MAO-A Inhibition by Metaxalone Reverts IL-1β-Induced Inflammatory Phenotype in Microglial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Treatment
2.2. MTT Assay
2.3. Real-Time Quantitative PCR Amplification (RTqPCR)
2.4. Measurements of Cytokines by Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. MAO-A Activity Assay
2.6. Malondialdehyde Assay
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
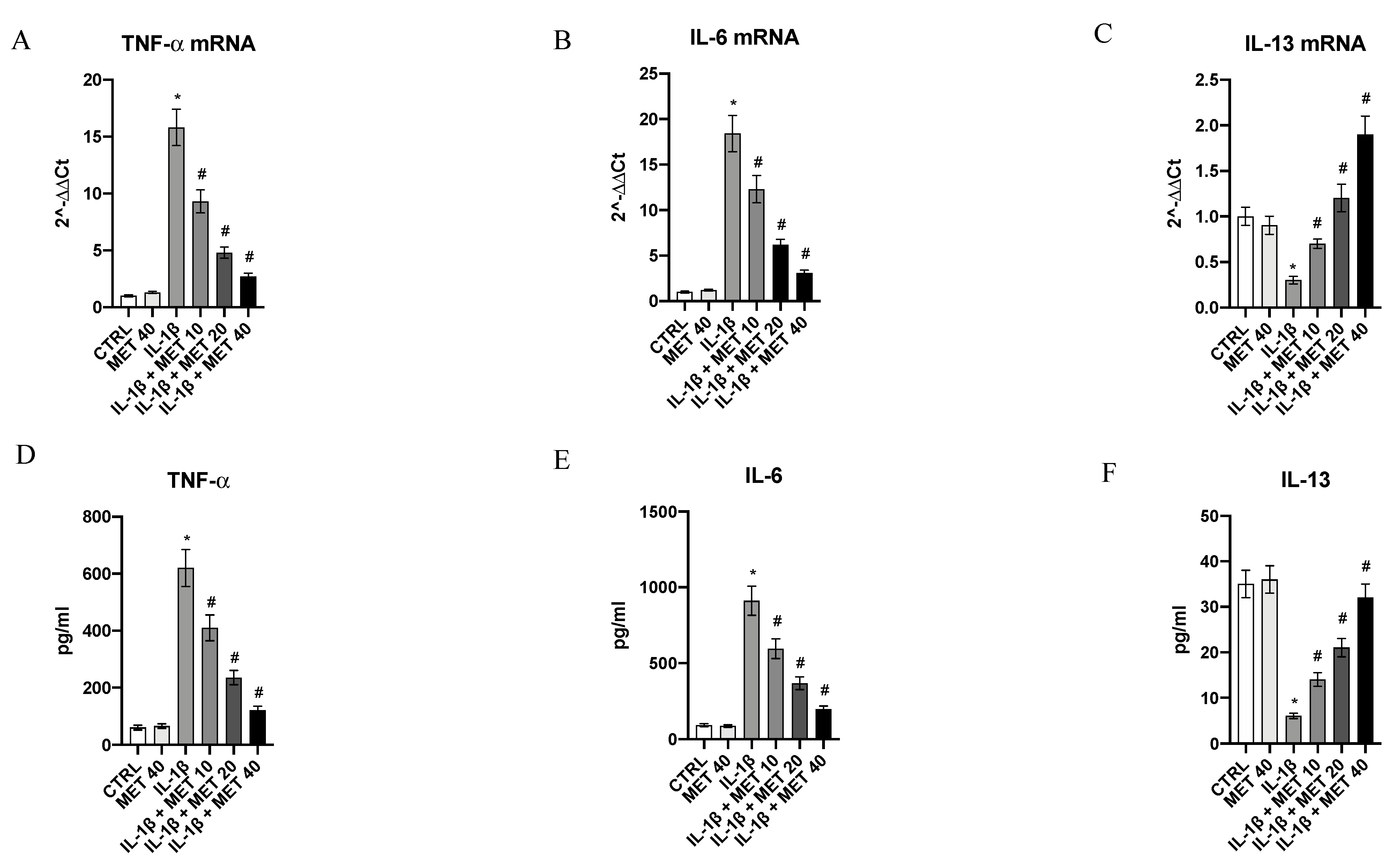
3.1. Metaxalone Reverts the Inflammatory Phenotype Induced by IL-1β in HMC3 Microglial Cells
3.2. Effects of Metaxalone on Oxidative Stress
3.3. Metaxalone Targets Upstream Signals That Trigger the Inflammatory Phenotype
3.4. Metaxalone Reduces the Augmented MAO-A Expression and Activity Induced by IL-1β in Microglia Cells
3.5. Effect of Metaxalone on HMC3 Microglial Cells Viability
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Clauw, D.J. Fibromyalgia: A clinical review. JAMA 2014, 311, 1547–1555. [Google Scholar] [CrossRef]
- Vincent, A.; Lahr, B.D.; Wolfe, F.; Clauw, D.J.; Whipple, M.O.; Oh, T.H.; Barton, D.L.; St Sauver, J. Prevalence of fibromyalgia: A population-based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology Project. Arthritis Care Res. 2013, 65, 786–792. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, D.S.; MacKie, P.J.; Kareken, D.A.; Hutchins, G.D.; Chumin, E.J.; Christian, B.T.; Yoder, K.K. Differential dopamine function in fibromyalgia. Brain Imaging Behav. 2016, 10, 829–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oaklander, A.L.; Herzog, Z.D.; Downs, H.M.; Klein, M.M. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain 2013, 154, 2310–2316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Littlejohn, G.; Guymer, E. Neurogenic inflammation in fibromyalgia. Semin. Immunopathol. 2018, 40, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Vaeroy, H.; Helle, R.; Forre, O.; Kass, E.; Terenius, L. Elevated CSF levels of substance P and high incidence of Raynaud phenomenon in patients with fibromyalgia: New features for diagnosis. Pain 1988, 32, 21–26. [Google Scholar] [CrossRef]
- Russell, I.J.; Orr, M.D.; Littman, B.; Vipraio, G.A.; Alboukrek, D.; Michalek, J.E.; Lopez, Y.; MacKillip, F. Elevated cerebrospinal fluid levels of substance P in patients with the fibromyalgia syndrome. Arthritis Rheum. 1994, 37, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pinto, I.; Agmon-Levin, N.; Howard, A.; Shoenfeld, Y. Fibromyalgia and cytokines. Immunol. Lett. 2014, 161, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Generaal, E.; Vogelzangs, N.; Macfarlane, G.J.; Geenen, R.; Smit, J.H.; Dekker, J.; Penninx, B.W. Basal inflammation and innate immune response in chronic multisite musculoskeletal pain. Pain 2014, 155, 1605–1612. [Google Scholar] [CrossRef]
- Uceyler, N.; Hauser, W.; Sommer, C. Systematic review with meta-analysis: Cytokines in fibromyalgia syndrome. BMC Musculoskelet Disord. 2011, 12, 245. [Google Scholar] [CrossRef] [Green Version]
