Melatonin and Glycine Reduce Uterus Ischemia/Reperfusion Injury in a Rat Model of Warm Ischemia
Abstract
1. Introduction
2. Results
2.1. General Data
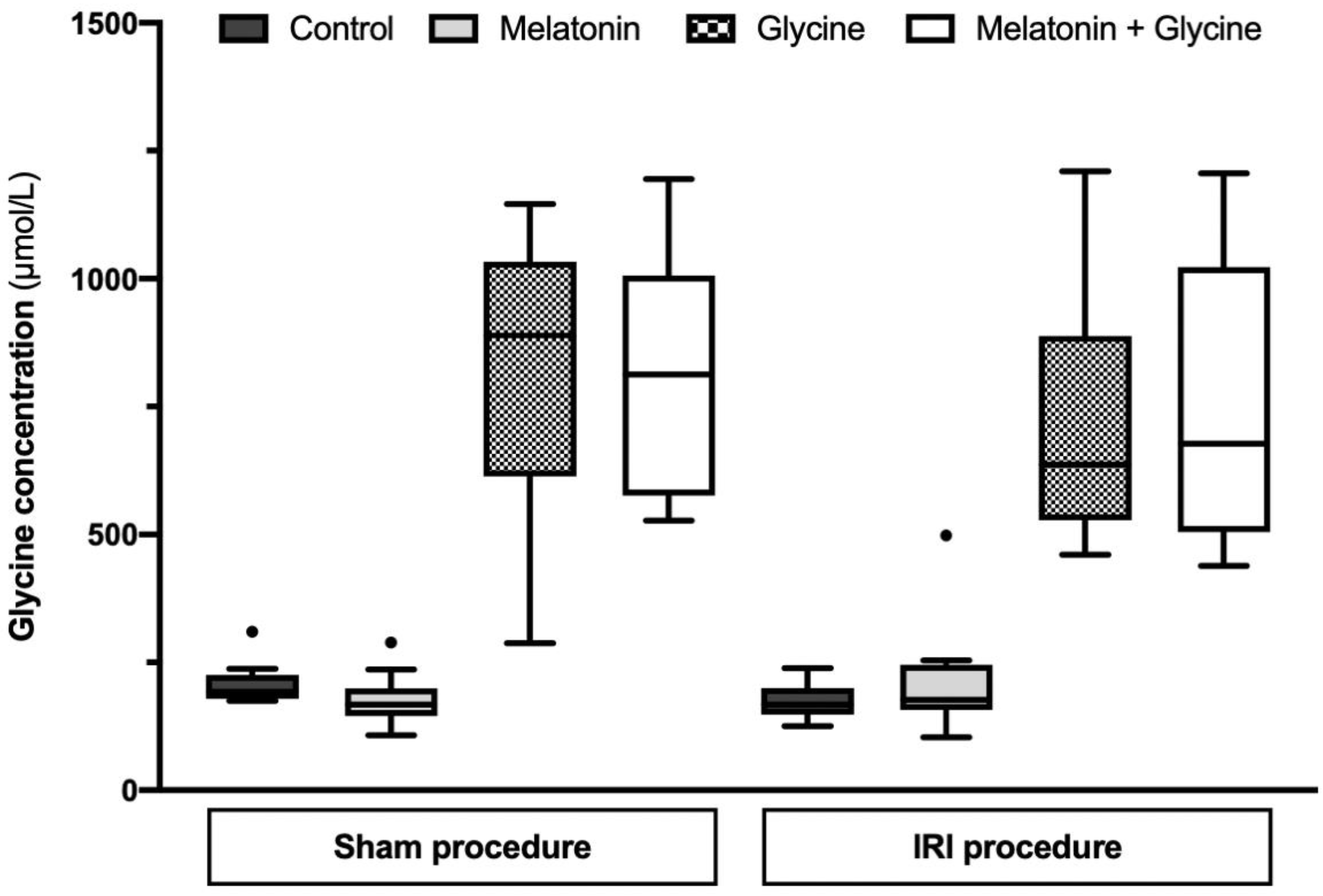
2.2. Glycine Concentration
2.3. Histology
2.4. Tissue MPO Expression
2.5. Tissue SOD Activity
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Animal Groups and Experimental Design
4.3. IRI and Sham Procedure
4.4. Histology
4.5. IHC Staining
4.6. Biochemistry
4.7. Blood Sample Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brannstrom, M.; Enskog, A.; Kvarnstrom, N.; Ayoubi, J.M.; Dahm-Kahler, P. Global results of human uterus transplantation and strategies for pre-transplantation screening of donors. Fertil. Steril. 2019, 112, 3–10. [Google Scholar] [CrossRef]
- Jones, B.P.; Saso, S.; Bracewell-Milnes, T.; Thum, M.Y.; Nicopoullos, J.; Diaz-Garcia, C.; Friend, P.; Ghaem-Maghami, S.; Testa, G.; Johannesson, L.; et al. Human uterine transplantation: A review of outcomes from the first 45 cases. BJOG 2019, 126, 1310–1319. [Google Scholar] [CrossRef]
- Ozkan, O.; Dogan, N.U.; Ozkan, O.; Mendilcioglu, I.; Dogan, S.; Aydinuraz, B.; Simsek, M. Uterus transplantation: From animal models through the first heart beating pregnancy to the first human live birth. Women’s Health 2016, 12, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Zitkute, V.; Kvietkauskas, M.; Leber, B.; Strupas, K.; Stiegler, P.; Schemmer, P. Ischemia and reperfusion injury in uterus transplantation: A comprehensive review. Transplant. Rev. 2020, 34, 100550. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, L.; Williams, N.J.; Wilkinson, S. Ethical and policy issues raised by uterus transplants. Br. Med. Bull. 2019, 131, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Brannstrom, M.; Dahm-Kahler, P. Uterus transplantation and fertility preservation. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 55, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Castellon, L.A.R.; Amador, M.I.G.; Gonzalez, R.E.D.; Eduardo, M.S.J.; Diaz-Garcia, C.; Kvarnstrom, N.; Branstrom, M. The history behind successful uterine transplantation in humans. JBRA Assist. Reprod. 2017, 21, 126–134. [Google Scholar] [CrossRef]
- Halladin, N.L. Oxidative and inflammatory biomarkers of ischemia and reperfusion injuries. Dan. Med. J. 2015, 62, B5054. [Google Scholar]
- Wu, M.Y.; Yiang, G.T.; Liao, W.T.; Tsai, A.P.; Cheng, Y.L.; Cheng, P.W.; Li, C.Y.; Li, C.J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell. Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion--from mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef]
- Horvat, M.; Iltis, A. What Are Good Guidelines for Evaluating Uterus Transplantation? AMA J. Ethics 2019, 21, 988–995. [Google Scholar] [CrossRef]
