Rootstocks Overexpressing StNPR1 and StDREB1 Improve Osmotic Stress Tolerance of Wild-Type Scion in Transgrafted Tobacco Plants
Abstract
:1. Introduction
2. Results
2.1. Expression Profiles of ORGs in the Homografted Potato Plants
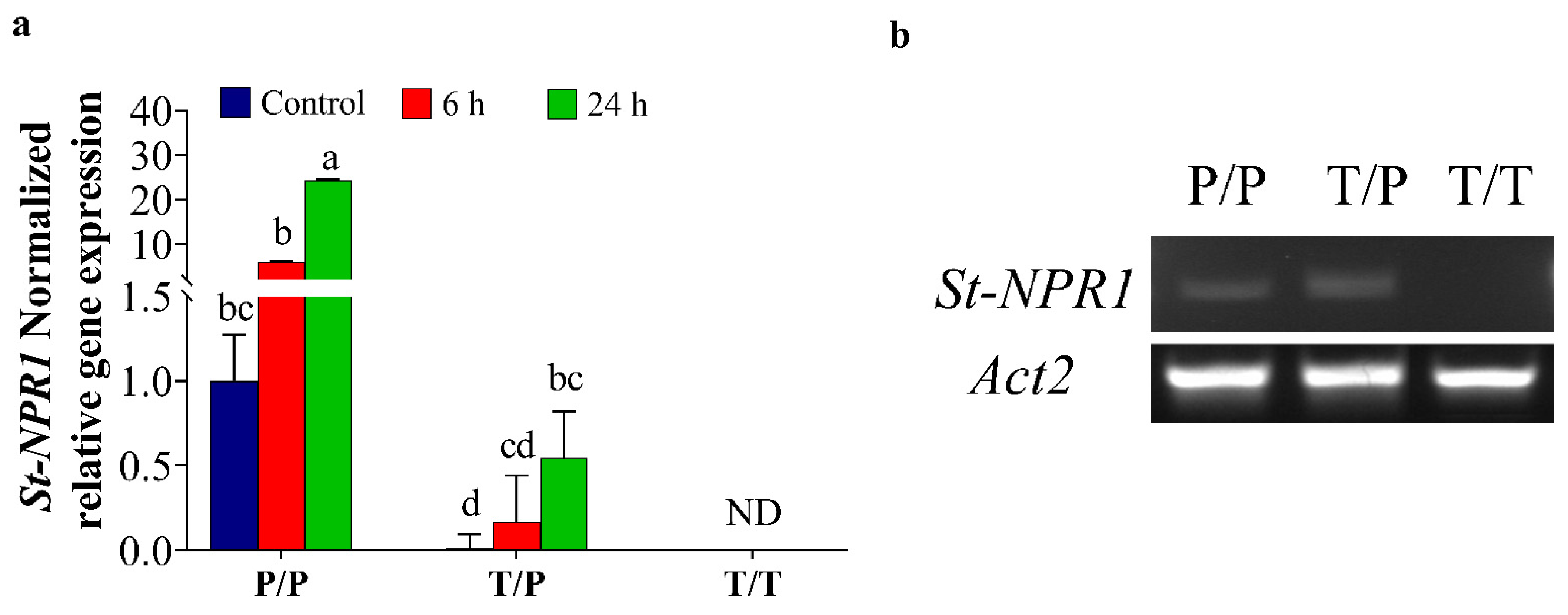
2.2. The Movement of ORGs Transcripts across the Graft Union under Osmotic Stress Conditions
2.3. Potato Rootstock Increased ABA Content in Tomato Scion
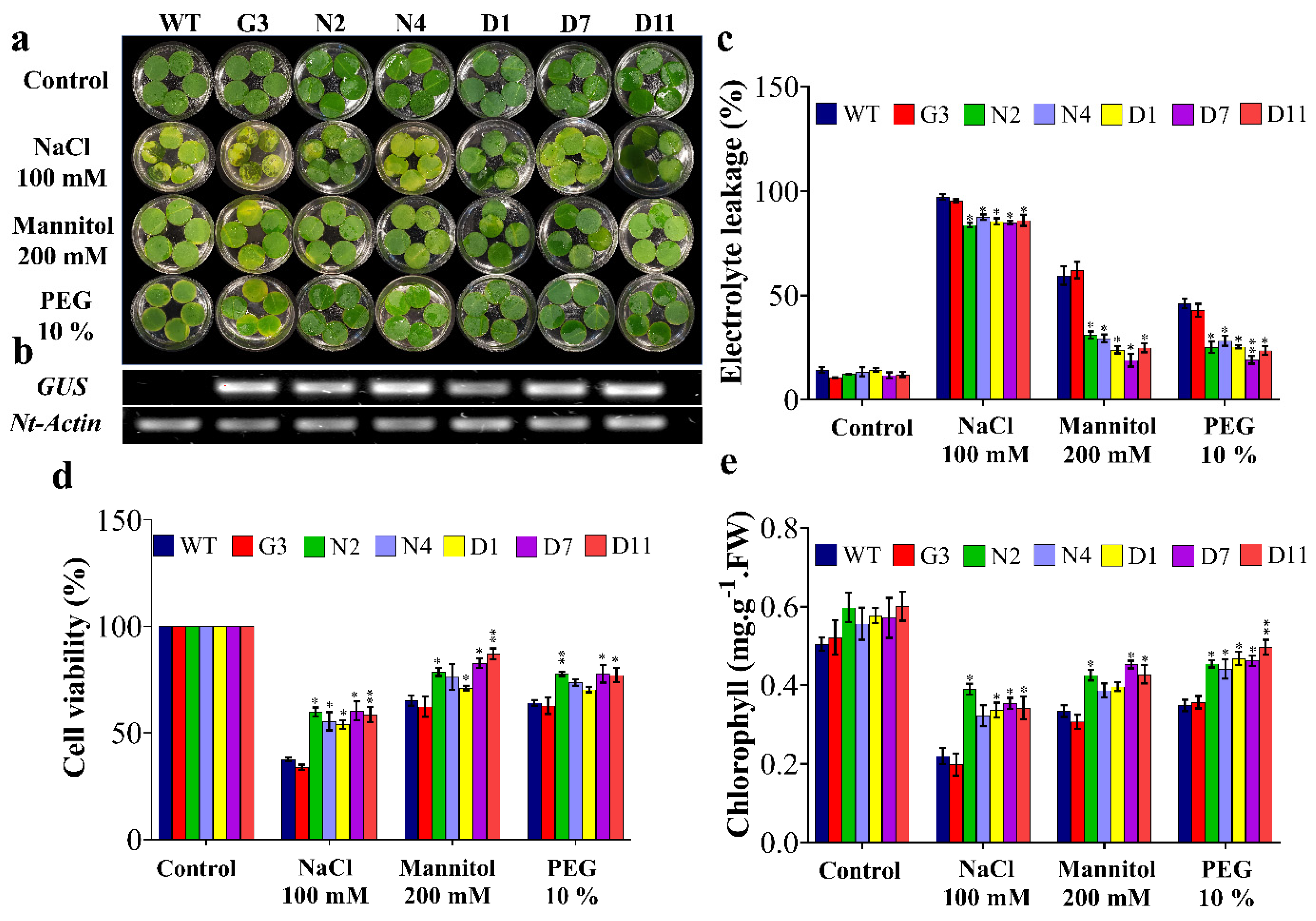
2.4. Molecular Analyses of Transgenic Tobacco Plants
2.5. Overexpression of StNPR1 and StDREB1 Confers Enhanced Growth under Stress Conditions
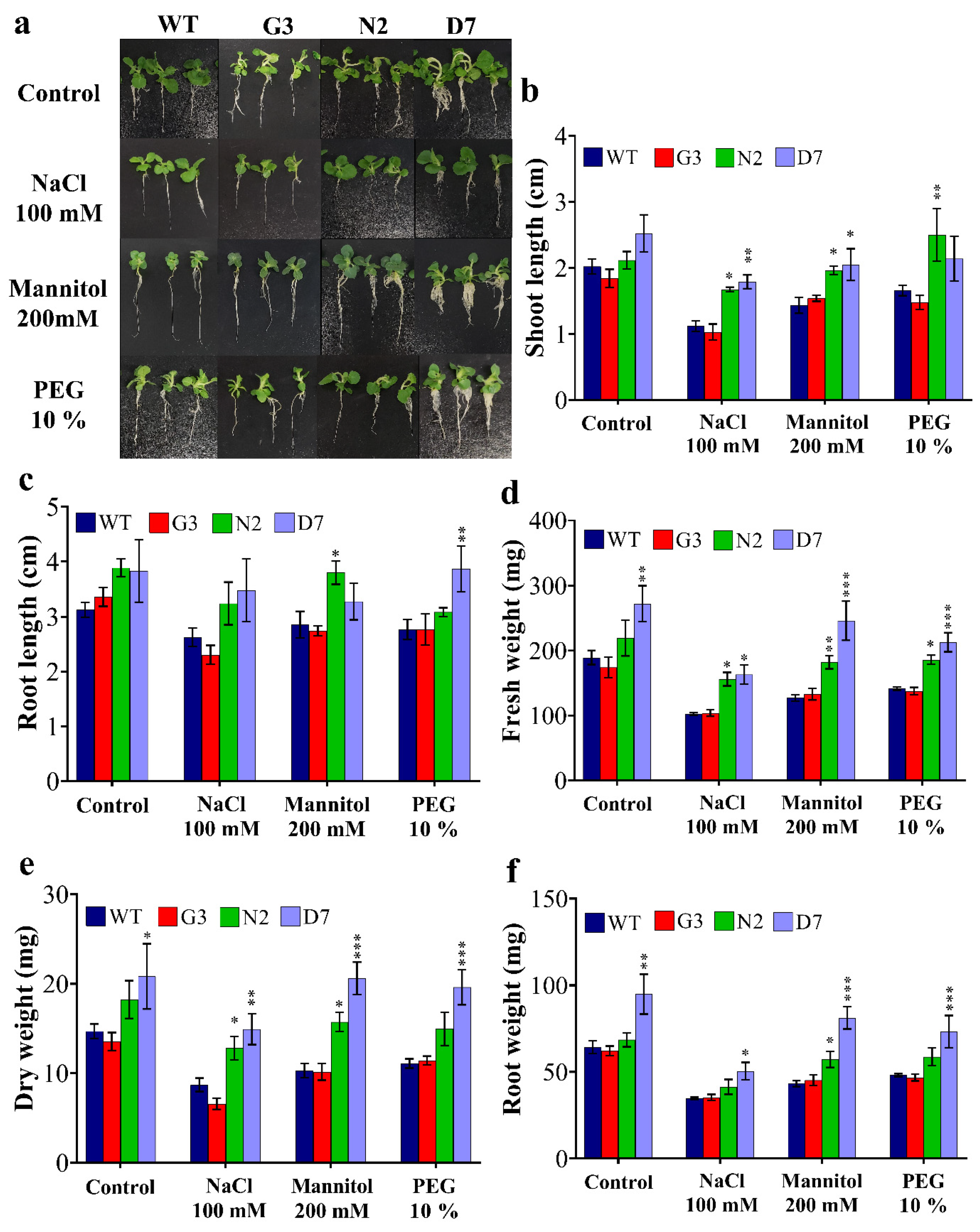
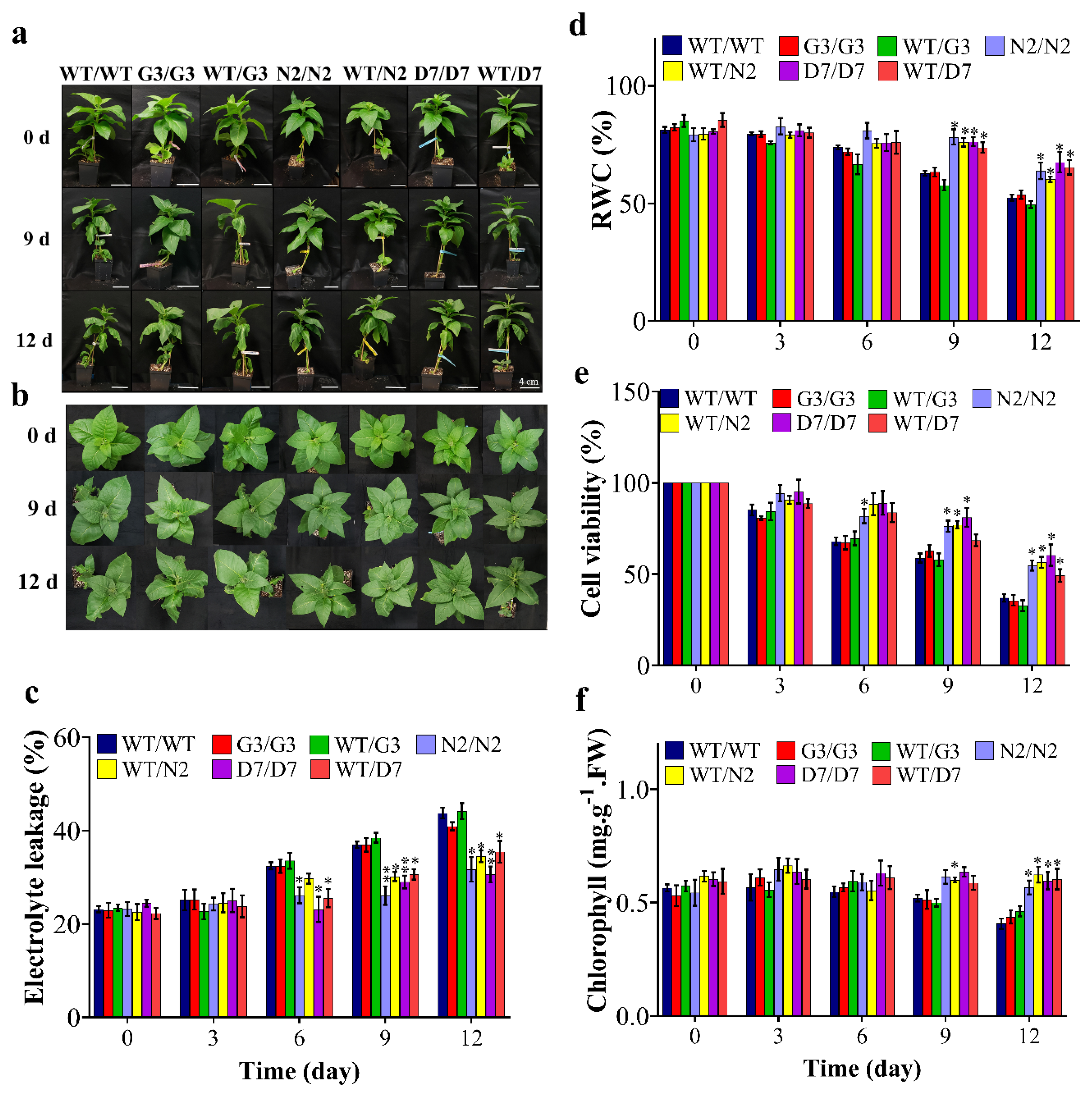
2.6. Transgenic Rootstocks Improved the Growth of WT Scion in Transgrafted Tobacco Plants
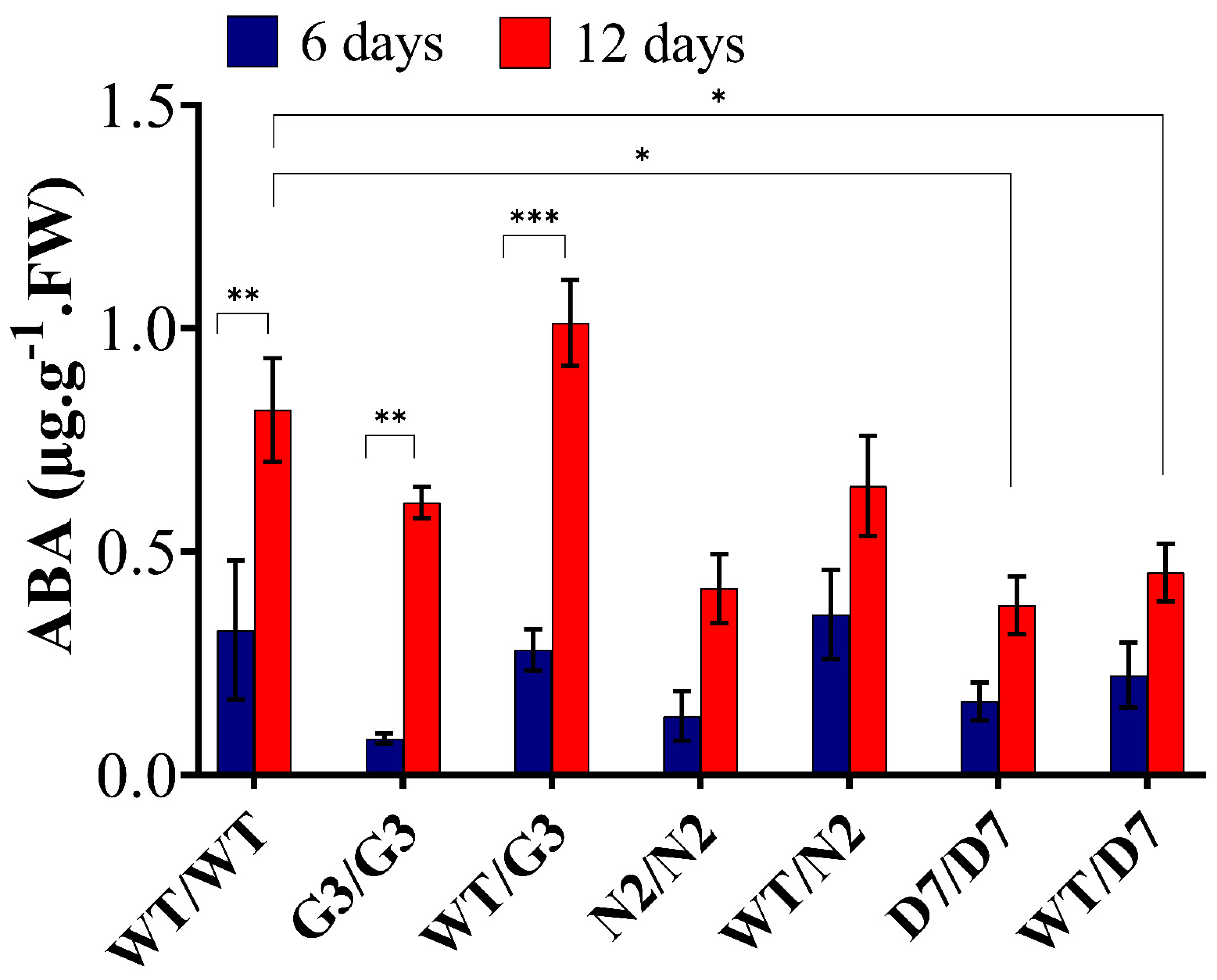
2.7. Evaluation of ABA Content in Transgrafted Tobacco Plants Growing under Osmotic Stress
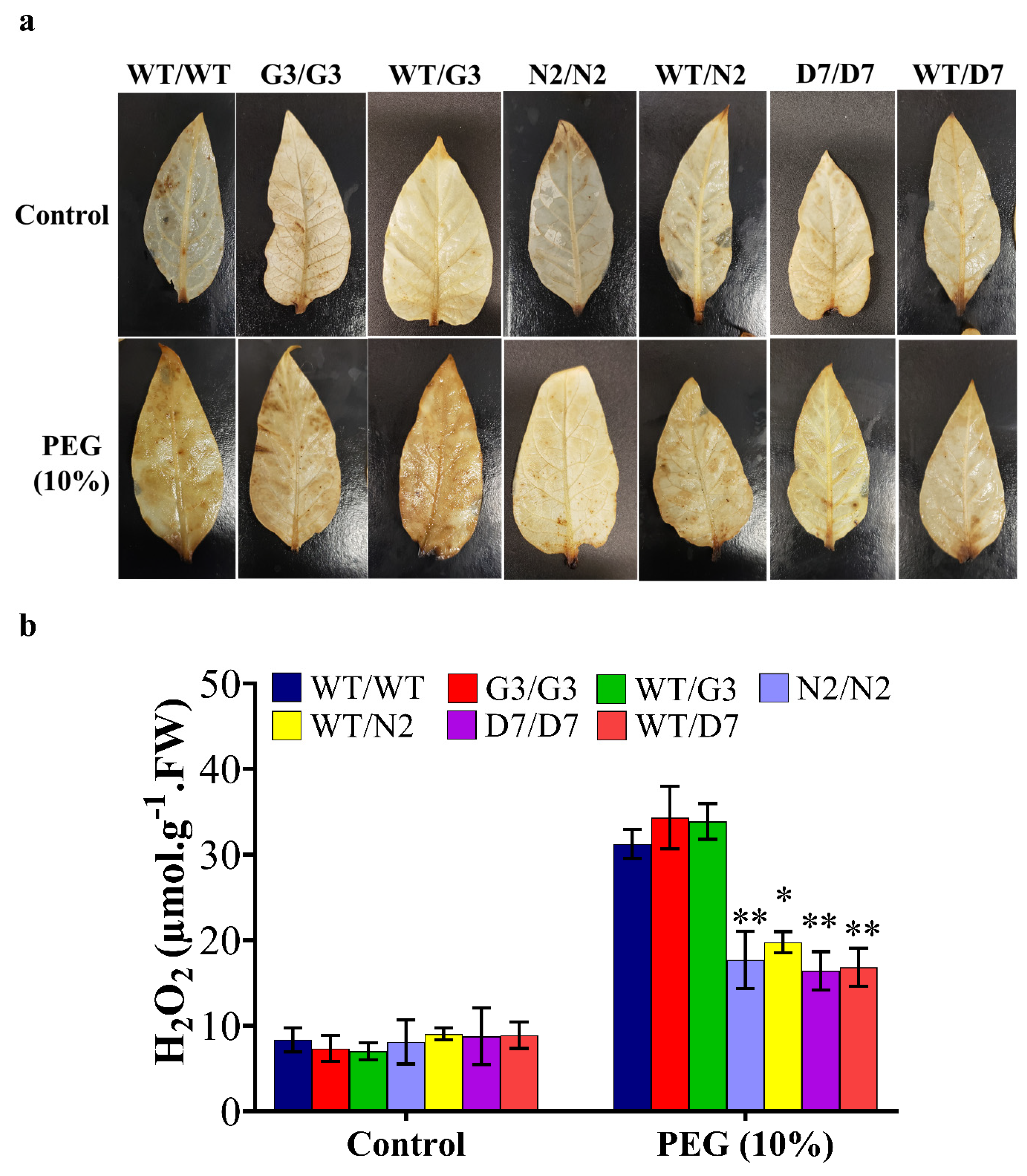
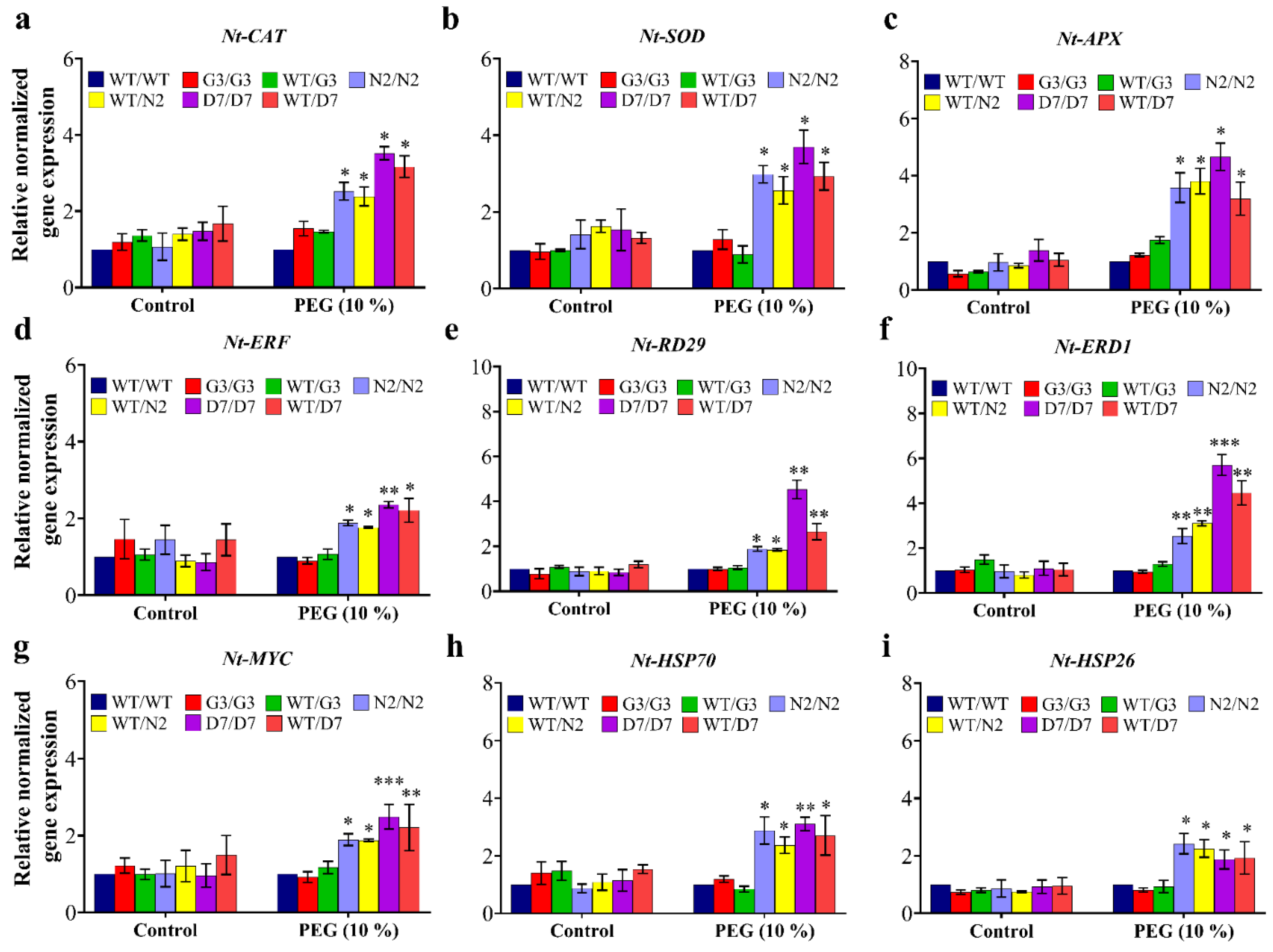
2.8. Evaluation of Osmotic-Responsive Gene Expression in Transgrafted Tobacco Plants Grown under Osmotic Stress
3. Discussion
3.1. Osmotic Stress Altered Expression Levels of Various ORG Genes
3.2. The Transport of NPR1 Transcripts through Graft-Union
3.3. Overexpression of StNPR1 and StDREB1 Improved Tobacco Tolerance to Osmotic Stress
3.4. The N2 and D7 Transgenic Rootstocks Improved the Growth Parameters of WT-Scions under Osmotic Stress
3.5. ABA Level in the Scion Is Rootstock Dependent
3.6. Transgenic Rootstocks Improve WT-Scion via Up-Regulation of Various ORGs
4. Conclusions
5. Materials and Methods
5.1. Grafting, Transgrafting and Growth Conditions
5.2. Plant Materials and Sample Collection for Analysis of mRNA Transport
5.3. Tobacco Transformation and Molecular Confirmation of Transgenics
5.4. Analyses of F1 and F2 Transgenic Plants Exposed to Abiotic Stresses
5.5. Stress Tolerance of Transgrafted Tobacco Plants
5.6. Measurement of Electrolyte Leakage, Cell Viability, Relative Water Content and Chlorophyll Content
5.7. In Situ Localization and Estimation of Hydrogen Peroxide (H2O2)
5.8. Extraction and Analysis of ABA
5.9. DNA and RNA Extraction and Expression Analysis of Abiotic Stress-Responsive Genes
5.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OS | Osmotic stress |
| ORGs | Osmotic-responsive genes |
| PEG | Polyethylene glycol |
| SA | Salicylic acid |
| ABA | Abscisic acid |
| HSP | Heat shock protein |
| NPR1 | non-expressor of pathogenesis-related (PR) gene 1 |
| DREB | Dehydration responsive element binding |
| WT | Wild type |
| CV | Cell viability |
| EL | Electrolyte leakage |
| RWC | Relative water content |
| ROS | Reactive oxygen species |
| H2O2 | Hydrogen peroxide |
References
- Gaupels, F.; Vlot, A.C. Plant Defense and Long-Distance Signaling in the Phloem. In Ploem: Molecular Cell Biology, Systemic Communication, Biotic Interactions; Thompson, G.A., van Bel, A.J.E., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 227–247. [Google Scholar]
- Notaguchi, M.; Okamoto, S. Dynamics of long-distance signaling via plant vascular tissues. Front. Plant Sci. 2015, 6, 161. [Google Scholar] [CrossRef] [Green Version]
- Shabala, S.; White, R.G.; Djordjevic, M.A.; Ruan, Y.; Mathesius, U. Root-to-shoot signalling: Integration of diverse molecules, pathways and functions. Funct. Plant Biol. 2016, 43, 87–104. [Google Scholar] [CrossRef] [Green Version]
- Anjan, K.; Suh, S.; Banerjee, A.K.; Chatterjee, M.; Yu, Y.; Suh, S.; Miller, W.A.; Hannapel, D.J. Dynamics of a Mobile RNA of Potato Involved in a Long-Distance Signaling Pathway. Plant Cell 2006, 18, 3443–3457. [Google Scholar] [CrossRef] [Green Version]
- Kudo, H.; Harada, T. A graft-transmissible RNA from tomato rootstock changes leaf morphology of potato scion. HortScience 2007, 42, 225–226. [Google Scholar] [CrossRef] [Green Version]
