Receptor–Receptor Interactions and Glial Cell Functions with a Special Focus on G Protein-Coupled Receptors
Abstract
:1. Introduction
2. Structural Biology and Signaling of Receptor Complexes
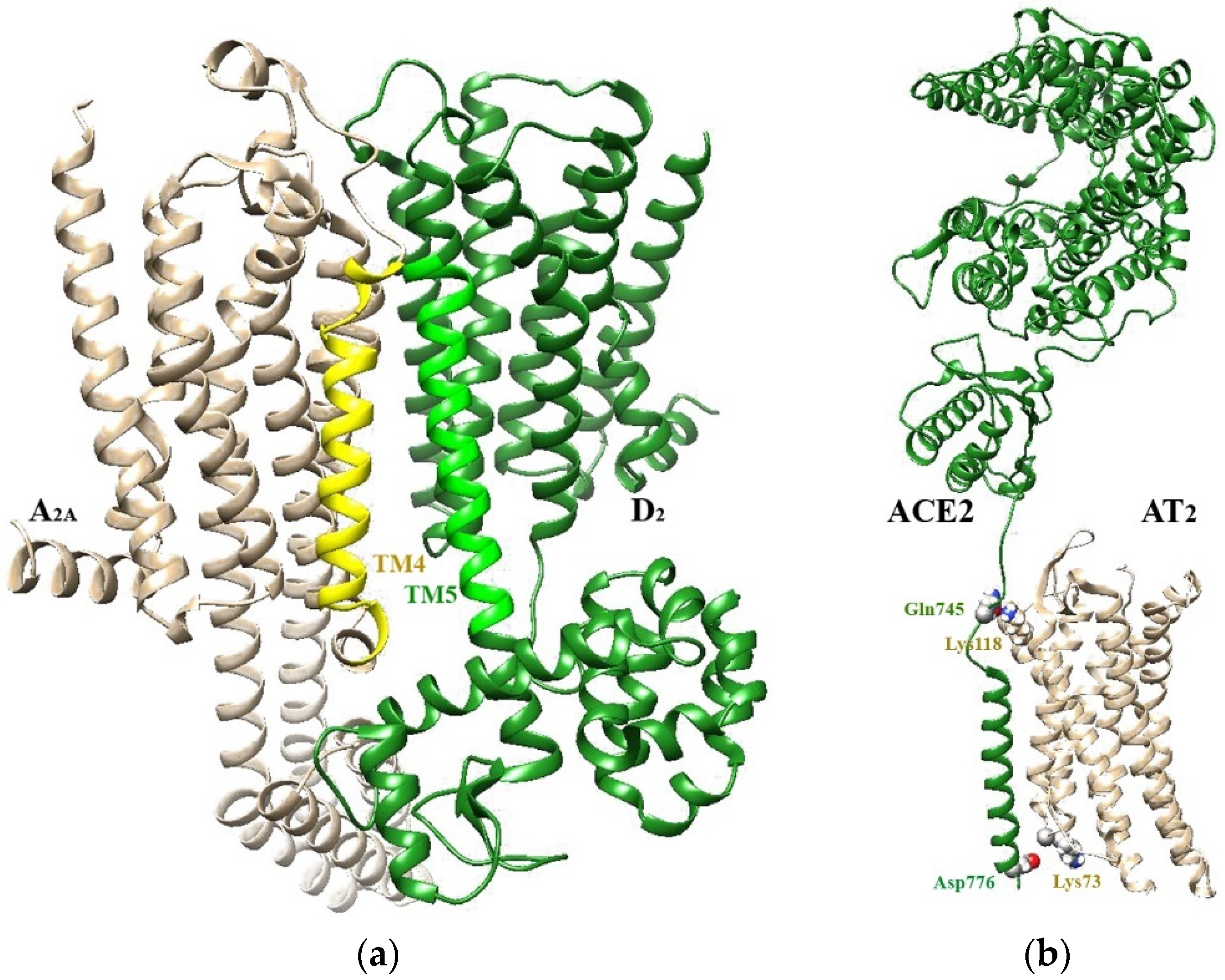
2.1. RRI as Allosteric Interactions
2.2. Signaling from Receptor Complexes
3. Receptor–Receptor Interactions in Glial Cells
3.1. Astrocytes
3.2. Microglia
3.3. Oligodendrocytes and Schwann Cells
4. Concluding Remarks and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lefkowitz, R.J. Seven transmembrane receptors: A brief personal retrospective. Biochim. Biophys. Acta 2007, 1768, 748–755. [Google Scholar] [CrossRef] [Green Version]
- Humphrey, P.P.A.; Barnard, E.A. International union of pharmacology. XIX The IUPHAR receptor code: A proposal for an alphanumeric classification system. Pharmacol. Rev. 1998, 50, 271–277. [Google Scholar] [PubMed]
- Foord, S.M.; Jupe, S.; Holbrook, J. Bioinformatics and type II G-protein-coupled receptors. Biochem. Soc. Trans. 2002, 30, 473–479. [Google Scholar] [CrossRef]
- Rosenbaum, D.; Rasmussen, S.G.F.; Kobilka, B.K. The structure and function of G-protein-coupled receptors. Nature 2009, 459, 356–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayburt, T.H.; Leitz, A.J.; Xie, G.; Oprian, D.D.; Sligar, S.G. Transducin activation by nanoscale lipid bilayers containing one and two rhodopsins. J. Biol. Chem. 2007, 282, 14875–14881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whorton, M.R.; Bokoch, M.P.; Rasmussen, S.G.; Huang, B.; Zare, R.N.; Kobilka, B.; Sunahara, R.K. A monomeric G protein-coupled receptor isolated in high-density lipoprotein particle efficiently activates its G protein. Proc. Natl. Acad. Sci. USA 2007, 104, 7682–7687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuszak, A.J.; Pitchiaya, S.; Anand, J.P.; Mosberg, H.I.; Walter, N.G.; Sunahara, R.K. Purification and functional reconstitution of monomeric μ-opioid receptors: Allosteric modulation of agonist binding by Gi2. J. Biol. Chem. 2009, 284, 26732–26741. [Google Scholar] [CrossRef] [Green Version]
- Zidar, D.A.; Violin, J.D.; Whalen, E.J.; Lefkowitz, R.J. Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc. Natl. Acad. Sci. USA 2009, 106, 9649–9654. [Google Scholar] [CrossRef] [Green Version]
- Kenakin, T. Functional selectivity and biased receptor signalling. J. Pharm. Exp. 2011, 336, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agnati, L.F.; Fuxe, K.; Zini, I.; Lenzi, P.; Hökfelt, T. Aspects on receptor regulation and isoreceptor identification. Med. Biol. 1980, 58, 182–187. [Google Scholar]
- Agnati, L.F.; Fuxe, K.; Benfenati, F.; Battistini, N.; Härfstrand, A.; Takemoto, K.; Hökfelt, T.; Mutt, V. Neuropeptide Y in vitro selectivity increases the number of α2-adrenergic binding sites in membranes of the medulla oblongata of the rat. Acta Physiol. Scand. 1983, 118, 293–295. [Google Scholar] [CrossRef]
- Fuxe, K.; Agnati, L.F.; Benfenati, F.; Celani, M.; Zini, I.; Zoli, M.; Mutt, V. Evidence for the existence of receptor-receptor interactions in the central nervous system. Studies on the regulation of monoamine receptors by neuropeptides. J. Neural Transm. 1983, 18, 165–179. [Google Scholar]
- Fuxe, K.; Marcellino, D.; Borroto-Escuela, D.O.; Frankowska, M.; Ferraro, L.; Guidolin, D.; Ciruela, F.; Agnati, L.F. The changing world of G protein-coupled receptors: From monomers to dimers and receptor mosaics with allosteric receptor-receptor interactions. J. Recept. Signal Transduct. Res. 2010, 30, 272–283. [Google Scholar] [CrossRef]
- Farran, B. An update on the physiological and therapeutic relevance of GPCR oligomers. Pharm. Res. 2017, 117, 303–327. [Google Scholar] [CrossRef] [PubMed]
- Guidolin, D.; Marcoli, M.; Tortorella, C.; Maura, G.; Agnati, L.F. G protein-coupled receptor-receptor interactions give integrative dynamics to intercellular communication. Rev. Neurosci. 2018, 29, 703–726. [Google Scholar] [CrossRef] [PubMed]
- Agnati, L.F.; Guidolin, D.; Vilardaga, J.P.; Ciruela, F.; Fuxe, K. On the expanding terminology in the GPCR field: The meaning of receptor mosaics and receptor heteromers. J. Recept. Signal Transduct. Res. 2010, 30, 287–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuxe, K.; Ferré, S.; Zoli, M.; Agnati, L.F. Integrated events in central dopamine transmission as analyzed at multiple levels. Evidence for intramembrane adenosine A2A/dopamine D2 and adenosine A1/dopamine D1 receptor interactions in the basal ganglia. Brain Res. Rev. 1998, 26, 258–273. [Google Scholar] [CrossRef]
