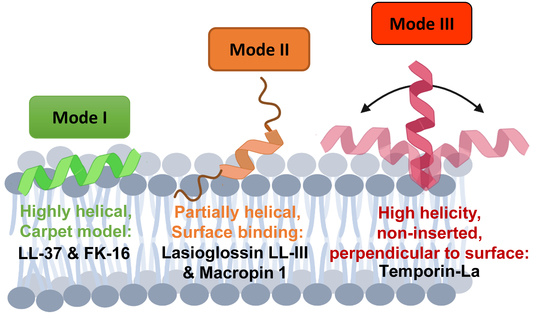
Membrane Association Modes of Natural Anticancer Peptides: Mechanistic Details on Helicity, Orientation, and Surface Coverage
Abstract
Share and Cite
Quemé-Peña, M.; Juhász, T.; Kohut, G.; Ricci, M.; Singh, P.; Szigyártó, I.C.; Papp, Z.I.; Fülöp, L.; Beke-Somfai, T. Membrane Association Modes of Natural Anticancer Peptides: Mechanistic Details on Helicity, Orientation, and Surface Coverage. Int. J. Mol. Sci. 2021, 22, 8613. https://doi.org/10.3390/ijms22168613
Quemé-Peña M, Juhász T, Kohut G, Ricci M, Singh P, Szigyártó IC, Papp ZI, Fülöp L, Beke-Somfai T. Membrane Association Modes of Natural Anticancer Peptides: Mechanistic Details on Helicity, Orientation, and Surface Coverage. International Journal of Molecular Sciences. 2021; 22(16):8613. https://doi.org/10.3390/ijms22168613
Chicago/Turabian StyleQuemé-Peña, Mayra, Tünde Juhász, Gergely Kohut, Maria Ricci, Priyanka Singh, Imola Cs. Szigyártó, Zita I. Papp, Lívia Fülöp, and Tamás Beke-Somfai. 2021. "Membrane Association Modes of Natural Anticancer Peptides: Mechanistic Details on Helicity, Orientation, and Surface Coverage" International Journal of Molecular Sciences 22, no. 16: 8613. https://doi.org/10.3390/ijms22168613
APA StyleQuemé-Peña, M., Juhász, T., Kohut, G., Ricci, M., Singh, P., Szigyártó, I. C., Papp, Z. I., Fülöp, L., & Beke-Somfai, T. (2021). Membrane Association Modes of Natural Anticancer Peptides: Mechanistic Details on Helicity, Orientation, and Surface Coverage. International Journal of Molecular Sciences, 22(16), 8613. https://doi.org/10.3390/ijms22168613