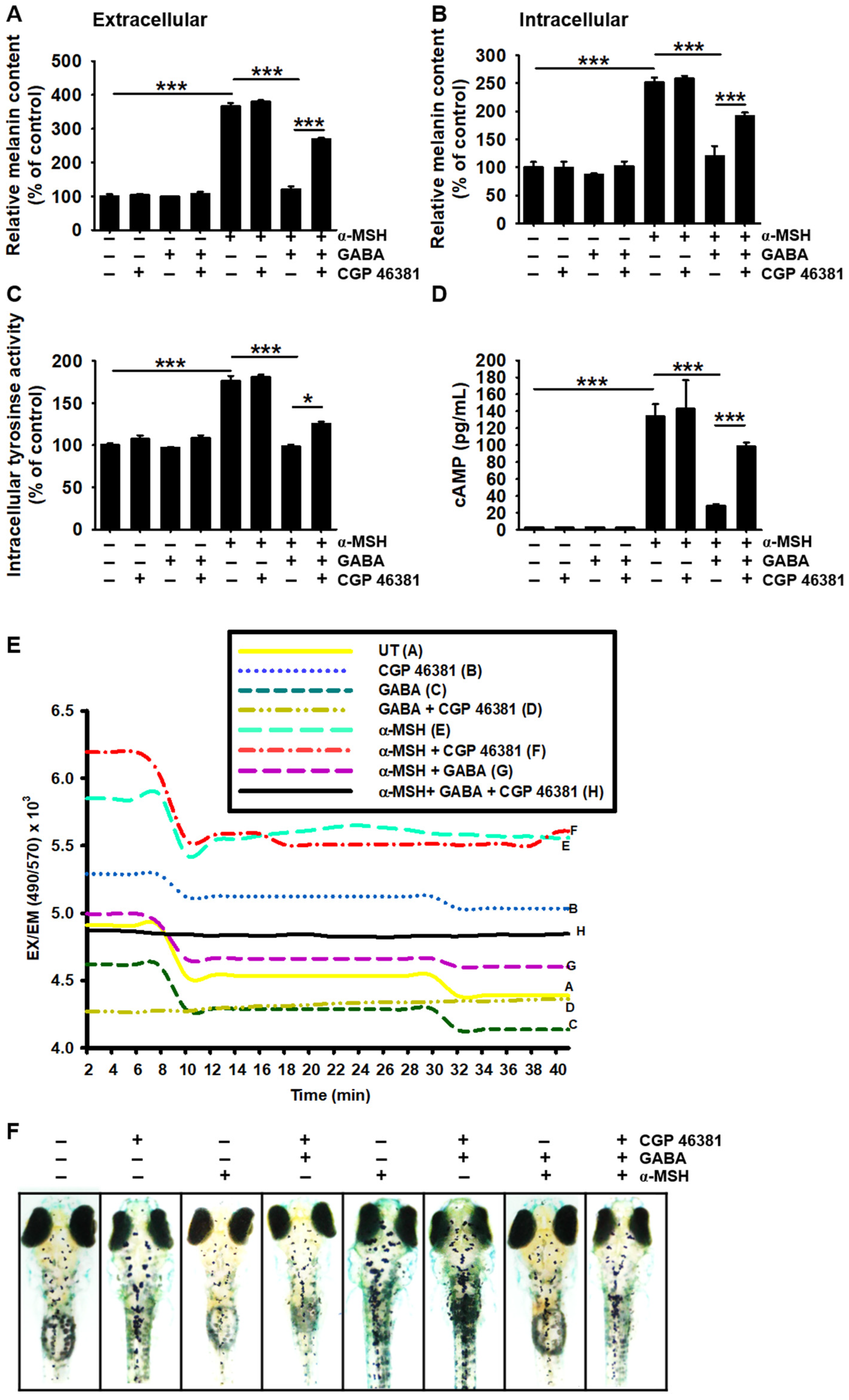
2.2. GABA Inhibits α-MSH-Induced Melanogenesis in B16F10 Melanoma Cells
2.3. GABA Inhibits Melanogenesis in Zebrafish Larvae
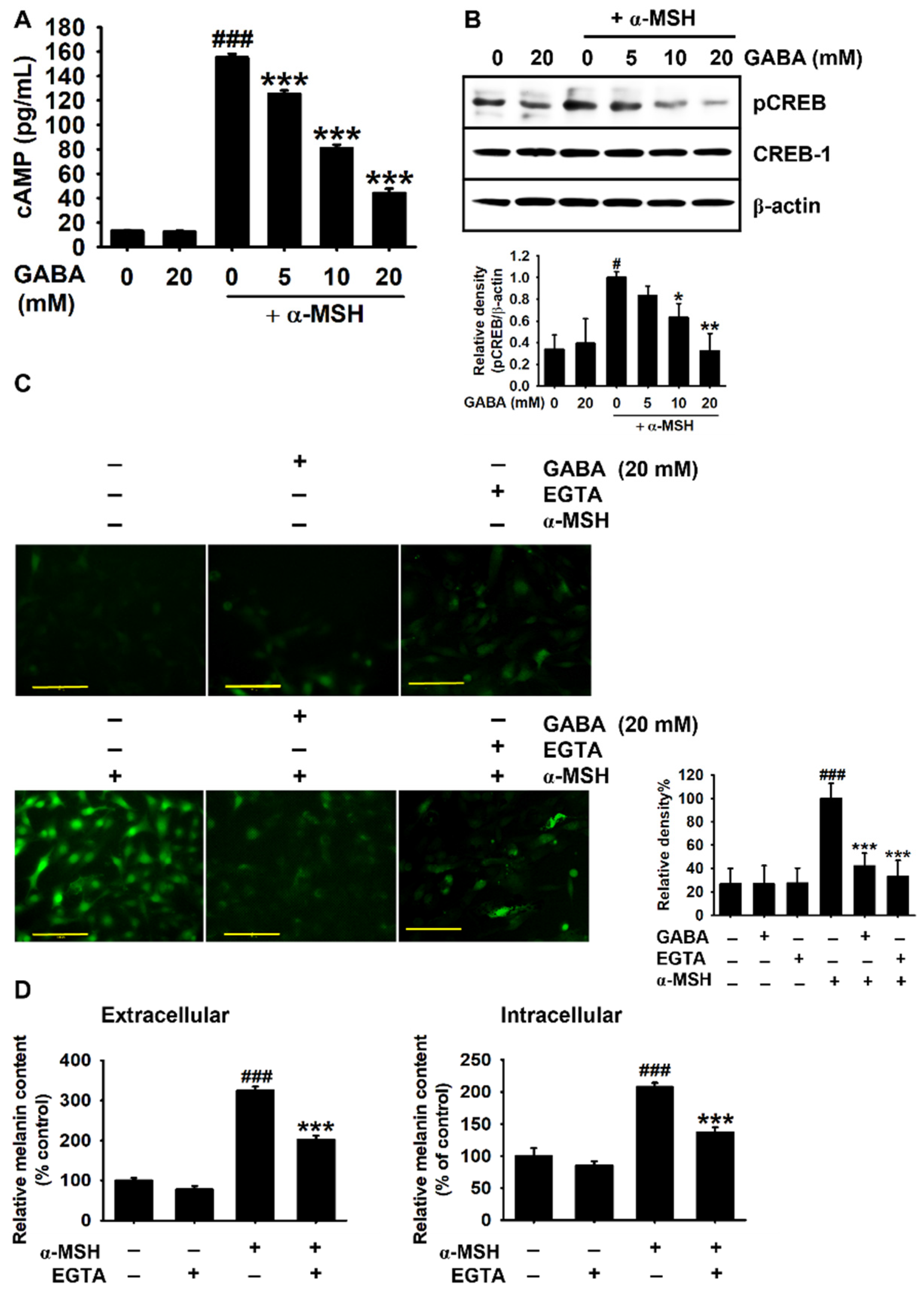
2.4. GABA Inhibits α-MSH-Induced Melanogenesis by Downregulating the cAMP–CREB Axis and Ca2+ Levels
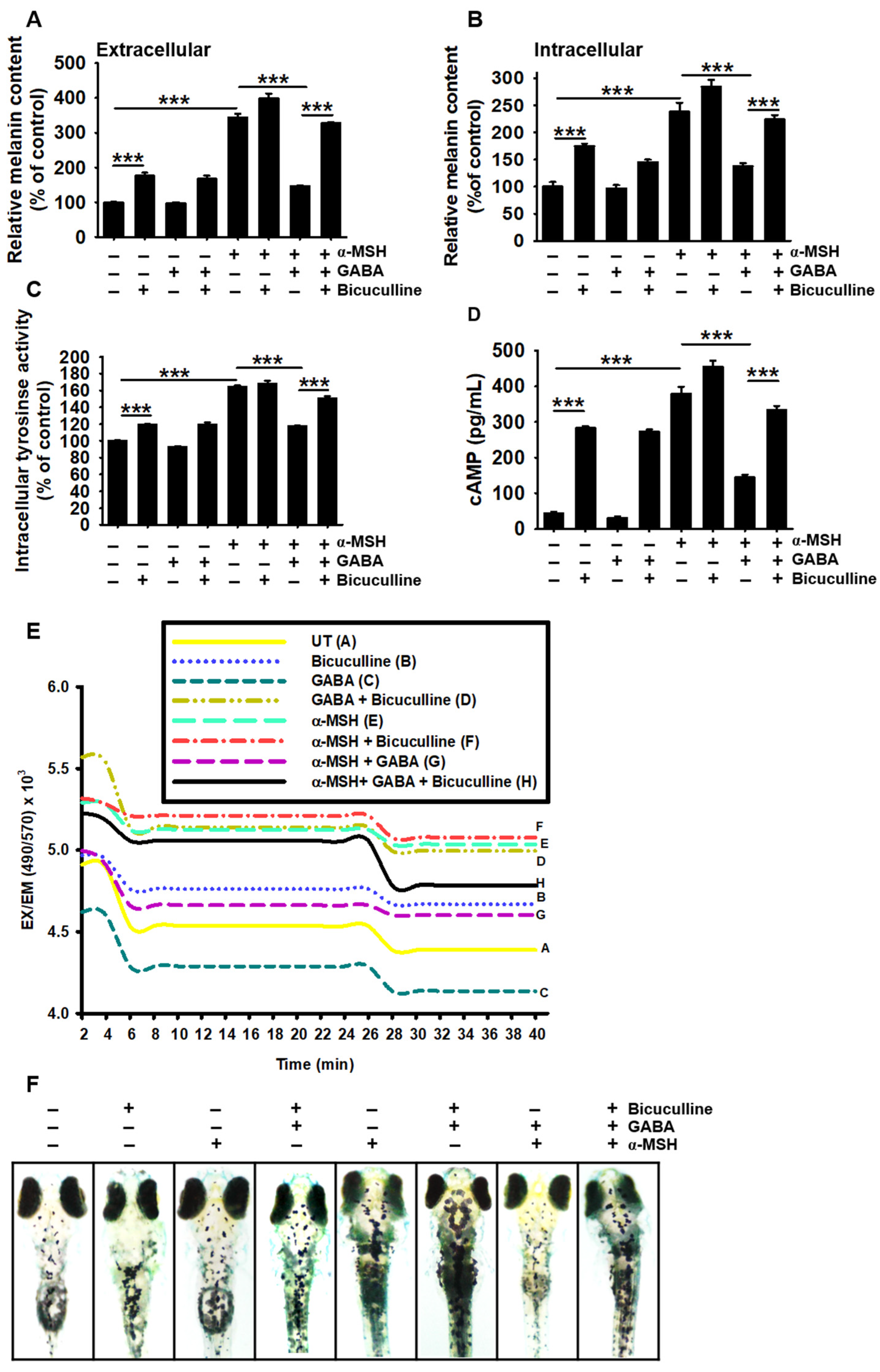
2.5. GABAA Receptor Is Responsible for the Antimelanogenic Properties of GABA
2.6. GABAB Receptor Regulates GABA-Mediated Antimelanogenic Properties
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture and Viability
4.3. Flow Cytometry Analysis
4.4. Measurement of Extracellular and Intracellular Melanin Content
4.5. cAMP Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.7. Western Blotting
4.8. Measurement of Intracellular Tyrosinase Activity
4.9. Staining of Intracellular Tyrosinase
4.10. Kinetics Scan of Intracellular Ca2+ Changes
4.11. Imaging for Intracellular Ca2+
4.12. Evaluation of Antimelanogenic Properties of GABA in Zebrafish Larvae
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Agostino, G.; Diano, S. Alpha-melanocyte stimulating hormone: Production and degradation. J. Mol. Med. 2010, 88, 1195–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Borrón, J.C.; Abdel-Malek, Z.; Jiménez-Cervantes, C. MC1R, the cAMP pathway, and the response to solar UV: Extending the horizon beyond pigmentation. Pigment Cell Melanoma Res. 2014, 27, 699–720. [Google Scholar] [CrossRef] [PubMed]
- Motiani, R.K.; Tanwar, J.; Raja, D.A.; Vashisht, A.; Khanna, S.; Sharma, S.; Srivastava, S.; Sivasubbu, S.; Natarajan, V.T.; Gokhale, R.S. STIM1 activation of adenylyl cyclase 6 connects Ca2+ and cAMP signaling during melanogenesis. EMBO J. 2018, 37, e97597. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.M. Store-operated Ca²⁺-entry and adenylyl cyclase. Cell Calcium 2015, 58, 368–375. [Google Scholar] [CrossRef]