- Mendieta, D.; De la Cruz-Aguilera, D.L.; Barrera-Villalpando, M.I.; Becerril-Villanueva, E.; Arreola, R.; Hernandez-Ferreira, E.; PerezTapia, S.M.; Perez-Sanchez, G.; Garces-Alvarez, M.E.; Aguirre-Cruz, L.; et al. IL-8 and IL-6 primarily mediate the inflammatory response in fibromyalgia patients. J. Neuroimmunol. 2016, 290, 22–25. [Google Scholar] [CrossRef]
- Gota, C.E. What you can do for your fibromyalgia patient. Cleve. Clin. J. Med. 2018, 85, 367–376. [Google Scholar] [CrossRef]
- Whiteside, N.; Sarmanova, A.; Chen, X.; Zou, K.; Abdullah, N.; Doherty, M.; Zhang, W. Proportion of contextual effects in the treatment of fibromyalgia-a meta-analysis of randomised controlled trials. Clin. Rheumatol. 2018, 37, 1375–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, B.L.; Whittle, S.L.; Buchbinder, R. Muscle relaxants for pain management in rheumatoid arthritis. Cochrane Database Syst. Rev. 2012, 1, CD008922. [Google Scholar] [CrossRef]
- Bosak, A.R.; Skolnik, A.B. Serotonin syndrome associated with metaxalone overdose. J. Med. Toxicol. 2014, 10, 402–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherrington, B.; Englich, U.; Niruntari, S.; Grant, W.; Hodgman, M. Monoamine oxidase A inhibition by toxic concentrations of metaxalone. Clin. Toxicol. 2020, 58, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Deshwal, S.; Forkink, M.; Hu, C.H.; Buonincontri, G.; Antonucci, S.; Di Sante, M.; Murphy, M.P.; Paolocci, N.; Mochly-Rosen, D.; Krieg, T.; et al. Monoamine oxidase-dependent endoplasmic reticulum-mitochondria dysfunction and mast cell degranulation lead to adverse cardiac remodeling in diabetes. Cell Death Differ. 2018, 25, 1671–1685. [Google Scholar] [CrossRef] [Green Version]
- Rațiu, C.; Uțu, D.; Petruș, A.; Norbert, P.; Olariu, S.; Duicu, O.; Sturza, A.; Muntean, D.M. Monoamine oxidase inhibition improves vascular function and reduces oxidative stress in rats with lipopolysaccharide-induced inflammation. Gen. Physiol. Biophys. 2018, 37, 687–694. [Google Scholar] [CrossRef]
- Ekuni, D.; Firth, J.D.; Nayer, T.; Tomofuji, T.; Sanbe, T.; Irie, K.; Yamamoto, T.; Oka, T.; Liu, Z.; Vielkind, J.; et al. Lipopolysaccharide-induced epithelial monoamine oxidase mediates alveolar bone loss in a rat chronic wound model. Am. J. Pathol. 2009, 175, 1398–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuohelainen, V.; Hämäläinen, M.; Paavonen, T.; Karlsson, S.; Moilanen, E.; Mennander, A. Inhibition of monoamine oxidase A increases recovery after experimental cardiac arrest. Interact Cardiovasc. Thorac Surg. 2015, 21, 441–449. [Google Scholar] [CrossRef]
- Cathcart, M.K.; Bhattacharjee, A. Monoamine oxidase A (MAO-A): A signature marker of alternatively activated monocytes/macrophages. Inflamm. Cell Signal. 2014, 1, e161. [Google Scholar] [PubMed]
- Bhattacharjee, A.; Shukla, M.; Yakubenko, V.P.; Mulya, A.; Kundu, S.; Cathcart, M.K. IL-4 and IL-13 employ discrete signaling pathways for target gene expression in alternatively activated monocytes/macrophages. Free Radic. Biol. Med. 2013, 54, 1–16. [Google Scholar] [CrossRef] [Green Version]
