Trace Amine-Associated Receptor 1 (TAAR1) Is a Positive Prognosticator for Epithelial Ovarian Cancer
Abstract
1. Introduction
2. Results
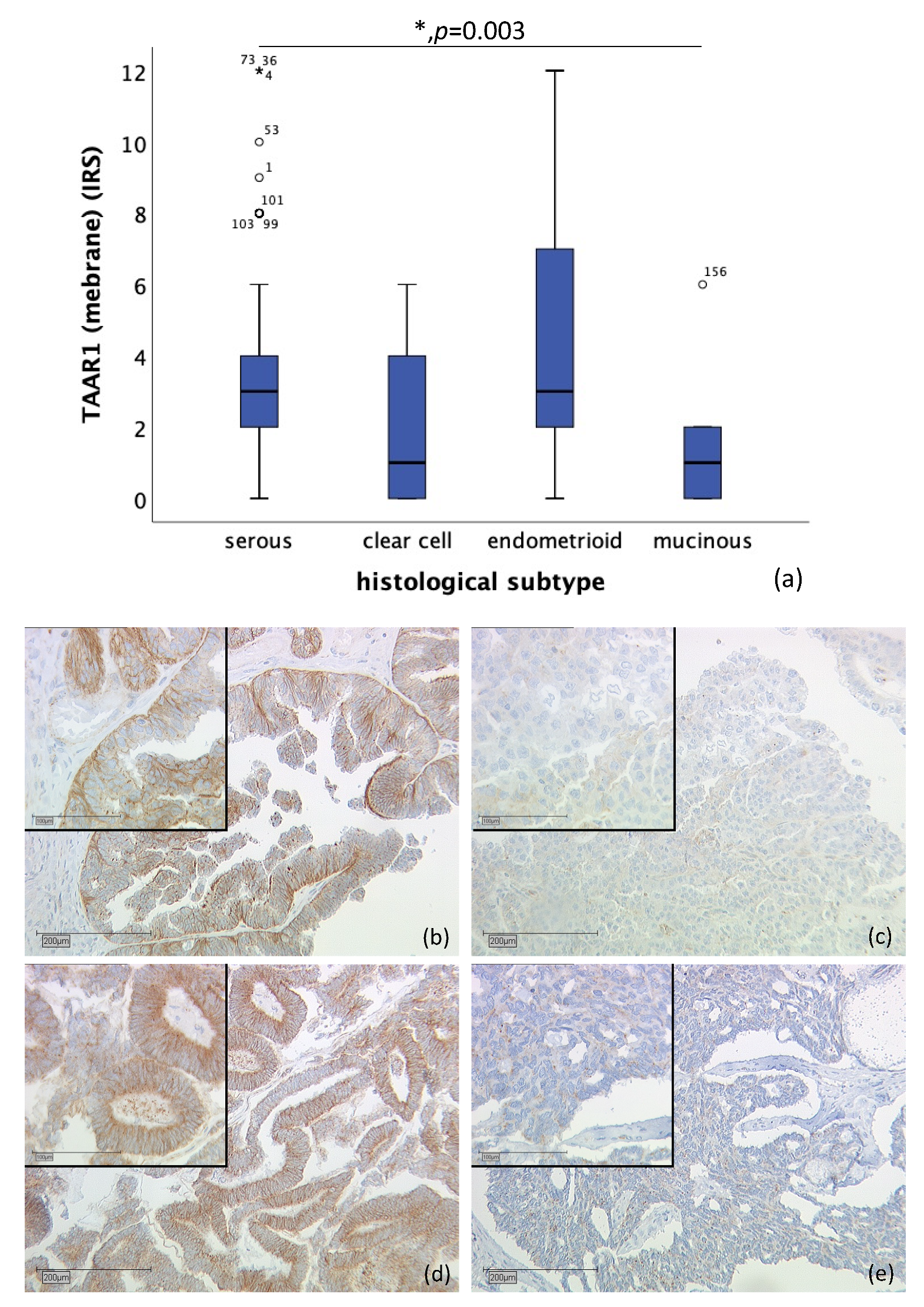
2.1. Differences of TAAR1 Expression in Histological Subtypes of Ovarian Cancer
2.2. TAAR1 Expression in High-Grade and Low-Grade Serous Ovarian Cancer
2.3. TAAR1 Expression Correlated with TNM Classification and FIGO
2.4. Correlation of Membrane and Cytoplasmic TAAR1 Expression with Overall and Progression-Free Survival of Ovarian Cancer Patients
2.5. Correlations of TAAR1 and Other Variables
2.6. Correlations of TAAR1 Gene with Overall Survival and Progression Free Survival of Large Independent Ovarian Cancer Cohorts
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Immunohistochemistry
4.3. Quantification
4.4. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TAAR1 | trace amine-associated receptor |
| T1AM | 3-iodothyronamine |
| OC | ovarian cancer |
| ODC | ornithine decarboxylase |
| GPCR | G-protein-coupled receptor |
| GPER | G-protein-coupled estrogen receptor |
| LHR | luteinizing hormone receptor |
| FSHR | follicle-stimulating hormone receptor |
| ER-α | estrogen receptor-α |
| ER-β | estrogen receptor-β |
| PR-A | progesterone receptor-A |
| PR-B | progesterone receptor-B |
| VDR | vitamin D receptor |
| Gd | Glycodelin |
| GPR30 | G-protein-coupled receptor 30 |
| MUC1 | Mucin-1 |
| D2R | dopamine receptor D2 |
| OS | overall survival |
| HEK-293 | human embryonic kidney 293 cells |
| IRS | immunoreactive score |
References
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): S3-Leitlinie Diagnostik, Therapie und Nachsorge Maligner Ovarialtumoren, Langversion 4.0, 2020, AWMF-Registernummer: 032/035OL. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/ovarialkarzinom/ (accessed on 1 May 2021).
- Thibault, B.; Castells, M.; Delord, J.P.; Couderc, B. Ovarian cancer microenvironment: Implications for cancer dissemination and chemoresistance acquisition. Cancer Metastasis Rev. 2014, 33, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.; Trope, C.G. Ovarian cancer: Diagnostic, biological and prognostic aspects. Womens Health 2014, 10, 519–533. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Survival Rates for Ovarian Cancer. Available online: https://www.cancer.org/cancer/ovarian-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 3 August 2021).
- Allemani, C.; Weir, H.K.; Carreira, H.; Harewood, R.; Spika, D.; Wang, X.S.; Bannon, F.; Ahn, J.V.; Johnson, C.J.; Bonaventure, A.; et al. Global surveillance of cancer survival 1995-2009: Analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015, 385, 977–1010. [Google Scholar] [CrossRef]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Neal, R.D.; Kehoe, S. Diagnosis of ovarian cancer. BMJ Br. Med. J. 2015, 351, h4443. [Google Scholar] [CrossRef]
