Polyphenol Containing Sorghum Brans Exhibit an Anti-Cancer Effect in Apc Min/+ Mice Treated with Dextran Sodium Sulfate
Abstract
:1. Introduction
2. Results
2.1. High Phenolic Sorghum Bran Extracts Repress Proliferation and Induce Apoptosis in Human Colon Cancer Cells
2.2. High-Phenolic Sorghum Bran Extracts Modulate NF-κB Activity in Human Colon Cancer Cells
2.3. High Phenolic Sorghum Bran Extracts Downregulate the Transcriptional Activity of β-Catenin and Its Expression in Human Colon Cancer Cells
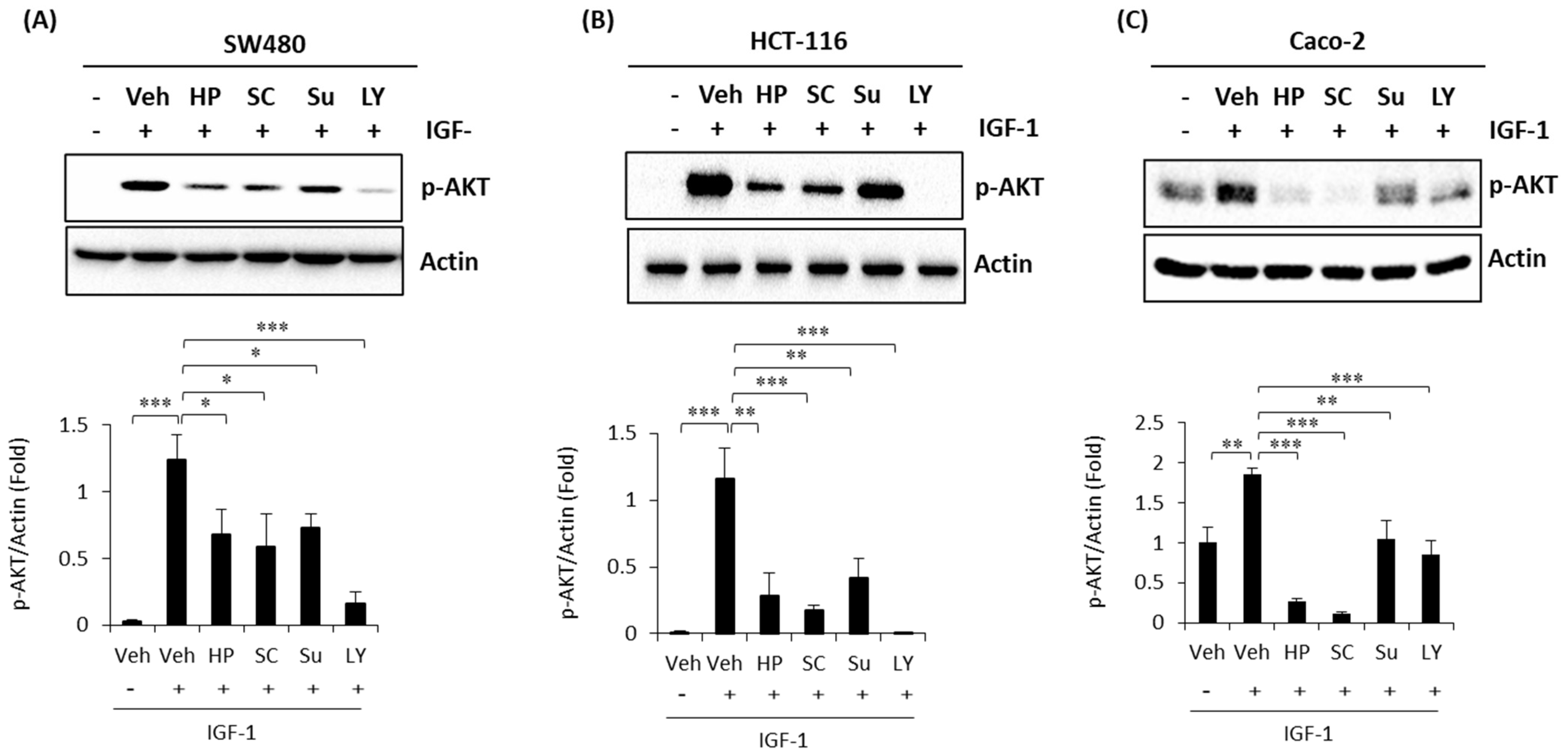
2.4. High Phenolic Sorghum Bran Extracts Weaken the IGF-1 (Insulin-Like Growth Factor-1)-Stimulated PI3K/AKT Pathway in Human Colon Cancer Cells
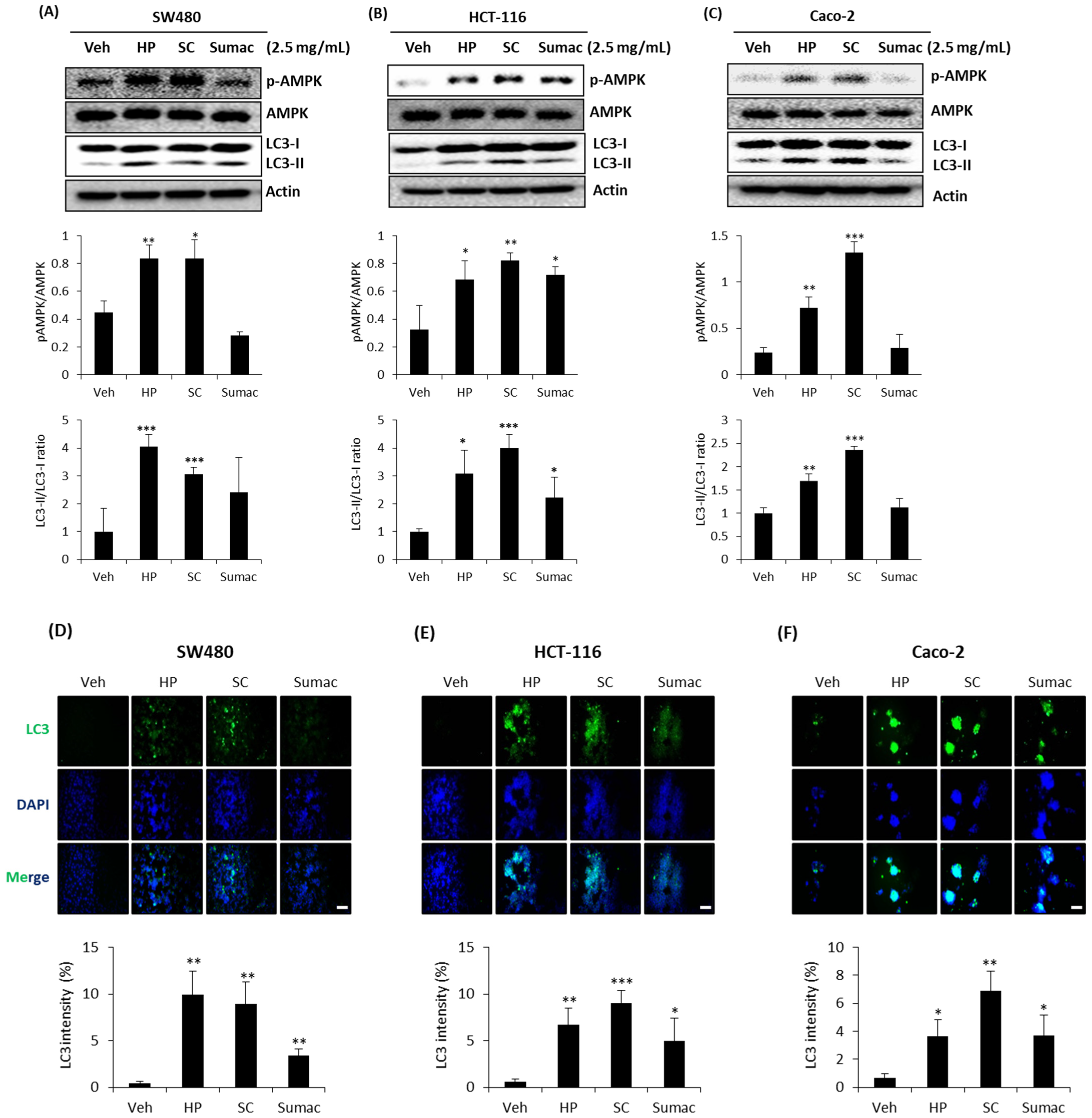
2.5. High Phenolic Sorghum Bran Extracts Activate AMPK and Autophagy in Human Colon Cancer Cells
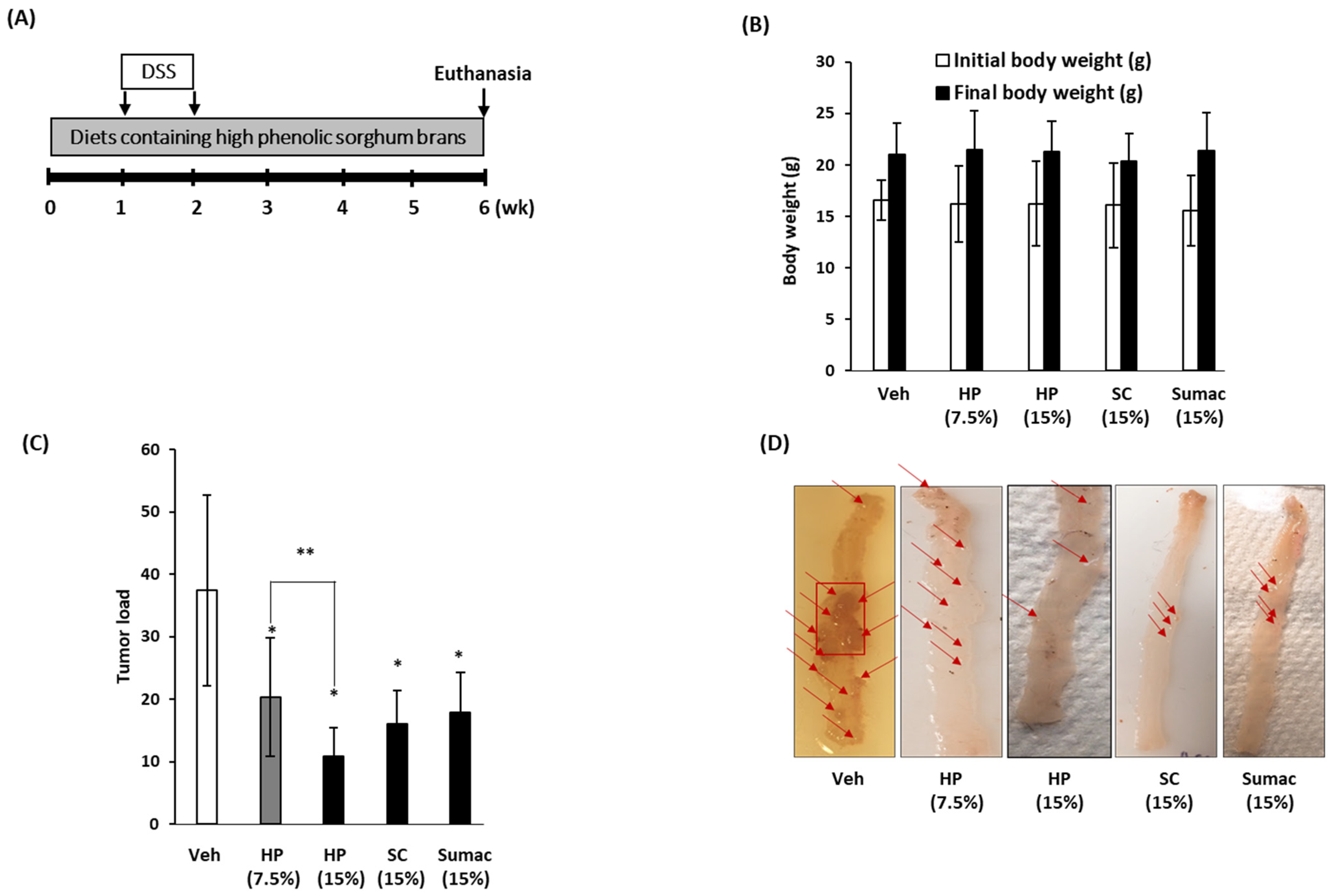
2.6. High Phenolic Sorghum Brans Repress the Formation of Colon Tumors in APC Min/+Mice
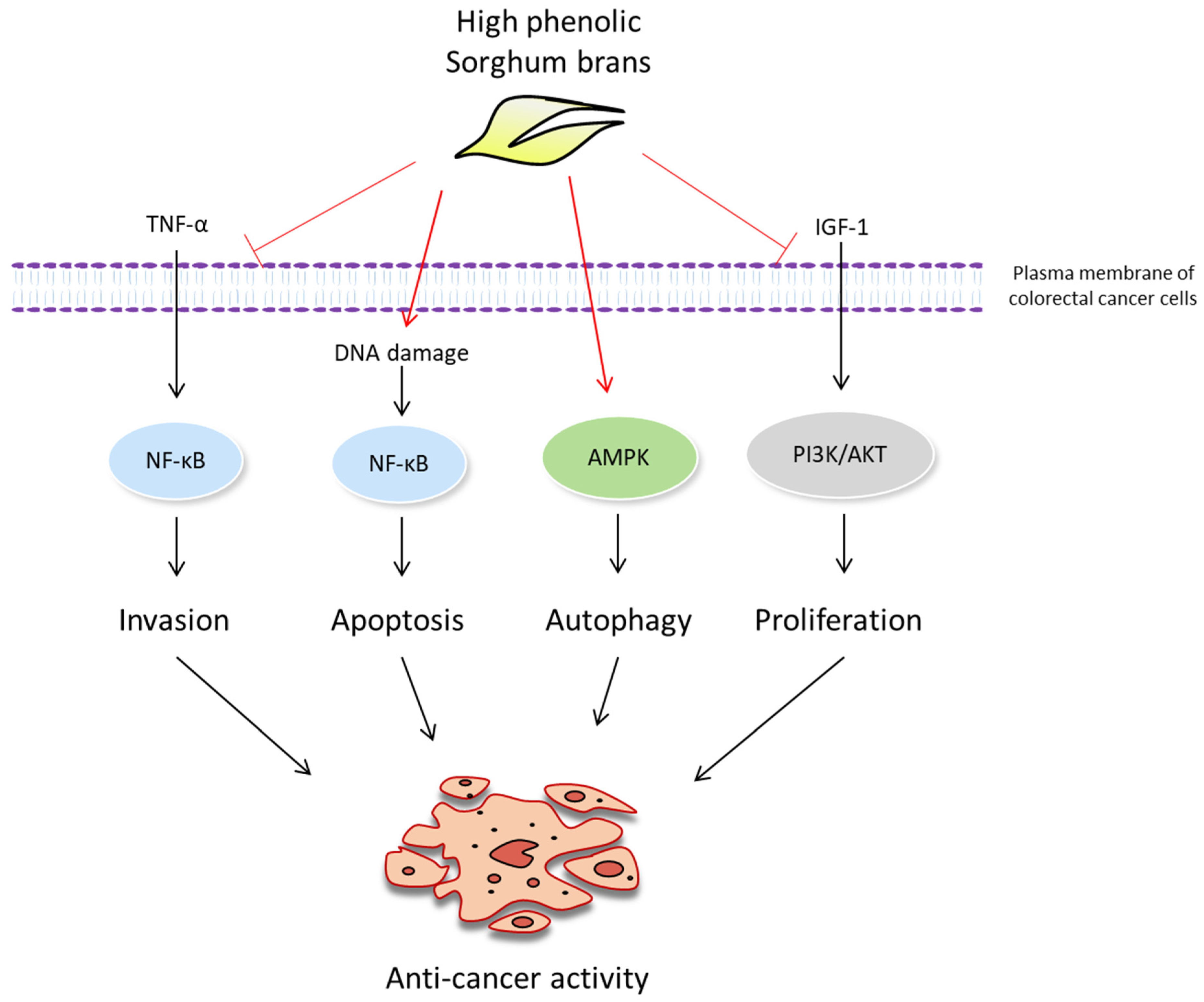
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sorghum Grain Processing
4.3. Preparation of High Phenolic Sorghum Bran Extract
4.4. Cell Culture and Treatment
4.5. Cell Proliferation and Apoptosis
4.6. Western Blotting
4.7. Immunofluorescence
4.8. Transient Transfection and Luciferase Assay
4.9. Formulation of Mouse Diets
4.10. In Vivo Study
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kothari, K.; Ale, S.; Bordovsky, J.P.; Munster, C.L. Assessing the Climate Change Impacts on Grain Sorghum Yield and Irrigation Water Use under Full and Deficit Irrigation Strategies. Trans. ASABE 2020, 63, 81–94. [Google Scholar] [CrossRef]