- Bockaert, J.; Pin, J.P. Molecular tinkering of G proteincoupled receptors: An evolutionary success. EMBO J. 1999, 18, 1723–1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, F.H.; White, J.; Main, M.; Green, A.; Wise, A. GABA(B) receptors function as heterodimers. Biochem. Soc. Trans. 1999, 27, 530–535. [Google Scholar] [CrossRef]
- Xie, Z.; Lee, S.P.; O’Dowd, B.F.; George, S.R. Serotonin 5-HT1B and 5-HT1D receptors form homodimers when expressed alone and heterodimers when co-expressed. FEBS Lett. 1999, 456, 63–67. [Google Scholar] [CrossRef] [Green Version]
- Franco, R.; Ferré, S.; Agnati, L.F.; Torvinen, M.; Ginés, S.; Hilion, J.; Casadò, V.; Lledò, P.; Zoli, M.; Lluis, C.; et al. Evidence for adenosine/dopamine receptor interactions: Indications for heteromerization. Neuropsychopharmacology 2000, 23, S50–S59. [Google Scholar] [CrossRef]
- Overton, M.C.; Blumer, K.J. G protein-coupled receptors function as oligomers in vivo. Curr. Biol. 2000, 10, 341–344. [Google Scholar] [CrossRef] [Green Version]
- Zeng, F.; Wess, J. Molecular aspects of muscarinic receptor dimerization. Neuropsychopharmacology 2000, 23, S19–S31. [Google Scholar] [CrossRef]
- Angers, S.; Salahpour, A.; Bouvier, M. Biochemical and biophysical demonstration of GPCR oligomerization in mammalian cells. Life Sci. 2001, 68, 2243–2250. [Google Scholar] [CrossRef]
- Dean, M.K.; Higgs, C.; Smith, R.E.; Bywater, R.P.; Snell, C.R.; Scott, P.D.; Upton, G.J.; Howe, T.J.; Reynolds, C.A. Dimerization of G protein-coupled receptors. J. Med. Chem. 2001, 44, 4595–4614. [Google Scholar] [CrossRef]
- Kenakin, T. Drug efficacy at G protein-coupled receptors. Annu. Rev. Pharm. Toxicol. 2002, 42, 349–379. [Google Scholar] [CrossRef] [Green Version]
- Waldhoer, M.; Fong, J.; Jones, R.M.; Lunzer, M.M.; Sharma, S.K.; Kostenis, E.; Portoghese, P.S.; Whistler, J.L. A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. Proc. Natl. Acad. Sci. USA 2005, 102, 9050–9055. [Google Scholar] [CrossRef] [Green Version]
- Trifilieff, P.; Rives, M.L.; Urizar, E.; Piskorowski, R.A.; Vishwasrao, H.D.; Castrillon, J.; Schmauss, C.; Stätmann, M.; Gullberg, M.; Javitch, J.A. Detection of antigen interactions ex vivo by proximity ligation assay: Endogenous dopamine D2-adenosine A2A receptor complexes in the striatum. Biotechniques 2011, 51, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Petazzi, R.A.; Aji, A.K.; Chiantia, S. Fluorescence microscopy methods for the study of protein oligomerization. In Progress in Molecular Biology and Translational Science; Giraldo, J., Ciruela, F., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 169, pp. 1–42. [Google Scholar]
- Guidolin, D.; Marcoli, M.; Tortorella, C.; Maura, G.; Agnati, L.F. Receptor-receptor interactions as a widespread phenomenon: Novel targets for drug development? Front. Endocrinol. 2019, 10, 53. [Google Scholar] [CrossRef] [Green Version]
- Corringer, P.-J.; Baaden, M.; Bocquet, N.; Delarue, M.; Dufresne, V.; Nury, H.; Prevost, M.; Van Renterghem, C. Atomic structure and dynamics of pentameric ligand-gated ion channels: New insights from bacterial homologs. J. Physiol. 2010, 588, 565–572. [Google Scholar] [CrossRef]
- Burris, T.P.; Solt, L.A.; Wang, Y.; Crumbley, C.; Banerjee, S.; Griffet, K.; Lundasen, T.; Hughes, T.; Kojetin, D.J. Nuclear receptors and their selective pharmacological modulators. Pharm. Rev. 2013, 65, 710–778. [Google Scholar] [CrossRef] [Green Version]
- Zahavi, E.E.; Steinberg, N.; Altman, T.; Chein, M.; Joshi, Y.; Gradus-Pery, T.; Perlson, E. The receptor tyrosine kinase TrkB signals without dimerization at the plasma membrane. Sci. Signal. 2018, 11, eaao4006. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, A.; Schlessinger, J. Signal transduction by receptors with tyrosine kinase activity. Cell 1990, 61, 203–212. [Google Scholar] [CrossRef]
- Changeaux, J.P.; Christopoulos, A. Allosteric modulation as a unifying mechanism for receptor function and regulation. Diabetes Obes. Metab. 2017, 19, 4–21. [Google Scholar] [CrossRef] [Green Version]
