Keratin-Alginate Sponges Support Healing of Partial-Thickness Burns
Abstract
:1. Introduction
2. Results
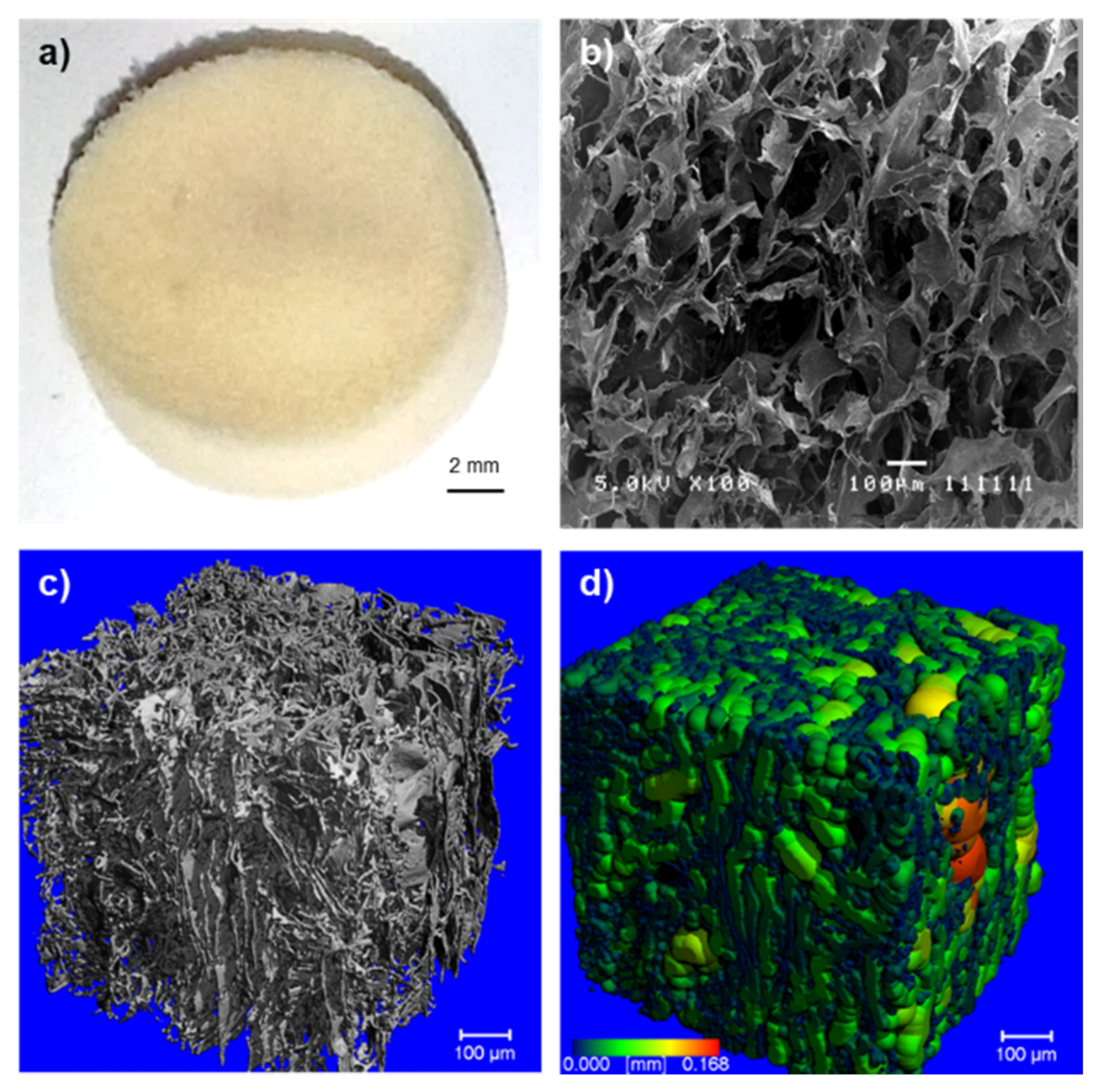
2.1. Morphology of the Crosslinked Keratin–Alginate Sponge
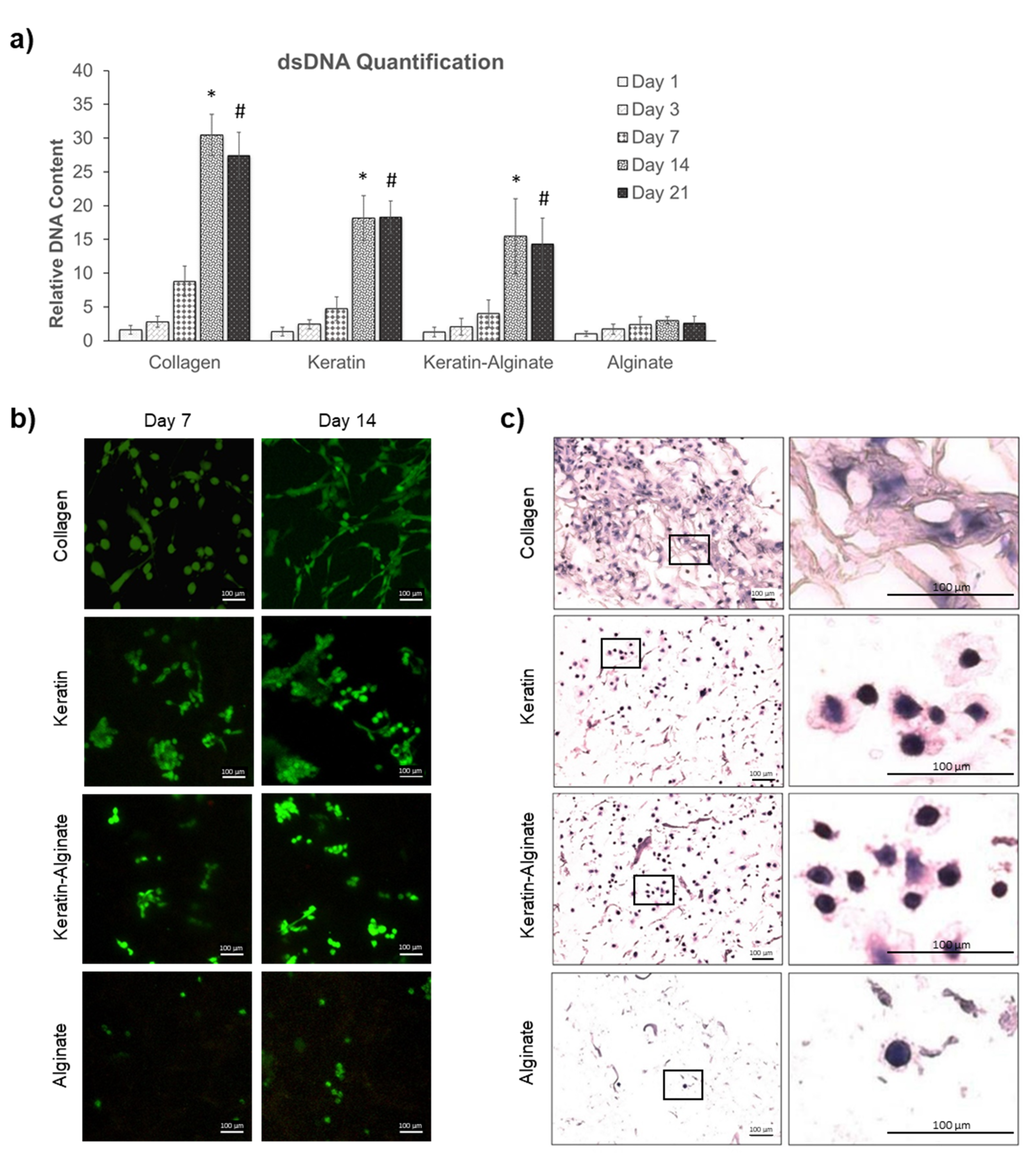
2.2. Culture of Human Dermal Fibroblasts
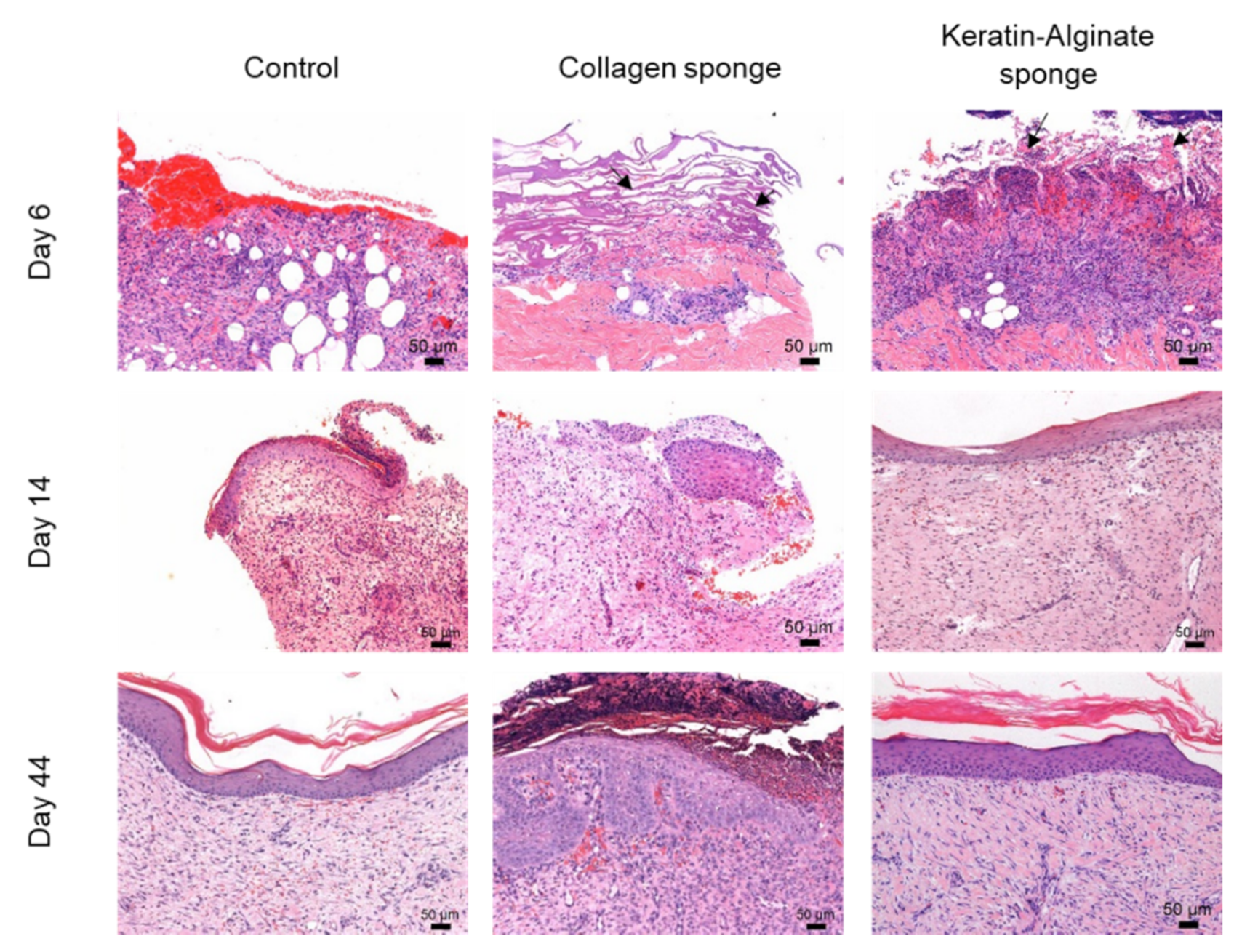
2.3. Evaluation of Keratin–Alginate Sponge in Burn Wound Study
3. Discussion
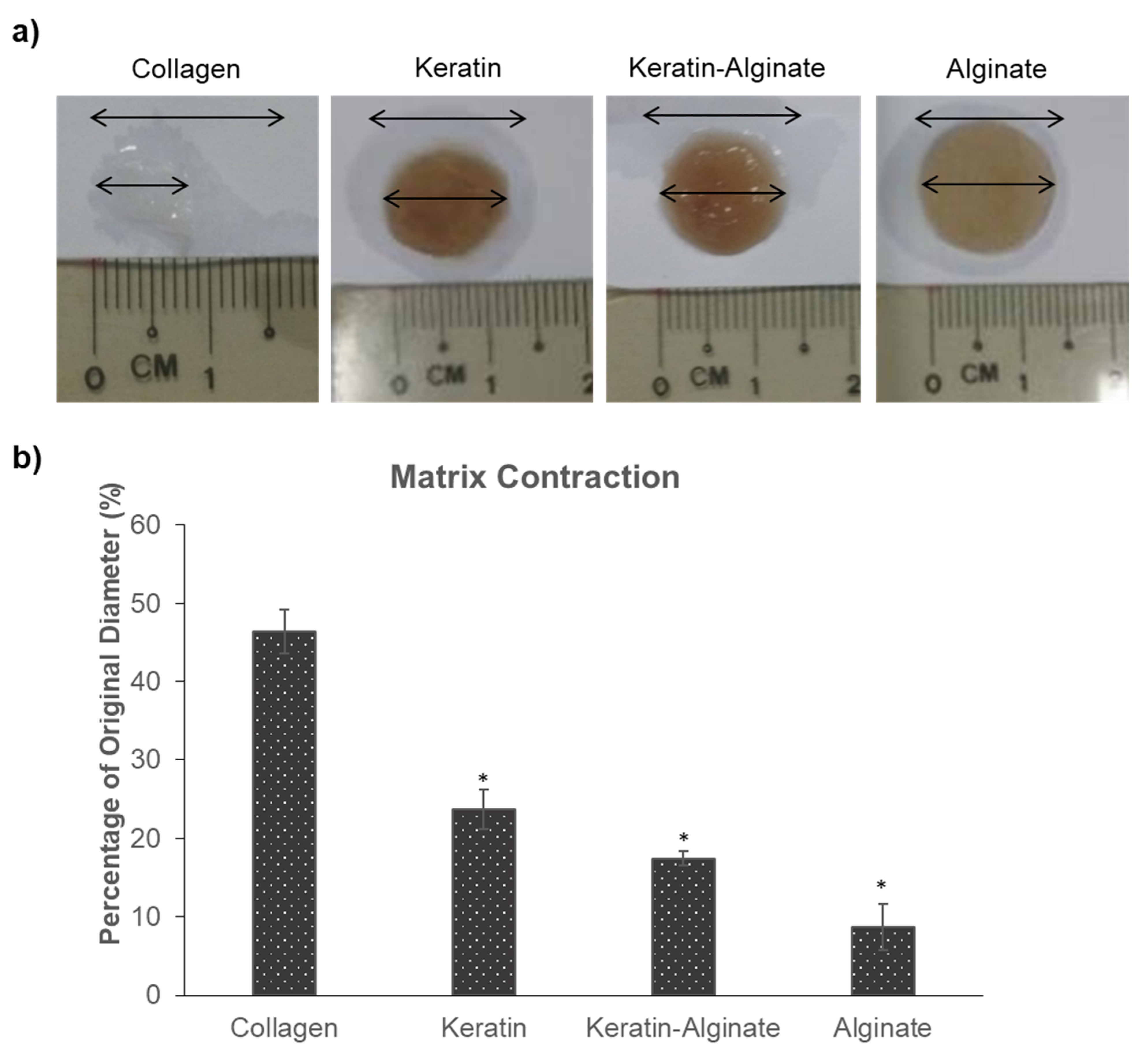
3.1. Keratin–Alginate Sponges Support Cell Proliferation and ECM Formation
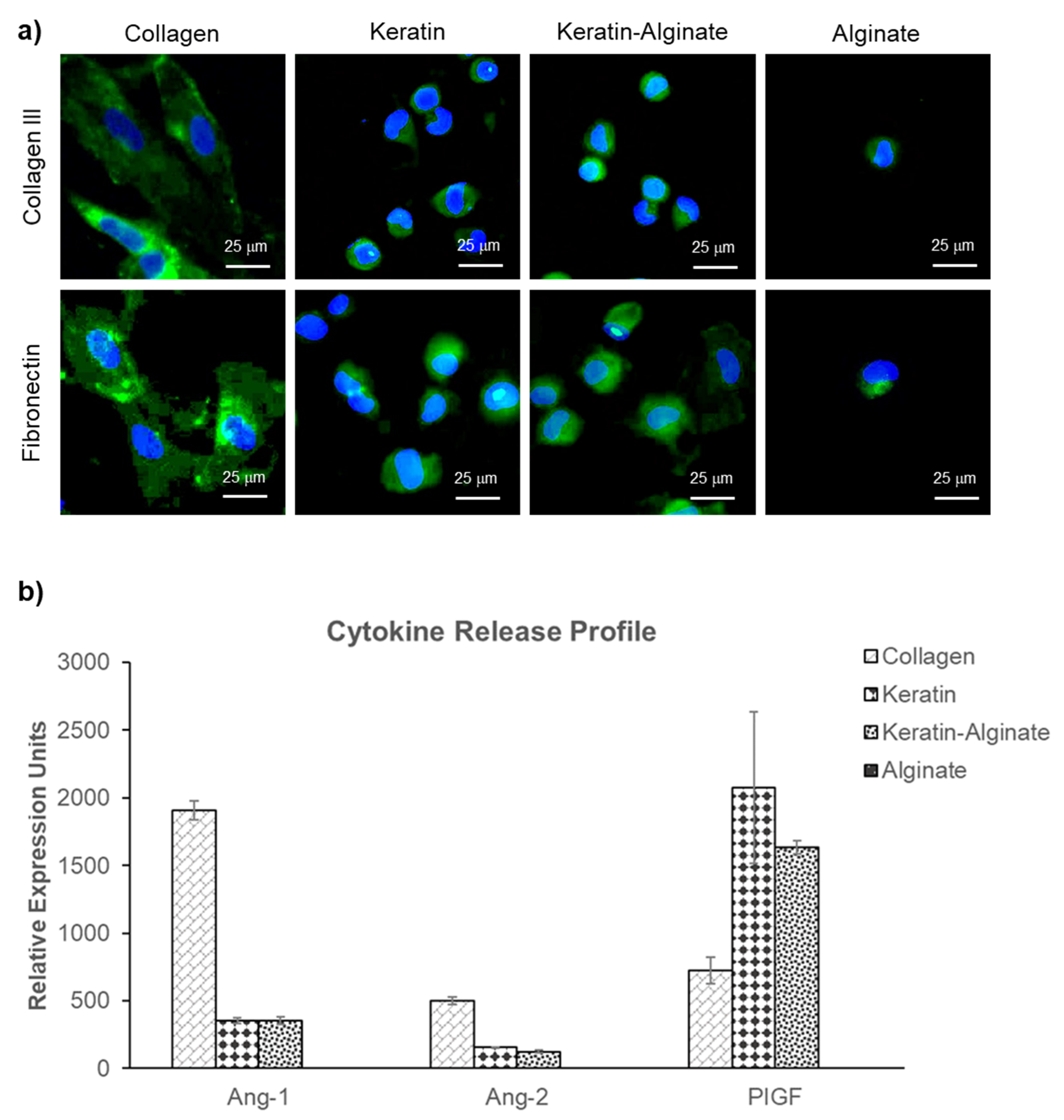
3.2. Angiogenic Ability of Keratin–Alginate Sponges
3.3. Epidermis Formation on Keratin–Alginate Sponges
4. Materials and Methods
4.1. Sponge Fabrication
4.2. Scanning Electron Microscopy Analysis
4.3. MicroCT Analysis
4.4. Human Dermal Fibroblast Culture
4.5. Hematoxylin and Eosin (H&E) Staining
4.6. Immunofluorescence Staining of Cultured Sponges
4.7. Evaluation of Cytokine Release
4.8. Matrix Contraction
4.9. Burn Wound Study
4.10. Immunohistochemistry Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Monzack, E.L.; Rodriguez, K.J.; McCoy, C.M.; Gu, X.X.; Masters, K.S. Natural Materials in Tissue Engineering Applications; Springer: Vienna, Austria, 2011. [Google Scholar]
- Abou Neel, E.A.; Bozec, L.; Knowles, J.C.; Syed, O.; Mudera, V.; Day, R.; Hyun, J.K. Collagen—Emerging collagen based therapies hit the patient. Adv. Drug Deliv. Rev. 2013, 65, 429–456. [Google Scholar] [CrossRef] [PubMed]
