Quantitative Assessment of Point-of-Care 3D-Printed Patient-Specific Polyetheretherketone (PEEK) Cranial Implants
Abstract
:1. Introduction
2. Results
2.1. Geometric Characteristics of the FFF 3D-Printed PEEK Patient-Specific Cranial Implants
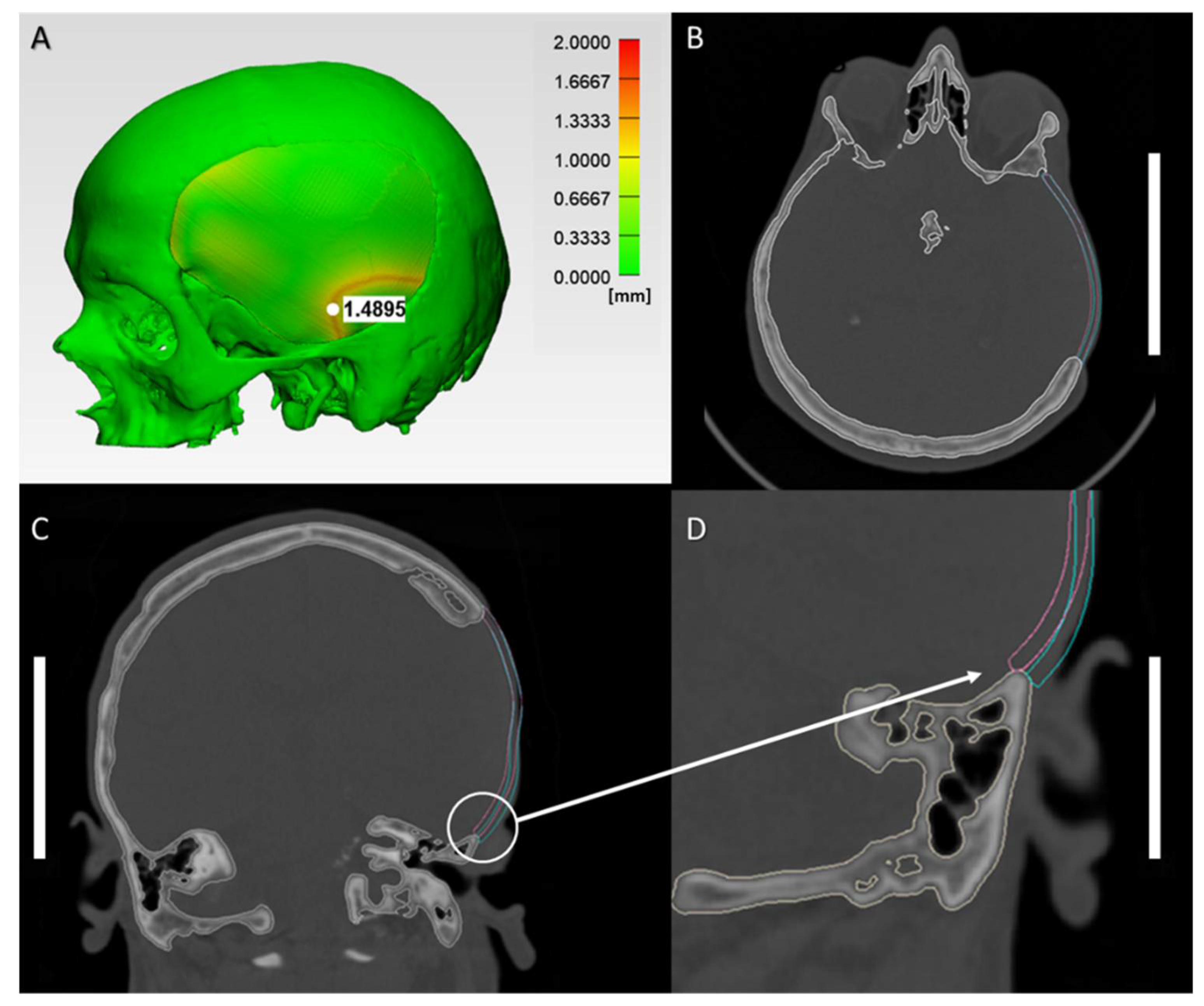
2.2. Morphological Characteristics of Anatomical Reconstructions with 3D-Printed PEEK Patient-Specific Cranial Implants
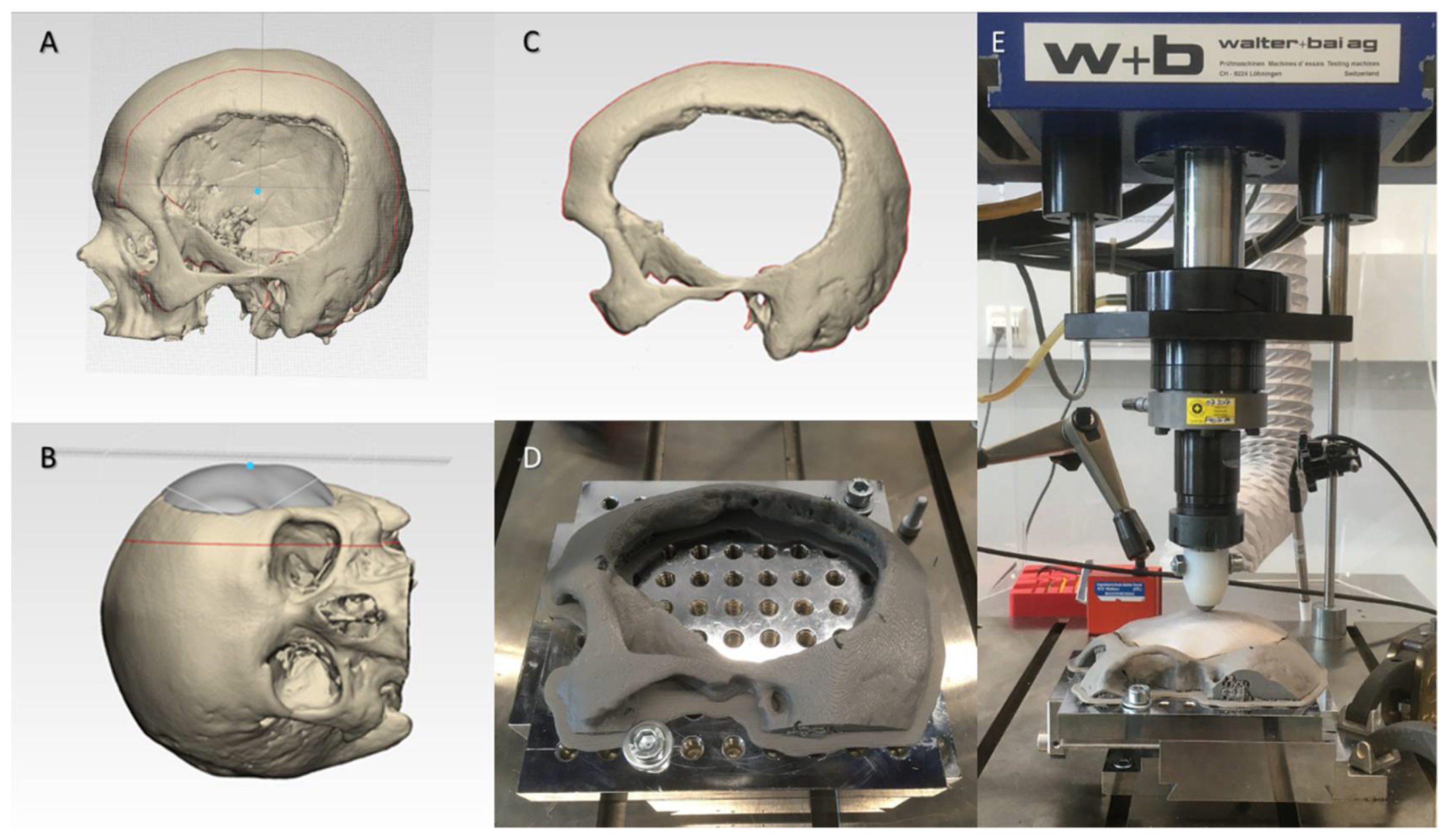
2.3. Biomechanical Characteristics of the 3D-Printed PEEK Patient-Specific Cranial Implants
3. Discussion
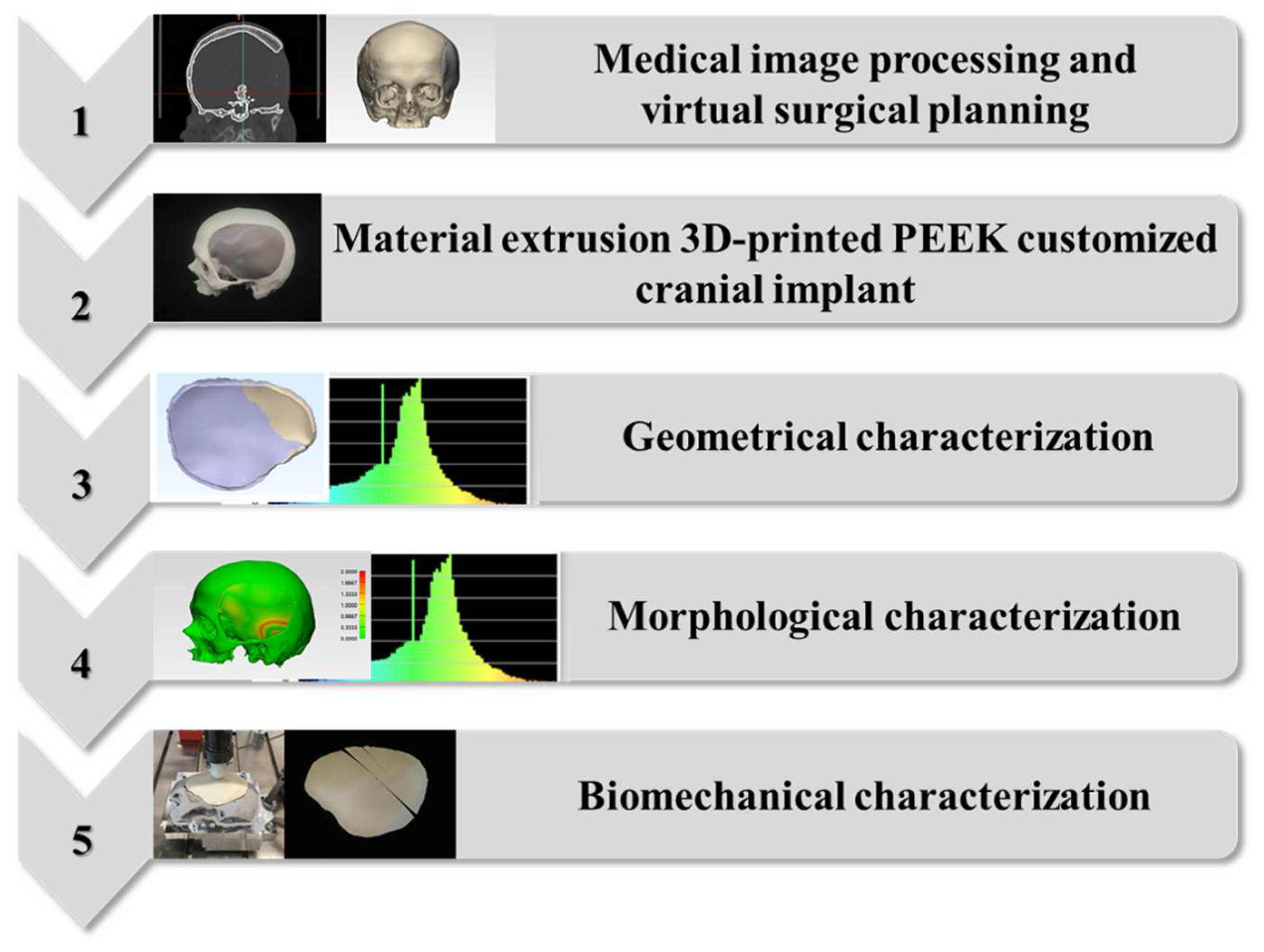
4. Materials and Methods
4.1. Medical Image Processing and Virtual Surgical Planning (VSP) Protocol
4.2. Material Extrusion 3D Printing Protocol of PEEK Patient-Specific Cranial Implants
4.3. Geometrical Characterization Protocol for the 3D-Printed PEEK Patient-Specific Cranial Implants
4.4. Morphological Characterization Protocol for the Anatomical Reconstruction with 3D-Printed PEEK Patient-Specific Cranial Implants
4.5. Biomechanical Characterization Protocol for the 3D-Printed PEEK Patient-Specific Cranial Implants
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| 3D | Three-Dimensional |
| AM | Additive Manufacturing |
| ASTM | American Society for Testing and Materials |
| CAD | Computer-Aided Design |
| CAM | Computer-Aided Manufacturing |
| CT | Computed Tomography |
| DfAM | Design for Additive Manufacturing |
| DICOM | Digital Imaging and Communications in Medicine |
| DSC | Dice Similarity Coefficient |
| EBM | Electron Beam Melting |
| FFF | Fused Filament Fabrication |
| HU | Hounsfield Units |
| ICP | Iterative Closest Point |
| ISO | International Organization for Standardization |
| PLA | Polylactic Acid |
| PEEK | Polyetheretherketone |
| POC | Point-of-Care |
| PSIs | Patient-Specific Implants |
| RSI | Reconstruction Symmetry Index |
| RMSE | Root Mean Square Error |
| SD | Standard Deviation |
| SLS | Selective Laser Sintering |
| STL | Standard Tessellation Language |
| VSP | Virtual Surgical Planning |
References
- Moreira-Gonzalez, A.; Jackson, I.T.; Miyawaki, T.; Barakat, K.; DiNick, V. Clinical outcome in cranioplasty: Critical review in long-term follow-up. J. Craniofac. Surg. 2003, 14, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Alkhaibary, A.; Alharbi, A.; Alnefaie, N.; Almubarak, A.O.; Aloraidi, A.; Khairy, S. Cranioplasty: A comprehensive review of the history, materials, surgical aspects, and complications. World Neurosurg. 2020, 139, 445–452. [Google Scholar] [CrossRef]