- Stiegler, P.; Bausys, A.; Leber, B.; Strupas, K.; Schemmer, P. Impact of Melatonin in Solid Organ Transplantation—Is It Time for Clinical Trials? A Comprehensive Review. Int. J. Mol. Sci. 2018, 19, 3509. [Google Scholar] [CrossRef]
- Zitkute, V.; Kvietkauskas, M.; Maskoliunaite, V.; Leber, B.; Ramasauskaite, D.; Strupas, K.; Stiegler, P.; Schemmer, P. Custodiol-N Is Superior to Custodiol((R)) Solution in Experimental Rat Uterus Preservation. Int. J. Mol. Sci. 2020, 21, 8015. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell. Endocrinol. 2012, 351, 152–166. [Google Scholar] [CrossRef]
- Crupi, R.; Mazzon, E.; Marino, A.; La Spada, G.; Bramanti, P.; Spina, E.; Cuzzocrea, S. Melatonin’s stimulatory effect on adult hippocampal neurogenesis in mice persists after ovariectomy. J. Pineal Res. 2011, 51, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Zubero, E.; Garcia-Gil, F.A.; Lopez-Pingarron, L.; Alatorre-Jimenez, M.A.; Inigo-Gil, P.; Tan, D.X.; Garcia, J.J.; Reiter, R.J. Potential benefits of melatonin in organ transplantation: A review. J. Endocrinol. 2016, 229, R129–R146. [Google Scholar] [CrossRef] [PubMed]
- Campolo, M.; Ahmad, A.; Crupi, R.; Impellizzeri, D.; Morabito, R.; Esposito, E.; Cuzzocrea, S. Combination therapy with melatonin and dexamethasone in a mouse model of traumatic brain injury. J. Endocrinol. 2013, 217, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Siracusa, R.; D’Amico, R.; Peritore, A.F.; Cordaro, M.; Gugliandolo, E.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Melatonin Plus Folic Acid Treatment Ameliorates Reserpine-Induced Fibromyalgia: An Evaluation of Pain, Oxidative Stress, and Inflammation. Antioxidants 2019, 8, 628. [Google Scholar] [CrossRef]
- Olcese, J.M. Melatonin and Female Reproduction: An Expanding Universe. Front. Endocrinol. 2020, 11, 85. [Google Scholar] [CrossRef]
- Saat, N.; Risvanli, A.; Dogan, H.; Onalan, E.; Akpolat, N.; Seker, I.; Sahna, E. Effect of melatonin on torsion and reperfusion induced pathogenesis of rat uterus. Biotech. Histochem. 2019, 94, 533–539. [Google Scholar] [CrossRef]
- Turkoz, Y.; Celik, O.; Hascalik, S.; Cigremis, Y.; Hascalik, M.; Mizrak, B.; Yologlu, S. Melatonin reduces torsion-detorsion injury in rat ovary: Biochemical and histopathologic evaluation. J. Pineal Res. 2004, 37, 137–141. [Google Scholar] [CrossRef]
- Aslaner, A.; Gunal, O.; Turgut, H.T.; Celik, E.; Yildirim, U.; Demirci, R.K.; Gunduz, U.R.; Calis, H.; Dogan, S. Effect of melatonin on kidney cold ischemic preservation injury. Int. J. Clin. Exp. Med. 2013, 6, 794–798. [Google Scholar] [PubMed]
