PPARγ-Induced Global H3K27 Acetylation Maintains Osteo/Cementogenic Abilities of Periodontal Ligament Fibroblasts
Abstract
1. Introduction
2. Results
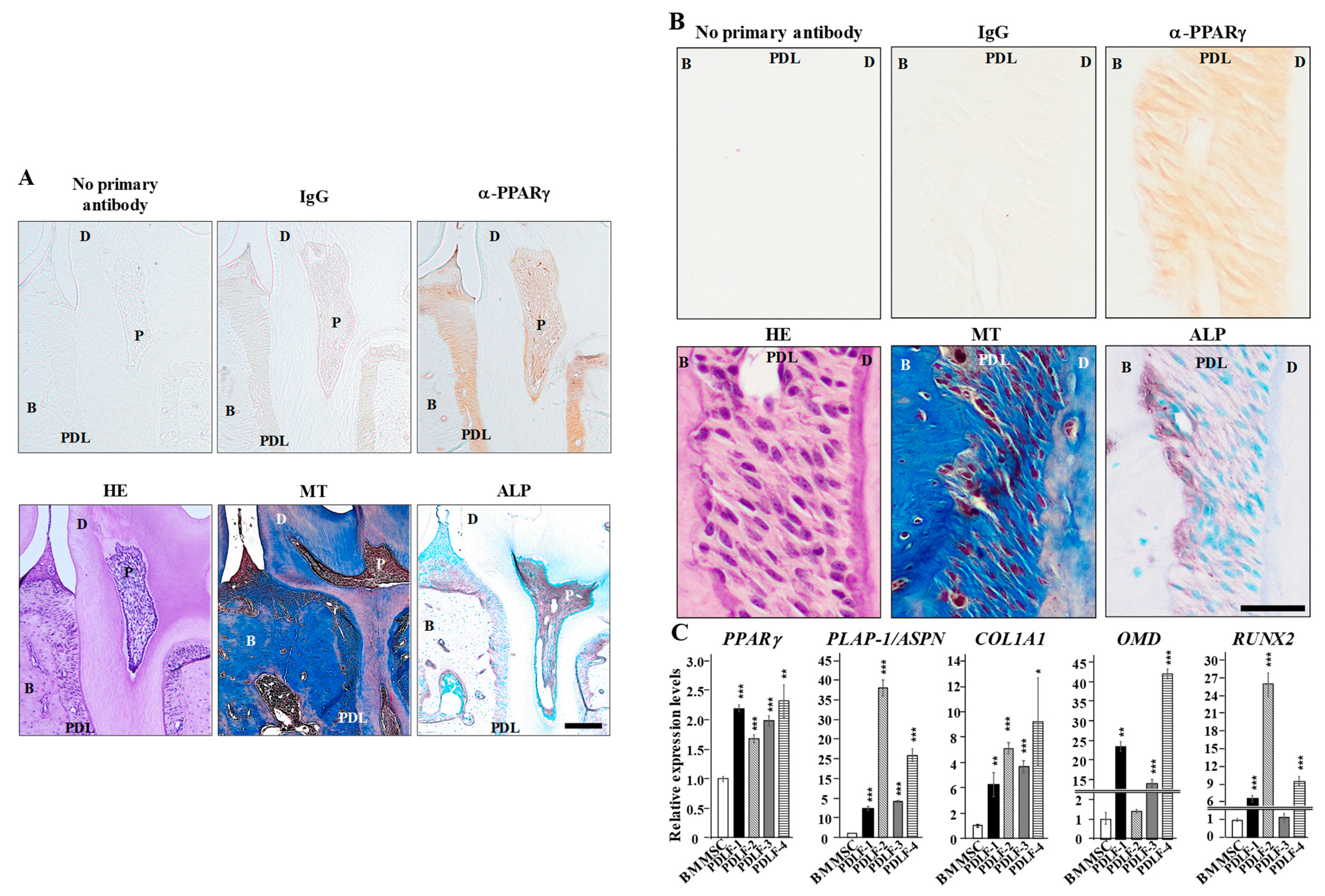
2.1. PPARγ Expression in PDL Tissue and PDLF
2.2. Ligand-Dependent Modulation of PPARγ Activity Positively Correlates with the Osteo/Cementogenic Potential of PDLF
2.3. PDLF Lose Osteo/Cementogenic Gene Expression Due to the Suppressed Expression of PPARγ
2.4. The Knockdown of PPARγ Inhibits the Global Acetylation of H3K9 and H3K27
2.5. The Knockdown of PPARγ Comprehensively Alters Gene Expression Profiles
2.6. The Whole-Genomic Identification of H3K27ac Peaks Reveals the Enrichment of RUNX2-Binding Sites in Peaks Retained by PPARγ
2.7. Sodium Acetate Supplementation Restores si-PPARγ-Suppressed ALP Activity More Effectively Than the HDAC Inhibitor
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Histology
4.3. Cell Culture
4.4. Quantitative PCR (qPCR) Analysis
4.5. ALP Activity
4.6. Alizarin Red S Staining
4.7. Transient Transfection of siRNA
4.8. Histone Purification
4.9. Immunoblotting
4.10. Subcellular Fractionation
4.11. RNA-seq
4.12. Chromatin Immunoprecipitation (ChIP)-seq
4.13. Accession Numbers
4.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


| Primer Name | Direction | Sequence |
|---|---|---|
| PPARγ | forward | GAGCCCAAGTTTGAGTTTGC |
| reverse | GGCGGTCTCCACTGAGAATA | |
| COL1A1 | forward | GTGCTAAAGGTGCCAATGGT |
| reverse | ACCAGGTTCACCGCTGTTAC | |
| PLAP-1/ASPN | forward | GAGAGCCAAGAAGCCATTTTT |
| reverse | AATGTTGGTTGGGACTGAGG | |
| RUNX2 | forward | CGCATTCCTCATCCCAGTAT |
| reverse | GCCTGGGGTCTGTAATATGA | |
| ALPL | forward | GACAAGAAGCCCTTCACTGC |
| reverse | AGACTGCGCCTGGTAGTTGT | |
| BGLAP | forward | GGCGCTACCTGTATCAATGG |
| reverse | TCAGCCAACTCGTCACAGTC | |
| SULF2 | forward | GAGCTGCAAGGGTTACAAGC |
| reverse | CTTCCCACAGTTGTCCCAGT | |
| RCAN2 | forward | GGGCTGTCACTCGTTGTTTT |
| reverse | GTCCGAAACAGTCCCTCAAA | |
| RGMA | forward | GAACGTGCAGGTCACCAAC |
| reverse | TGTCCCCACCGTTCTTAGAG | |
| OMD | forward | AGGCTGTGTCAGTGAATGCTT |
| reverse | GCTGAATGTGCATCGGAATA | |
| HPRT | forward | TGGCGTCGTGATTAGTGATG |
| reverse | CGAGCAAGACGTTCAGTCCT |
| Target Sequences for siRNA Duplex | ||
|---|---|---|
| Oligo Name | Sense Strand (5′ → 3′) | Antisense Strand (5′ → 3′) |
| si-PPARγ | CACUAUUCUGAGGGAAAAUCU | AUUUUCCCUCAGAAUAGUGCA |
| si-PPARγ-2 | UGUAUGAGUCAUACAAAUGUU | CAUUUGUAUGACUCAUACAUA |
| si-PPARγ-3 | AAUUCAUGUCAUAGAUAACGA | GUUAUCUAUGACAUGAAUUCC |
| si-PPARγ-4 | UCUUGAAUGUCUUCAAUGGGC | CCAUUGAAGACAUUCAAGACA |
| si-control | GUACCGCACGUCAUUCGUAUC | UACGAAUGACGUGCGGUACGU |
References
- Bruce, L.P.; Bryan, S.M.; Newell, W.J. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Petersen, P.E.; Ogawa, H. Strengthening the Prevention of Periodontal Disease: The WHO Approach. J. Periodontol. 2005, 76, 2187–2193. [Google Scholar] [CrossRef]