- Glowacki, J.; Mizuno, S. Collagen scaffolds for tissue engineering. Biopolymers 2008, 89, 338–344. [Google Scholar] [CrossRef]
- Cen, L.; Liu, W.; Cui, L.; Zhang, W.; Cao, Y. Collagen Tissue Engineering: Development of Novel Biomaterials and Applications. Pediatric Res. 2008, 63, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Juncosa-Melvin, N.; Shearn, J.T.; Boivin, G.P.; Gooch, C.; Galloway, M.T.; West, J.R.; Nirmalanandhan, V.S.; Bradica, G.; Butler, D.L. Effects of Mechanical Stimulation on the Biomechanics and Histology of Stem Cell–Collagen Sponge Constructs for Rabbit Patellar Tendon Repair. Tissue Eng. 2006, 12, 2291–2300. [Google Scholar] [CrossRef]
- Hemmrich, K.; von Heimburg, D.; Rendchen, R.; Di Bartolo, C.; Milella, E.; Pallua, N. Implantation of preadipocyte-loaded hyaluronic acid-based scaffolds into nude mice to evaluate potential for soft tissue engineering. Biomaterials 2005, 26, 7025–7037. [Google Scholar] [CrossRef]
- Solchaga, L.A.; Temenoff, J.S.; Gao, J.; Mikos, A.G.; Caplan, A.I.; Goldberg, V.M. Repair of osteochondral defects with hyaluronan- and polyester-based scaffolds. Osteoarthr. Cartil. 2005, 13, 297–309. [Google Scholar] [CrossRef] [Green Version]
- Rouse, J.G.; Van Dyke, M.E. A Review of Keratin-Based Biomaterials for Biomedical Applications. Materials 2010, 3, 999–1014. [Google Scholar] [CrossRef] [Green Version]