- Aydin, S.; Kucukyuruk, B.; Abuzayed, B.; Aydin, S.; Sanus, G.Z. Cranioplasty: Review of materials and techniques. J. Neurosci. Rural Pract. 2011, 2, 162–167. [Google Scholar] [CrossRef]
- Bonda, D.J.; Manjila, S.; Selman, W.R.; Dean, D. The Recent Revolution in the Design and Manufacture of Cranial Implants: Modern Advancements and Future Directions. Neurosurgery 2015, 77, 814–824. [Google Scholar] [CrossRef] [Green Version]
- D’Urso, P.S.; Earwaker, W.J.; Barker, T.M.; Redmond, M.J.; Thompson, R.G.; Effeney, D.J.; Tomlinson, F.H. Custom cranioplasty using stereolithography and acrylic. Br. J. Plast. Surg. 2000, 53, 200–204. [Google Scholar] [CrossRef]
- Huys, S.E.; Van Gysel, A.; Mommaerts, M.Y.; Vander Sloten, J. Evaluation of Patient-Specific Cranial Implant Design Using Finite Element Analysis. World Neurosurg. 2021, 148, 198–204. [Google Scholar] [CrossRef]
- Scolozzi, P.; Martinez, A.; Jaques, B. Complex orbito-fronto-temporal reconstruction using computer-designed PEEK implant. J. Craniofac. Surg. 2007, 18, 224–228. [Google Scholar] [CrossRef]
- Eppley, B.L.; Kilgo, M.; Coleman, J.J., 3rd. Cranial reconstruction with computer-generated hard-tissue replacement patient-matched implants: Indications, surgical technique, and long-term follow-up. Plast. Reconstr. Surg. 2002, 109, 864–871. [Google Scholar] [CrossRef]
- Chim, H.; Schantz, J.T. New frontiers in calvarial reconstruction: Integrating computer-assisted design and tissue engineering in cranioplasty. Plast. Reconstr. Surg. 2005, 116, 1726–1741. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, G.; Piccininni, A.; Ambrogio, G.; Sgambitterra, E. Design of custom cranial prostheses combining manufacturing and drop test finite element simulations. Int. J. Adv. Manuf. Technol. 2020, 111, 1627–1641. [Google Scholar] [CrossRef]
- Ghai, S.; Sharma, Y.; Jain, N.; Satpathy, M.; Pillai, A.K. Use of 3-D printing technologies in craniomaxillofacial surgery: A review. Oral Maxillofac. Surg. 2018, 22, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Msallem, B.; Beiglboeck, F.; Honigmann, P.; Jaquiéry, C.; Thieringer, F. Craniofacial Reconstruction by a Cost-Efficient Template-Based Process Using 3D Printing. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jindal, S.; Manzoor, F.; Haslam, N.; Mancuso, E. 3D printed composite materials for craniofacial implants: Current concepts, challenges and future directions. Int. J. Adv. Manuf. Technol. 2021, 112, 635–653. [Google Scholar] [CrossRef]