- Wilson, M.K.; Pujade-Lauraine, E.; Aoki, D.; Mirza, M.R.; Lorusso, D.; Oza, A.M.; du Bois, A.; Vergote, I.; Reuss, A.; Bacon, M.; et al. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: Recurrent disease. Ann. Oncol. 2017, 28, 727–732. [Google Scholar] [CrossRef]
- Trillsch, F.; Mahner, S.; Czogalla, B.; Rottmann, M.; Chekerov, R.; Braicu, E.I.; Oskay-Öczelik, G.; Wimberger, P.; Richter, R.; Sehouli, J. Primary platinum resistance and its prognostic impact in patients with recurrent ovarian cancer: An analysis of three prospective trials from the NOGGO study group. J. Gynecol. Oncol. 2021, 32, e37. [Google Scholar] [CrossRef]
- Salminen, L.; Braicu, E.I.; Lääperi, M.; Jylhä, A.; Oksa, S.; Hietanen, S.; Sehouli, J.; Kulbe, H.; Bois, A.D.; Mahner, S.; et al. A Novel Two-Lipid Signature Is a Strong and Independent Prognostic Factor in Ovarian Cancer. Cancers 2021, 13, 1764. [Google Scholar] [CrossRef] [PubMed]
- Fraungruber, P.; Kaltofen, T.; Heublein, S.; Kuhn, C.; Mayr, D.; Burges, A.; Mahner, S.; Rathert, P.; Jeschke, U.; Trillsch, F. G Protein-Coupled Estrogen Receptor Correlates With Dkk2 Expression and Has Prognostic Impact in Ovarian Cancer Patients. Front. Endocrinol. 2021, 12, 564002. [Google Scholar] [CrossRef] [PubMed]
- Czogalla, B.; Partenheimer, A.; Jeschke, U.; von Schönfeldt, V.; Mayr, D.; Mahner, S.; Burges, A.; Simoni, M.; Melli, B.; Benevelli, R.; et al. β-arrestin 2 Is a Prognostic Factor for Survival of Ovarian Cancer Patients Upregulating Cell Proliferation. Front. Endocrinol. 2020, 11, 554733. [Google Scholar] [CrossRef]
- Shinderman-Maman, E.; Cohen, K.; Moskovich, D.; Hercbergs, A.; Werner, H.; Davis, P.J.; Ellis, M.; Ashur-Fabian, O. Thyroid hormones derivatives reduce proliferation and induce cell death and DNA damage in ovarian cancer. Sci. Rep. 2017, 7, 16475. [Google Scholar] [CrossRef]
- Shinderman-Maman, E.; Weingarten, C.; Moskovich, D.; Werner, H.; Hercbergs, A.; Davis, P.J.; Ellis, M.; Ashur-Fabian, O. Molecular insights into the transcriptional regulatory role of thyroid hormones in ovarian cancer. Mol. Carcinog. 2018, 57, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Mousa, S.A.; Lin, H.Y.; Tang, H.Y.; Hercbergs, A.; Luidens, M.K.; Davis, P.J. Modulation of angiogenesis by thyroid hormone and hormone analogues: Implications for cancer management. Angiogenesis 2014, 17, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.K.; Nayak, B. Management of Ovarian Cancer in Elderly. Rev. Recent Clin. Trials 2015, 10, 270–275. [Google Scholar] [CrossRef]
- Hollowell, J.G.; Staehling, N.W.; Flanders, W.D.; Hannon, W.H.; Gunter, E.W.; Spencer, C.A.; Braverman, L.E. Serum TSH, T4, and Thyroid Antibodies in the United States Population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J. Clin. Endocrinol. Metab. 2002, 87, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.N.; Albrecht, D.; Scholz, A.; Gutierrez-Buey, G.; Lazarus, J.H.; Dayan, C.M.; Okosieme, O.E. Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 2018, 14, 301–316. [Google Scholar] [CrossRef]