- Jaeger, K.E.; Wigge, P.A.; Lane, C. Report FT Protein Acts as a Long-Range Signal in Arabidopsis. Curr. Biol. 2007, 17, 1050–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turgeon, R.; Wolf, S. Phloem Transport: Cellular Pathways and Molecular Trafficking. Annu. Rev. Plant Biol. 2009, 60, 207–221. [Google Scholar] [CrossRef] [Green Version]
- Notaguchi, M.; Wolf, S.; Lucas, W.J. Phloem-Mobile Aux/IAA Transcripts Target to the Root Tip and Modify Root Architecture. J. Integr. Plant Biol. 2012, 54, 760–772. [Google Scholar] [CrossRef]
- Zhao, D.; Song, G.Q. Rootstock-to-scion transfer of transgene-derived small interfering RNAs and their effect on virus resistance in nontransgenic sweet cherry. Plant Biotechnol. J. 2014, 12, 1319–1328. [Google Scholar] [CrossRef] [Green Version]
- Spiegelman, Z.; Golan, G.; Wolf, S. Macromolecules Trafficking in the Phloem and Interorgan Communication. Signal. Commun. Plants 2013, 19, 275–290. [Google Scholar] [CrossRef]
- Kanehira, A.; Yamada, K.; Iwaya, T.; Ryo, T.; Kasai, A.; Mikio, N.; Harada, T. Apple phloem cells contain some mRNAs transported over long distances. Tree Genet. Genomes 2010, 6, 635–642. [Google Scholar] [CrossRef]
- Yang, Y.; Mao, L.; Jittayasothorn, Y.; Kang, Y.; Jiao, C.; Fei, Z. Messenger RNA exchange between scions and rootstocks in grafted grapevines. BMC Plant Biol. 2015, 15, 251. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; Zhang, W.; Huang, J.; Hao, L.; Wang, S.; Wang, A.; Meng, D.; Zhang, Q.; Chen, Q.; Li, T. PbWoxT1 mRNA from pear (Pyrus betulaefolia) undergoes long-distance transport assisted by a polypyrimidine tract binding protein. New Phytol. 2016, 210, 511–524. [Google Scholar] [CrossRef]
- Hannapel, D.J. A Model System of Development Regulated by the Long-distance Transport of mRNA. J. Integr. Plant Biol. 2010, 52, 40–52. [Google Scholar] [CrossRef]
- Hannapel, D.J.; Banerjee, A.K. Multiple mobile mRNA signals regulate tuber development in potato. Plants 2017, 6, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.K.; Sharma, P.; Butler, N.M.; Kang, I.; Shah, S.; Rao, A.G.; Hannapel, D.J. Polypyrimidine tract-binding proteins of potato mediate tuberization through an interaction with StBEL5 RNA. J. Exp. Bot. 2015, 66, 6835–6847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, L.; Zhu, J.K. Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ. 2002, 25, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Verslues, P.E.; Agarwal, M.; Katiyar-agarwal, S.; Zhu, J.; Zhu, J. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Cheong, M.S.; Yun, D. Salt-Stress Signaling. J. Plant Biol. 2007, 50, 148–155. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, R.K.; Lal, M.K.; Naga, K.C.; Kumar, R.; Chourasia, K.N.; Subhash, S.; Kumar, D.; Sharma, S. Emerging roles of melatonin in mitigating abiotic and biotic stresses of horticultural crops. Sci. Hortic. 2020, 272, 109592. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Lal, M.K.; Kumar, R.; Chourasia, K.N.; Naga, K.C.; Kumar, D.; Das, S.K.; Zinta, G. Mechanistic insights on melatonin-mediated drought stress mitigation in plants. Physiol. Plant. 2021, 172, 1212–1226. [Google Scholar] [CrossRef]
- Chourasia, K.N.; Lal, M.K.; Tiwari, R.K.; Dev, D.; Kardile, H.B.; Patil, V.U.; Kumar, A.; Vanishree, G.; Kumar, D.; Bhardwaj, V.; et al. Salinity Stress in Potato: Understanding Physiological, Biochemical and Molecular Responses. Life 2021, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Besseau, S.; Törönen, P.; Sipari, N.; Kollist, H.; Holm, L.; Palva, E.T. Defense-related transcription factors WRKY70 and WRKY54 modulate osmotic stress tolerance by regulating stomatal aperture in Arabidopsis. New Phytol. 2013, 200, 457–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajabpoor, S.; Kiani, S.; Sorkheh, K.; Tavakoli, F. Changes induced by osmotic stress in the morphology, biochemistry, physiology, anatomy and stomatal parameters of almond species (Prunus L. spp.) grown in vitro. J. For. Res. 2014, 25, 523–534. [Google Scholar] [CrossRef]