- Hartrianti, P.; Ling, L.; Goh, L.M.; Ow, K.S.; Samsonraj, R.M.; Sow, W.T.; Wang, S.; Nurcombe, V.; Cool, S.M.; Ng, K.W. Modulating Mesenchymal Stem Cell Behavior Using Human Hair Keratin-Coated Surfaces. Stem Cells Int. 2015, 2015, 752424. [Google Scholar] [CrossRef] [Green Version]
- Taraballi, F.; Wang, S.; Li, J.; Lee, F.Y.Y.; Venkatraman, S.S.; Birch, W.R.; Teoh, S.H.; Boey, F.Y.C.; Ng, K.W. Understanding the Nano-topography Changes and Cellular Influences Resulting from the Surface Adsorption of Human Hair Keratins. Adv. Healthc. Mater. 2012, 1, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Z.; Foo, S.E.M.; Tan, N.S.; Yuan, Y.; Lin, W.; Zhang, Z.; Ng, K.W. Culturing Fibroblasts in 3D Human Hair Keratin Hydrogels. ACS Appl. Mater. Interfaces 2015, 7, 5187–5198. [Google Scholar] [CrossRef]
- Chua, H.M.; Zhao, Z.; Ng, K.W. Cryogelation of Human Hair Keratins. Macromol. Rapid Commun. 2020, 41, 2000254. [Google Scholar] [CrossRef]
- Hartrianti, P.; Nguyen, L.T.H.; Johanes, J.; Chou, S.M.; Zhu, P.; Tan, N.S.; Tang, M.B.Y.; Ng, K.W. Fabrication and characterization of a novel crosslinked human keratin-alginate sponge. J. Tissue Eng. Regen. Med. 2017, 11, 2590–2602. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Moay, Z.K.; Lai, H.Y.; Goh, B.H.R.; Chua, H.M.; Setyawati, M.I.; Ng, K.W. Characterization of Anisotropic Human Hair Keratin Scaffolds Fabricated via Directed Ice Templating. Macromol. Biosci. 2020, 2000314. [Google Scholar] [CrossRef] [PubMed]