- Scanlan, T.S.; Suchland, K.L.; Hart, M.E.; Chiellini, G.; Huang, Y.; Kruzich, P.J.; Frascarelli, S.; Crossley, D.A.; Bunzow, J.R.; Ronca-Testoni, S.; et al. 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat. Med. 2004, 10, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Hoefig, C.S.; Zucchi, R.; Kohrle, J. Thyronamines and Derivatives: Physiological Relevance, Pharmacological Actions, and Future Research Directions. Thyroid 2016, 26, 1656–1673. [Google Scholar] [CrossRef]
- Burchett, S.A.; Hicks, T.P. The mysterious trace amines: Protean neuromodulators of synaptic transmission in mammalian brain. Prog. Neurobiol. 2006, 79, 223–246. [Google Scholar] [CrossRef] [PubMed]
- Gozal, E.A.; O’Neill, B.E.; Sawchuk, M.A.; Zhu, H.; Halder, M.; Chou, C.C.; Hochman, S. Anatomical and functional evidence for trace amines as unique modulators of locomotor function in the mammalian spinal cord. Front. Neural. Circuits 2014, 8, 134. [Google Scholar] [CrossRef]
- Babusyte, A.; Kotthoff, M.; Fiedler, J.; Krautwurst, D. Biogenic amines activate blood leukocytes via trace amine-associated receptors TAAR1 and TAAR2. J. Leukoc. Biol. 2013, 93, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Vattai, A.; Akyol, E.; Kuhn, C.; Hofmann, S.; Heidegger, H.; von Koch, F.; Hermelink, K.; Wuerstlein, R.; Harbeck, N.; Mayr, D.; et al. Increased trace amine-associated receptor 1 (TAAR1) expression is associated with a positive survival rate in patients with breast cancer. J. Cancer Res. Clin. Oncol. 2017, 143, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, A.; Lam, B.Y.; Billing, L.; Skeffington, K.; Sewing, S.; Reimann, F.; Gribble, F. A Transcriptome-Led Exploration of Molecular Mechanisms Regulating Somatostatin-Producing D-Cells in the Gastric Epithelium. Endocrinology 2015, 156, 3924–3936. [Google Scholar] [CrossRef] [PubMed]
- Regard, J.B.; Kataoka, H.; Cano, D.A.; Camerer, E.; Yin, L.; Zheng, Y.W.; Scanlan, T.S.; Hebrok, M.; Coughlin, S.R. Probing cell type-specific functions of Gi in vivo identifies GPCR regulators of insulin secretion. J. Clin. Investig. 2007, 117, 4034–4043. [Google Scholar] [CrossRef][Green Version]
- Borowsky, B.; Adham, N.; Jones, K.A.; Raddatz, R.; Artymyshyn, R.; Ogozalek, K.L.; Durkin, M.M.; Lakhlani, P.P.; Bonini, J.A.; Pathirana, S.; et al. Trace amines: Identification of a family of mammalian G protein-coupled receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 8966–8971. [Google Scholar] [CrossRef] [PubMed]