- Baldoni, E.; Genga, A.; Cominelli, E. Plant MYB transcription factors: Their role in drought response mechanisms. Int. J. Mol. Sci. 2015, 16, 15811–15851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.S.; Mizoi, J.; Yoshida, T.; Fujita, Y.; Nakajima, J.; Ohori, T.; Todaka, D.; Nakashima, K.; Hirayama, T.; Shinozaki, K.; et al. An ABRE promoter sequence is involved in osmotic stress-responsive expression of the DREB2A gene, which encodes a transcription factor regulating drought-inducible genes in Arabidopsis. Plant Cell Physiol. 2011, 52, 2136–2146. [Google Scholar] [CrossRef]
- Li, C.; Wei, Z.; Liang, D.; Zhou, S.; Li, Y.; Liu, C.; Ma, F. Enhanced salt resistance in apple plants overexpressing a Malus vacuolar Na+/H+ antiporter gene is associated with differences in stomatal behavior and photosynthesis. Plant Physiol. Biochem. 2013, 70, 164–173. [Google Scholar] [CrossRef]
- Dong, Q.; Zheng, W.; Duan, D.; Huang, D.; Wang, Q.; Liu, C.; Li, C.; Gong, X.; Li, C.; Mao, K.; et al. MdWRKY30, a group IIa WRKY gene from apple, confers tolerance to salinity and osmotic stresses in transgenic apple callus and Arabidopsis seedlings. Plant Sci. 2020, 299, 110611. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhao, L.; Zhao, S.; Wang, J.; Shi, H. Biochemical and transcriptomic analyses of drought stress responses of LY1306 tobacco strain. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Park, H.Y.; Seok, H.Y.; Park, B.K.; Kim, S.H.; Goh, C.H.; Lee, B.H.; Lee, C.H.; Moon, Y.H. Overexpression of Arabidopsis ZEP enhances tolerance to osmotic stress. Biochem. Biophys. Res. Commun. 2008, 375, 80–85. [Google Scholar] [CrossRef]
- Soares-Cavalcanti, N.M.; Belarmino, L.C.; Kido, E.A.; Wanderley-Nogueira, A.C.; Bezerra-Neto, J.P.; Cavalcanti-Lira, R.; Pandolfi, V.; Nepomuceno, A.L.; Abdelnoor, R.V.; Nascimento, L.C.; et al. In silico identification of known osmotic stress responsive genes from Arabidopsis in Soybean and Medicago. Genet. Mol. Biol. 2012, 35, 315–321. [Google Scholar] [CrossRef]
- Osakabe, Y.; Arinaga, N.; Umezawa, T.; Katsura, S.; Nagamachi, K.; Tanaka, H.; Ohiraki, H.; Yamada, K.; Seo, S.U.; Abo, M.; et al. Osmotic stress responses and plant growth controlled by potassium transporters in Arabidopsis. Plant Cell 2013, 25, 609–624. [Google Scholar] [CrossRef] [Green Version]
- Mahouachi, J.; Arbona, V.; Gomez-Cadenas, A. Hormonal changes in papaya seedlings subjected to progressive water stress and re-watering. Plant Growth Regul. 2007, 53, 43–51. [Google Scholar] [CrossRef]
- Marcińska, I.; Czyczyło-Mysza, I.; Skrzypek, E.; Grzesiak, M.T.; Janowiak, F.; Filek, M.; Dziurka, M.; Dziurka, K.; Waligórski, P.; Juzoń, K.; et al. Alleviation of osmotic stress effects by exogenous application of salicylic or abscisic acid on wheat seedlings. Int. J. Mol. Sci. 2013, 14, 13171–13193. [Google Scholar] [CrossRef] [Green Version]
- Schachtman, D.P.; Goodger, J.Q.D. Chemical root to shoot signaling under drought. Trends Plant Sci. 2008, 13, 281–287. [Google Scholar] [CrossRef]
- McAdam, S.A.M.; Brodribb, T.J.; Ross, J.J. Shoot-derived abscisic acid promotes root growth. Plant Cell Environ. 2016, 39, 652–659. [Google Scholar] [CrossRef]
- Dong, H.; Niu, Y.; Li, W.; Zhang, D. Effects of cotton rootstock on endogenous cytokinins and abscisic acid in xylem sap and leaves in relation to leaf senescence. J. Exp. Bot. 2008, 59, 1295–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; de Ollas, C.; Dodd, I.C. Long-distance ABA transport can mediate distal tissue responses by affecting local ABA concentrations. J. Integr. Plant Biol. 2018, 60, 16–33. [Google Scholar] [CrossRef] [Green Version]