- Sow, W.T.; Lui, Y.S.; Ng, K.W. Electrospun human keratin matrices as templates for tissue regeneration. Nanomedicine 2013, 8, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Burnett, L.R.; Richter, J.G.; Rahmany, M.B.; Soler, R.; Steen, J.A.; Orlando, G.; Abouswareb, T.; Van Dyke, M.E. Novel keratin (KeraStat™) and polyurethane (Nanosan®-Sorb) biomaterials are hemostatic in a porcine lethal extremity hemorrhage model. J. Biomater. Appl. 2014, 28, 869–879. [Google Scholar] [CrossRef]
- Richter, J.R.; de Guzman, R.C.; Van Dyke, M.E. Mechanisms of hepatocyte attachment to keratin biomaterials. Biomaterials 2011, 32, 7555–7561. [Google Scholar] [CrossRef]
- Gailit, J.; Pierschbacher, M.; Clark, R.A. Expression of functional alpha 4 beta 1 integrin by human dermal fibroblasts. J. Investig. Dermatol. 1993, 100, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Spiekstra, S.W.; Breetveld, M.; Rustemeyer, T.; Scheper, R.J.; Gibbs, S. Wound-healing factors secreted by epidermal keratinocytes and dermal fibroblasts in skin substitutes. Wound Repair Regen. 2007, 15, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.R.; Navarro, J.; Coburn, J.C.; Mahadik, B.; Molnar, J.; Holmes IV, J.H.; Nam, A.J.; Fisher, J.P. Current and Future Perspectives on Skin Tissue Engineering: Key Features of Biomedical Research, Translational Assessment, and Clinical Application. Adv. Healthc. Mater. 