- Strydom, H.; Maltha, J.C.; Kuijpers-Jagtman, A.M.; Von den Hoff, J.W. The oxytalan fibre network in the periodontium and its possible mechanical function. Arch. Oral Biol. 2012, 57, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- de Jong, T.; Bakker, A.D.; Everts, N.; Smit, T.H. The intricate anatomy of the periodontal ligament and its development: Lessons for periodontal regeneration. J. Periodontal Res. 2017, 52, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Ivanovski, S.; Haase, H.R.; Bartold, P.M. Expression of bone matrix Protein mRNAs by Primary and Cloned Cultures of the Regenerative Phenotype of Human Periodontal Fibroblasts. J. Dent. Res. 2001, 80, 1665–1671. [Google Scholar] [CrossRef]
- Lekic, P.; McCulloch, C.A.G. Periodontal ligament cell populations: The central role of fibroblasts in creating a unique tissue. Anat. Rec. 1996, 245, 327–341. [Google Scholar] [CrossRef]
- Brunette, D.M.; Melcher, A.H.; Moe, H.K. Culture and origin of epithelium-like and fibroblast-like cells from porcine periodontal ligament explants and cell suspensions. Arch. Oral. Bio. 1975, 21, 393–400. [Google Scholar] [CrossRef]
- Gould, T.R.L. Ultrastructural Characteristics of Progenitor Cell Populations in the Periodontal Ligament. J. Dent. Res. 1983, 62, 873–876. [Google Scholar] [CrossRef]
- Prateeptongkum, E.; Klingelhöffer, C.; Müller, S.; Ettl, T.; Morsczeck, C. Characterization of progenitor cells and stem cells from the periodontal ligament tissue derived from a single person. J. Periodontal Res. 2016, 51, 265–272. [Google Scholar] [CrossRef]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.J.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Zepp, J.A.; Zacharias, W.J.; Frank, D.B.; Cavanaugh, C.A.; Zhou, S.; Morley, M.P.; Morrisey, E.E. Distinct Mesenchymal Lineages and Niches Promote Epithelial Self-Renewal and Myofibrogenesis in the Lung. Cell 2017, 170, 1134–1148. [Google Scholar] [CrossRef]
- Tsukui, T.; Sun, K.W.; Wetter, J.B.; Wilson-Kanamori, J.R.; Hazelwood, L.A.; Henderson, N.C.; Adams, T.S.; Schupp, J.C.; Poli, S.D.; Rosas, I.O.; et al. Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nat. Commun. 2020, 11, 1920. [Google Scholar] [CrossRef]
- Zhou, Y.; Hutmacher, D.W.; Sae-Lim, V.; Zhou, Z.; Woodruff, M.; Lim, T.M. Osteogenic and Adipogenic Induction Potential of Human Periodontal Cells. J. Periodontol. 2008, 79, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Boyko, G.A.; Melcher, A.H.; Brunette, D.M. Formation of new periodontal ligament by periodontal ligament cells implanted in vivo after culture in vitro: A preliminary study of transplanted roots in the dog. J. Periodontal. Res. 1981, 16, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.H.; Liu, B.; Cheng, D.; Williams, B.O.; Mah, S.J.; Helms, J.A. Wnt signaling regulates homeostasis of the periodontal ligament. J. Periodontal. Res. 2014, 49, 751–759. [Google Scholar] [CrossRef]