- Singare, S.; Dichen, L.; Bingheng, L.; Yanpu, L.; Zhenyu, G.; Yaxiong, L. Design and fabrication of custom mandible titanium tray based on rapid prototyping. Med. Eng. Phys. 2004, 26, 671–676. [Google Scholar] [CrossRef]
- Ghantous, Y.; Nashef, A.; Mohanna, A.; Abu-El-Naaj, I. Three-Dimensional Technology Applications in Maxillofacial Reconstructive Surgery: Current Surgical Implications. Nanomaterials 2020, 10, 2523. [Google Scholar] [CrossRef]
- Maniar, R.N.; Singhi, T. Patient specific implants: Scope for the future. Curr. Rev. Musculoskelet. Med. 2014, 7, 125–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alasseri, N.; Alasraj, A. Patient-specific implants for maxillofacial defects: Challenges and solutions. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 15. [Google Scholar] [CrossRef]
- Louvrier, A.; Marty, P.; Barrabé, A.; Euvrard, E.; Chatelain, B.; Weber, E.; Meyer, C. How useful is 3D printing in maxillofacial surgery? J. Stomatol. Oral Maxillofac. Surg. 2017, 118, 206–212. [Google Scholar] [CrossRef]
- Hoang, D.; Perrault, D.; Stevanovic, M.; Ghiassi, A. Surgical applications of three-dimensional printing: A review of the current literature & how to get started. Ann. Transl. Med. 2016, 4, 456. [Google Scholar] [CrossRef] [Green Version]
- Chamo, D.; Msallem, B.; Sharma, N.; Aghlmandi, S.; Kunz, C.; Thieringer, F.M. Accuracy Assessment of Molded, Patient-Specific Polymethylmethacrylate Craniofacial Implants Compared to Their 3D Printed Originals. J. Clin. Med. 2020, 9, 832. [Google Scholar] [CrossRef] [Green Version]
- Schön, S.N.; Skalicky, N.; Sharma, N.; Zumofen, D.W.; Thieringer, F.M. 3D-Printer-Assisted Patient-Specific Polymethyl Methacrylate Cranioplasty: A Case Series of 16 Consecutive Patients. World Neurosurg. 2021, 148, e356–e362. [Google Scholar] [CrossRef] [PubMed]
- Tel, A.; Tuniz, F.; Fabbro, S.; Sembronio, S.; Costa, F.; Robiony, M. Computer-Guided In-House Cranioplasty: Establishing a Novel Standard for Cranial Reconstruction and Proposal of an Updated Protocol. J. Oral Maxillofac. Surg. 2020, 78, 2297.e1–2297.e16. [Google Scholar] [CrossRef] [PubMed]