2019, 8, 1801471. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Hunt, N.C.; Shelton, R.M.; Grover, L.M. Reversible mitotic and metabolic inhibition following the encapsulation of fibroblasts in alginate hydrogels. Biomaterials 2009, 30, 6435–6443. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Singh, R.; Sarker, B.; Papageorgiou, D.G.; Juhasz, J.A.; Roether, J.A.; Cicha, I.; Kaschta, J.; Schubert, D.W.; Chrissafis, K.; et al. Hybrid hydrogels based on keratin and alginate for tissue engineering. J. Mater. Chem. B 2014, 2, 5441–5451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Taraballi, F.; Tan, L.P.; Ng, K.W. Human keratin hydrogels support fibroblast attachment and proliferation in vitro. Cell Tissue Res. 2012, 347, 795–802. [Google Scholar] [CrossRef]
- Singh, V.; Wang, S.; Ng, K.W. 2.25 Keratin as a Biomaterial. In Comprehensive Biomaterials II; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2017; pp. 542–557. [Google Scholar]
- Dubin-Thaler, B.J.; Hofman, J.M.; Cai, Y.; Xenias, H.; Spielman, I.; Shneidman, A.V.; David, L.A.; Döbereiner, H.-G.; Wiggins, C.H.; Sheetz, M.P. Quantification of Cell Edge Velocities and Traction Forces Reveals Distinct Motility Modules during Cell Spreading. PLoS ONE 2008, 3, e3735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinz, B. Formation and function of the myofibroblast during tissue repair. J. Investig. Dermatol. 2007, 127, 526–537. [Google Scholar] [CrossRef]