- Aruna, C.; Visarada, K.; Bhat, B.V.; Tonapi, V.A. (Eds.) Chapter 1—Sorghum: A Bundle of Opportunities in the 21st Century. In Breeding Sorghum for Diverse End Uses; Woodhead Publishing: Sawston, UK, 2019; pp. 1–14. [Google Scholar]
- Ratnavathi, C.V. Chapter 12—Grain Structure, Quality, and Nutrition. In Breeding Sorghum for Diverse End Uses; Woodhead Publishing: Sawston, UK, 2019; pp. 193–207. [Google Scholar]
- Wu, G.; Bennett, S.J.; Bornman, J.F.; Clarke, M.; Fang, Z.; Johnson, S.K. Phenolic profile and content of sorghum grains under different irrigation managements. Food Res. Int. 2017, 97, 347–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Li, M.; Gao, H.; Wang, B.; Tongcheng, X.; Gao, B.; Yu, L. Triacylglycerol, fatty acid, and phytochemical profiles in a new red sorghum variety (Ji Liang No. 1) and its antioxidant and anti-inflammatory properties. Food Sci. Nutr. 2019, 7, 949–958. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Xu, T.; Zheng, W.; Gao, B.; Zhu, H.; Xu, R.; Deng, H.; Wang, B.; Wu, Y.; Sun, X.; et al. Triacylglycerols compositions, soluble and bound phenolics of red sorghums, and their radical scavenging and anti-inflammatory activities. Food Chem. 2021, 340, 128123. [Google Scholar] [CrossRef]
- Yang, L.; Browning, J.D.; Awika, J.M. Sorghum 3-deoxyanthocyanins possess strong phase II enzyme inducer activity and cancer cell growth inhibition properties. J. Agric. Food Chem. 2009, 57, 1797–1804. [Google Scholar] [CrossRef]