- Bunzow, J.R.; Sonders, M.S.; Arttamangkul, S.; Harrison, L.M.; Zhang, G.; Quigley, D.I.; Darland, T.; Suchland, K.L.; Pasumamula, S.; Kennedy, J.L.; et al. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol. Pharmacol. 2001, 60, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Heublein, S.; Page, S.; Mayr, D.; Schmoeckel, E.; Trillsch, F.; Marmé, F.; Mahner, S.; Jeschke, U.; Vattai, A. Potential Interplay of the Gatipotuzumab Epitope TA-MUC1 and Estrogen Receptors in Ovarian Cancer. Int. J. Mol. Sci. 2019, 20, 295. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, U.; Wiest, I.; Schumacher, A.L.; Kupka, M.; Rack, B.; Stahn, R.; Karsten, U.; Mayr, D.; Friese, K.; Dian, D. Determination of MUC1 in sera of ovarian cancer patients and in sera of patients with benign changes of the ovaries with CA15-3, CA27.29, and PankoMab. Anticancer Res. 2012, 32, 2185–2189. [Google Scholar]
- Huang, L.; Chen, D.; Liu, D.; Yin, L.; Kharbanda, S.; Kufe, D. MUC1 oncoprotein blocks glycogen synthase kinase 3beta-mediated phosphorylation and degradation of beta-catenin. Cancer Res. 2005, 65, 10413–10422. [Google Scholar] [CrossRef]
- Li, Y.; Kuwahara, H.; Ren, J.; Wen, G.; Kufe, D. The c-Src tyrosine kinase regulates signaling of the human DF3/MUC1 carcinoma-associated antigen with GSK3 beta and beta-catenin. J. Biol. Chem. 2001, 276, 6061–6064. [Google Scholar] [CrossRef]
- Li, Y.; Bharti, A.; Chen, D.; Gong, J.; Kufe, D. Interaction of glycogen synthase kinase 3beta with the DF3/MUC1 carcinoma-associated antigen and beta-catenin. Mol. Cell. Biol. 1998, 18, 7216–7224. [Google Scholar] [CrossRef] [PubMed]
- Mommers, E.C.; Leonhart, A.M.; von Mensdorff-Pouilly, S.; Schol, D.J.; Hilgers, J.; Meijer, C.J.; Baak, J.P.; van Diest, P.J. Aberrant expression of MUC1 mucin in ductal hyperplasia and ductal carcinoma In situ of the breast. Int. J. Cancer 1999, 84, 466–469. [Google Scholar] [CrossRef]
- Gaemers, I.C.; Vos, H.L.; Volders, H.H.; van der Valk, S.W.; Hilkens, J. A stat-responsive element in the promoter of the episialin/MUC1 gene is involved in its overexpression in carcinoma cells. J. Biol. Chem. 2001, 276, 6191–6199. [Google Scholar] [CrossRef] [PubMed]
- Engelstaedter, V.; Heublein, S.; Schumacher, A.L.; Lenhard, M.; Engelstaedter, H.; Andergassen, U.; Guenthner-Biller, M.; Kuhn, C.; Rack, B.; Kupka, M.; et al. Mucin-1 and its relation to grade, stage and survival in ovarian carcinoma patients. BMC Cancer 2012, 12, 600. [Google Scholar] [CrossRef] [PubMed]
- Dian, D.; Lenhard, M.; Mayr, D.; Heublein, S.; Karsten, U.; Goletz, S.; Kuhn, C.; Wiest, I.; Friese, K.; Weissenbacher, T.; et al. Staining of MUC1 in ovarian cancer tissues with PankoMab-GEX detecting the tumour-associated epitope, TA-MUC1, as compared to antibodies HMFG-1 and 115D8. Histol. Histopathol. 2013, 28, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Kaplan-Meier Plotter. Available online: https://kmplot.com/analysis/ (accessed on 24 July 2021).