- Di Liberto, V.; Borroto-Escuela, D.O.; Frinchi, M.; Verdi, V.; Fuxe, K.; Belluardo, N.; Mudò, G. Evidence of muscarinic acetylcholine receptor (mAChR) and fibroblast growth factor receptor (FGFR) heteroreceptor complexes and their enhancement of neurite outgrowth in neural hippocampal cultures. Biochim. Biophys. Acta 2017, 1861, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Tòth, A.D.; Turu, G.; Hunyady, L.; Balla, A. Novel mechanisms of GPCR functions: AT1 angiotensin receptor acts as a signaling hub and focal point of receptor cross-talk. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 69–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stocco, E.; Sfriso, M.M.; Borile, G.; Contran, M.; Barbon, S.; Romanato, F.; Macchi, V.; Guidolin, D.; De Caro, R.; Porzionato, A. Experimental Evidence of A2A–D2 Receptor–Receptor Interactions in the Rat and Human Carotid Body. Front. Physiol. 2021, 12, 645723. [Google Scholar] [CrossRef] [PubMed]
- Kleinau, G.; Miller, A.; Biebermann, H. Oligomerization of GPCRs involved in endocrine regulation. J. Mol. Endocrinol. 2016, 57, 859–880. [Google Scholar] [CrossRef] [Green Version]
- Moreno, E.; Cavic, M.; Krivocuca, A.; Casadò, V.; Canela, E. The endocannabinoid system as a target in cancer diseases: Are we there yet? Front. Pharm. 2019, 10, 339. [Google Scholar] [CrossRef] [Green Version]
- Guidolin, D.; Marcoli, M.; Maura, G.; Agnati, L.F. New dimensions of connectomics and network plasticity in the central nervous system. Rev. Neurosci. 2017, 28, 113–132. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Carlsson, J.; Ambrogini, P.; Narvàez, M.; Wydra, K.; Tarakanov, A.; Li, X.; Millón, C.; Ferraro, L.; Cuppini, R.; et al. Understanding the role of GPCR heteroreceptor complexes in modulating the brain networks in health and disease. Front. Cell. Neurosci. 2017, 11, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferré, S.; Casadò, V.; Devi, L.A.; Filizola, M.; Jockers, R.; Lohse, M.J.; Milligan, G.; Pin, J.P.; Guitart, J. G protein-coupled receptor oligomerization revisited: Functional and pharmacological perspectives. Pharm. Rev. 2014, 66, 413–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuxe, K.; Borroto-Escuela, D.O. Heteroreceptor complexes and their allosteric receptor-receptor interactions as a novel biological principle for integration of communication in the CNS: Targets for drug development. Neuropsychopharmacology 2016, 41, 380–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guidolin, D.; Marcoli, M.; Tortorella, C.; Maura, G.; Agnati, L.F. Adenosine A2A-Dopamine D2 Receptor-Receptor Interaction in Neurons and Astrocytes: Evidence and Perspectives. Prog. Med. Biol. Transl. Sci. 2020, 169, 247–277. [Google Scholar]
- Borroto-Escuela, D.O.; Ambrogini, P.; Cruscicka, B.; Lindskog, M.; Crespo-Ramirez, M.; Hernàndez-Mondragòn, J.C.; Perez de la Mora, M.; Schellekens, H.; Fuxe, K. The role of central serotonin neurons and 5HT heteroreceptor complexes in the pathophysiology of depression: A historical perspective and future prospects. Int. J. Mol. Sci. 2021, 22, 1927. [Google Scholar] [CrossRef]
- Perez de la Mora, M.; Hernandez-Mondragon, J.C.; Crespo-Ramirez, M.; Regon-Orantes, J.; Borroto-Escuela, D.O.; Fuxe, K. Conventional and novel pharmacological approaches to treat dopamine-related disorders: Focus on Parkinson’s disease and schizophrenia. Neuroscience 2020, 439, 301–318. [Google Scholar] [CrossRef]
- Allen, N.J.; Lyons, D.A. Glia as arquitects of central nervous system formation an function. Science 2018, 362, 181–185. [Google Scholar] [CrossRef] [Green Version]
- Prezeau, L.; Rives, M.L.; Comps-Agrar, L.; Maurel, D.; Knlazeff, J.; Pin, J.P. Functional crosstalk between GPCRs: With or without oligomerization. Curr. Opin. Pharm. 2010, 10, 6–13. [Google Scholar] [CrossRef]
- Kenakin, T.; Agnati, L.F.; Caron, M.; Fredholm, B.; Guidolin, D.; Kobilka, B.; Lefkowitz, R.J.; Lohse, M.; Woods, A.; Fuxe, K. International workshop at the Nobel Forum, Karolinska Institutet on G protein-coupled receptors: Finding the words to describe monomers, oligomers and their molecular mechanisms and defining their meaning. J. Recept. Signal Transduct. Res. 2010, 30, 284–286. [Google Scholar] [CrossRef]
- Kenakin, T.; Miller, I.J. Seven transmembrane receptors as shape shifting proteins: The impact of allosteric modulation and functional selectivity on new drug discovery. Pharm. Rev. 2010, 62, 265–304. [Google Scholar] [CrossRef] [Green Version]
- Smith, N.J.; Milligan, G. Allostery of G protein-coupled receptors homo- and heteromers: Uncharted pharmacological landscapes. Pharm. Rev. 2010, 62, 701–725. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Nussinov, R. Allostery: An overview of its history, concepts, methods and applications. PLoS Comput. Biol. 2016, 12, e1004966. [Google Scholar] [CrossRef]