- Louws, F.J.; Rivard, C.L.; Kubota, C. Grafting fruiting vegetables to manage soilborne pathogens, foliar pathogens, arthropods and weeds. Sci. Hortic. 2010, 127, 127–146. [Google Scholar] [CrossRef]
- Koepke, T.; Dhingra, A. Rootstock scion somatogenetic interactions in perennial composite plants. Plant Cell Rep. 2013, 32, 1321–1337. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.; Rouphael, Y.; Colla, G.; Henk, J. Grafting as a tool to improve tolerance of vegetables to abiotic stresses: Thermal stress, water stress and organic pollutants. Sci. Hortic. 2010, 127, 162–171. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Romero, L.; Ruiz, J.M. Role of Grafting in Resistance to Water Stress in Tomato Plants: Ammonia Production and Assimilation. J. Plant Growth Regul. 2013, 32, 831–842. [Google Scholar] [CrossRef]
- Smolka, A.; Li, X.-Y.; Heiklt, C.; Welander, M.; Zhu, L. Effects of transgenic rootstocks on growth and development of non-transgenic scion cultivars in apple. Transgen. Res. 2010, 19, 933–948. [Google Scholar] [CrossRef] [PubMed]
- Albacete, A.; Martínez-Andújar, C.; Martínez-Pérez, A.; Thompson, A.J.; Dodd, I.C.; Pérez-Alfocea, F. Unravelling rootstock×scion interactions to improve food security. J. Exp. Bot. 2015, 66, 2211–2226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artlip, T.S.; Wisniewski, M.E.; Arora, R.; Norelli, J.L. An apple rootstock overexpressing a peach CBF gene alters growth and flowering in the scion but does not impact cold hardiness or dormancy. Hortic. Res. 2016, 3, 16006. [Google Scholar] [CrossRef] [Green Version]
- Jeannine, K.; Piyum, A.; James, H. Cross-Species Translocation of mRNA from Host Plants into the Parasitic Plant Dodder1. Plant Physiol. 2007, 143, 1037–1043. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Zhang, W.; Li, M.; Harada, T.; Han, Z.; Li, T. Gibberellic acid insensitive mRNA transport in both directions between stock and scion in Malus. Tree Genet. Genomes 2010, 6, 1013–1019. [Google Scholar] [CrossRef]
- Xu, H.; Iwashiro, R.; Li, T.; Harada, T. Long-distance transport of Gibberellic Acid Insensitive mRNA in Nicotiana benthamiana. BMC Plant Biol. 2013, 13, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haroldsen, V.M.; Szczerba, M.W.; Aktas, H.; Lopez-Baltazar, J.; Odias, M.J.; Chi-ham, C.L.; Labavitch, J.M.; Bennett, A.B.; Powell, A.L.T. Mobility of transgenic nucleic acids and proteins within grafted rootstocks for agricultural improvement. Front. Plant Sci. 2012, 3, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, K.; Yamaguchi-Shinozaki, K. Regulons involved in osmotic stress-responsive and cold stress-responsive gene expression in plants. Physiol. Plant 2006, 126, 62–71. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Penfield, S. Temperature perception and signal transduction in plants. New Phytol. 2008, 179, 615–628. [Google Scholar] [CrossRef]
- Frank, G.; Pressman, E.; Ophir, R.; Althan, L.; Shaked, R.; Freedman, M.; Shen, S.; Firon, N. Transcriptional profiling of maturing tomato (Solanum lycopersicum L.) microspores reveals the involvement of heat shock proteins, ROS scavengers, hormones, and sugars in the heat stress response. J. Exp. Bot. 2009, 60, 3891–3908. [Google Scholar] [CrossRef] [PubMed]