- Desmoulière, A.; Chaponnier, C.; Gabbiani, G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005, 13, 7–12. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.H. Fibroblasts and myofibroblasts in wound healing: Force generation and measurement. J. Tissue Viability 2011, 20, 108–120. [Google Scholar] [CrossRef] [Green Version]
- Gillard, J.; Reed, M.; Buttle, D.; Cross, S.; Brown, N. Matrix metalloproteinase activity and immunohistochemical profile of matrix metalloproteinase-2 and -9 and tissue inhibitor of metalloproteinase-1 during human dermal wound healing. Wound Repair Regen. 2004, 12, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Kim, H.; Liu, X.; Sugiura, H.; Kohyama, T.; Fang, Q.; Wen, F.-Q.; Abe, S.; Wang, X.; Atkinson, J.J.; et al. Matrix metalloproteinase-9 activates TGF-β and stimulates fibroblast contraction of collagen gels. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 306, L1006–L1015. [Google Scholar] [CrossRef] [Green Version]
- Van Wart, H.E.; Birkedal-Hansen, H. The cysteine switch: A principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc. Natl. Acad. Sci. USA 1990, 87, 5578–5582. [Google Scholar] [CrossRef] [Green Version]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123 (Pt 24), 4195–4200. [Google Scholar] [CrossRef] [Green Version]