- Xie, Z.; Vallender, E.J.; Yu, N.; Kirstein, S.L.; Yang, H.; Bahn, M.E.; Westmoreland, S.V.; Miller, G.M. Cloning, expression, and functional analysis of rhesus monkey trace amine-associated receptor 6: Evidence for lack of monoaminergic association. J. Neurosci. Res. 2008, 86, 3435–3446. [Google Scholar] [CrossRef] [PubMed]
- Tremmel, E.; Hofmann, S.; Kuhn, C.; Heidegger, H.; Heublein, S.; Hermelink, K.; Wuerstlein, R.; Harbeck, N.; Mayr, D.; Mahner, S.; et al. Thyronamine regulation of TAAR1 expression in breast cancer cells and investigation of its influence on viability and migration. Breast Cancer 2019, 11, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; de Lange, P.; Lombardi, A.; Silvestri, E.; Lanni, A.; Goglia, F. Metabolic effects of thyroid hormone derivatives. Thyroid 2008, 18, 239–253. [Google Scholar] [CrossRef]
- Bai, W.; Oliveros-Saunders, B.; Wang, Q.; Acevedo-Duncan, M.E.; Nicosia, S.V. Estrogen stimulation of ovarian surface epithelial cell proliferation. Vitr. Cell Dev. Biol. Anim. 2000, 36, 657–666. [Google Scholar] [CrossRef]
- Sharma, G.; Prossnitz, E.R. G-Protein-Coupled Estrogen Receptor (GPER) and Sex-Specific Metabolic Homeostasis. Adv. Exp. Med. Biol. 2017, 1043, 427–453. [Google Scholar] [CrossRef]
- Symonds, D.A.; Merchenthaler, I.; Flaws, J.A. Methoxychlor and estradiol induce oxidative stress DNA damage in the mouse ovarian surface epithelium. Toxicol. Sci. 2008, 105, 182–187. [Google Scholar] [CrossRef]
- Laviolette, L.A.; Garson, K.; Macdonald, E.A.; Senterman, M.K.; Courville, K.; Crane, C.A.; Vanderhyden, B.C. 17beta-estradiol accelerates tumor onset and decreases survival in a transgenic mouse model of ovarian cancer. Endocrinology 2010, 151, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Laviolette, L.A.; Hodgkinson, K.M.; Minhas, N.; Perez-Iratxeta, C.; Vanderhyden, B.C. 17beta-estradiol upregulates GREB1 and accelerates ovarian tumor progression in vivo. Int. J. Cancer 2014, 135, 1072–1084. [Google Scholar] [CrossRef]
- Czogalla, B.; Kahaly, M.; Mayr, D.; Schmoeckel, E.; Niesler, B.; Kolben, T.; Burges, A.; Mahner, S.; Jeschke, U.; Trillsch, F. Interaction of ERα and NRF2 Impacts Survival in Ovarian Cancer Patients. Int. J. Mol. Sci. 2018, 20, 112. [Google Scholar] [CrossRef]
- Smith, H.O.; Arias-Pulido, H.; Kuo, D.Y.; Howard, T.; Qualls, C.R.; Lee, S.J.; Verschraegen, C.F.; Hathaway, H.J.; Joste, N.E.; Prossnitz, E.R. GPR30 predicts poor survival for ovarian cancer. Gynecol. Oncol. 2009, 114, 465–471. [Google Scholar] [CrossRef]
- Kolkova, Z.; Casslen, V.; Henic, E.; Ahmadi, S.; Ehinger, A.; Jirstrom, K.; Casslen, B. The G protein-coupled estrogen receptor 1 (GPER/GPR30) does not predict survival in patients with ovarian cancer. J. Ovarian Res. 2012, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Heublein, S.; Mayr, D.; Vrekoussis, T.; Friese, K.; Hofmann, S.S.; Jeschke, U.; Lenhard, M. The G-protein coupled estrogen receptor (GPER/GPR30) is a gonadotropin receptor dependent positive prognosticator in ovarian carcinoma patients. PLoS ONE 2013, 8, e71791. [Google Scholar] [CrossRef] [PubMed]