- da Silva Júnior, E.B.; de Aragão, A.H.; de Paula Loureiro, M.; Lobo, C.S.; Oliveti, A.F.; de Oliveira, R.M.; Ramina, R. Cranioplasty with three-dimensional customised mould for polymethylmethacrylate implant: A series of 16 consecutive patients with cost-effectiveness consideration. 3D Print Med. 2021, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Haro, J.A.; Pascau, J.; Asencio-Pascual, J.M.; Calvo-Manuel, F.; Cancho-Gil, M.J.; Del Cañizo López, J.F.; Fanjul-Gómez, M.; García-Leal, R.; González-Casaurrán, G.; González-Leyte, M.; et al. Point-of-care manufacturing: A single university hospital’s initial experience. 3D Print Med. 2021, 7, 11. [Google Scholar] [CrossRef]
- Msallem, B.; Sharma, N.; Cao, S.; Halbeisen, F.S.; Zeilhofer, H.F.; Thieringer, F.M. Evaluation of the Dimensional Accuracy of 3D-Printed Anatomical Mandibular Models Using FFF, SLA, SLS, MJ, and BJ Printing Technology. J. Clin. Med. 2020, 9, 817. [Google Scholar] [CrossRef] [Green Version]
- Meglioli, M.; Naveau, A.; Macaluso, G.M.; Catros, S. 3D printed bone models in oral and cranio-maxillofacial surgery: A systematic review. 3D Print Med. 2020, 6, 30. [Google Scholar] [CrossRef]
- Honigmann, P.; Sharma, N.; Okolo, B.; Popp, U.; Msallem, B.; Thieringer, F.M. Patient-Specific Surgical Implants Made of 3D Printed PEEK: Material, Technology, and Scope of Surgical Application. Biomed Res. Int. 2018, 19, 4520636. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Sharma, N.; Xu, Z.; Scheideler, L.; Geis-Gerstorfer, J.; Rupp, F.; Thieringer, F.M.; Spintzyk, S. An In Vitro Study of Osteoblast Response on Fused-Filament Fabrication 3D Printed PEEK for Dental and Cranio-Maxillofacial Implants. J. Clin. Med. 2019, 8, 771. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Honigmann, P.; Cao, S.; Thieringer, F. Dimensional characteristics of FDM 3D printed PEEK implant for craniofacial reconstructions. Trans. AMMM 2020, 2. [Google Scholar] [CrossRef]
- Asaad, M.; Taslakian, E.N.; Banuelos, J.; Abu-Ghname, A.; Bite, U.; Mardini, S.; Van Gompel, J.J.; Sharaf, B. Surgical and Patient-Reported Outcomes in Patients with PEEK Versus Titanium Cranioplasty Reconstruction. J. Craniofac. Surg. 2021, 32, 193–197. [Google Scholar] [CrossRef]
- Wilcox, B.; Mobbs, R.J.; Wu, A.M.; Phan, K. Systematic review of 3D printing in spinal surgery: The current state of play. J. Spine Surg. 2017, 3, 433–443. [Google Scholar] [CrossRef] [Green Version]
- Honigmann, P.; Sharma, N.; Schumacher, R.; Rueegg, J.; Haefeli, M.; Thieringer, F. In-Hospital 3D Printed Scaphoid Prosthesis Using Medical-Grade Polyetheretherketone (PEEK) Biomaterial. Biomed Res. Int. 2021, 11, 1301028. [Google Scholar] [CrossRef]