- Staton, C.A.; Valluru, M.; Hoh, L.; Reed, M.W.; Brown, N.J. Angiopoietin-1, angiopoietin-2 and Tie-2 receptor expression in human dermal wound repair and scarring. Br. J. Dermatol. 2010, 163, 920–927. [Google Scholar] [PubMed]
- Breitkreutz, D.; Mirancea, N.; Nischt, R. Basement membranes in skin: Unique matrix structures with diverse functions? Histochem. Cell Biol. 2009, 132, 1–10. [Google Scholar] [CrossRef]
- Ghahary, A.; Ghaffari, A. Role of keratinocyte-fibroblast cross-talk in development of hypertrophic scar. Wound Repair Regen. 2007, 15 (Suppl. 1), S46–S53. [Google Scholar] [CrossRef]
- Hildebrand, T.; Rüegsegger, P. A new method for the model-independent assessment of thickness in three-dimensional images. J. Microsc. 1997, 185, 67–75. [Google Scholar] [CrossRef]
- Aptum Biologics Ltd. How to Run the Antigen-Unmasking Cycle. Available online: https://www.antigen-retriever.com/about (accessed on 22 July 2020).
- Cuttle, L.; Kempf, M.; Phillips, G.E.; Mill, J.; Hayes, M.T.; Fraser, J.F.; Wang, X.Q.; Kimble, R.M. A porcine deep dermal partial thickness burn model with hypertrophic scarring. Burns 2006, 32, 806–820. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, C.; Zhang, A.; Wang, H.; Rao, H.; Zhang, Z. Fabrication and biological evaluation in vivo of an injectable keratin hydrogel as filler materials. J. Bioact. Compat. Polym. 