- Lenhard, M.; Tereza, L.; Heublein, S.; Ditsch, N.; Himsl, I.; Mayr, D.; Friese, K.; Jeschke, U. Steroid hormone receptor expression in ovarian cancer: Progesterone receptor B as prognostic marker for patient survival. BMC Cancer 2012, 12, 553. [Google Scholar] [CrossRef]
- Kaldawy, A.; Segev, Y.; Lavie, O.; Auslender, R.; Sopik, V.; Narod, S.A. Low-grade serous ovarian cancer: A review. Gynecol. Oncol. 2016, 143, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Lam, V.M.; Espinoza, S.; Gerasimov, A.S.; Gainetdinov, R.R.; Salahpour, A. In-vivo pharmacology of Trace-Amine Associated Receptor 1. Eur. J. Pharmacol. 2015, 763, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, S.; Salahpour, A.; Masri, B.; Sotnikova, T.D.; Messa, M.; Barak, L.S.; Caron, M.G.; Gainetdinov, R.R. Functional interaction between trace amine-associated receptor 1 and dopamine D2 receptor. Mol. Pharmacol. 2011, 80, 416–425. [Google Scholar] [CrossRef]
- Yong, M.; Yu, T.; Tian, S.; Liu, S.; Xu, J.; Hu, J.; Hu, L. DR2 blocker thioridazine: A promising drug for ovarian cancer therapy. Oncol. Lett. 2017, 14, 8171–8177. [Google Scholar] [CrossRef]
- Mao, M.; Yu, T.; Hu, J.; Hu, L. Dopamine D2 receptor blocker thioridazine induces cell death in human uterine cervical carcinoma cell line SiHa. J. Obstet. Gynaecol. Res. 2015, 41, 1240–1245. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; He, S.; Shen, G.; Ye, T.; Guo, F.; Wang, Y. Dopamine receptor antagonist thioridazine inhibits tumor growth in a murine breast cancer model. Mol. Med. Rep. 2015, 12, 4103–4108. [Google Scholar] [CrossRef] [PubMed]
- Sachlos, E.; Risueno, R.M.; Laronde, S.; Shapovalova, Z.; Lee, J.H.; Russell, J.; Malig, M.; McNicol, J.D.; Fiebig-Comyn, A.; Graham, M.; et al. Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells. Cell 2012, 149, 1284–1297. [Google Scholar] [CrossRef]
- Lu, M.; Li, J.; Luo, Z.; Zhang, S.; Xue, S.; Wang, K.; Shi, Y.; Zhang, C.; Chen, H.; Li, Z. Roles of dopamine receptors and their antagonist thioridazine in hepatoma metastasis. OncoTargets Ther. 2015, 8, 1543–1552. [Google Scholar] [CrossRef]
- Basu, S.; Nagy, J.A.; Pal, S.; Vasile, E.; Eckelhoefer, I.A.; Bliss, V.S.; Manseau, E.J.; Dasgupta, P.S.; Dvorak, H.F.; Mukhopadhyay, D. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat. Med. 2001, 7, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Chakroborty, D.; Sarkar, C.; Basu, B.; Dasgupta, P.S.; Basu, S. Catecholamines regulate tumor angiogenesis. Cancer Res. 2009, 69, 3727–3730. [Google Scholar] [CrossRef]