- Ritchie, L.E.; Sturino, J.M.; Carroll, R.J.; Rooney, L.W.; Azcarate-Peril, M.A.; Turner, N.D. Polyphenol-rich sorghum brans alter colon microbiota and impact species diversity and species richness after multiple bouts of dextran sodium sulfate-induced colitis. FEMS Microbiol. Ecol. 2015, 91, fiv008. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, L.E.; Taddeo, S.S.; Weeks, B.R.; Carroll, R.J.; Dykes, L.; Rooney, L.W.; Turner, N.D. Impact of Novel Sorghum Bran Diets on DSS-Induced Colitis. Nutrients 2017, 9, 330. [Google Scholar] [CrossRef] [Green Version]
- Turner, N.; Diaz, A.; Taddeo, S.; Vanamala, J.; McDonough, C.; Dykes, L.; Murphy, M.; Carroll, R.; Rooney, L. Bran from black or brown sorghum suppresses colon carcinogenesis (ABSTRACT). FASEB J. 2006, 20, A599. [Google Scholar] [CrossRef]
- Park, J.H.; Darvin, P.; Lim, E.J.; Joung, Y.H.; Hong, D.Y.; Park, E.U.; Park, S.H.; Choi, S.K.; Moon, E.-S.; Cho, B.W.; et al. Hwanggeumchal sorghum induces cell cycle arrest, and suppresses tumor growth and metastasis through Jak2/STAT pathways in breast cancer xenografts. PLoS ONE 2012, 7, e40531. [Google Scholar] [CrossRef]
- Smolensky, D.; Rhodes, D.; McVey, D.S.; Fawver, Z.; Perumal, R.; Herald, T.; Noronha, L. High-Polyphenol Sorghum Bran Extract Inhibits Cancer Cell Growth Through ROS Induction, Cell Cycle Arrest, and Apoptosis. J. Med. Food 2018, 21, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.; Noronha, L.; Herald, T.; Bean, S.; Lee, S.-H.; Perumal, R.; Wang, W.; Smolensky, D. Evaluation of ethanol-based extraction conditions of sorghum bran bioactive compounds with downstream anti-proliferative properties in human cancer cells. Heliyon 2019, 5, e01589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-H.; Lee, J.; Herald, T.; Cox, S.; Noronha, L.; Perumal, R.; Lee, H.