- Wright, P.E.; Dyson, H.J. Intrinsically disordered proteins in cellular signalling and regulation. Nat. Rev. Mol. Cell Biol. 2015, 16, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Agnati, L.F.; Guidolin, D.; Leo, G.; Carone, C.; Genedani, S.; Fuxe, K. Receptor-receptor interactions: A novel concept in brain integration. Prog. Neurobiol. 2010, 90, 157–175. [Google Scholar] [CrossRef] [PubMed]
- Filizola, M.; Weinstein, H. The study of G-protein coupled receptor oligomerization with computational modeling and bioinformatics. FEBS J. 2005, 272, 2926–2938. [Google Scholar] [CrossRef]
- Simpson, L.M.; Taddese, B.; Wall, I.D.; Reynolds, C.A. Bioinformatics and molecular modelling approaches to GPCR oligomerization. Curr. Opin. Pharm. 2010, 10, 30–37. [Google Scholar] [CrossRef]
- Guidolin, D.; Ciruela, F.; Genedani, S.; Guescini, M.; Tortorella, C.; Albertin, G.; Fuxe, K.; Agnati, L.F. Bioinformatics and mathematical modeling in the study of receptor-receptor interactions and receptor oligomerization. Focus on adenosine receptors. Biochim. Biophys. Acta 2011, 1808, 1267–1283. [Google Scholar] [CrossRef]
- Agnati, L.F.; Leo, G.; Genedani, S.; Andreoli, N.; Marcellino, D.; Woods, A.; Piron, L.; Guidolin, D.; Fuxe, K. Structural plasticity in G-protein coupled receptors as demonstrated by the allosteric actions of homocysteine and computer-assisted analysis of disordered domains. Brain Res. Rev. 2008, 58, 459–474. [Google Scholar] [CrossRef]
- Tovo-Rodrigues, L.; Roux, A.; Hutz, M.H.; Rohde, L.A.; Woods, A.S. Functional characterization of G-protein-coupled receptors: A bioinformatics approach. Neuroscience 2014, 277, 764–779. [Google Scholar] [CrossRef] [Green Version]
- Pagadala, N.S.; Khajamohiddin, S.; Tuszynski, J. Software for molecular docking: A review. Biophys. Rev. 2017, 9, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Krieger, E.; Joo, K.; Lee, J.; Raman, S.; Thompson, J.; Tyka, M.; Baker, D.; Karplus, K. Improving physical realism, stereochemistry and side-chain accuracy in homology modeling. Four approaches that performed well in CASP8. Proteins 2009, 77, 114–122. [Google Scholar] [PubMed] [Green Version]
- Latorraca, N.R.; Venkatakrishnan, A.J.; Dror, R.O. GPCR dynamics: Structures in motion. Chem. Rev. 2017, 117, 139–155. [Google Scholar]
- Borroto-Escuela, D.O.; Rodriguez, D.; Romero-Fernandez, W.; Kapla, J.; Jalteh, M.; Ranganathan, A.; Lazarova, T.; Fuxe, K.; Carlsson, J. Mapping the interface of a GPCR dimer: A structural model of A2A adenosine and D2 dopamine receptor heteromer. Front. Pharm. 2018, 9, 829. [Google Scholar]
- Guidolin, D.; Tortorella, C.; Anderlini, D.; Marcoli, M.; Maura, G.; Agnati, L.F. Heteromerization as a mechanism modulating the affinity of the ACE2 receptor to the receptor binding domain of SARS-CoV-2 spike protein. Curr. Proteom. 2021, 18. [Google Scholar] [CrossRef]
- Grisshammer, R. New approaches towards the understanding of integral membrane proteins: A structural perspective on G protein-coupled receptors. Prot. Sci. 2017, 26, 1493–1504. [Google Scholar]
- Jonas, K.C.; Fanelli, F.; Huhtaniemi, I.T.; Hanyaloglu, A.C. Single molecule analysis of functionally asymmetric G protein-coupled receptor (GPCR) oligomers reveals diverse spatial and structural assemblies. J. Biol. Chem. 2015, 290, 3875–3892. [Google Scholar]
- Woods, A.S.; Ciruela, F.; Fuxe, K.; Agnati, L.F.; Lluis, C.; Franco, R.; Ferré, S. Role of electrostatic interaction in receptor-receptor heteromerization. J. Mol. Neurosci. 2005, 26, 125–132. [Google Scholar]
- Sarabipour, S.; Hristova, K. Mechanism of FGF receptor dimerization and activation. Nat. Commun. 2016, 7, 10262. [Google Scholar]
- Lock, A.; Forfar, R.; Weston, C.; Bowsher, L.; Upton, G.J.G.; Reynolds, C.A.; Ladds, G.; Dixon, A.M. One motif to bind them: A small-XXX-small motif affects transmembrane domain 1 oligomerization, function, localization and cross-talk between two yeast GPCR. Biochim. Biophys. Acta Biomembr. 2014, 1838, 3036–3051. [Google Scholar]
- Gurevich, V.V.; Gurevich, E.V. How and why do GPCRs dimerize? Trends Pharm. Sci. 2008, 29, 234–240. [Google Scholar]
- Agnati, L.F.; Guidolin, D.; Leo, G.; Fuxe, K. A Boolean network modelling of receptor mosaics: Relevance of topology and cooperativity. J. Neural Transm. 2007, 114, 77–92. [Google Scholar] [CrossRef]