- D’Mello, S.A.N.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [Green Version]
- Cestari, T.F.; Dantas, L.P.; Boza, J.C. Acquired hyperpigmentations. An. Bras. Dermatol. 2014, 89, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Nuss, P. Anxiety disorders and GABA neurotransmission: A disturbance of modulation. Neuropsychiatr. Dis. Treat. 2015, 11, 165–175. [Google Scholar]
- Diana, M.; Quílez, J.; Rafecas, M. Gamma-aminobutyric acid as a bioactive compound in foods: A review. J. Funct. Foods 2014, 10, 407–420. [Google Scholar] [CrossRef]
- Lin, S.-D.; Mau, J.-L.; Hsu, C.-A. Bioactive components and antioxidant properties of γ-aminobutyric acid (GABA) tea leaves. Food Sci. Technol. 2012, 46, 64–70. [Google Scholar] [CrossRef]
- Roohinejad, S.; Omidizadeh, A.; Mirhosseini, H.; Saari, N.; Mustafa, S.; Mohd Yusof, R.; Meor Hussin, A.S.; Hamid, A.; Abd Manap, M.Y. Effect of pre-germination time of brown rice on serum cholesterol levels of hypercholesterolaemic rats. J. Sci. Food Agric. 2010, 90, 245–251. [Google Scholar] [CrossRef]
- Tian, J.; Lu, Y.; Zhang, H.; Chau, C.H.; Dang, H.N.; Kaufman, D.L. γ-Aminobutyric acid inhibits T cell autoimmunity and the development of inflammatory responses in a mouse type 1 diabetes model. J. Immunol. 2004, 173, 5298–5304. [Google Scholar] [CrossRef]
- Oh, S.-H.; Soh, J.-R.; Cha, Y.-S. Germinated brown rice extract shows a nutraceutical effect in the recovery of chronic alcohol-related symptoms. J. Med. Food 2003, 6, 115–121. [Google Scholar] [CrossRef]
- Smith, T.A. Type A gamma-aminobutyric acid (GABAA) receptor subunits and benzodiazepine binding: Significance to clinical syndromes and their treatment. Br. J. Biomed. Sci. 2001, 58, 111–121. [Google Scholar]