- Sharma, N.; Aghlmandi, S.; Cao, S.; Kunz, C.; Honigmann, P.; Thieringer, F.M. Quality characteristics and clinical relevance of in-house 3D-printed customized polyetheretherketone (PEEK) implants for craniofacial reconstruction. J. Clin. Med. 2020, 9, 2818. [Google Scholar] [CrossRef]
- Nout, E.; Mommaerts, M.Y. Considerations in computer-aided design for inlay cranioplasty: Technical note. Oral Maxillofac. Surg. 2018, 22, 65–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Vijfeijken, S.E.C.M.; Schreurs, R.; Dubois, L.; Becking, A.G.; CranioSafe, Group. The use of cranial resection templates with 3D virtual planning and PEEK patient-specific implants: A 3 year follow-up. J. Craniomaxillofac. Surg. 2019, 47, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Basgul, C.; Spece, H.; Sharma, N.; Thieringer, F.M.; Kurtz, S.M. Structure, properties, and bioactivity of 3D printed PAEKs for implant applications: A systematic review. J. Biomed Mater. Res. B Appl. Biomater. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pijpker, P.A.J.; Wagemakers, M.; Kraeima, J.; Vergeer, R.A.; Kuijlen, J.M.A.; Groen, R.J.M. Three-dimensional printed polymethylmethacrylate Casting molds for Posterior Fossa reconstruction in the surgical Treatment of Chiari I Malformation: Technical note and Illustrative cases. World Neurosurg. 2019, 129, 148–156. [Google Scholar] [CrossRef]
- Basu, B.; Bhaskar, N.; Barui, S.; Sharma, V.; Das, S.; Govindarajan, N.; Hegde, P.; Perikal, P.J.; Antharasanahalli Shivakumar, M.; Khanapure, K.; et al. Evaluation of implant properties, safety profile and clinical efficacy of patient-specific acrylic prosthesis in cranioplasty using 3D binderjet printed cranium model: A pilot study. J. Clin. Neurosci. 2021, 85, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Stieglitz, L.H.; Gerber, N.; Schmid, T.; Mordasini, P.; Fichtner, J.; Fung, C.; Murek, M.; Weber, S.; Raabe, A.; Beck, J. Intraoperative fabrication of patient-specific moulded implants for skull reconstruction: Single-centre experience of 28 cases. Acta Neurochir. 2014, 156, 793–803. [Google Scholar] [CrossRef] [Green Version]
- Berretta, S.; Evans, K.; Ghita, O. Additive manufacture of PEEK cranial implants: Manufacturing considerations versus accuracy and mechanical performance. Mater. Des. 2018, 139, 141–152. [Google Scholar] [CrossRef]