- Aida, Y.; Kurihara, H.; Kato, K. Wnt3a promotes differentiation of human bone marrow-derived mesenchymal stem cells into cementoblast-like cells In. Vitro. Cell. Dev. Biol. Anim. 2018, 54, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Dristi, B.; Ahmed, E.; Karen, S.K. The human WNT5A isoforms display similar patterns of expression but distinct and overlapping activities in normal human osteoblasts. J. Cell. Biochem. 2021. [Google Scholar] [CrossRef]
- Naruphong, P.; Nittaya, B.; Pakpoom, K.; Kanoknetr, S.; Sirikul, M.; Chairat, T.; Duangrat, T. Andrographolide promotes proliferative and osteogenic potentials of human placenta-derived mesenchymal stem cells through the activation of Wnt/β-catenin signaling. Stem. Cell. Res. Ther. 2021, 12, 241. [Google Scholar] [CrossRef]
- Lima, M.L.S.; Medeiros, C.A.C.X.; Guerra, G.C.B.; Santos, R.; Bader, M.; Pirih, F.Q.; Araújo Júnior, R.F.; Chan, A.B.; Cruz, L.J.; Brito, G.A.C.; et al. AT1 and AT2 Receptor Knockout Changed Osteonectin and Bone Density in Mice in Periodontal Inflammation Experimental Model. Int. J. Mol. Sci. 2021, 22, 5217. [Google Scholar] [CrossRef]
- Patricia, C.; Chui, H.P.J.; Michael, L.; Mitchell, A.L. PPARγ regulates adipocyte cholesterol metabolism via oxidized LDL receptor 1. J. Clin. Investig. 2005, 115, 2244–2256. [Google Scholar] [CrossRef]
- Umeno, A.; Sakashita, M.; Sugino, S.; Murotomi, K.; Okuzawa, T.; Morita, N.; Tomii, K.; Tsuchiya, Y.; Yamasaki, K.; Horie, M.; et al. Comprehensive analysis of PPARgamma agonist activities of stereo-, regio-, and enantio-isomers of hydroxyoctadecadienoic acids. Biosci. Rep. 2020, 40, 1–40. [Google Scholar] [CrossRef]
- Jiang, C.; Ting, A.T.; Seed, B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature 1998, 391, 82–86. [Google Scholar] [CrossRef]
- Jung, W.K.; Park, I.S.; Park, S.J.; Yea, S.S.; Choi, Y.H.; Oh, S.; Park, S.G.; Choi, I.W. The 15-deoxy-Delta12,14-prostaglandin J2 inhibits LPS-stimulated AKT and NF-kappaB activation and suppresses interleukin-6 in osteoblast-like cells MC3T3E-1. Life Sci. 2009, 85, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Hassumi, M.Y.; Silva-Filho, V.J.; Campos-Júnior, J.C.; Vieira, S.M.; Cunha, F.Q.; Alves, P.M.; Alves, J.B.; Kawai, T.; Gonçalves, R.B.; Napimoga, M.H. PPAR-gamma agonist rosiglitazone prevents inflammatory periodontal bone loss by inhibiting osteoclastogenesis. Int. Immunopharmacol. 2009, 9, 1150–1158. [Google Scholar] [CrossRef]
- Akune, T.; Ohba, S.; Kamekura, S.; Yamaguchi, M.; Chung, U.; Kubota, N.; Terauchi, Y.; Harada, Y.; Azuma, Y.; Nakmura, K.; et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J. Clin. Investig. 2004, 113, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Soccio1, R.E.; Chen, E.R.; Lazar, M.A. Thiazolidinediones and the Promise of Insulin Sensitization in Type 2 Diabetes. Cell Metab. 2014, 20, 573–591. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Oh, Y.J.; Park, B.; Lee, S.; Jeong, C.H.; Lee, S.; Seo, J.H.; Seo, Y.H. Development of Thiazolidinedione-Based HDAC6 Inhibitors to Overcome Methamphetamine Addiction. Int. J. Mol. Sci. 2019, 20, 6213. [Google Scholar] [CrossRef]