- Chebib, M.; Johnston, G.A. The ’ABC’ of GABA receptors: A brief review. Clin. Exp. Pharmacol. Physiol. 1999, 26, 937–940. [Google Scholar] [CrossRef]
- Sigel, E.; Steinmann, M.E. Structure, function, and modulation of GABAA receptors. J. Biol. Chem. 2012, 287, 40224–40231. [Google Scholar] [CrossRef] [Green Version]
- Cheng, T.; Wallace, D.M.; Ponteri, B.; Tuli, M. Valium without dependence? Individual GABAA receptor subtype contribution toward benzodiazepine addiction, tolerance, and therapeutic effects. Neuropsychiatr. Dis. Treat. 2018, 14, 1351–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, R.W.; Sieghart, W. GABA A receptors: Subtypes provide diversity of function and pharmacology. Neuropharmacology 2009, 56, 141–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sieghart, W. Subunit composition and structure of GABAA-receptor subtypes. In The GABA Receptors; Enna, S.J., Möhler, H., Eds.; Humana Press: Totowa, NJ, USA, 2007; pp. 69–86. [Google Scholar] [CrossRef]
- Olsen, R.W.; Gordey, M. GABAA Receptor Chloride Ion Channels. In Pharmacology of Ionic Channel Function: Activators and Inhibitors; Endo, M., Kurachi, Y., Mishina, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 499–517. [Google Scholar] [CrossRef]
- Terunuma, M. Diversity of structure and function of GABAB receptors: A complexity of GABA(B)-mediated signaling. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2018, 94, 390–411. [Google Scholar] [CrossRef] [Green Version]
- Wood, J.M.; Hoogduijn, M.J.; Smit, N.P.; Thody, A.J.; Van Der Laarse, A.; Van Nieuwpoort, A.F. Melanin has a role in Ca2+ homeostasis in human melanocytes. Pigment Cell Res. 2003, 16, 127–132. [Google Scholar]
- Pomeranz Krummel, D.A.; Nasti, T.H.; Kaluzova, M.; Kallay, L.; Bhattacharya, D.; Melms, J.C.; Izar, B.; Xu, M.; Burnham, A.; Ahmed, T.; et al. Melanoma cell intrinsic GABAA receptor enhancement potentiates radiation and immune checkpoint inhibitor response by promoting direct and T cell-mediated antitumor activity. Int. J. Radiat. Oncol. Biol. Phys. 2020, 109, 1040–1053. [Google Scholar] [CrossRef]