- Kung, W.M.; Chen, S.T.; Lin, C.H.; Lu, Y.M.; Chen, T.H.; Lin, M.S. Verifying three-dimensional skull model reconstruction using cranial index of symmetry. PLoS ONE 2013, 8, e74267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, E.T.; Ling, J.M.; Dinesh, S.K. The feasibility of producing patient-specific acrylic cranioplasty implants with a low-cost 3D printer. J. Neurosurg. 2016, 124, 1531–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moser, M.; Schmid, R.; Schindel, R.; Hildebrandt, G. Patient-specific polymethylmethacrylate prostheses for secondary reconstruction of large calvarial defects: A retrospective feasibility study of a new intraoperative moulding device for cranioplasty. J Craniomaxillofac. Surg. 2017, 45, 295–303. [Google Scholar] [CrossRef]
- Motherway, J.A.; Verschueren, P.; Van der Perre, G.; Vander Sloten, J.; Gilchrist, M.D. The mechanical properties of cranial bone: The effect of loading rate and cranial sampling position. J. Biomech. 2009, 42, 2129–2135. [Google Scholar] [CrossRef]
- Lethaus, B.; Safi, Y.; ter Laak-Poort, M.; Kloss-Brandstätter, A.; Banki, F.; Robbenmenke, C.; Steinseifer, U.; Kessler, P. Cranioplasty with customized titanium and PEEK implants in a mechanical stress model. J. Neurotrauma 2012, 29, 1077–1083. [Google Scholar] [CrossRef]
- Vaezi, M.; Yang, S. Extrusion-based additive manufacturing of PEEK for biomedical applications. Virtual Phys. Prototyp. 2015, 10, 123–135. [Google Scholar] [CrossRef]
- Basgul, C.; Thieringer, F.M.; Kurtz, S.M. Heat transfer-based non-isothermal healing model for the interfacial bonding strength of fused filament fabricated polyetheretherketone. Addit. Manuf. 2021, 46, 102097. [Google Scholar] [CrossRef]
- El Halabi, F.; Rodriguez, J.F.; Rebolledo, L.; Hurtós, E.; Doblaré, M. Mechanical characterization and numerical simulation of polyether-ether-ketone (PEEK) cranial implants. J. Mech. Behav. Biomed Mater. 2011, 4, 1819–1832. [Google Scholar] [CrossRef] [PubMed]
- Ono, I.; Tateshita, T.; Nakajima, T.; Ogawa, T. Determinations of strength of synthetic hydroxyapatite ceramic implants. Plast. Reconstr. Surg. 1998, 102, 807–813. [Google Scholar] [CrossRef]
- Stefini, R.; Zanotti, B.; Nataloni, A.; Martinetti, R.; Scafuto, M.; Colasurdo, M.; Tampieri, A. The efficacy of custom-made porous hydroxyapatite prostheses for cranioplasty: Evaluation of postmarketing data on 2697 patients. J. Appl. Biomater. Funct. Mater. 2015, 13, e136–e144. [Google Scholar] [CrossRef]