- Petrat, F.; Boengler, K.; Schulz, R.; de Groot, H. Glycine, a simple physiological compound protecting by yet puzzling mechanism(s) against ischaemia-reperfusion injury: Current knowledge. Br. J. Pharmacol. 2012, 165, 2059–2072. [Google Scholar] [CrossRef]
- Habib, M.M.; Hodgson, H.J.; Davidson, B.R. The role of glycine in hepatic ischemia-reperfusion injury. Curr. Pharm. Des. 2006, 12, 2953–2967. [Google Scholar] [CrossRef]
- Petrat, F.; Drowatzky, J.; Boengler, K.; Finckh, B.; Schmitz, K.J.; Schulz, R.; de Groot, H. Protection from glycine at low doses in ischemia-reperfusion injury of the rat small intestine. Eur. Surg. Res. 2011, 46, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Li, X.; Qian, L.; Xu, Y.; Lu, Y.; Zhang, J.; Li, N.; Zhu, X.; Ben, J.; Yang, Q.; et al. Glycine attenuates myocardial ischemia-reperfusion injury by inhibiting myocardial apoptosis in rats. J. Biomed. Res. 2012, 26, 346–354. [Google Scholar] [CrossRef]
- Maneikyte, J.; Bausys, A.; Leber, B.; Feldbacher, N.; Hoefler, G.; Kolb-Lenz, D.; Strupas, K.; Stiegler, P.; Schemmer, P. Dietary Glycine Prevents FOLFOX Chemotherapy-Induced Heart Injury: A Colorectal Cancer Liver Metastasis Treatment Model in Rats. Nutrients 2020, 12, 2634. [Google Scholar] [CrossRef] [PubMed]
- Maneikyte, J.; Bausys, A.; Leber, B.; Horvath, A.; Feldbacher, N.; Hoefler, G.; Strupas, K.; Stiegler, P.; Schemmer, P. Dietary glycine decreases both tumor volume and vascularization in a combined colorectal liver metastasis and chemotherapy model. Int. J. Biol. Sci. 2019, 15, 1582–1590. [Google Scholar] [CrossRef]
- Brannstrom, M.; Wranning, C.A.; Altchek, A. Experimental uterus transplantation. Hum. Reprod. Update 2010, 16, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Haddad, C.F.; Haddad, J.M.; Veiga, E.C.A.; Sorpreso, I.C.E.; Simoes, R.S.; Baracat, E.C.; Soares Junior, J.M. Melatonin and organ transplantation: What is the relationship? Rev. Assoc. Med. Bras. (1992) 2020, 66, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Jung, F.J.; Yang, L.; Harter, L.; Inci, I.; Schneiter, D.; Lardinois, D.; Keel, M.; Weder, W.; Korom, S. Melatonin in vivo prolongs cardiac allograft survival in rats. J. Pineal Res. 2004, 37, 36–41. [Google Scholar] [CrossRef]
- Liu, C.; Hong, T.; Shao, M.; Chen, Z.; Wang, C. Melatonin synergized with cyclosporine A improves cardiac allograft survival by suppressing inflammation and apoptosis. Mol. Med. Rep. 2014, 10, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ma, Q.; Zhu, P.; Ren, J.; Reiter, R.J.; Chen, Y. Protective role of melatonin in cardiac ischemia-reperfusion injury: From pathogenesis to targeted therapy. J. Pineal Res. 2018, 64, e12471. [Google Scholar] [CrossRef] [PubMed]