- O’Mahony, L.F.; Srivastava, A.; Mehta, P.; Ciurtin, C. Is fibromyalgia associated with a unique cytokine profile? Rheumatology 2021, 12, 146. [Google Scholar]
- Hankittichai, P.; Lou, H.J.; Wikan, N.; Smith, D.R.; Potikanond, S.; Nimlamool, W. Oxyresveratrol Inhibits IL-1β-Induced Inflammation via Suppressing AKT and ERK1/2 Activation in Human Microglia, HMC3. Int. J. Mol. Sci. 2020, 21, 6054. [Google Scholar] [CrossRef]
- D’Ascola, A.; Irrera, N.; Ettari, R.; Bitto, A.; Pallio, G.; Mannino, F.; Atteritano, M.; Campo, G.M.; Minutoli, L.; Arcoraci, V.; et al. Exploiting Curcumin Synergy with Natural Products Using Quantitative Analysis of Dose-Effect Relationships in an Experimental In Vitro Model of Osteoarthritis. Front. Pharmacol. 2019, 10, 1347. [Google Scholar] [CrossRef] [Green Version]
- Pallio, G.; Micali, A.; Benvenga, S.; Antonelli, A.; Marini, H.R.; Puzzolo, D.; Macaione, V.; Trichilo, V.; Santoro, G.; Irrera, N.; et al. Myo-inositol in the protection from cadmium-induced toxicity in mice kidney: An emerging nutraceutical challenge. Food Chem. Toxicol. 2019, 132, 110675. [Google Scholar] [CrossRef]
- Pizzino, G.; Bitto, A.; Pallio, G.; Irrera, N.; Galfo, F.; Interdonato, M.; Mecchio, A.; De Luca, F.; Minutoli, L.; Squadrito, F.; et al. Blockade of the JNK signalling as a rational therapeutic approach to modulate the early and late steps of the inflammatory cascade in polymicrobial sepsis. Mediat. Inflamm. 2015, 2015, 591572. [Google Scholar] [CrossRef]
- Irrera, N.; Arcoraci, V.; Mannino, F.; Vermiglio, G.; Pallio, G.; Minutoli, L.; Bagnato, G.; Anastasi, G.P.; Mazzon, E.; Bramanti, P.; et al. Activation of A2A Receptor by PDRN Reduces Neuronal Damage and Stimulates WNT/β-CATENIN Driven Neurogenesis in Spinal Cord Injury. Front. Pharmacol. 2018, 9, 506. [Google Scholar] [CrossRef] [Green Version]
- Pizzino, G.; Irrera, N.; Galfo, F.; Pallio, G.; Mannino, F.; D’amore, A.; Pellegrino, E.; Ieni, A.; Russo, G.T.; Calapai, M.; et al. Effects of the antagomiRs 15b and 200b on the altered healing pattern of diabetic mice. Br. J. Pharmacol. 2018, 175, 644–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irrera, N.; D’Ascola, A.; Pallio, G.; Bitto, A.; Mazzon, E.; Mannino, F.; Squadrito, V.; Arcoraci, V.; Minutoli, L.; Campo, G.M.; et al. β-Caryophyllene Mitigates Collagen Antibody Induced Arthritis (CAIA) in Mice Through a Cross-Talk between CB2 and PPAR-γ Receptors. Biomolecules 2019, 9, 326. [Google Scholar] [CrossRef] [Green Version]
- Minutoli, L.; Marini, H.; Rinaldi, M.; Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Calò, M.; Adamo, E.B.; Trichilo, V.; et al. A dual inhibitor of cyclooxygenase and 5-lipoxygenase protects against kainic acid-induced brain injury. Neuromol. Med. 2015, 17, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Chang, Y.T.; Campbell, M.; Lin, T.P.; Pan, C.C.; Lee, H.C.; Shih, J.C.; Chang, P.C. MAOA-A novel decision maker of apoptosis and autophagy in hormone refractory neuroendocrine prostate cancer cells. Sci. Rep. 2017, 7, 46338. [Google Scholar] [CrossRef] [PubMed]