- Piitulainen, J.M.; Mattila, R.; Moritz, N.; Vallittu, P.K. Load-bearing capacity and fracture behavior of glass fiber-reinforced composite cranioplasty implants. J. Appl. Biomater. Funct. Mater. 2017, 15, e356–e361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linder, L.K.; Birgersson, U.; Lundgren, K.; Illies, C.; Engstrand, T. Patient-Specific Titanium-Reinforced Calcium Phosphate Implant for the Repair and Healing of Complex Cranial Defects. World Neurosurg. 2019, 122, e399–e407. [Google Scholar] [CrossRef] [PubMed]
- Lewin, S.; Åberg, J.; Neuhaus, D.; Engqvist, H.; Ferguson, S.J.; Öhman-Mägi, C.; Helgason, B.; Persson, C. Mechanical behaviour of composite calcium phosphate–titanium cranial implants: Effects of loading rate and design. J. Mech. Behav. Biomed Mater. 2020, 104, 103701. [Google Scholar] [CrossRef]
- Lewin, S.; Fleps, I.; Åberg, J.; Ferguson, S.J.; Engqvist, H.; Öhman-Mägi, C.; Helgason, B.; Persson, C. Additively manufactured mesh-type titanium structures for cranial implants: E-PBF vs. L-PBF. Mater. Des. 2021, 197, 109207. [Google Scholar] [CrossRef]
- Poukens, J.; Laeven, P.; Beerens, M.; Nijenhuis, G.; Sloten, J.V.; Stoelinga, P.; Kessler, P. A classification of cranial implants based on the degree of difficulty in computer design and manufacture. Int. J. Med. Robot. 2008, 4, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Ostas, D.; Rotar, H.; Brantner, P.; Thieringer, F.M. Design and Additive Manufacturing of a Biomimetic Customized Cranial Implant Based on Voronoi Diagram. Front. Physiol. 2021, 12, 647923. [Google Scholar] [CrossRef]
- Garcia-Leiner, M.; Ghita, O.; McKay, R.; Kurtz, S. Additive Manufacturing of Polyaryletherketones. In PEEK Biomaterials Handbook, 2nd ed.; Kurtz, S., Ed.; William Andrew Publishing: New York, NY, USA, 2019; pp. 89–103. [Google Scholar]
- Graham, J.; Peck, J. FDA Regulation of PEEK Implants. In PEEK Biomaterials Handbook, 2nd ed.; Kurtz, S., Ed.; William Andrew Publishing: New York, NY, USA, 2019; pp. 431–445. [Google Scholar]
- International Organization for Standardization. ISO 5725-2:2019. Accuracy (Trueness and Precision) of Measurement Methods and Results—Part 2: Basic Method for the Determination of Repeatability and Reproducibility of a Standard Measurement Method. 2019. Available online: https://www.iso.org/standard/69419.html (accessed on 10 July 2021).