- Aneja, B.; Yadav, N.R.; Kumar, N.; Yadav, R.C. HSP transcript induction is correlated with physiological changes under drought stress in Indian mustard. Physiol. Mol. Biol. Plants 2015, 21, 305–316. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Jaishankar, J.; Muthamilarasan, M.; Shweta, S.; Dangi, A.; Prasad, M. Genome-wide analysis of heat shock proteins in C4 model, foxtail millet identifies potential candidates for crop improvement under abiotic stress. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Sun, C.; Li, Z.; Hu, Q.; Han, L.; Luo, H. AsHSP17, A creeping bentgrass small heat shock protein modulates plant photosynthesis and ABA-dependent and independent signalling to attenuate plant response to abiotic stress. Plant Cell Environ. 2016, 39, 1320–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, F.; Qin, C.; Gao, J.; Liu, M.; Luo, X.; Zhang, W.; Liu, H.; Liao, X.; Shen, Y.; Mao, L.; et al. Genome-Wide Identification and Analysis of Drought-Responsive Genes and MicroRNAs in Tobacco. Int. J. Mol. Sci. 2015, 16, 5714–5740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakashima, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front. Plant Sci. 2014, 5, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, H.; Zhang, S.; Ruan, M.; Wang, Y.; Wang, C. Analysis and application of RD29 genes in abiotic stress response. Acta Physiol. Plant. 2012, 34, 1239–1250. [Google Scholar] [CrossRef]
- Kasuga, M.; Liu, Q.; Miura, S.; Yamaguchi-shinozaki, K.; Shinozaki, K. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat. Biotechnol. 1999, 17, 287–291. [Google Scholar] [CrossRef]
- Maruyama, K.; Sakuma, Y.; Kasuga, M.; Ito, Y.; Seki, M.; Goda, H.; Shimada, Y.; Yoshida, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Identification of cold-inducible downstream genes of the Arabidopsis DREB1A/CBF3 transcriptional factor using two microarray systems. Plant J. 2004, 38, 982–993. [Google Scholar] [CrossRef]
- Rehman, S.; Mahmood, T. Functional role of DREB and ERF transcription factors: Regulating stress-responsive network in plants. Acta Physiol. Plant. 2015, 37, 178. [Google Scholar] [CrossRef]
- Chen, H.; Liu, L.; Wang, L.; Wang, S.; Cheng, X. VrDREB2A, a DREB—Binding transcription factor from Vigna radiata, increased drought and high-salt tolerance in transgenic Arabidopsis thaliana. J. Plant Res. 2016, 129, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Han, Y.; Feng, Y.; Xing, S.; Zhao, M.; Chen, Y.; Wang, W. Expression of wheat expansin driven by the RD29 promoter in tobacco confers water-stress tolerance without impacting growth and development. J. Biotechnol. 2013, 163, 281–291. [Google Scholar] [CrossRef]
- Kiyosue, T.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Cloning of cDNAs for genes that are early-responsive to dehydration stress (ERDs) in Arabidopsis thaliana L.: Identification of three ERDs as HSP cognate genes. Plant Mol. Biol. 1994, 25, 791–798. [Google Scholar] [CrossRef]
- Kimura, M.; Yamamoto, Y.Y.; Seki, M.; Sakurai, T.; Sato, M.; Abe, T.; Yoshida, S.; Manabe, K.; Shinozaki, K.; Matsui, M. Rapid Communication Identification of Arabidopsis Genes Regulated by High Light-Stress Using cDNA Microarray. Photochem. Photobiol. 2003, 77, 226–233. [Google Scholar]
- Munné-Bosch, S.; Alegre, L. Die and let live: Leaf senescence contributes to plant survival under drought stress. Funct. Plant Biol. 2004, 31, 203–216. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Agarwal, P.; Reddy, M.K.; Sopory, S.K. Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Rep. 2006, 25, 1263–1274. [Google Scholar] [CrossRef]
- Li, R.; Liu, C.; Zhao, R.; Wang, L.; Chen, L.; Yu, W.; Zhang, S.; Sheng, J.; Shen, L. CRISPR/Cas9-Mediated SlNPR1 mutagenesis reduces tomato plant drought tolerance. BMC Plant Biol. 2019, 19, 38. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zhang, D.; Chu, J.Y.; Boyle, P.; Wang, Y.; Brindle, I.D.; De Luca, V.; Després, C. The Arabidopsis NPR1 Protein Is a Receptor for the Plant Defense Hormone Salicylic Acid. Cell Rep. 2012, 1, 639–647. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Cominelli, E.; Bailey, P.; Parr, A.; Mehrtens, F.; Jones, J.; Tonelli, C.; Weisshaar, B.; Martin, C. Transcriptional repression by AtMYB4 controls production of UV-protecting sunscreens in Arabidopsis. EMBO J. 2000, 19, 6150–6161. [Google Scholar] [CrossRef] [Green Version]