- Lagosz, K.B.; Bysiek, A.; Macina, J.M.; Bereta, G.P.; Kantorowicz, M.; Lipska, W.; Sochalska, M.; Gawron, K.; Kacmarzyk, T.; Chomyszyn-Gajewska, M.; et al. HDAC3 Regulates Gingival Fibroblast Inflammatory Responses in Periodontitis. J. Dent. Res. 2020, 99, 98–106. [Google Scholar] [CrossRef]
- Huynh, N.C.; Everts, V.; Pavasant, P.; Ampornaramveth, R.S. Inhibition of Histone Deacetylases Enhances the Osteogenic Differentiation of Human Periodontal Ligament Cells. J. Cell Biochem. 2016, 117, 1384–1395. [Google Scholar] [CrossRef]
- Koda, N.; Sato, T.; Shinohara, M.; Ichinose, S.; Ito, Y.; Nakamichi, R.; Kayama, T.; Kataoka, K.; Suzuki, H.; Moriyama, K.; et al. The transcription factor mohawk homeobox regulates homeostasis of the periodontal ligament. Development 2017, 144, 313–320. [Google Scholar] [CrossRef]
- Yamada, S.; Tomoeda, M.; Ozawa, Y.; Yoneda, S.; Terashima, Y.; Ikezawa, K.; Ikegawa, S.; Saito, M.; Toyosawa, S.; Murakami, S. PLAP-1/asporin, a novel negative regulator of periodontal ligament mineralization. J. Biol. Chem. 2007, 282, 23070–23080. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, A.; Kawatsu, M.; Yoshimoto, Y.; Kawamoto, T.; Seiryu, M.; Takano-Yamamoto, T.; Hiraki, Y.; Shukunami, C. Scleraxis and osterix antagonistically regulate tensile force-responsive remodeling of the periodontal ligament and alveolar bone. Development 2015, 142, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Watt, J.; Schlezinger, J.J. Structurally-diverse, PPARγ-activating environmental toxicants induce adipogenesis and suppress osteogenesis in bone marrow mesenchymal stromal cells. Toxicology 2015, 331, 66–77. [Google Scholar] [CrossRef]
- Fu, S.; Wang, Q.; Moore, J.E.; Purcaro, M.J.; Pratt, H.E.; Fan, K.; Gu, C.; Jiang, C.; Zhu, R.; Kundaje, A.; et al. Differential analysis of chromatin accessibility and histone modifications for predicting mouse developmental enhancers. Nucleic Acids Res. 2018, 46, 11184–11201. [Google Scholar] [CrossRef] [PubMed]
- Kawane, T.; Komori, H.; Liu, W.; Moriishi, T.; Miyazaki, T.; Mori, M.; Matsuo, Y.; Takada, Y.; Izumi, S.; Jiang, Q.; et al. Dlx5 and Mef2 Regulate a Novel Runx2 Enhancer for Osteoblast-Specific Expression. J. Bone Miner. Res. 2014, 29, 1960–1969. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Whitfield, T.W.; Gordon, J.A.; Dobson, J.R.; Tai, P.W.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; Lian, J.B. Genomic occupancy of Runx2 with global expression profiling identifies a novel dimension to control of osteoblastogenesis. Genome Biol. 2014, 15, R52. [Google Scholar] [CrossRef] [PubMed]