2016, 31, 179–190. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moay, Z.K.; Nguyen, L.T.H.; Hartrianti, P.; Lunny, D.P.; Leavesley, D.; Kok, Y.O.; Chong, S.J.; Chua, A.W.C.; Tee, S.-I.; Ng, K.W. Keratin-Alginate Sponges Support Healing of Partial-Thickness Burns. Int. J. Mol. Sci. 2021, 22, 8594. https://doi.org/10.3390/ijms22168594
Moay ZK, Nguyen LTH, Hartrianti P, Lunny DP, Leavesley D, Kok YO, Chong SJ, Chua AWC, Tee S-I, Ng KW. Keratin-Alginate Sponges Support Healing of Partial-Thickness Burns. International Journal of Molecular Sciences. 2021; 22(16):8594. https://doi.org/10.3390/ijms22168594
Chicago/Turabian StyleMoay, Zi Kuang, Luong T. H. Nguyen, Pietradewi Hartrianti, Declan P. Lunny, David Leavesley, Yee Onn Kok, Si Jack Chong, Alvin Wen Choong Chua, Shang-Ian Tee, and Kee Woei Ng. 2021. "Keratin-Alginate Sponges Support Healing of Partial-Thickness Burns" International Journal of Molecular Sciences 22, no. 16: 8594. https://doi.org/10.3390/ijms22168594
APA StyleMoay, Z. K., Nguyen, L. T. H., Hartrianti, P., Lunny, D. P., Leavesley, D., Kok, Y. O., Chong, S. J., Chua, A. W. C., Tee, S.-I., & Ng, K. W. (2021). Keratin-Alginate Sponges Support Healing of Partial-Thickness Burns. International Journal of Molecular Sciences, 22(16), 8594. https://doi.org/10.3390/ijms22168594