- Shen, W.; Li, Y.; Li, B.; Zheng, L.; Xie, X.; Le, J.; Lu, Y.; Li, T.; Chen, F.; Jia, L. Downregulation of KCTD12 contributes to melanoma stemness by modulating CD271. Cancer Biol. Med. 2019, 16, 498–513. [Google Scholar] [PubMed]
- Nakamura, H.; Takishima, T.; Kometani, T.; Yokogoshi, H. Psychological stress-reducing effect of chocolate enriched with γ-aminobutyric acid (GABA) in humans: Assessment of stress using heart rate variability and salivary chromogranin A. Int. J. Food Sci. Nutr. 2009, 60, 106–113. [Google Scholar] [CrossRef]
- Kanehira, T.; Nakamura, Y.; Nakamura, K.; Horie, K.; Horie, N.; Furugori, K.; Sauchi, Y.; Yokogoshi, H. Relieving occupational fatigue by consumption of a beverage containing γ-amino butyric acid. J. Nutr. Sci. Vitaminol. 2011, 57, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Arredouani, A.; Yu, F.; Sun, L.; Machaca, K. Regulation of store-operated Ca2+ entry during the cell cycle. J. Cell Sci. 2010, 123, 2155–2162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gudermann, T.; Bader, M. Receptors, G proteins, and integration of calcium signalling. J. Mol. Med. 2015, 93, 937–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, A.R.; Almeida, H.; Gouveia, A.M. Intracellular signaling mechanisms of the melanocortin receptors: Current state of the art. Cell. Mol. Life Sci. 2015, 72, 1331–1345. [Google Scholar] [CrossRef] [PubMed]
- Carsberg, C.J.; Jones, K.T.; Sharpe, G.R.; Friedmann, P.S. Intracellular calcium modulates the responses of human melanocytes to melanogenic stimuli. J. Dermatol. Sci. 1995, 9, 157–164. [Google Scholar] [CrossRef]