- Harmeier, A.; Obermueller, S.; Meyer, C.A.; Revel, F.G.; Buchy, D.; Chaboz, S.; Dernick, G.; Wettstein, J.G.; Iglesias, A.; Rolink, A.; et al. Trace amine-associated receptor 1 activation silences GSK3beta signaling of TAAR1 and D2R heteromers. Eur. Neuropsychopharmacol. 2015, 25, 2049–2061. [Google Scholar] [CrossRef]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar]





| Variable | Significance | Hazard Ratio | 95% Confidence Interval | |
|---|---|---|---|---|
| Lower | Higher | |||
| Age | 0.040 | 1.022 | 1.001 | 1.044 |
| Histological subtype | 0.990 | 0.998 | 0.743 | 1.342 |
| Grading | 0.001 | 1.775 | 1.262 | 2.496 |
| FIGO | 0.001 | 1.963 | 1.323 | 2.913 |
| TAAR1 | 0.344 | 0.807 | 0.518 | 1.258 |
| Number of Cases (Total Number of Cases: n = 156) | % | |
|---|---|---|
| Histopathological tumor subtype | ||
| Serous | 110 | 70.5 |
| Clear cell | 12 | 7.7 |
| Endometrioid | 21 | 13.5 |
| Mucinous | 13 | 8.3 |
| Tumor grading | ||
| G1 | 36 | 23.1 |
| G2 | 11 | 7.1 |
| G3 | 97 | 62.2 |
| Unknown | 12 | 7.7 |
| Extent of primary tumor (pT) | ||
| pT1 | 40 | 25.6 |
| pT2 | 18 | 11.5 |
| pT3 | 93 | 59.6 |
| pT4 | 4 | 2.6 |
| Unknown | 1 | 0.6 |
| Regional lymph node involvement (pN) | ||
| pN0 | 43 | 27.6 |
| pN1 | 52 | 33.3 |
| Unknown | 61 | 39.1 |
| Presence of distant metastatic spread (pM) | ||
| pM0 | 3 | 1.9 |
| pM1 | 6 | 3.9 |
| Unknown | 147 | 94.2 |
| FIGO classification | ||
| FIGO I | 35 | 22.4 |
| FIGO II | 10 | 6.4 |
| FIGO III | 103 | 66.0 |
| FIGO IV | 3 | 1.9 |
| Unknown | 5 | 3.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogelsang, T.L.R.; Vattai, A.; Schmoeckel, E.; Kaltofen, T.; Chelariu-Raicu, A.; Zheng, M.; Mahner, S.; Mayr, D.; Jeschke, U.; Trillsch, F. Trace Amine-Associated Receptor 1 (TAAR1) Is a Positive Prognosticator for Epithelial Ovarian Cancer. Int. J. Mol. Sci. 2021, 22, 8479. https://doi.org/10.3390/ijms22168479
Vogelsang TLR, Vattai A, Schmoeckel E, Kaltofen T, Chelariu-Raicu A, Zheng M, Mahner S, Mayr D, Jeschke U, Trillsch F. Trace Amine-Associated Receptor 1 (TAAR1) Is a Positive Prognosticator for Epithelial Ovarian Cancer. International Journal of Molecular Sciences. 2021; 22(16):8479. https://doi.org/10.3390/ijms22168479
Chicago/Turabian StyleVogelsang, Tilman L. R., Aurelia Vattai, Elisa Schmoeckel, Till Kaltofen, Anca Chelariu-Raicu, Mingjun Zheng, Sven Mahner, Doris Mayr, Udo Jeschke, and Fabian Trillsch. 2021. "Trace Amine-Associated Receptor 1 (TAAR1) Is a Positive Prognosticator for Epithelial Ovarian Cancer" International Journal of Molecular Sciences 22, no. 16: 8479. https://doi.org/10.3390/ijms22168479
APA StyleVogelsang, T. L. R., Vattai, A., Schmoeckel, E., Kaltofen, T., Chelariu-Raicu, A., Zheng, M., Mahner, S., Mayr, D., Jeschke, U., & Trillsch, F. (2021). Trace Amine-Associated Receptor 1 (TAAR1) Is a Positive Prognosticator for Epithelial Ovarian Cancer. International Journal of Molecular Sciences, 22(16), 8479. https://doi.org/10.3390/ijms22168479