- Takechi, M.; Tatehara, S.; Satomura, K.; Fujisawa, K.; Nagayama, M. Effect of FGF-2 and melatonin on implant bone healing: A histomorphometric study. J. Mater. Sci. Mater. Med. 2008, 19, 2949–2952. [Google Scholar] [CrossRef]
- Inci, I.; Inci, D.; Dutly, A.; Boehler, A.; Weder, W. Melatonin Attenuates Posttransplant Lung Ischemia-Reperfusion Injury. Ann. Thorac. Surg. 2002, 73, 220–225. [Google Scholar] [CrossRef]
- Lin, G.J.; Huang, S.H.; Chen, Y.W.; Hueng, D.Y.; Chien, M.W.; Chia, W.T.; Chang, D.M.; Sytwu, H.K. Melatonin prolongs islet graft survival in diabetic NOD mice. J. Pineal Res. 2009, 47, 284–292. [Google Scholar] [CrossRef]
- Munoz-Casares, F.C.; Padillo, F.J.; Briceno, J.; Collado, J.A.; Munoz-Castaneda, J.R.; Ortega, R.; Cruz, A.; Tunez, I.; Montilla, P.; Pera, C.; et al. Melatonin reduces apoptosis and necrosis induced by ischemia/reperfusion injury of the pancreas. J. Pineal Res. 2006, 40, 195–203. [Google Scholar] [CrossRef]
- Panah, F.; Ghorbanihaghjo, A.; Argani, H.; Haiaty, S.; Rashtchizadeh, N.; Hosseini, L.; Dastmalchi, S.; Rezaeian, R.; Alirezaei, A.; Jabarpour, M.; et al. The effect of oral melatonin on renal ischemia-reperfusion injury in transplant patients: A double-blind, randomized controlled trial. Transpl. Immunol. 2019, 57, 101241. [Google Scholar] [CrossRef]
- Shi, S.; Lei, S.; Tang, C.; Wang, K.; Xia, Z. Melatonin attenuates acute kidney ischemia/reperfusion injury in diabetic rats by activation of the SIRT1/Nrf2/HO-1 signaling pathway. Biosci. Rep. 2019, 39, BSR20181614. [Google Scholar] [CrossRef]
- Li, Z.; Nickkholgh, A.; Yi, X.; Bruns, H.; Gross, M.L.; Hoffmann, K.; Mohr, E.; Zorn, M.; Buchler, M.W.; Schemmer, P. Melatonin protects kidney grafts from ischemia/reperfusion injury through inhibition of NF-kB and apoptosis after experimental kidney transplantation. J. Pineal Res. 2009, 46, 365–372. [Google Scholar] [CrossRef]
- Esteban-Zubero, E.; Garcia-Gil, F.A.; Lopez-Pingarron, L.; Alatorre-Jimenez, M.A.; Ramirez, J.M.; Tan, D.X.; Garcia, J.J.; Reiter, R.J. Melatonin role preventing steatohepatitis and improving liver transplantation results. Cell. Mol. Life Sci. 2016, 73, 2911–2927. [Google Scholar] [CrossRef]
- Mortezaee, K.; Khanlarkhani, N. Melatonin application in targeting oxidative-induced liver injuries: A review. J. Cell. Physiol. 2018, 233, 4015–4032. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.M. Cytoprotective effects of melatonin against necrosis and apoptosis induced by ischemia/reperfusion injury in rat liver. J. Pineal Res. 2008, 44, 165–171. [Google Scholar] [CrossRef]