- Ando, H.; Niki, Y.; Ito, M.; Akiyama, K.; Matsui, M.S.; Yarosh, D.B.; Ichihashi, M. Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J. Investig. Dermatol. 2012, 132, 1222–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.Y.; Perez, J.M.; Laursen, R.; Hara, M.; Gilchrest, B.A. Protein kinase C-beta activates tyrosinase by phosphorylating serine residues in its cytoplasmic domain. J. Biol. Chem. 1999, 274, 16470–16478. [Google Scholar] [CrossRef] [Green Version]
- Park, H.Y.; Wu, H.; Killoran, C.E.; Gilchrest, B.A. The receptor for activated C-kinase-I (RACK-I) anchors activated PKC-beta on melanosomes. J. Cell Sci. 2004, 117, 3659–3668. [Google Scholar] [CrossRef] [Green Version]
- Buffey, J.A.; Edgecombe, M.; Mac Neil, S. Calcium plays a complex role in the regulation of melanogenesis in murine B16 melanoma cells. Pigment Cell Res. 1993, 6, 385–393. [Google Scholar] [CrossRef]
- Allen, J.R.; Skeath, J.B.; Johnson, S.L. GABA-A receptor and mitochondrial TSPO signaling act in parallel to regulate melanocyte stem cell quiescence in larval zebrafish. Pigment Cell Melanoma Res. 2020, 33, 416–425. [Google Scholar] [CrossRef]
- Allen, J.R.; Skeath, J.B.; Johnson, S.L. Maintenance of melanocyte stem cell quiescence by GABA-A signaling in larval zebrafish. Genetics 2019, 213, 555–566. [Google Scholar] [CrossRef]
- Yokogawa, T.; Kim, S.U.; Krieger, C.; Puil, E. Analysis of GABA(A)- and GABA(B)-receptor mediated effects on intracellular Ca2+ in DRG hybrid neurones. Br. J. Pharmacol. 2001, 134, 98–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mestdagh, N.; Wulfert, E. Bicuculline increases Ca2+ transients in rat cerebellar granule cells through non-GABAA receptor associated mechanisms. Neurosci. Lett. 1999, 265, 95–98. [Google Scholar] [CrossRef]