- Jackson, M.B. Molecular and Cellular Biophysics; Cambridge University Press: Cambridge, UK, 2006; pp. 111–141. [Google Scholar]
- Kenakin, T. Cellular assays as portals to seven-transmembrane receptor-based drug discovery. Nat. Rev. Drug Discov. 2009, 8, 617–626. [Google Scholar] [CrossRef]
- Romero, G.G. The role of the cell background in biased signaling. In Biased Signaling in Physiology, Pharmacology and Therapeutics; Arey, B.J., Ed.; Elsevier: San Diego, CA, USA, 2014; pp. 41–79. [Google Scholar]
- Andersen, M.; Nargaard-Pedersen, D.; Brandt, J.; Pettersson, I.; Slaabi, R. IGF1 and IGF2 specificities to the two insulin receptor isoforms are determined by insulin receptor amino acid 718. PLoS ONE 2017, 12, e0178885. [Google Scholar] [CrossRef] [Green Version]
- Ferrada, C.; Moreno, E.; Casadò, V.; Bongers, G.; Cortés, A.; Mallol, J.; Canela, E.I.; Leurs, R.; Ferré, S.; Lluís, C.; et al. Marked changes in signal transduction upon heteromerization of dopamine D1 and histamine H3 receptors. Br. J. Pharm. 2009, 157, 64–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Naour, M.; Lunzer, M.M.; Powers, M.D.; Kalyuzhny, A.E.; Benneyworth, M.A.; Thomas, M.J.; Portoghese, P.S. Putative Kappa Opioid Heteromers as Targets for Developing Analgesics Free of Adverse Effects. J. Med. Chem. 2014, 57, 6383–6392. [Google Scholar] [CrossRef]
- Allen, N.J.; Barres, B.A. Signaling between glia and neurons: Focus on synaptic plasticity. Curr. Opin. Neurobiol. 2005, 15, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Sancho, L.; Contreras, M.; Allen, N.J. Glia as sculptors of synaptic plasticity. Neurosci. Res. 2021, 167, 17–29. [Google Scholar] [CrossRef]
- Cervetto, C.; Venturini, A.; Passalacqua, M.; Guidolin, D.; Genedani, S.; Fuxe, K.; Borroto-Esquela, D.O.; Cortelli, P.; Woods, A.; Maura, G.; et al. A2A-D2 receptor-receptor interaction modulates gliotransmitter release from striatal astrocyte processes. J. Neurochem. 2016, 140, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Pelassa, S.; Guidolin, D.; Venturini, A.; Averna, M.; Frumento, G.; Campanini, L.; Bernardi, R.; Cortelli, P.; Buonaura, G.C.; Maura, G.; et al. A2A-D2 Heteromers on Striatal Astrocytes: Biochemical and Biophysical Evidence. Int. J. Mol. Sci. 2019, 20, 2457. [Google Scholar] [CrossRef] [Green Version]
- Cervetto, C.; Venturini, A.; Guidolin, D.; Maura, G.; Passalacqua, M.; Tacchetti, C.; Cortelli, P.; Genedani, S.; Candiani, S.; Ramoino, P.; et al. Homocysteine and A2A-D2 Receptor-Receptor Interaction at Striatal Astrocyte Processes. J. Mol. Neurosci. 2018, 65, 456–466. [Google Scholar] [CrossRef]
- García-Gutiérrez, M.S.; Navarrete, F.; Navarro, G.; Reyes-Resina, I.; Franco, R.; Lanciego, J.L.; Giner, S.; Manzanares, J. Alterations in Gene and Protein Expression of Cannabinoid CB2 and GPR55 Receptors in the Dorsolateral Prefrontal Cortex of Suicide Victims. Neurotherapeutics 2018, 15, 796–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narváez, M.; Andrade-Talavera, Y.; Valladolid-Acebes, I.; Fredriksson, M.; Siegele, P.; Hernandez-Sosa, A.; Fisahn, A.; Fuxe, K.; Borroto-Escuela, D.O. Existence of FGFR1-5-HT1AR heteroreceptor complexes in hippocampal astrocytes. Putative link to 5-HT and FGF2 modulation of hippocampal gamma oscillations. Neuropharmacology 2020, 170, 108070. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, L.; Losi, G.; Lia, A.; Melone, M.; Chiavegato, A.; Gómez-Gonzalo, M.; Sessolo, M.; Bovetti, S.; Forli, A.; Zonta, M.; et al. Interneuron-specific signaling evokes distinctive somatostatin-mediated responses in adult cortical astrocytes. Nat. Commun. 2018, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Cristovão-Ferreira, S.; Navarro, G.; Brugarolas, M.; Pérez-Capote, K.; Vaz, S.H.; Fattorini, G.; Conti, F.; Lluis, C.; Ribeiro, J.A.; McCormick, P.J.; et al. A1R-A2AR heteromers coupled to Gs and Gi/0 proteins modulate GABA transport into astrocytes. Purinergic Signal. 2013, 9, 433–449. [Google Scholar] [CrossRef] [Green Version]
- Kolasa, M.; Solich, J.; Faron-Gòreka, A.; Zurawek, D.; Pabian, P.; Lukasiewicz, S.; Kuśmider, M.; Szafran-Pilch, K.; Szlachta, M.; Dziedzicka-Wasylewska, M. Paroxetine and low-dose risperidone induce serotonin 5-HT1A and Dopamine D2 receptor heteromerization in the mouse prefrontal cortex. Neuroscience 2018, 377, 184–196. [Google Scholar] [CrossRef]