- Zaman, G.; Staines, K.A.; Farquharson, C.; Newton, P.T.; Dudhia, J.; Chenu, C.; Pitsillides, A.A.; Dhoot, G.K. Expression of Sulf1 and Sulf2 in cartilage, bone and endochondral fracture healing. Histochem. Cell Biol. 2016, 145, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Hayano, S.; Kurosaka, H.; Yanagita, T.; Kalus, I.; Milz, F.; Ishihara, Y.; Islam, M.N.; Kawanabe, N.; Saito, M.; Kamoka, H.; et al. Roles of Heparan Sulfate Sulfation in Dentinogenesis. J. Biol. Chem. 2012, 287, 12217–12229. [Google Scholar] [CrossRef] [PubMed]
- Bassett, J.H.; Logan, J.G.; Boyde, A.; Cheung, M.S.; Evans, H.; Croucher, P.; Sun, X.; Xu, S.; Murata, Y.; Williams, G.R. Mice Lacking the Calcineurin Inhibitor Rcan2 Have an Isolated Defect of Osteoblast Function. Endocrinology 2012, 153, 3537–3548. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yu, P.B.; Sidis, Y.; Beppu, H.; Bloch, K.D.; Schneyer, A.L.; Lin, H.Y. Repulsive guidance molecule RGMa alters utilization of bone morphogenetic protein (BMP) type II receptors by BMP2 and BMP4. J. Biol. Chem. 2007, 282, 18129–18140. [Google Scholar] [CrossRef]
- Iwata, T.; Mizuno, N.; Nagahara, T.; Kaneda-Ikeda, E.; Kajiya, M.; Kitagawa, M.; Takeda, K.; Yoshioka, M.; Yagi, R.; Takata, T.; et al. Identification of regulatory mRNA and microRNA for differentiation into cementoblasts and periodontal ligament cells. J. Periodontal Res. 2021, 56, 69–82. [Google Scholar] [CrossRef]
- Ziros, P.G.; Gil, A.P.; Georgakopoulos, T.; Habeos, I.; Kletsas, T.; Basdra, E.K.; Papavassiliou, A.G. The Bone-spcific Transcriptional Regulator Cbfa1 Is a Target of Mechanical Signals in Osteoblastic Cells. J. Biol. Chem. 2002, 277, 23934–23941. [Google Scholar] [CrossRef]
- Ishibashi, O.; Ikegame, M.; Takizawa, F.; Yoshizawa, T.; Moksed, M.A.; Iizawa, F.; Mera, H.; Matsuda, A.; Kawashima, H. Endoglin is involved in BMP-2-induced osteogenic differentiation of periodontal ligament cells through a pathway independent of Smad-1/5/8 phosphorylation. J. Cell Physiol. 2010, 222, 465–473. [Google Scholar] [CrossRef]
- Fuchigami, S.; Nakamura, T.; Furue, K.; Sena, K.; Shinohara, Y.; Noguchi, K. Recombinant human bone morphogenetic protein-9 potently induces osteogenic differentiation of human periodontal ligament fibroblasts. Eur. J. Oral Sci. 2016, 124, 151–157. [Google Scholar] [CrossRef]
- Bian, Y.; Xiang, J. Salvianolic acid B promotes the osteogenic differentiation of human periodontal ligament cells through Wnt/β-catenin signaling pathway. Arch. Oral Biol. 2020, 113, 104693. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, B.; He, J.; Cui, Z.; Liu, Y. Silver nanoparticles promote osteogenic differentiation of human periodontal ligament fibroblasts by regulating the RhoA–TAZ axis. Cell Biol. Int. 2019, 43, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Kei, H.; Saori, F.; Daisuke, K. Metabolic Reprogramming Induces Germinal Center B Cell Differentiation through Bcl6 Locus Remodeling. Cell Rep. 2020, 33, 108333. [Google Scholar] [CrossRef]