- Ender, A.; Mehl, A. Accuracy of complete-arch dental impressions: A new method of measuring trueness and precision. J. Prosthet. Dent. 2013, 109, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Zonenshayn, M.; Kronberg, E.; Souweidane, M.M. Cranial index of symmetry: An objective semiautomated measure of plagiocephaly. Technical note. J. Neurosurg. 2004, 100, 537–540. [Google Scholar] [CrossRef]
- Davies, J.C.; Chan, H.H.L.; Jozaghi, Y.; Goldstein, D.P.; Irish, J.C. Analysis of simulated mandibular reconstruction using a segmental mirroring technique. J. Craniomaxillofac. Surg. 2019, 47, 468–472. [Google Scholar] [CrossRef]
- Pagedar, N.A.; Gilbert, R.W.; Chan, H.; Daly, M.J.; Irish, J.C.; Siewerdsen, J.H. Maxillary reconstruction using the scapular tip free flap: A radiologic comparison of 3D morphology. Head Neck 2012, 34, 1377–1382. [Google Scholar] [CrossRef] [PubMed]




| PSI 1 | Mean RMSE 2 ± SD 3 | Median RMSE (Q1 to Q3) |
|---|---|---|
| 1 | 0.143 ± 0.075 | 0.121 (0.092 to 0.143) |
| 2 | 0.143 ± 0.090 | 0.123 (0.080 to 0.153) |
| 3 | 0.129 ± 0.079 | 0.115 (0.076 to 0.138) |
| 4 | 0.133 ± 0.087 | 0.114 (0.096 to 0.120) |
| 5 | 0.129 ± 0.072 | 0.109 (0.083 to 0.120) |
| 6 | 0.219 ± 0.024 | 0.227 (0.214 to 0.232) |
| 7 | 0.259 ± 0.038 | 0.270 (0.259 to 0.278) |
| 8 | 0.134 ± 0.069 | 0.118 (0.103 to 0.130) |
| 9 | 0.133 ± 0.081 | 0.113 (0.099 to 0.116) |
| 10 | 0.124 ± 0.074 | 0.098 (0.087 to 0.119) |
| PSI 1 | PSI 2 | PSI 3 | PSI 4 | PSI 5 | PSI 6 | PSI 7 | PSI 8 | PSI 9 | |
|---|---|---|---|---|---|---|---|---|---|
| PSI 2 | 1.00 | ||||||||
| PSI 3 | 1.00 | 1.00 | |||||||
| PSI 4 | 1.00 | 1.00 | 1.00 | ||||||
| PSI 5 | 1.00 | 1.00 | 1.00 | 1.00 | |||||
| PSI 6 | 1.00 | 1.00 | 0.51 | 1.00 | 0.51 | ||||
| PSI 7 | 0.29 | 0.29 | 0.16 | 0.22 | 0.11 | 0.11 | |||
| PSI 8 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.51 | 0.03 a | ||
| PSI 9 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.22 | 1.00 | |
| PSI 10 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.51 | 0.05 b | 1.00 | 1.00 |
| PSI 1 | Peak Force (N) | Displacement at Peak Force (mm) |
|---|---|---|
| 1 | 877.50 | 2.74 |
| 2 | 1000.31 | 2.96 |
| 3 | 732.92 | 2.34 |
| 4 | 626.51 | 2.59 |
| 5 | 933.93 | 2.70 |
| 6 | 522.92 | 1.79 |
| 7 | 541.10 | 1.87 |
| 8 | 679.72 | 2.53 |
| 9 | 786.11 | 2.14 |
| 10 | 1182.91 | 3.72 |
| Extruder | Infill | ||
| Nozzle Diameter (mm) | 0.4 | Internal Fill Pattern | Rectilinear |
| Temperature | External Fill Pattern | Rectilinear | |
| Extruder Temperature (°C) | 485 | Infill Percentage | 100% |
| Airflow Temperature (°C) | 130–280 | Raster angle | 45/−45 |
| Layer | Support | ||
| Layer Height (mm) | 0.15 | Support Infill (%) | 40 |
| Top Solid Layers | 3 | Support Pillar Resolution (mm) | 4 |
| Bottom Solid Layer | 3 | Speed (mm/min) | |
| Outline/Perimeter Shells | 2 | Printing speed | 2000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, N.; Aghlmandi, S.; Dalcanale, F.; Seiler, D.; Zeilhofer, H.-F.; Honigmann, P.; Thieringer, F.M. Quantitative Assessment of Point-of-Care 3D-Printed Patient-Specific Polyetheretherketone (PEEK) Cranial Implants. Int. J. Mol. Sci. 2021, 22, 8521. https://doi.org/10.3390/ijms22168521
Sharma N, Aghlmandi S, Dalcanale F, Seiler D, Zeilhofer H-F, Honigmann P, Thieringer FM. Quantitative Assessment of Point-of-Care 3D-Printed Patient-Specific Polyetheretherketone (PEEK) Cranial Implants. International Journal of Molecular Sciences. 2021; 22(16):8521. https://doi.org/10.3390/ijms22168521
Chicago/Turabian StyleSharma, Neha, Soheila Aghlmandi, Federico Dalcanale, Daniel Seiler, Hans-Florian Zeilhofer, Philipp Honigmann, and Florian M. Thieringer. 2021. "Quantitative Assessment of Point-of-Care 3D-Printed Patient-Specific Polyetheretherketone (PEEK) Cranial Implants" International Journal of Molecular Sciences 22, no. 16: 8521. https://doi.org/10.3390/ijms22168521
APA StyleSharma, N., Aghlmandi, S., Dalcanale, F., Seiler, D., Zeilhofer, H.-F., Honigmann, P., & Thieringer, F. M. (2021). Quantitative Assessment of Point-of-Care 3D-Printed Patient-Specific Polyetheretherketone (PEEK) Cranial Implants. International Journal of Molecular Sciences, 22(16), 8521. https://doi.org/10.3390/ijms22168521