- Di Menna, L.; Joffe, M.E.; Iacovelli, L.; Orlando, R.; Lindsley, C.W.; Mairesse, J.; Gressèns, P.; Cannella, M.; Caraci, F.; Copani, A.; et al. Functional partnership between mGlu3 and mGlu5 metabotropic glutamate receptors in the central nervous system. Neuropharmacology 2017, 128, 301–313. [Google Scholar] [CrossRef]
- Tonazzini, I.; Trincavelli, M.L.; Montali, M.; Martini, C. Regulation of A1adenosine receptor functioning induced by P2Y1 purinergic receptor activation in human astroglial cells. J. Neurosci. Res. 2008, 86, 2857–2866. [Google Scholar] [CrossRef]
- Guo, C.; Masin, M.; Qureshi, O.S.; Murrell-Lagnado, R. Evidence for Functional P2X4/P2X7 Heteromeric Receptors. Mol. Pharmacol. 2007, 72, 1447–1456. [Google Scholar] [CrossRef]
- Callén, L.; Moreno, E.; Barroso-Chinea, P.; Moreno-Delgado, D.; Cortés, A.; Mallol, J.; Casadó, V.; Lanciego, J.; Franco, R.; Lluis, C.; et al. Cannabinoid Receptors CB1 and CB2 Form Functional Heteromers in Brain. J. Biol. Chem. 2012, 287, 20851–20865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, G.; Borroto-Escuela, D.O.; Angelats, E.; Etayo, I.; Reyes-Resina, I.; Pulido-Salgado, M.; Rodrìguez-Peréz, A.I.; Canela, E.I.; Saura, J.; Lanciego, J.L.; et al. Receptor-heteromer mediated regulation of endocannabinoid signaling in activated microglia. Role of CB1 and CB2 receptors and relevance for Alzheimer’s disease and levodopa-induced dyskinesia. Brain. Behav. Immun. 2018, 67, 139–151. [Google Scholar] [CrossRef]
- Franco, R.; Reyes-Resina, I.; Aguinaga, D.; Lillo, A.; Jiménez, J.; Raïch, I.; Borroto-Escuela, D.O.; Ferreiro-Vera, C.; Canela, E.I.; De Medina, V.S.; et al. Potentiation of cannabinoid signaling in microglia by adenosine A2A receptor antagonists. Glia 2019, 67, 2410–2423. [Google Scholar] [CrossRef]
- Reyes-Resina, I.; Navarro, G.; Aguinaga, D.; Canela, E.I.; Schoeder, C.T.; Załuski, M.; Kieć-Kononowicz, K.; Saura, C.A.; Müller, C.E.; Franco, R. Molecular and functional interaction between GPR18 and cannabinoid CB2 G-protein-coupled receptors. Relevance in neurodegenerative diseases. Biochem. Pharmacol. 2018, 157, 169–179. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Kirchhoff, F.; Scheller, A. Oligodendroglial GABAergic Signaling: More rhan Inhibition! Neurosci. Bull. 2021, 37, 1039–1050. [Google Scholar] [CrossRef]
- Vartanian, T.; Goodearl, A.; Viehöver, A.; Fischbach, G. Axonal neuregulin signal cells of the oligodendrocyte lineage throughactivation of HER4 and Schwann cells through HER2 and HER3. J. Cell Biol. 1997, 137, 211–220. [Google Scholar] [CrossRef]
- Hubbard, J.A.; Szu, J.I.; Binder, D.K. The role of aquaporin-4 in synaptic plasticity, memory and disease. Brain Res. Bull. 2018, 136, 118–129. [Google Scholar] [CrossRef]
- Suzuki, A.; Stern, S.A.; Bozdagi, O.; Huntley, G.W.; Walker, R.H.; Magistretti, P.J.; Alberini, C.M. Astrocyte-Neuron Lactate Transport Is Required for Long-Term Memory Formation. Cell 2011, 144, 810–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999, 22, 208–215. [Google Scholar] [CrossRef]
- Nedergaard, M.; Verkhratsky, A. Artifact versus reality-How astrocytes contribute to synaptic events. Glia 2012, 60, 1013–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, N.J. Astrocyte Regulation of Synaptic Behavior. Annu. Rev. Cell Dev. Biol. 2014, 30, 439–463. [Google Scholar] [CrossRef]
- Hirase, H.; Qian, I.; Barth, P.; Buzsàki, G. Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol. 2004, 2, e96. [Google Scholar] [CrossRef] [Green Version]
- Fellin, T.; Carmignoto, G. Neurone-to-astrocyte signalling in the brain represents a distinct multifunctional unit. J. Physiol. 2004, 559, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Savtchouk, I.; Volterra, A. Gliotransmission: Beyond Black-and-White. J. Neurosci. 2018, 38, 14–25. [Google Scholar] [CrossRef]
- Martin-Fernandez, M.; Jamison, S.; Robin, L.M.; Zhao, Z.; Martín, E.D.; Aguilar, J.; Benneyworth, M.A.; Marsicano, G.; Araque, A. Synapse-specific astrocyte gating of amygdala-related behavior. Nat. Neurosci. 2017, 20, 1540–1548. [Google Scholar] [CrossRef] [Green Version]
- Ascoli, G.A. Petilla terminology: Nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat. Rev. Neurosci. 2008, 9, 557–568. [Google Scholar]
- Hamilton, N.; Attwell, D. Do astrocytes really exocytose neurotransmitters? Nat. Rev. Neurosci. 2010, 11, 227–238. [Google Scholar] [CrossRef]