-S.; Smolensky, D. Anticancer Activity of a Novel High Phenolic Sorghum Bran in Human Colon Cancer Cells. Oxidative Med. Cell. Longev. 2020, 2020, 2890536. [Google Scholar] [CrossRef] [PubMed]
- Awika, J.M.; Rooney, L.W. Sorghum phytochemicals and their potential impact on human health. Phytochemistry 2004, 65, 1199–1221. [Google Scholar] [CrossRef]
- Rhodes, D.; Gadgil, P.; Perumal, R.; Tesso, T.; Herald, T.J. Natural Variation and Genome-Wide Association Study of Antioxidants in a Diverse Sorghum Collection. Cereal Chem. 2017, 94, 190–198. [Google Scholar] [CrossRef]
- Uchimiya, M. Proton-Coupled Electron Transfers of Defense Phytochemicals in Sorghum (Sorghum bicolor (L.) Moench). J. Agric. Food Chem. 2020, 68, 12978–12983. [Google Scholar] [CrossRef]
- Soleimani, A.; Rahmani, F.; Ferns, G.A.; Ryzhikov, M.; Avan, A.; Hassanian, S.M. Role of the NF-kappaB signaling pathway in the pathogenesis of colorectal cancer. Gene 2020, 726, 144132. [Google Scholar] [CrossRef]
- Yu, W.-K.; Xu, Z.Y.; Yuan, L.; Mo, S.; Xu, B.; Cheng, X.-D.; Qin, J.-J. Targeting beta-Catenin Signaling by Natural Products for Cancer Prevention and Therapy. Front. Pharmacol. 2020, 11, 984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Roberts, T.M.; Shivdasani, R.A. Targeting PI3K signaling as a therapeutic approach for colorectal cancer. Gastroenterology 2011, 141, 50–61. [Google Scholar] [CrossRef]