- Liang, R.; Nickkholgh, A.; Hoffmann, K.; Kern, M.; Schneider, H.; Sobirey, M.; Zorn, M.; Buchler, M.W.; Schemmer, P. Melatonin protects from hepatic reperfusion injury through inhibition of IKK and JNK pathways and modification of cell proliferation. J. Pineal Res. 2009, 46, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Koh, P.O. Melatonin prevents hepatic injury-induced decrease in Akt downstream targets phosphorylations. J. Pineal Res. 2011, 51, 214–219. [Google Scholar] [CrossRef]
- Hadj Ayed Tka, K.; Mahfoudh Boussaid, A.; Zaouali, M.A.; Kammoun, R.; Bejaoui, M.; Ghoul Mazgar, S.; Rosello Catafau, J.; Ben Abdennebi, H. Melatonin modulates endoplasmic reticulum stress and Akt/GSK3-beta signaling pathway in a rat model of renal warm ischemia reperfusion. Anal. Cell. Pathol. 2015, 2015, 635172. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kang, S.M.; Lee, W.T.; Park, K.A.; Lee, K.M.; Lee, J.E. The beneficial effect of melatonin in brain endothelial cells against oxygen-glucose deprivation followed by reperfusion-induced injury. Oxid. Med. Cell. Longev. 2014, 2014, 639531. [Google Scholar] [CrossRef] [PubMed]
- Schemmer, P.; Bradford, B.U.; Rose, M.L.; Bunzendahl, H.; Raleigh, J.A.; Lemasters, J.J.; Thurman, R.G. Intravenous glycine improves survival in rat liver transplantation. Am. J. Physiol. 1999, 276, G924–G932. [Google Scholar] [CrossRef]
- Wheeler, M.D.; Ikejema, K.; Enomoto, N.; Stacklewitz, R.F.; Seabra, V.; Zhong, Z.; Yin, M.; Schemmer, P.; Rose, M.L.; Rusyn, I.; et al. Glycine: A new anti-inflammatory immunonutrient. Cell. Mol. Life Sci. CMLS 1999, 56, 843–856. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; Lin, G.; Hu, S.; Wang, B.; Dai, Z.; Wu, G. Glycine stimulates protein synthesis and inhibits oxidative stress in pig small intestinal epithelial cells. J. Nutr. 2014, 144, 1540–1548. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, J.; Ma, B.; Li, K.; Li, X.; Bai, H.; Yang, Q.; Zhu, X.; Ben, J.; Chen, Q. Glycine attenuates cerebral ischemia/reperfusion injury by inhibiting neuronal apoptosis in mice. Neurochem. Int. 2012, 61, 649–658. [Google Scholar] [CrossRef]
- Kvietkauskas, M.; Leber, B.; Strupas, K.; Stiegler, P.; Schemmer, P. Machine Perfusion of Extended Criteria Donor Organs: Immunological Aspects. Front. Immunol. 2020, 11, 192. [Google Scholar] [CrossRef]
- Kvietkauskas, M.; Zitkute, V.; Leber, B.; Strupas, K.; Stiegler, P.; Schemmer, P. Dietary Melatonin and Glycine Decrease Tumor Growth through Antiangiogenic Activity in Experimental Colorectal Liver Metastasis. Nutrients 2021, 13, 2035. [Google Scholar] [CrossRef] [PubMed]
- Mikalauskas, S.; Mikalauskiene, L.; Bruns, H.; Nickkholgh, A.; Hoffmann, K.; Longerich, T.; Strupas, K.; Buchler, M.W.; Schemmer, P. Dietary glycine protects from chemotherapy-induced hepatotoxicity. Amino Acids 2011, 40, 1139–1150. [Google Scholar] [CrossRef]