- Khan, Z.U.; Koulen, P.; Rubinstein, M.; Grandy, D.K.; Goldman-Rakic, P.S. An astroglia-linked dopamine D2-receptor action in prefrontal cortex. Proc. Natl. Acad. Sci. USA 2001, 98, 1964–1969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santana, N.; Bortolozzi, A.; Serrats, J.; Mengod, G.; Artigas, F. Expression of Serotonin1A and Serotonin2A Receptors in Pyramidal and GABAergic Neurons of the Rat Prefrontal Cortex. Cereb. Cortex 2004, 14, 1100–1109. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Nan, G. The extracellular signal-regulated kinase 1/2 pathway in neurological diseases: A potential therapeutic target. Int. J. Mol. Med. 2017, 39, 1338–1346. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Bendito, G.; Shigemoto, R.; Fairén, A.; Luján, R. Differential distribution of group I metabotropic glutamate receptors during rat cortical development. Cereb. Cortex 2002, 12, 625–638. [Google Scholar] [CrossRef] [Green Version]
- Guidolin, D.; Fede, C.; Tortorella, C. Nerve cells developmental processes and the dynamic role of cytokine signaling. Int. J. Dev. Neurosci. 2018, 77, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Köles, L.; Leichsenring, A.; Rubini, P.; Illes, P. P2 Receptor Signaling in Neurons and Glial Cells of the Central Nervous System. Adv. Pharmacol. 2011, 61, 441–493. [Google Scholar] [CrossRef] [PubMed]
- Trang, M.; Schmalzing, G.; Müller, C.E.; Markwardt, F. Dissection of P2X4 and P2X7 receptor current components in BV-2 microglia. Int. J. Mol. Sci. 2020, 21, 8489. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Fernández-Suárez, D. Alternatively activated microglia and macrophages in the central nervous system. Prog. Neurobiol. 2015, 131, 65–86. [Google Scholar] [CrossRef]
- Zhang, T.; Dong, K.; Liang, W.; Xu, D.; Xia, H.; Geng, J.; Najafov, A.; Liu, M.; Li, Y.; Han, X.; et al. G-protein coupled receptors regulate autophagy by ZBTB16-mediated ubiquitination and proteasomal degradation of adaptor protein Atg14L. eLife 2015, 4, e06734. [Google Scholar] [CrossRef]
- Stella, N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia 2010, 58, 1017–1030. [Google Scholar] [CrossRef] [Green Version]
- Bisogno, T.; Di Marzo, V. Cannabinoid receptors and endocannabinoids: Role in neuroinflammatory and neurodegenerative disorders. CNS Neurol. Disord. Drug Targets 2010, 9, 564–573. [Google Scholar] [CrossRef]
- Lin, S.-C.; Bergles, D. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat. Neurosci. 2003, 7, 24–32. [Google Scholar] [CrossRef]
- Marshall, F.H.; Jones, K.A.; Kaupmann, K.; Bettler, B. GABAB receptors—The first 7TM heterodimers. Trends Pharmacol. Sci. 1999, 20, 396–399. [Google Scholar] [CrossRef]
- Morrissey, T.K.; Levi, A.D.; Nuijens, A.; Sliwkowski, M.X.; Bunge, R.P. Axon-induced mitogenesis of human Schwann cells involves heregulin and p185erbB2. Proc. Natl. Acad. Sci. USA 1995, 92, 1431–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khelashvili, G.; Dorff, K.; Shan, J.; Camacho-Artacho, M.; Skrabanek, L.; Vroling, B.; Bouvier, M.; Devi, L.A.; George, S.R.; Javitch, J.; et al. GPCR-OKB: The G Protein Coupled Receptor Oligomer Knowledge Base. Bioinformatics 2010, 26, 1804–1805. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Britto, I.; Romero-Fernandez, W.; Di Palma, M.; Oflijan, J.; Skieterska, K.; Duchou, J.; Van Craenenbroeck, K.; Suárez-Boomgaard, D.; Rivera, A.; et al. The G protein-coupled heterodimer network (GPCR-HetNet) and its hub components. Int. J. Mol. Sci. 2014, 15, 8570–8590. [Google Scholar] [CrossRef]
- Durkee, C.A.; Araque, A. Diversity and Specificity of Astrocyte–neuron Communication. Neuroscience 2019, 396, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Agnati, L.F.; Guidolin, D.; Guescini, M.; Genedani, S.; Fuxe, K. Understanding wiring and volume transmission. Brain Res. Rev. 2010, 64, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Syková, E.; Nicholson, C. Diffusion in Brain Extracellular Space. Physiol. Rev. 2008, 88, 1277–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Färber, K.; Kettenmann, H. Physiology of microglial cells. Brain Res. Rev. 2005, 48, 133–143. [Google Scholar] [CrossRef]
- Hirase, H.; Iwai, Y.; Takata, N.; Shinohara, Y.; Mishima, T. Volume transmission signalling via astrocytes. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130604. [Google Scholar] [CrossRef] [Green Version]
- Rózsa, M.; Baka, J.; Bordé, S.; Rózsa, B.; Katona, G.; Tamás, G. Unitary GABAergic volume transmission from individual interneurons to astrocytes in the cerebral cortex. Brain Struct. Funct. 2015, 222, 651–659. [Google Scholar] [CrossRef] [Green Version]