- Storozhenko, S.; De Pauw, P.; Van Montagu, M.; Inze, D.; Kushnir, S. The Heat-Shock Element Is a Functional Component of the Arabidopsis APX1 Gene Promoter 1. Plant Physiol. 1998, 118, 1005–1014. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef]
- Sakamoto, H.; Maruyama, K.; Sakuma, Y.; Meshi, T.; Iwabuchi, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis Cys2/His2-Type Zinc-Finger Proteins Function as Transcription Repressors under Drought. Plant Physiol. 2004, 136, 2734–2746. [Google Scholar] [CrossRef] [Green Version]
- Bowles, D.; Lim, E.; Poppenberger, B.; Vaistij, E. Glycosyltransferases of Lipophilic Small Molecules. Annu. Rev. Plant Biol. 2006, 57, 568–597. [Google Scholar] [CrossRef]
- Shi, H.; Liu, W.; Yao, Y.; Wei, Y.; Chan, Z. Alcohol dehydrogenase 1 (ADH1) confers both abiotic and biotic stress resistance in Arabidopsis. Plant Sci. 2017, 262, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Vogt, T. Glycosyltransferases in secondary plant metabolism: Tranquilizers and stimulant controllers. Planta 2001, 213, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Bowles, D.; Isayenkova, J.; Lim, E. Glycosyltransferases: Managers of small molecules. Curr. Opin. Plant Biol. 2005, 8, 254–263. [Google Scholar] [CrossRef]
- Sun, X.P.; Yan, H.L.; Kang, X.Y.; Ma, F.W. Growth, gas exchange, and water-use efficiency response of two young apple cultivars to drought stress in two scion-one rootstock grafting system. Photosynthetica 2013, 51, 404–410. [Google Scholar] [CrossRef]
- Abe, H.; Yamaguchi-shinozaki, K.; Urao, T.; Hosokawa, C.D. Role of Arabidopsis MYC and MYB Homologs in Drought- and Abscisic Acid-Regulated Gene Expression. Plant Cell 1997, 9, 1859–1869. [Google Scholar]
- Harada, T. Grafting and RNA transport via phloem tissue in horticultural plants. Sci. Hortic. 2010, 125, 545–550. [Google Scholar] [CrossRef]
- Zhang, W.N.; Duan, X.W.; Ma, C.; Harada, T.; Li, T.Z. Transport of mRNA molecules coding NAC domain protein in grafted pear and transgenic tobacco. Biol. Plant. 2013, 57, 224–230. [Google Scholar] [CrossRef]
- Thieme, C.J.; Rojas-Triana, M.; Stecyk, E.; Schudoma, C.; Zhang, W.; Yang, L.; Miñambres, M.; Walther, D.; Schulze, W.X.; Paz-Ares, J.; et al. Endogenous Arabidopsis messenger RNAs transported to distant tissues. Nat. Plants 2015, 1, 15025. [Google Scholar] [CrossRef]
- Notaguchi, M.; Higashiyama, T.; Suzuki, T. Identification of mRNAs that move over long distances using an RNA-Seq analysis of Arabidopsis/Nicotiana benthamiana heterografts. Plant Cell Physiol. 2015, 56, 311–321. [Google Scholar] [CrossRef] [Green Version]
- Pallas, V.; Gómez, G. Phloem RNA-binding proteins as potential components of the long-distance RNA transport system. Front. Plant Sci. 2013, 4, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, A.K.; Lin, T.; Hannapel, D.J. Untranslated Regions of a Mobile Transcript Mediate. Plant Physiol. 2009, 151, 1831–1843. [Google Scholar] [CrossRef] [Green Version]
- Hannapel, D.J. Long-Distance Systemic Signaling and Communication in Plants; Long-Distance Signaling via Mobile RNAs; Springer Nature Switzerland AG: Cham, Switzerland, 2013; pp. 53–70. ISBN 9783642364709. [Google Scholar]
- Cao, H.; Glazebrook, J.; Clarke, J.D.; Volko, S.; Dong, X. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 1997, 88, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, M.; Ishikawa, A.; Jikumaru, Y.; Seki, M.; Umezawa, T.; Asami, T.; Maruyama-Nakashita, A.; Kudo, T.; Shinozaki, K.; Yoshida, S.; et al. Antagonistic interaction between systemic acquired resistance and the abscisic acid-mediated abiotic stress response in Arabidopsis. Plant Cell 2008, 20, 1678–1692. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Sun, T.; Ao, K.; Peng, Y.; Zhang, Y.; Li, X.; Zhang, Y. Opposite Roles of Salicylic Acid Receptors NPR1 and NPR3/NPR4 in Transcriptional Regulation of Plant Immunity. Cell 2018, 173, 1454–1467.e10. [Google Scholar] [CrossRef]