- Danielsen, S.A.; Eide, P.W.; Nesbakken, A.; Guren, T.; Leithe, E.; Lothe, R.A. Portrait of the PI3K/AKT pathway in colorectal cancer. Biochim. Biophys. Acta 2015, 1855, 104–121. [Google Scholar] [CrossRef]
- Suvarna, V.; Murahari, M.; Khan, T.; Chaubey, P.; Sangave, P. Phytochemicals and PI3K Inhibitors in Cancer-An Insight. Front. Pharmacol. 2017, 8, 916. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Saud, S.M.; Young, M.R.; Chen, G.; Hua, B. Targeting AMPK for cancer prevention and treatment. Oncotarget 2015, 6, 7365–7378. [Google Scholar] [CrossRef] [Green Version]
- Codina, N.S.; Mancias, J.D.; Kimmelman, A.C. The Role of Autophagy in Cancer. Annu. Rev. Cancer Biol. 2017, 1, 19–39. [Google Scholar] [CrossRef]
- Hasima, N.; Ozpolat, B. Regulation of autophagy by polyphenolic compounds as a potential therapeutic strategy for cancer. Cell Death Disease 2014, 5, e1509. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amarakoon, D.; Lou, Z.; Lee, W.; Smolensky, D.; Lee, S. A mechanistic review: Potential chronic disease-preventive properties of sorghum. J. Sci. Food Agric. 2020, 101, 2641–2649. [Google Scholar] [CrossRef]
- de Morais Cardoso, L.; Pinheiro, S.S.; Martino, H.S.; Pinheiro-Sant’Ana, H.M. Sorghum (Sorghum bicolor L.): Nutrients, bioactive compounds, and potential impact on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 372–390. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.D. NF-kappaB: Tumor promoter or suppressor? Trends Cell Biol. 2004, 14, 64–69. [Google Scholar] [CrossRef]