- Heidelberger, R.; Matthews, G. Inhibition of calcium influx and calcium current by gamma-aminobutyric acid in single synaptic terminals. Proc. Natl. Acad. Sci. USA 1991, 88, 7135–7139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Z.Y.; Wang, X.P.; Schmid, K.L.; Han, X.G. GABAB1 and GABAB2 receptor subunits co-expressed in cultured human RPE cells regulate intracellular Ca2+ via Gi/o-protein and phospholipase C pathways. Neuroscience 2014, 280, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Strøbaek, D.; Jørgensen, T.D.; Christophersen, P.; Ahring, P.K.; Olesen, S.P. Pharmacological characterization of small-conductance Ca2+-activated K+ channels stably expressed in HEK 293 cells. Br. J. Pharmacol. 2000, 129, 991–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Nakayama, Y.; Hattori, N.; Liu, B.; Inagaki, C. GABAC-receptor stimulation activates cAMP-dependent protein kinase via A-kinase anchoring protein 220. J. Pharmacol. Sci. 2008, 160, 578–584. [Google Scholar] [CrossRef] [Green Version]
- Isoldi, M.C.; Pereira, E.A.; Visconti, M.A.; Castrucci, A.M. The role of calcium, calcium-activated K+ channels, and tyrosine/kinase in psoralen-evoked responses in human melanoma cells. Braz. J. Med. Biol. Res. 2004, 37, 559–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molagoda, I.M.N.; Karunarathne, W.; Park, S.R.; Choi, Y.H.; Park, E.K.; Jin, C.Y.; Yu, H.; Jo, W.S.; Lee, K.T.; Kim, G.Y. GSK-3β-targeting fisetin promotes melanogenesis in B16F10 melanoma cells and zebrafish larvae through β-catenin activation. Int. J. Mol. Sci. 2020, 21, 312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molagoda, I.M.N.; Choi, Y.H.; Lee, S.; Sung, J.; Lee, C.R.; Lee, H.G.; Lim, J.; Lee, K.-J.; Jeon, Y.-J.; Ma, J. Ethanolic extract of Hippocampus abdominalis exerts anti-melanogenic effects in B16F10 melanoma cells and zebrafish larvae by activating the ERK signaling pathway. Cosmetics 2020, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]