- Pajerowska-Mukhtar, K.M.; Emerine, D.K.; Mukhtar, M.S. Tell me more: Roles of NPRs in plant immunity. Trends Plant Sci. 2013, 18, 402–411. [Google Scholar] [CrossRef]
- Sharma, V.; Goel, P.; Kumar, S.; Singh, A.K. An apple transcription factor, MdDREB76, confers salt and drought tolerance in transgenic tobacco by activating the expression of stress-responsive genes. Plant Cell Rep. 2019, 38, 221–241. [Google Scholar] [CrossRef]
- Singh, V.K.; Mishra, A.; Haque, I.; Jha, B. A novel transcription factor-like gene SbSDR1 acts as a molecular switch and confers salt and osmotic endurance to transgenic tobacco. Sci. Rep. 2016, 6, 31686. [Google Scholar] [CrossRef] [Green Version]
- Bouaziz, D.; Pirrello, J.; Charfeddine, M.; Hammami, A.; Jbir, R.; Dhieb, A.; Bouzayen, M.; Gargouri-bouzid, R. Overexpression of StDREB1 Transcription Factor Increases Tolerance to Salt in Transgenic Potato Plants. Mol Biotechnol 2013, 54, 803–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, A.; Jha, B. Overexpression of a Cytosolic Abiotic Stress Responsive Universal Stress Protein (Sb USP) Mitigates Salt and Osmotic Stress in Transgenic Tobacco Plants. Front. Plant Sci. 2016, 7, 518. [Google Scholar] [CrossRef] [Green Version]
- Jayakannan, M.; Bose, J.; Babourina, O.; Shabala, S.; Massart, A.; Poschenrieder, C.; Rengel, Z. The NPR1-dependent salicylic acid signalling pathway is pivotal for enhanced salt and oxidative stress tolerance in Arabidopsis. J. Exp. Bot. 2015, 66, 1865–1875. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Li, X.; Zhang, D.; Gao, B.; Yang, H.; Wang, Y.; Guan, K.; Wood, A.J. Plant Physiology and Biochemistry ScDREB8, a novel A-5 type of DREB gene in the desert moss Syntrichia caninervis, confers salt tolerance to Arabidopsis. Plant Physiol. Biochem. 2017, 120, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gupta, A.; Soni, D.; Garg, R.; Pathre, U. V Ectopic expression of a tomato DREB gene affects several ABA processes and in fluences plant growth and root architecture in an age-dependent manner. J. Plant Physiol. 2017, 214, 97–107. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Taiz, L.; Zeiger, E. Plant Physiology, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 2003; ISBN 0878938230. [Google Scholar]
- Van Breusegem, F.; Dat, J.F. Reactive Oxygen Species in Plant Cell Death 1. Plant Physiol. 2006, 141, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Soar, C.J.; Dry, P.R.; Loveys, B.R. Scion photosynthesis and leaf gas exchange in Vitis vinifera L. cv. Shiraz: Mediation of rootstock effects via xylem sap ABA. Aust. J. Grapes Wine Res. 2006, 12, 82–96. [Google Scholar] [CrossRef]
- Ke, Y.G.; Yang, Z.J.; Yu, S.W.; Li, T.F.; Wu, J.H.; Gao, H.; Fu, Y.P.; Luo, L.J. Characterization of OsDREB6 responsive to osmotic and cold stresses in rice. J. Plant Biol. 2014, 57, 150–161. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, Y.; Lan, W.; Zhou, Q.; Li, F.; Chen, F.; Bao, M.; Liu, G. LlDREB1G, a novel DREB subfamily gene from Lilium longiflorum, can enhance transgenic Arabidopsis tolerance to multiple abiotic stresses. Plant Cell Tissue Organ Cult. 2019, 138, 489–506. [Google Scholar] [CrossRef]
- Mehrotra, R.; Bhalothia, P.; Bansal, P.; Basantani, M.K.; Bharti, V.; Mehrotra, S. Abscisic acid and abiotic stress tolerance—Different tiers of regulation. J. Plant Physiol. 2014, 171, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Timperio, A.M.; Egidi, M.G.; Zolla, L. Proteomics applied on plant abiotic stresses: Role of heat shock proteins (HSP). J. Proteom. 2008, 71, 391–411. [Google Scholar] [CrossRef]
- Jumali, S.S.; Said, I.M.; Ismail, I.; Zainal, Z. Genes induced by high concentration of salicylic acid in Mitragyna speciosa. Aust. J. Crop Sci. 2011, 5, 296–303. [Google Scholar]