- Arts, R.J.; Novakovic, B.; Ter Horst, R.; Carvalho, A.; Bekkering, S.; Lachmandas, E.; Rodrigues, F.; Silvestre, R.; Cheng, S.C.; Wang, S.Y.; et al. Glutaminolysis and Fumarate Accumulation Integrate Immunometabolic and Epigenetic Programs in Trained Immunity. Cell. Metab. 2016, 24, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Takai, R.; Uehara, O.; Harada, F.; Utsunomiya, M.; Chujo, T.; Yoshida, K.; Sato, J.; Nishimura, M.; Chiba, I.; Abiko, Y. DNA hypermethylation of extracellular matrix-related genes in human periodontal fibroblasts induced by stimulation for a prolonged period with lipopolysaccharide derived from Porphyromonas gingivalis. J. Periodont. Res. 2015, 51, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.V.; Berry, C.T.; Kim, K.; Sen, P.; Kim, T.; Carrer, A.; Trefely, S.; Zhao, S.; Fernandez, S.; Barney, L.E.; et al. Acetyl-CoA promotes glioblastoma cell adhesion and migration through Ca2+–NFAT signaling. Genes Dev. 2018, 32, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Villa, M.; Sanin, D.E.; Buck, M.D.; O’Sullivan, D.; Ching, R.; Matsushita, M.; Grzes, K.M.; Winkler, F.; Chang, C.H.; et al. Acetate Promotes T Cell Effector Function during Glucose Restriction. Cell Rep. 2019, 27, 2063–2074. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, P. Acetyl-CoA Metabolism and Histone Acetylation in the Regulation of Aging and Lifespan. Antioxidants 2021, 10, 572. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Suh, J.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Tu, B.P. Acetyl-CoA and the regulation of metabolism: Mechanisms and consequences. Curr. Opin. Cell. Biol. 2015, 33, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Furuhata, R.; Kabe, Y.; Kanai, A.; Sugiura, y.; Tsugawa, H.; Sugiyama, E.; Hirai, M.; Yamamoto, T.; Koike, I.; Yoshikawa, N.; et al. Progesterone receptor membrane associated component 1 enhances obesity progression in mice by facilitating lipid accumulation in adipocytes. Commu. Biol. 2020, 3, 479. [Google Scholar] [CrossRef]
- Berahim, Z.; Moharamzadeh, K.; Jowett, A.; Rawlinson, A. Evaluation of Osteogenic and Cementogenic Potential of Periodontal Ligament Fibroblast Spheroids Using a Three-Dimensional In Vitro Model of Periodontium. Int. J. Dent. 2015, 2005, 605813. [Google Scholar] [CrossRef][Green Version]
- McIntyre, R.; Soczynska, J.; Woldeyohannes, H.; Lewis, G.; Leiter, L.; Macqueen, G.; Miranda, A.; Fulgosi, D.; Konarski, J.; Kennedy, S. Thiazolidinediones: Novel treatments for cognitive deficits in mood disorders? Expert. Opin. Pharmacother 2007, 8, 1615–1628. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, J.S.; Kim, J.E.; Lee, M.H.; Jeon, J.G.; Park, I.S.; Yi, H.K. Nanoparticle mediated PPARγ gene delivery on dental implants improves osseointegration via mitochondrial biogenesis in diabetes mellitus rat model. Nanomedicine 2017, 13, 1821–1832. [Google Scholar] [CrossRef]