- Hoesel, B.; Schmid, J.A. The complexity of NF-kappaB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zhou, B.P. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br. J. Cancer 2010, 102, 639–644. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Xia, W.; Miller, S.A.; Wen, Y.; Wang, H.Y.; Hung, M.C. Crossregulation of NF-kappaB by the APC/GSK-3beta/beta-catenin pathway. Mol. Carcinog. 2004, 39, 139–146. [Google Scholar] [CrossRef]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef]
- Walter, E.; Kissel, T. Heterogeneity in the human intestinal cell line Caco-2 leads to differences in transepithelial transport. Eur. J. Pharm. Sci. 1995, 3, 215–230. [Google Scholar] [CrossRef]
- Rhodes, D.H.; Hoffmann, L., Jr.; Rooney, W.L.; Ramu, P.; Morris, G.P.; Kresovich, S. Genome-wide association study of grain polyphenol concentrations in global sorghum [Sorghum bicolor (L.) Moench] germplasm. J. Agric. Food Chem. 2014, 62, 10916–10927. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.; Lee, J.; Lee, S.H. Synergistic anticancer activity of capsaicin and 3,3′-diindolylmethane in human colorectal cancer. J. Agric. Food Chem. 2015, 63, 4297–4304. [Google Scholar] [CrossRef]
- Lee, S.-H.; Bahn, J.H.; Whitlock, N.C.; Baek, S.J. Activating transcription factor 2 (ATF2) controls tolfenamic acid-induced ATF3 expression via MAP kinase pathways. Oncogene 2010, 29, 5182–5192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-H.; Richardson, R.L.; Dashwood, R.H.; Baek, S.J. Capsaicin represses transcriptional activity of beta-catenin in human colorectal cancer cells. J. Nutr. Biochem. 2012, 23, 646–655. [Google Scholar] [CrossRef] [Green Version]