- Olate, E.; Jiménez-Gómez, J.M.; Holuigue, L.; Salinas, J. Acclimation by interacting with HSFA1 factors. Nat. Plants 2018, 4, 811–823. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Expósito-Rodríguez, M.; Borges, A.A.; Borges-Pérez, A.; Pérez, J.A. Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. BMC Plant Biol. 2008, 8, 131. [Google Scholar] [CrossRef] [Green Version]
- Horsch, R.B.; Fraley, R.T.; Rogers, S.G.; Sanders, P.R.; Lloyd, A.; Hoffmann, N. Inheritance of functional foreign genes in plants. Am. Assoc. Adv. Sci. 1984, 223, 496–498. [Google Scholar] [CrossRef] [PubMed]
- Horsch, A.R.B.; Fry, J.E.; Hoffman, N.L.; Eichholtz, D.; Rogers, S.G.; Fraley, R.T. A simple and general method for transferring genes into plants. Am. Assoc. Adv. Sci. 1985, 227, 1229. [Google Scholar]
- Singla-Pareek, S.L.; Reddy, M.K.; Sopory, S.K. Genetic engineering of the glyoxalase pathway in tobacco leads to enhanced salinity tolerance. Proc. Natl. Acad. Sci. USA 2003, 100, 14672–14677. [Google Scholar] [CrossRef] [Green Version]
- Bajji, M.; Kinet, J.M.; Lutts, S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 2002, 36, 61–70. [Google Scholar] [CrossRef]
- Hema, R.; Vemanna, R.S.; Sreeramulu, S.; Reddy, C.P.; Senthil-Kumar, M.; Udayakumar, M. Stable expression of mtlD gene imparts multiple stress tolerance in finger millet. PLoS ONE 2014, 9, e99110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtenthaler, H. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Daudi, A.; O’Brien, J.A. Detection of Hydrogen Peroxide by DAB Staining in Arabidopsis Leaves. Bio-Protocol 2012, 2, e263. [Google Scholar] [CrossRef] [Green Version]
- Patterson, B.D.; MacRae, E.A.; Ferguson, I.B. Estimation of hydrogen peroxide in plant extracts using titanium(IV). Anal. Biochem. 1984, 139, 487–492. [Google Scholar] [CrossRef]
- Ayyanath, M.M.; Shukla, M.R.; Saxena, P.K. Role of water percolation in reproductive physiology of hazelnut (Corylus spp.). Environ. Exp. Bot. 2021, 182, 104278. [Google Scholar] [CrossRef]
- Erland, L.A.E.; Shukla, M.R.; Glover, W.B.; Saxena, P.K. A simple and efficient method for analysis of plant growth regulators: A new tool in the chest to combat recalcitrance in plant tissue culture. Plant Cell. Tissue Organ Cult. 2017, 131, 459–470. [Google Scholar] [CrossRef]
- Gasic, K.; Hernandez, A.; Korban, S.S. RNA Extraction From Different Apple Tissues Rich in Polyphenols and Polysaccharides for cDNA. Plant Mol. Biol. Report. 2004, 22, 437a–437g. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hezema, Y.S.; Shukla, M.R.; Goel, A.; Ayyanath, M.M.; Sherif, S.M.; Saxena, P.K. Rootstocks Overexpressing StNPR1 and StDREB1 Improve Osmotic Stress Tolerance of Wild-Type Scion in Transgrafted Tobacco Plants. Int. J. Mol. Sci. 2021, 22, 8398. https://doi.org/10.3390/ijms22168398
Hezema YS, Shukla MR, Goel A, Ayyanath MM, Sherif SM, Saxena PK. Rootstocks Overexpressing StNPR1 and StDREB1 Improve Osmotic Stress Tolerance of Wild-Type Scion in Transgrafted Tobacco Plants. International Journal of Molecular Sciences. 2021; 22(16):8398. https://doi.org/10.3390/ijms22168398
Chicago/Turabian StyleHezema, Yasmine S., Mukund R. Shukla, Alok Goel, Murali M. Ayyanath, Sherif M. Sherif, and Praveen K. Saxena. 2021. "Rootstocks Overexpressing StNPR1 and StDREB1 Improve Osmotic Stress Tolerance of Wild-Type Scion in Transgrafted Tobacco Plants" International Journal of Molecular Sciences 22, no. 16: 8398. https://doi.org/10.3390/ijms22168398
APA StyleHezema, Y. S., Shukla, M. R., Goel, A., Ayyanath, M. M., Sherif, S. M., & Saxena, P. K. (2021). Rootstocks Overexpressing StNPR1 and StDREB1 Improve Osmotic Stress Tolerance of Wild-Type Scion in Transgrafted Tobacco Plants. International Journal of Molecular Sciences, 22(16), 8398. https://doi.org/10.3390/ijms22168398