- Ceravolo, I.; Mannino, F.; Irrera, N.; Squadrito, F.; Altavilla, D.; Ceravolo, G.; Pallio, G.; Minutoli, L. Health Potential of Aloe vera against Oxidative Stress Induced Corneal Damage: An “In Vitro” Study. Antioxidants 2021, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Irrera, N.; D’Ascola, A.; Pallio, G.; Bitto, A.; Mannino, F.; Arcoraci, V.; Rottura, M.; Ieni, A.; Minutoli, L.; Metro, D.; et al. β-Caryophyllene Inhibits Cell Proliferation through a Direct Modulation of CB2 Receptors in Glioblastoma Cells. Cancers 2020, 12, 1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abeles, M.; Solitar, B.M.; Pillinger, M.H.; Abeles, A.M. Update on fibromyalgia therapy. Am. J. Med. 2008, 121, 555–561. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Tsilioni, I.; Arbetman, L.; Panagiotidou, S.; Stewart, J.M.; Gleason, R.M.; Russell, I.J. Fibromyalgia, a syndrome in search of pathogenesis and therapy. J. Pharmacol. Exp. Ther. 2015, 355, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Kadetoff, D.; Lampa, J.; Westman, M.; Andersson, M.; Kosek, E. Evidence of central inflammation in fibromyalgia-increased cerebrospinal fluid interleukin-8 levels. J. Neuroimmunol. 2012, 242, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Harry, G.J.; Kraft, A.D. Microglia in the developing brain: A potential target with lifetime effects. Neurotoxicology 2012, 33, 191–206. [Google Scholar] [CrossRef] [Green Version]
- Park, S.E.; Sapkota, K.; Kim, S.; Kim, H.; Kim, S.J. Kaempferol acts throughmitogen-activated protein kinases and protein kinase B/AKT to elicit protection in a model of neuroinflammation in BV2 microglial cells. Br. J. Pharmacol. 2011, 164, 3008–3025. [Google Scholar] [CrossRef] [Green Version]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The role and consequences. Neurosci. Res. 2013, 79, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation pathways: A general review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Vuletić, L.; Khan, M.Z.I.; Špoljarić, D.; Radić, M.; Cetina-Čižmek, B.; Filipović-Grčić, J. Development of a Clinically Relevant Dissolution Method for Metaxalone Immediate Release Formulations Based on an IVIVC Model. Pharm. Res. 2018, 35, 163. [Google Scholar] [CrossRef]
- Schwartz, T.L. A neuroscientific update on monoamine oxidase and its inhibitors. CNS Spectr. 2013, 18, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, P.; Kunduzova, O.; Masini, E.; Cambon, C.; Bani, D.; Raimondi, L.; Seguelas, M.H.; Nistri, S.; Colucci, W.; Leducq, N.; et al. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation 2005, 112, 3297–3305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, M.; Levy, R.M. Metaxalone Suppresses Production of Inflammatory Cytokines Associated with Painful Conditions in Mouse Macrophages RAW264.7 cells in Vitro: Synergistic Effect with β-caryophyllene. Curr. Mol. Med. 2020, 20, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Ji, L.; Chen, Y. TSPO Modulates IL-4-induced Microglia/Macrophage M2 Polarization via PPAR-γ Pathway. J. Mol. Neurosci. 2020, 70, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Sarathlal, K.C.; Kakoty, V.; Marathe, S.; Chitkara, D.; Taliyan, R. Exploring the Neuroprotective Potential of Rosiglitazone Embedded Nanocarrier System on Streptozotocin Induced Mice Model of Alzheimer’s Disease. Nurotox. Res. 2021, 39, 240–255. [Google Scholar]