| Gene * | Primer Sequence (5′-3′) | Size | Tm | Cycle No. |
|---|---|---|---|---|
| MITF | F: 5′-CCCGTCTCTGGAAACTTGATCG-3′ | 767 bp | 60 °C | 27 |
| R: 5′-CTGTACTCTGAGCAGCAGGTC-3′ | ||||
| Tyrosinase | F: 5′-GTCGTCACCCTGAAAATCCTAACT-3′ | 110 bp | 60 °C | 27 |
| R: 5′-CATCGCATAAAACCTGATGGC-3′ | ||||
| GAPDH | F: 5′-ACCACAGTCCATGCCATCAC-3′ | 480 bp | 60 °C | 25 |
| R: 5′-CACCACCCTGTTGCTGTAGC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molagoda, I.M.N.; Kavinda, M.H.D.; Ryu, H.W.; Choi, Y.H.; Jeong, J.-W.; Kang, S.; Kim, G.-Y. Gamma-Aminobutyric Acid (GABA) Inhibits α-Melanocyte-Stimulating Hormone-Induced Melanogenesis through GABAA and GABAB Receptors. Int. J. Mol. Sci. 2021, 22, 8257. https://doi.org/10.3390/ijms22158257
Molagoda IMN, Kavinda MHD, Ryu HW, Choi YH, Jeong J-W, Kang S, Kim G-Y. Gamma-Aminobutyric Acid (GABA) Inhibits α-Melanocyte-Stimulating Hormone-Induced Melanogenesis through GABAA and GABAB Receptors. International Journal of Molecular Sciences. 2021; 22(15):8257. https://doi.org/10.3390/ijms22158257
Chicago/Turabian StyleMolagoda, Ilandarage Menu Neelaka, Mirissa Hewage Dumindu Kavinda, Hyung Won Ryu, Yung Hyun Choi, Jin-Woo Jeong, Sanghyuck Kang, and Gi-Young Kim. 2021. "Gamma-Aminobutyric Acid (GABA) Inhibits α-Melanocyte-Stimulating Hormone-Induced Melanogenesis through GABAA and GABAB Receptors" International Journal of Molecular Sciences 22, no. 15: 8257. https://doi.org/10.3390/ijms22158257
APA StyleMolagoda, I. M. N., Kavinda, M. H. D., Ryu, H. W., Choi, Y. H., Jeong, J.-W., Kang, S., & Kim, G.-Y. (2021). Gamma-Aminobutyric Acid (GABA) Inhibits α-Melanocyte-Stimulating Hormone-Induced Melanogenesis through GABAA and GABAB Receptors. International Journal of Molecular Sciences, 22(15), 8257. https://doi.org/10.3390/ijms22158257