- Bhattarai, G.; Lee, Y.H.; Lee, N.H.; Park, I.S.; Lee, M.H.; Yi, H.K. PPARγ delivered by Ch-GNPs onto titanium surfaces inhibits implant-induced inflammation and induces bone mineralization of MC-3T3E1 osteoblast-like cells. Clin. Oral Impl. Res. 2013, 24, 1101–1109. [Google Scholar] [CrossRef]
- Urata, M.; Kokabu, S.; Matsubara, T.; Sugiyama, G.; Nakatomi, C.; Takeuchi, H.; Hirata-Tsuchiya, S.; Aoki, K.; Tamura, Y.; Moriyama, Y.; et al. A peptide that blocks the interaction of NF-κB p65 subunit with Smad4 enhances BMP2-induced osteogenesis. J. Cell. Physiol. 2018, 233, 7356–7366. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Yuan, H.; Hirata-Tsuchiya, S.; Yoshida, K.; Sato, A.; Nemoto, E.; Shiba, H.; Yamada, S. DMP-1 promoter-associated antisense strand non-coding RNA, panRNA-DMP-1, physically associates with EGFR to repress EGF-induced squamous cell carcinoma migration. Mol. Cell. Biochem. 2021, 476, 1673–1690. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Heinz, S.; Benner, C.; Spann, N.; Bertolino, E.; Lin, Y.C.; Laslo, P.; Cheng, J.X.; Murre, C.; Singh, H.; Glass, C.K. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell. 2010, 38, 576–589. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Ramírez, F.; Ryan, D.P.; Grüning, B.; Bhardwaj, V.; Kilpert, F.; Richter, A.S.; Heyne, S.; Dündar, F.; Manke, T. deepTools2: A next generation web server for deep-sequencing data analysis. Nucleic. Acids Res. 2016, 44, 160–165. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Suzuki, S.; Hirata-Tsuchiya, S.; Sato, A.; Nemoto, E.; Saito, M.; Shiba, H.; Yamada, S. PPARγ-Induced Global H3K27 Acetylation Maintains Osteo/Cementogenic Abilities of Periodontal Ligament Fibroblasts. Int. J. Mol. Sci. 2021, 22, 8646. https://doi.org/10.3390/ijms22168646
Yuan H, Suzuki S, Hirata-Tsuchiya S, Sato A, Nemoto E, Saito M, Shiba H, Yamada S. PPARγ-Induced Global H3K27 Acetylation Maintains Osteo/Cementogenic Abilities of Periodontal Ligament Fibroblasts. International Journal of Molecular Sciences. 2021; 22(16):8646. https://doi.org/10.3390/ijms22168646
Chicago/Turabian StyleYuan, Hang, Shigeki Suzuki, Shizu Hirata-Tsuchiya, Akiko Sato, Eiji Nemoto, Masahiro Saito, Hideki Shiba, and Satoru Yamada. 2021. "PPARγ-Induced Global H3K27 Acetylation Maintains Osteo/Cementogenic Abilities of Periodontal Ligament Fibroblasts" International Journal of Molecular Sciences 22, no. 16: 8646. https://doi.org/10.3390/ijms22168646
APA StyleYuan, H., Suzuki, S., Hirata-Tsuchiya, S., Sato, A., Nemoto, E., Saito, M., Shiba, H., & Yamada, S. (2021). PPARγ-Induced Global H3K27 Acetylation Maintains Osteo/Cementogenic Abilities of Periodontal Ligament Fibroblasts. International Journal of Molecular Sciences, 22(16), 8646. https://doi.org/10.3390/ijms22168646