- Fuxe, K.; Agnati, L.F.; Marcoli, M.; Borroto-Escuela, D.O. Volume Transmission in Central Dopamine and Noradrenaline Neurons and Its Astroglial Targets. Neurochem. Res. 2015, 40, 2600–2614. [Google Scholar] [CrossRef] [PubMed]
- Vizi, E.S. Role of high-affinity receptors and membrane transporters in nonsynaptic communication and drug action in the CNS. Pharmacol. Rev. 2000, 52, 63–89. [Google Scholar]
- Lia, A.; Henriques, V.J.; Zonta, M.; Chiavegato, A.; Carmignoto, G.; Gómez-Gonzalo, M.; Losi, G. Calcium Signals in Astrocyte Microdomains, a Decade of Great Advances. Front. Cell. Neurosci. 2021, 15, 177. [Google Scholar] [CrossRef]
- Arizono, M.; Inavalli, V.V.G.K.; Panatier, A.; Pfeiffer, T.; Angibaud, J.; Levet, F.; Ter Veer, M.J.T.; Stobart, J.; Bellocchio, L.; Mikoshiba, K.; et al. Author Correction: Structural basis of astrocytic Ca2+ signals at tripartite synapses. Nat. Commun. 2020, 11, 1906. [Google Scholar] [CrossRef] [Green Version]
- Fuxe, K.; Borroto-Escuela, D.O.; Romero-Fernandez, W.; Palkovits, M.; Tarakanov, A.O.; Ciruela, F.; Agnati, L.F. Moonlighting Proteins and Protein–Protein Interactions as Neurotherapeutic Targets in the G Protein-Coupled Receptor Field. Neuropsychopharmacology 2013, 39, 131–155. [Google Scholar] [CrossRef] [Green Version]
- Hiller, C.; Kühhorn, J.; Gmeiner, P. Class A G-Protein-Coupled Receptor (GPCR) Dimers and Bivalent Ligands. J. Med. Chem. 2013, 56, 6542–6559. [Google Scholar] [CrossRef] [PubMed]
- Daniels, D.J.; Lenard, N.R.; Etienne, C.L.; Law, P.-Y.; Roerig, S.C.; Portoghese, P.S. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc. Natl. Acad. Sci. USA 2005, 102, 19208–19213. [Google Scholar] [CrossRef] [Green Version]
- Javanainen, M. Universal Method for Embedding Proteins into Complex Lipid Bilayers for Molecular Dynamics Simulations. J. Chem. Theory Comput. 2014, 10, 2577–2582. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Espigares, I.; Torrens-Fontanals, M.; Tiemann, J.K.S.; Aranda-García, D.; Ramírez-Anguita, J.M.; Stepniewski, T.M.; Worp, N.; Varela-Rial, A.; Morales-Pastor, A.; Medel-Lacruz, B.; et al. GPCRmd uncovers the dynamics of the 3D-GPRCome. Nat. Methods 2020, 17, 777–787. [Google Scholar] [CrossRef]
- Manev, H.; Uz, T.; Manev, R. Glia as a putative target for antidepressant treatments. J. Affect. Disord. 2003, 75, 59–64. [Google Scholar] [CrossRef]


| Glial Cell Population | Receptor Complex | Cellular Localization | Reference |
|---|---|---|---|
| Astrocytes | A2A–D2 | Striatal astrocyte processes | [81,82,83] |
| CB2–GPR55 | Plasma membrane | [84] | |
| FGFR1–5HT1A | Plasma membrane | [85] | |
| GABAB–SSTR4 (probable) | Cortical astrocyte processes | [86] | |
| A1–A2A | Plasma membrane | [87] | |
| 5HT1A–D2 | Mainly cell soma | [88] | |
| mGluR3–mGluR5 (putative) | Not reported | [89] | |
| A1–P2Y1 | Plasma membrane | [90] | |
| Microglia | P2X4–P2X7 | Plasma membrane | [91] |
| CB1–CB2 | Plasma membrane | [92,93] | |
| A2A–CB2 | Plasma membrane | [94] | |
| GPR18–CB2 | Plasma membrane | [95] | |
| Myelinating cells | GABAB1–GABAB2 | OPC–neuron contacts | [96] |
| erb2–erb3 | Not reported | [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guidolin, D.; Tortorella, C.; Marcoli, M.; Cervetto, C.; Maura, G.; Agnati, L.F. Receptor–Receptor Interactions and Glial Cell Functions with a Special Focus on G Protein-Coupled Receptors. Int. J. Mol. Sci. 2021, 22, 8656. https://doi.org/10.3390/ijms22168656
Guidolin D, Tortorella C, Marcoli M, Cervetto C, Maura G, Agnati LF. Receptor–Receptor Interactions and Glial Cell Functions with a Special Focus on G Protein-Coupled Receptors. International Journal of Molecular Sciences. 2021; 22(16):8656. https://doi.org/10.3390/ijms22168656
Chicago/Turabian StyleGuidolin, Diego, Cinzia Tortorella, Manuela Marcoli, Chiara Cervetto, Guido Maura, and Luigi F. Agnati. 2021. "Receptor–Receptor Interactions and Glial Cell Functions with a Special Focus on G Protein-Coupled Receptors" International Journal of Molecular Sciences 22, no. 16: 8656. https://doi.org/10.3390/ijms22168656
APA StyleGuidolin, D., Tortorella, C., Marcoli, M., Cervetto, C., Maura, G., & Agnati, L. F. (2021). Receptor–Receptor Interactions and Glial Cell Functions with a Special Focus on G Protein-Coupled Receptors. International Journal of Molecular Sciences, 22(16), 8656. https://doi.org/10.3390/ijms22168656