- Baek, S.; Okazaki, R.; Lee, S.-H.; Martinez, J.; Kim, J.-S.; Yamaguchi, K.; Mishina, Y.; Martin, D.W.; Shoieb, A.; McEntee, M.; et al. Nonsteroidal anti-inflammatory drug-activated gene-1 over expression in transgenic mice suppresses intestinal neoplasia. Gastroenterology 2006, 131, 1553–1560. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-H.; Lee, H.-S.; Lee, J.; Amarakoon, D.; Lou, Z.; Noronha, L.E.; Herald, T.J.; Perumal, R.; Smolensky, D. Polyphenol Containing Sorghum Brans Exhibit an Anti-Cancer Effect in Apc Min/+ Mice Treated with Dextran Sodium Sulfate. Int. J. Mol. Sci. 2021, 22, 8286. https://doi.org/10.3390/ijms22158286
Lee S-H, Lee H-S, Lee J, Amarakoon D, Lou Z, Noronha LE, Herald TJ, Perumal R, Smolensky D. Polyphenol Containing Sorghum Brans Exhibit an Anti-Cancer Effect in Apc Min/+ Mice Treated with Dextran Sodium Sulfate. International Journal of Molecular Sciences. 2021; 22(15):8286. https://doi.org/10.3390/ijms22158286
Chicago/Turabian StyleLee, Seong-Ho, Hee-Seop Lee, Jihye Lee, Darshika Amarakoon, Zhiyuan Lou, Leela E. Noronha, Thomas J. Herald, Ramasamy Perumal, and Dmitriy Smolensky. 2021. "Polyphenol Containing Sorghum Brans Exhibit an Anti-Cancer Effect in Apc Min/+ Mice Treated with Dextran Sodium Sulfate" International Journal of Molecular Sciences 22, no. 15: 8286. https://doi.org/10.3390/ijms22158286
APA StyleLee, S. -H., Lee, H. -S., Lee, J., Amarakoon, D., Lou, Z., Noronha, L. E., Herald, T. J., Perumal, R., & Smolensky, D. (2021). Polyphenol Containing Sorghum Brans Exhibit an Anti-Cancer Effect in Apc Min/+ Mice Treated with Dextran Sodium Sulfate. International Journal of Molecular Sciences, 22(15), 8286. https://doi.org/10.3390/ijms22158286