- Beheshti, F.; Hosseini, M.; Hashemzehi, M.; Soukhtanloo, M.; Khazaei, M.; Shafei, M.N. The effects of PPAR-γ agonist pioglitazone on hippocampal cytokines, brain-derived neurotrophic factor, memory impairment, and oxidative stress status in lipopolysaccharide-treated rats. Iran. J. Basic. Med. Sci. 2019, 22, 940–948. [Google Scholar]
- Thangaleela, S.; Ragu Varman, D.; Sivasangari, K.; Rajan, K.E. Inhibition of monoamine oxidase attenuates social defeat-induced memory impairment in goldfish (Corassius auratus): A possible involvement of synaptic proteins and BDNF. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 239, 108873. [Google Scholar] [CrossRef]
- Liu, W.; Rabinovich, A.; Nash, Y.; Frenkel, D.; Wang, Y.; Youdim, M.B.H.; Weinreb, O. Anti-inflammatory and protective effects of MT-031, a novel multitarget MAO-A and AChE/BuChE inhibitor in scopolamine mouse model and inflammatory cells. Neuropharmacology 2017, 113, 445–456. [Google Scholar] [CrossRef]




| Gene | Sequence |
|---|---|
| β-actin | Fw:5′AGAGCTACGAGCTGCCTGAC3′ |
| Rw:5′AGCACTGTGTTGGCGTACAG3′ | |
| IL-6 | Fw:5′TTCGGTCCAGTTGCCTTCTC3′ |
| Rw:5′CAGCTCTGGCTTGTTCCTCA3′ | |
| IL-13 | Fw:5′CATGGCGCTTTTGTTGACCA 3′ |
| Rw:5′AGCTGTCAGGTTGATGCTCC3′ | |
| NF-kB | Fw:5′CCTGGATGACTCTTGGGAAA3′ |
| Rw:5′TCAGCCAGCTGTTTCATGTC3′ | |
| PPAR-γ | Fw:5′TCGACCAGCTGAATCCAGAG3′ |
| Rw:5′GGGGGTGATGTGTTTGAACTTG3′ | |
| PGC-1a | Fw:5′CATGTGCAACCAGGACTCTGA3′ |
| Rw:5 GCGCATCAAATGAGGGCAAT3′ | |
| TNF-α | Fw:5′CAGAGGGCCTGTACCTCATC3′ |
| Rw:5′GGAAGACCCCTCCCAGATAG3′ | |
| MAO-A | Fw:5′ATGACACCAAGCCAGATGGG3′ |
| Rw:5′TCAGCAGGCCAGAAACAGAG3′ | |
| Nrf2 | Fw:5′CTCCACAGAAGACCCCAACC3′ |
| Rw:5′TCTGCAATTCTGAGCAGCCA3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pallio, G.; D’Ascola, A.; Cardia, L.; Mannino, F.; Bitto, A.; Minutoli, L.; Picciolo, G.; Squadrito, V.; Irrera, N.; Squadrito, F.; et al. MAO-A Inhibition by Metaxalone Reverts IL-1β-Induced Inflammatory Phenotype in Microglial Cells. Int. J. Mol. Sci. 2021, 22, 8425. https://doi.org/10.3390/ijms22168425
Pallio G, D’Ascola A, Cardia L, Mannino F, Bitto A, Minutoli L, Picciolo G, Squadrito V, Irrera N, Squadrito F, et al. MAO-A Inhibition by Metaxalone Reverts IL-1β-Induced Inflammatory Phenotype in Microglial Cells. International Journal of Molecular Sciences. 2021; 22(16):8425. https://doi.org/10.3390/ijms22168425
Chicago/Turabian StylePallio, Giovanni, Angela D’Ascola, Luigi Cardia, Federica Mannino, Alessandra Bitto, Letteria Minutoli, Giacomo Picciolo, Violetta Squadrito, Natasha Irrera, Francesco Squadrito, and et al. 2021. "MAO-A Inhibition by Metaxalone Reverts IL-1β-Induced Inflammatory Phenotype in Microglial Cells" International Journal of Molecular Sciences 22, no. 16: 8425. https://doi.org/10.3390/ijms22168425