- Johannesson, L.; Enskog, A.; Dahm-Kahler, P.; Hanafy, A.; Chai, D.C.; Mwenda, J.M.; Diaz-Garcia, C.; Olausson, M.; Brannstrom, M. Uterus transplantation in a non-human primate: Long-term follow-up after autologous transplantation. Hum. Reprod. 2012, 27, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S.; Ozakpinar, O.B.; Ak, K.; Eroglu, M.; Acikel, M.; Tetik, S.; Uras, F.; Cetinel, S. The protective effects of tacrolimus on rat uteri exposed to ischemia-reperfusion injury: A biochemical and histopathologic evaluation. Fertil. Steril. 2014, 101, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Atalay, Y.O.; Aktas, S.; Sahin, S.; Kucukodaci, Z.; Ozakpinar, O.B. Remifentanil protects uterus against ischemia-reperfusion injury in rats. Acta Cir. Bras. 2015, 30, 756–761. [Google Scholar] [CrossRef][Green Version]
- Siracusa, R.; Impellizzeri, D.; Cordaro, M.; Gugliandolo, E.; Peritore, A.F.; Di Paola, R.; Cuzzocrea, S. Topical Application of Adelmidrol + Trans-Traumatic Acid Enhances Skin Wound Healing in a Streptozotocin-Induced Diabetic Mouse Model. Front. Pharmacol. 2018, 9, 871. [Google Scholar] [CrossRef]
- Diaz-Garcia, C.; Akhi, S.N.; Martinez-Varea, A.; Brannstrom, M. The effect of warm ischemia at uterus transplantation in a rat model. Acta Obstet. Gynecol. Scand. 2013, 92, 152–159. [Google Scholar] [CrossRef]
- Sahin Ersoy, G.; Kurek Eken, M.; Cevik, O.; Cilingir, O.T.; Tal, R. Mycophenolate mofetil attenuates uterine ischaemia/reperfusion injury in a rat model. Reprod. Biomed. Online 2017, 34, 115–123. [Google Scholar] [CrossRef]
- Fusco, R.; Gugliandolo, E.; Siracusa, R.; Scuto, M.; Cordaro, M.; D’Amico, R.; Evangelista, M.; Peli, A.; Peritore, A.F.; Impellizzeri, D.; et al. Formyl Peptide Receptor 1 Signaling in Acute Inflammation and Neural Differentiation Induced by Traumatic Brain Injury. Biology 2020, 9, 238. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernandez, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]





| Sham Procedure | IRI Procedure | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | Melatonin | Glycine | Melatonin + Glycine | Control | Melatonin | Glycine | Melatonin + Glycine | |
| Inflammatory cells | 1 (0.75; 1) | 1 (0.75; 1) | 1 (1; 1) | 1 (0.5; 1) | 2 (1; 2) | 1 (1; 1.25) | 1 (1; 2) | 1 (1; 1.25) |
| Vasoconstriction | 0 (0; 0.25) | 0 (0; 0) | 0 (0; 0.25) | 0 (0; 0) | 0 (0; 1) | 0 (0; 1) | 0 (0; 0.25) | 0 (0; 0.25) |
| Hemorrhage | 0 (0; 0) | 0 (0; 0) | 0 (0; 0) | 0 (0; 0) | 0 (0; 0) | 0 (0; 0) | 0 (0; 0) | 0 (0; 0) |
| Necrosis | 1 (1; 1) | 1 (0.75; 1) | 1 (0.75; 1) | 1 (1; 1) | 1 (1; 2) | 1 (1; 1.25) | 1 (1; 2) | 1 (1; 1.25) |
| Edema | 1 (0.75; 1) | 1 (0; 1) | 1 (1; 1) | 1 (0; 1) | 2 (1; 2) | 1 (1; 2) | 1 (1; 2) | 1 (1; 1) |
| Thrombosis | 0 (0; 0) | 0 (0; 0) | 0 (0; 0) | 0 (0; 0) | 0 (0; 0.25) | 0 (0; 0) | 0 (0; 0) | 0 (0; 0) |
| Endometrial loss of cells | 1 (1; 1) | 1 (0.75; 1) | 1 (0.75; 1) | 1 (0.5; 1) | 1 (1; 2) | 1 (1; 1.25) | 1 (1; 2) | 1 (1; 1) |
| Smooth muscle contraction | 0 (0; 0) | 0 (0; 0) | 0 (0; 0) | 0 (0; 1) | 0 (0; 1) | 0 (0; 1) | 1 (0; 1) | 0 (0; 0.25) |
| Impaired basement membrane integrity | 1 (0; 1) | 0 (0; 1) | 0 (0; 0.25) | 0 (0; 1) | 1 (0; 1) | 0.5 (0; 1) | 1 (0; 1) | 1 (0.75; 1) |
| Perimeter thickening | 0 (0; 0.25) | 0 (0; 1) | 0.5 (0; 1) | 1 (0; 1) | 1 (0.75; 1) | 0.5 (0; 1) | 0 (0; 1) | 0.5 (0; 1) |
| Total score | 5 (4; 6) | 4 (3; 6) | 4.5 (4; 6) | 4 (3.5; 6) | 8.5 (7; 10.25) | 6 (5; 8) | 7 (6; 8) | 6 (6; 7) |
| Score | |||
|---|---|---|---|
| 0 | 1 | 2 | |
| Inflammatory cells | Absent | Moderate number of cells | Severe infiltration of cells |
| Vasoconstriction | Absent | <20% of small vessels | >20% of small vessels |
| Hemorrhage | Absent | Subendometrial | Myometrial and endometrial |
| Necrosis | Absent | <20% | >20% |
| Edema | Absent | <50% | >50% |
| Thrombosis | Absent | <50% of the vessels | >50% of the vessels |
| Endometrial loss of cells | Absent | <20% | >20% |
| Smooth muscle contraction | Absent | Present | |
| Impaired basement membrane integrity | Absent | Present | |
| Perimeter thickening | Absent | Present | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zitkute, V.; Kvietkauskas, M.; Maskoliunaite, V.; Leber, B.; Ramasauskaite, D.; Strupas, K.; Stiegler, P.; Schemmer, P. Melatonin and Glycine Reduce Uterus Ischemia/Reperfusion Injury in a Rat Model of Warm Ischemia. Int. J. Mol. Sci. 2021, 22, 8373. https://doi.org/10.3390/ijms22168373
Zitkute V, Kvietkauskas M, Maskoliunaite V, Leber B, Ramasauskaite D, Strupas K, Stiegler P, Schemmer P. Melatonin and Glycine Reduce Uterus Ischemia/Reperfusion Injury in a Rat Model of Warm Ischemia. International Journal of Molecular Sciences. 2021; 22(16):8373. https://doi.org/10.3390/ijms22168373
Chicago/Turabian StyleZitkute, Viktorija, Mindaugas Kvietkauskas, Vygante Maskoliunaite, Bettina Leber, Diana Ramasauskaite, Kestutis Strupas, Philipp Stiegler, and Peter Schemmer. 2021. "Melatonin and Glycine Reduce Uterus Ischemia/Reperfusion Injury in a Rat Model of Warm Ischemia" International Journal of Molecular Sciences 22, no. 16: 8373. https://doi.org/10.3390/ijms22168373
APA StyleZitkute, V., Kvietkauskas, M., Maskoliunaite, V., Leber, B., Ramasauskaite, D., Strupas, K., Stiegler, P., & Schemmer, P. (2021). Melatonin and Glycine Reduce Uterus Ischemia/Reperfusion Injury in a Rat Model of Warm Ischemia. International Journal of Molecular Sciences, 22(16), 8373. https://doi.org/10.3390/ijms22168373









